Abstract
The cattle tick Rhipicephalus microplus is a hematophagous ectoparasite that causes important economic losses in livestock. Different species of ticks harbor a symbiont bacterium of the genus Coxiella. It was showed that a Coxiella endosymbiont from R. microplus (CERM) is a vertically transmitted mutualist symbiont, comprising 98% of the 16S rRNA sequences in both eggs and larvae. Sequencing of the bacterial genome revealed genes for biosynthetic pathways for several vitamins and key metabolic cofactors that may provide a nutritional complement to the tick host. The CERM was abundant in ovary and Malpighian tubule of fully engorged female. Tetracycline treatment of either the tick or the vertebrate host reduced levels of bacteria in progeny in 74% for eggs and 90% for larvae without major impact neither on the reproductive fitness of the adult female or on embryo development. However, CERM proved to be essential for the tick to reach the adult life stage, as under antibiotic treatment no tick was able to progress beyond the metanymph stage. Data presented here suggest that interference in the symbiotic CERM-R. microplus relationship may be useful to the development of alternative control methods, highlighting the interdependence between ticks and their endosymbionts.
Introduction
The recent explosive growth of microbiome investigations have revealed an unsuspected dimension of the metazoan-associated microbial world, establishing the understanding of symbiotic interactions and their impact on host physiology as central issues in integrative biology. Mutualistic symbionts can provide nutrients for the host, defense against pathogens or natural enemies, and insecticide resistance1–4. Furthermore, the interaction with their invertebrate hosts has emerged as a potential target for arthropod control5,6.
Ticks are blood-sucking parasites of humans, pets, livestock and wildlife. They harbor a wide variety of microorganisms, such as bacteria, viruses and protozoa7,8. The cattle tick Rhipicephalus (Boophilus) microplus is responsible for large economic losses in livestock due to skin damage, decreases in animal weight gain and milk production, and transmission of pathogens9. Since the end of the 19th century, tick control has relied on acaricides10, which have lost efficiency due to the spread of resistance, justifying the search for alternative control methods11,12.
R. microplus is a one-host tick, presenting a free-living stage and a parasitic stage that lives on the host body. The ectoparasite feeds and molts twice on the same host (from larva to nymph and from nymph to adult) in a period that takes about three weeks. After mating, the adult female starts the slow feeding phase, when tick swallows moderate amounts of blood (about 5 days). The rapid engorgement phase takes about 2 days where a massive amount of blood is ingested13. After completing the blood meal, the fully engorged female detaches from the cattle host and performs the oviposition in the soil. Finished egg laying, the female dies and the hatched larva parasites a new host.
Several tick species have been shown to harbor pathogenic and non-pathogenic bacteria that are closely related to the Coxiella genus. The non-pathogenic bacterium (referred to as Coxiella endosymbiont, CE) were identified in ticks from the genera Rhipicephalus, Amblyomma, Haemaphysalis, Ornithodoros, Argas and Carios 14–20, being the most common maternally transmitted symbiont in ticks21. In some species, however, other bacterial species, such as Midichloria and Francisella, apparently have successfully replaced the CE21. Zhong et al.22 showed that treatment with antibiotics eliminated the CE from Amblyomma americanum, and compromised reproductive success by reducing hatching of the larvae. A genomic analysis of this symbiont showed that it forms a monophyletic clade with the pathogenic bacterium Coxiella burnetii 23. Despite sharing a common ancestor, very large genomic differences exist between these two organisms, with the symbiont showing a highly-reduced genome, which is less than one-third the size of the genome of C. burnetii. This work also revealed that the CE from A. americanum is a potential source of B complex vitamins and cofactors24.
Here, it was investigated the interaction between a CE and R. microplus. Tetracycline treatment of either the tick or the vertebrate host showed that Coxiella sp. is a mutualistic vertically transmitted symbiont that is essential to tick physiology. Coxiella endosymbiont from R. microplus (CERM) is crucial for the maturation of the metanymph to the adult stage, which indicated that the interaction between the CERM and R. microplus has a role in tick physiology that is distinct from conclusions of previous studies on CE-tick relationship. It is suggested that disruption of the symbiotic relationship between the CE and R. microplus is a target that may be used in the development of alternative control methods for this ectoparasite.
Results
A CERM is the predominant bacterium in R. microplus egg and larva
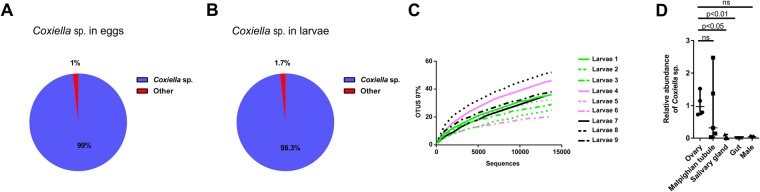
A 16S rRNA survey performed with R. microplus eggs revealed that ninety-five sequences out of ninety-six clones sequenced were identical and corresponded to a bacterium belonging to the genus Coxiella (Fig. 1A). To identify the bacteria found in newly hatched R. microplus larvae, a 16S rRNA survey was performed using Illumina technology, which showed that a single OTU (operational taxonomic unit) classified as a Coxiella sp. accounted for 98.3% of the reads from larval samples (Fig. 1B). The rarefaction curve (Fig. 1C) confirmed that sequencing coverage was sufficiently extensive to fully evaluate the microbial diversity. Therefore, these results reveal that the Coxiella species found in R. microplus eggs and larvae (CERM) is a vertically transmitted symbiont.
Figure 1.
CERM in R. microplus. (A) Bacterial genera in R. microplus eggs; (B) Bacterial genera in R. microplus larvae. The “Other” group includes Enterobacter (0.4%), Nocardiopsis (0.4%) and Rubrobacter (0.2%). Genera with frequencies below 0.1% are not represented; (C) Rarefaction curve of the 16S rRNA gene sequences in larvae based on OTUs determined at 97% similarity; (D) Quantification of CERM in ovary, Malpighian tubule, salivary gland and gut of fully engorged females by qPCR. The adult male was also analyzed. The levels of CERM were expressed as the median with 95% of CI of five biological samples with two technical replicates in each point. Statistical analyses: Kruskal-Wallis test for (D). ns: not statistically significant.
CERM is abundant in ovary and Malpighian tubule of fully engorged female R. microplus
The abundance of CERM in the adult male, ovary, salivary gland, gut and Malpighian tubule of fully engorged females was analyzed by qPCR (Fig. 1D). The CERM was present in all tissues analyzed, but the relative abundance was higher in ovary of fully engorged females, followed by Malpighian tubule. Low levels were detected in salivary gland of adult females, but the presence of the CERM in the saliva of fully engorged females was not detectable by PCR. Very low levels were found in adult males and gut of fully engorged females.
Genomic analysis of CERM
The genome sequencing showed an apparently complete CERM genome by comparison with other Coxiellas genomes (CRt – Coxiella symbiont of Rhipicephalus turanicus, CLEAA – Coxiella symbiont of Amblyomma americanum and C. burnetii RSA 493) (Table S1). A list of CERM genes identified here possibly involved in the interaction with R. microplus is shown in Table S2, revealing the presence of biosynthetic pathways for several vitamins and key metabolic cofactors. As exceptions are the biosynthetic pathway for Thiamine (B1), which is highly degraded in CERM and CRt, while a complete set of enzymes is found in CLEAA, and the pathway for Nicotinate (B3), which is not found in CERM and CRt, but present in CLEAA. The genome from CERM, as well as the other Coxiellas the also coded for genes involved in nitrogen metabolism (Table S3), such as those for the synthesis of essential amino acids phenylalanine, tryptophan, threonine, valine, leucine and isoleucine.
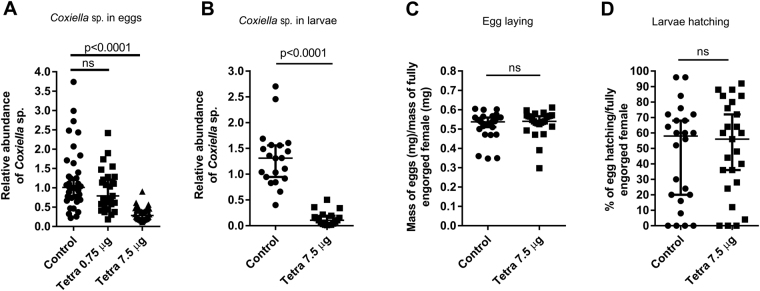
Levels of the CERM were affected in fully engorged females injected with tetracycline
Tetracycline in a dose of 0.75 µg/tick was not able to reduce the quantity of the CERM in the eggs (Fig. 2A). However, a higher dose (7.5 µg/tick) effectively reduced the levels of the bacterium in the eggs (Fig. 2A) and in the larvae (Fig. 2B) but did not affect oviposition or egg hatching (Fig. 2C and D).
Figure 2.
Reproductive fitness of fully engorged females injected with tetracycline and levels of CERM in eggs and larvae. (A) CERM in eggs by qPCR; (B) CERM in larvae by qPCR; (C) Oviposition index; D- Percentage of hatching. The levels of CERM were expressed as the median with 95% of CI of a biological triplicate with two technical replicates to each point. Statistical analyses: Kruskal-Wallis test for (A). Mann Whitney test for (B–D). ns: not statistically significant. The effect size calculated by Cohen’s d method was 2.9 (89%), −0.16 (−4%) and −0.13 (−8%) for (B–D), respectively.
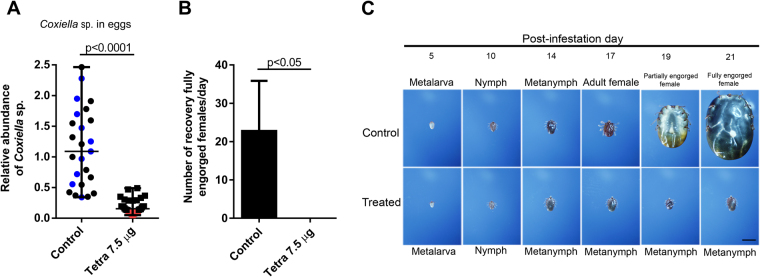
The development of larva with reduced levels of the CERM was arrested at the metanymph stage
The relative abundance of the CERM was reduced in eggs of tetracycline-treated fully engorged females compared with that in the eggs of non-treated ticks (Fig. 3A). One gram of eggs was chosen among the clutches of eggs presenting the most pronounced decrease in CERM levels after tetracycline administration (indicated by the red circles in Fig. 3A). A control group of eggs was chosen from eggs laid by control females from the same experiment (indicated by the blue circles). One head of cattle was seeded simultaneously with larvae hatched from tetracycline-treated and non-treated eggs. Each larva group was allowed to feed in a delimited space by closed cotton bags glued to the animal’s skin as described in the Methods. R. microplus development was followed through the collection of ticks at different post-infestation days (Fig. 3B and C). Ticks of both groups showed a similar time course of development until the 14th day, at the metanymph stage. After this day, metanymphs from the tetracycline-treated group did not develop further, whereas the non-treated ticks went through the regular developmental profile: appearance of the metanymphs at the 14th day and adult males and females on the 17th day, followed by full engorgement of the females by the 21st day (Fig. 3C). After the 14th day, only metanymphs were recovered in the tetracycline-treated group. These metanymphs could attach to the host, but were not able to mature further and did not molt into the adult life stage.
Figure 3.
Effect of tetracycline treatment of the tick on the development of the progeny of R. microplus: (A) Levels of CERM in eggs of fully engorged females injected with tetracycline. The selected batches of eggs whose hatched larvae were used for cattle infestation in control and treated groups are indicated in blue and red symbols, respectively; (B) Fully engorged females recovered at the 21st, 22nd, 23rd and 24th days post-infestation from the control and treated groups after infestation with 1 gram of 10-day-old larvae; (C) Phenotype of progeny collected on different days post-infestation. Scale bar: 2 mm. The levels of CERM are expressed as the median with 95% of CI of a biological triplicate with two technical replicates to each batch of eggs. Statistical analyses: Mann Whitney test for (A and B). The effect size calculated by Cohen’s d method was 2.3 (83%) for (A).
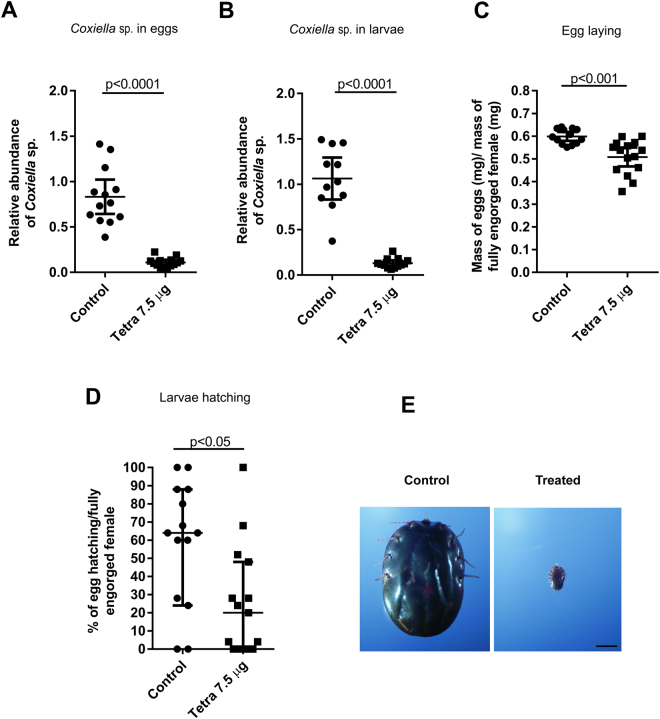
The progeny of fully engorged females fed on tetracycline-treated cattle blocked development at the metanymph stage
The results obtained with ticks injected with tetracycline, led us to hypothesize that if ticks fed on tetracycline-treated cattle, which is a situation more closely associated with the typical tick physiology, a similar effect would be achieved. One head of cattle was intramuscularly injected with three doses of 12 grams of tetracycline, applied at 12-hour intervals. This dosage was based on the treatment of fully engorged females at a dose of 7.5 µg/tick. Antibiotic levels found in both cattle blood (10.7 and 16.3 ng/ml) and in the gut of fully engorged females (7.2 and 20.3 ng/ml) were measured by mass spectrometry, confirming that effective concentrations were reached. Levels of the CERM were markedly reduced in the eggs and the larvae (Fig. 4A and B) from tick females fed on tetracycline-treated cattle. In contrast to the results obtained with the injection of the antibiotic into the tick, oviposition and hatching were also significantly reduced after treatment (Fig. 4C and D). The capacity of the larvae to develop on the host was also evaluated. One pool of larvae (hatched from one gram of eggs laid by 15 fully engorged females that were fed on tetracycline-treated cattle) was seeded onto one head of an untreated cattle. After 21 post-infestation days, the ticks were collected, and only metanymphs were found in the pool taken from the tetracycline-treated cattle, which agrees with the results shown in Fig. 4C and E). As a control, a head of an untreated cattle was seeded with larvae hatched from eggs laid by non-treated females, which, as expected, completed their life cycle on the 21st post-infestation day, when fully engorged females were recovered (Fig. 4E).
Figure 4.
Levels of CERM and reproductive fitness of fully engorged females fed on cattle treated with tetracycline. (A) CERM levels in eggs by qPCR; (B) CERM levels in larvae by qPCR; (C) Oviposition index; (D) Percentage of hatching; (E) Phenotype of progeny from treated and non-treated females collected on 21st day post-infestation. Scale bar: 2 mm. The levels of CERM were expressed as the mean with 95% of CI for (A) (B and C) and as the median with 95% of CI for (D) of a biological triplicate with two technical replicates to each point. Statistical analyses: t test for (A–C) and Mann Whitney test for (D). The effect size calculated by Cohen’s d method was 3.4 (87%), 3.1 (87%), 2 (15%) and 1.1 (57%) for (A–D), respectively.
Discussion
Although it is generally accepted that most arthropods are infected with heritable bacteria, in most cases the physiological role of these bacteria on their host’s biology has not been determined25. Maternally transmitted CE bacteria were identified in different tick species8,16,20,26,27. Here, using a 16S rRNA survey, we identified a CE in the cattle tick R. microplus as a dominant bacterium in eggs and larvae. One previous communication reported that R. microplus did not have a CE28, but the failure to detect this bacterium was probably due to the use of an PCR reaction based on a CE from Amblyomma cajennense. The bacterium found in our study is from the same genus found in R. microplus ovary by Andreotti et al.19, who speculated that the bacterium could be maternally transmitted, which has been experimentally demonstrated here and also by Duron et al.23.
Arthropods that feed exclusively on vertebrate host blood subsist in an unbalance diet, that although extremely rich in some nutrients, such as iron and amino acids, has a very low concentration of essential micronutrients, such as vitamins29,30. Symbionts can provide a nutritional complement to hosts as showed to the obligate hematophagous. Tsetse fly harbor the bacterium Wigglesworthia glossinidia that supplies vitamins that are lacking in the strictly hematophagous diet of the insect31. The presence in the CERM genome of a full complement of enzymes needed to synthesize several vitamins and enzyme cofactors supports the hypothesis that R. microplus obtain these molecules from the endosymbiont, as suggested for the Coxiella symbiont of A. americanum (CLEAA) and R. turanicus (CRt)24,32. The biosynthetic pathways for the vitamins Biotin (B7), Riboflavin (B2), Pyridoxine (B6), Folic acid (B9) and Pantothenate (B5) were found in all analyzed CEs. The Thiamine (B1) pathway is present is CLEAA but is highly degraded in CERM and CRt, while the Nicotinate (B3) is present in CLEAA but is not found in CERM and CRt. The coding genes for the cofactors Flavin adenine dinucleotide (FAD) and Coenzyme A (CoA) were found in all analyzed CLEs, while the pathway for biosynthesis of Nicotinamide adenine dinucleotide phosphate (NADP+) is degraded in the genomes of CERM and CRt, but present in CLEAA.
An early event in the evolution of metazoans was the loss of most of essential genes from the biosynthetic pathways for essential amino acids33. Some bacteria are able to provide essential amino acids to their hosts, as showed for the whitefly mutualistic symbiont, Candidatus Portiera aleyrodidarum 34. CERM genome also encodes genes involved in the synthesis of essential amino acids. Ticks feed large volumes of blood and the high protein content of vertebrate blood in a first glance makes it seems unlikely that providing additional amino acids would be of any physiological relevance for this ectoparasite. However, the initial developmental stages of the tick, the larva, metalarva, nymph and metanymph feed much lower volumes of blood than the following stages, and the data presented here indicate that the critical contribution of CERM occurs at some point before the tick starts to ingest massive amounts of blood. Therefore, an interesting possibility that deserves further investigation is that providing an extra supply of essential amino acids in early developmental stages might be one of the contributions of the CEs to the tick host.
Clearance of the bacteria from an arthropod is a common approach to interfere in host-symbiont interactions when testing for effects on host physiology. Tetracycline administration, either by injection into the tick hemocoel or through treatment of the vertebrate host decreased the CERM levels and demonstrated the mutualistic character of this symbiotic association. Both treatments resulted in the interruption of tick development at the metanymph stage, indicating a critical contribution by the bacterium shortly before the metanymph molting to the adult life stage. Although both treatments had a similar effect in the reduction of CERM levels, only the females that were fed on treated cattle showed a significant inhibition of oviposition and hatching. It is possible that the exact moment when the females were exposed to tetracycline dose has affected the results. Ticks fed on treated cattle received antibiotic in an earlier life stage when comparing with the tick injection that were performed in the fully engorged female. Thus, it is plausible that different aspects of the female physiology may be affected not just by number of CERM, but also by the moment when bacteria were eliminated. Different from our findings, in A. americanum, a lower but comparable dose of tetracycline injected in the hemocoel led to a decrease in oviposition and hatching22. However, the decrease in CE levels attained in that report was more pronounced than that obtained here. Therefore, it is possible that CERM has a different physiological role in R. microplus when compared to CE from A. americanum, or that the observed effects are dependent on the amount of bacteria remaining after antibiotic treatment. It is known that tetracycline acts through binding to the bacterial ribosome inhibiting protein synthesis. However, at high doses the antibiotic can also be toxic to animal cells. Although it was not possible to completely eliminate the CERM, it is likely that the interruption of the tick development was due to the reduced level of CERM and not to a toxic effect of tetracycline on tick cells, because the effects of the antibiotic were observed not in the adult female submitted to the treatment, but in its progeny.
As tissue distribution of the symbiont might be indicative of its role in the biology of the arthropod host, we investigated its levels in R. microplus. In our study, the CERM was identified in males as well as in several organs of fully engorged females. It was also found in egg and larva, showing the occurrence of transovarial transmission. The identification of a CERM in ovary is in accordance with other reports of heritable symbionts, where colonization of the reproductive tissue was shown to allow infection of the offspring2. Among all organs, ovary presented the higher levels of CERM, as has been shown in R. turanicus 35. The CERM was also identified in high levels in Malpighian tubule, which is in accordance with Lalzar et al.27 that showed the presence of a Coxiella sp. in R. turanicus and Klyachko et al.36 that found a bacterium from the same genus in A. americanum Malpighian tubule. The identification of the bacterium in Malpighian tubule suggest a role of CERM in the metabolism of nitrogen. The presence in salivary gland is also in agreement with the findings reported for other tick species28,36,37. The Ixodes ricinus symbiont Midichloria mitochondrii was found in the saliva, and antibodies against the bacteria were found in the sera of humans and animals exposed to tick bites38. However, although the CERM was found in the salivary gland, through the methodology used in this study it was able to show that the bacterium was not present in saliva of fully engorged female. The presence of bacterium in the salivary gland also raises the possibility that another physiological role for the CERM could be related to the development of the salivary gland, which might explain the blockage at the metanymph stage since tick saliva contains molecules with anti-inflammatory, immunosuppressive and anti-hemostatic properties that are essential for blood feeding39. Very low levels of CERM were identified in adult male, reinforcing the hypothesis of maternal transmission of CERM.
The reduction of the CERM in egg and larva of fully engorged females fed on tetracycline-treated cattle showed that the antibiotic circulating in the blood was able to cross the tick’s gut barrier and reach the hemolymph. The commercial use of antibiotics to control parasites is probably not feasible due to environmental and public health concerns, as an expected outcome would be the selection of resistant bacterial strains40. However, the comprehension of the role of the CERM in R. microplus biology could lead to alternative control methods that directly target the symbiont or tick proteins involved in the interaction with the bacterium8. Furthermore, blocking tick development at this early stage prevents most of the damage to cattle caused by massive blood loss that occur after a rapid engorgement phase that follows the metanymph stage, probably also limiting the transmission of pathogens to the next generations. Several groups are pursuing a vaccine against R. microplus using tick proteins as antigens and high levels of protection of the host against ticks have been achieved12,41. The description of the genome of CERM showed that the bacterium is a vitamin and cofactor provider, as well as other CEs. Description of the CERM genome together with the genomes of CE from A. americanum and R. turanicus provides useful information for understanding how Coxiella sp. bacteria interact with their hosts24,32. In this juncture, the data provided here suggest that the microbiota or tick molecules involved in the interaction with the mutualistic symbiont could be considered as a promising targets to vaccine development42.
Methods
R. microplus strain
R. microplus ticks (Porto Alegre strain) were maintained under laboratory conditions, through in Hereford cattle obtained from a naturally tick-free area43,44. The colony was kept isolated, without the introduction of wild ticks. All animals were housed in individual tick-proof pens on slatted floors. During the experiments, fully engorged females, eggs and larvae were kept under laboratory conditions in an incubator at 28 °C and 80% relative humidity. All animal care and experimental protocols were conducted following the guidelines of the institutional care and use committee (Ethics Committee on Animal Experimentation of the Federal University of Rio Grande do Sul) and were approved under the registry 14403/protocol 07.
16S rRNA survey and analysis from R. microplus eggs
A pool of 30 mg of newly laid eggs from R. microplus had the surface sterilized with 70% ethanol, followed by a washing with sterile water. The sample was homogenized in PBS followed by gDNA extraction and library construction45. Ninety-six clones from the 16S rRNA library were sequenced by capillary electrophoresis on a MegaBace 1000 DNA analysis system (GE Healthcare, Little Chalfont, UK). The partial 16S rRNA sequences (880-bp) were analyzed with the BLAST tool against the GenBank database. The partial CERM 16S rRNA gene sequence was deposited in GenBank under the accession number KT726373.
16S rRNA survey and analysis from R. microplus larvae
Nine pools of larvae hatched from 30 mg of eggs laid by three fully engorged females each were washed once in 70% ethanol and twice in sterile water. The larvae were homogenized in sterile water, and DNA was extracted from 100 µl of the homogenate with the Power Soil DNA Isolation Kit (MO BIO, Carlsbad, CA, EUA). PCR was performed with the AccuPrime Taq DNA Polymerase High Fidelity (Invitrogen, Carlsbad, CA, EUA) using primers targeted to the V4 region of the 16S rRNA gene in a dual-index strategy46. The amplicons were purified with PureLink Quick Gel Extraction & PCR Purification Combo kit Fidelity (Invitrogen, Carlsbad, CA, EUA). Equimolar concentrations of the nine purified PCR products were sequenced on an Illumina MiSeq platform. Fastq paired-end reads were assembled at the RDP pipeline website (version 11. 4) using their extended version of PANDAseq with a minimum read Q score of 25, sequence length between 240 and 275 bp and no ambiguities as parameters47. The assembled amplicon sequences were checked for chimeras using the UCHIME tool48 on the Fungene pipeline of the RDP website. After assembly and the first chimera check, samples were processed with Mothur following the MiSeq SOP46,49. Sequences were aligned to the SILVA bacterial 16S rRNA reference alignment, and those that did not align were discarded50. Unique sequences were identified and a preclustering step was performed to further reduce the sequencing errors obtained from clustering sequences with up to two nucleotide differences51. The resulting sequences were screened for chimeras using UCHIME. Sequences were then classified against the Mothur-formatted version of the RDP 16S rRNA gene training set (version 9) with a confidence score of 80%. Sequences that classified as Archea, Eukarya, chloroplast or mitochondria were excluded from further analysis. OTUs were assigned at a 3% dissimilarity level, and richness estimates were performed.
Genome sequencing and analysis of CERM
Genomic DNA was extracted from five ovaries of fully engorged R.microplus adult females, using DNeasy Blood & Tissue Kit from Quiagen (32.9 ng/uL) (Hilden, NRW, DE) and sequenced using the Illumina MiSeq platform (Paired-end Nextera XT library). The data comprised nearly 8 × 106 paired-end reads of 500 bp. Total raw reads were subjected to trimming using Trimmomatic version 0.3352 and filtered after mapping against the complete genome of Coxiella endosymbiont of R. turanicus (CRt - NZ_CP011126) and Coxiella endosymbiont of A. americanum (CLEAA - NZ_CP007541.1) using Bowtie 2 (version 2.2.3)53 to obtain a smaller set of 99232 paired-end reads. Flash (version 1.2.11)54 was used to merge pairs of mapped raw reads. The short sequence patterns in genomic data analysis (k-mer analysis) revealed a genome coverage of nearly 32X. Celera Assembler version 8.3rc2 was used for de novo assembly55 followed by the SPAdes genome assembler version 3.5.0 using the Celera resulting output as trusted contigs56. Further, a gap filling step (IMAGE tool from suite PAGIT, version 2.4)57 was performed using the whole set of original reads (8 × 106 paired-end reads), resulting into 215 contigs, after the elimination of less than 200 bps length ones. Additionally, to get rid of potential tick contamination, BLASTN (version 2.2.26+)58 was used for local alignment against Rhipicephalus microplus (LYUQ01000000). Finally, 171 contigs were obtained, which were submitted to NCBI GenBank (SRR5860283). The completeness of the assembled genome was inspected using a single-copy gene database59, and by comparison with CRt, CLEAA and C. burnetii RSA 493 (NC_002971.4), using the same single-copy gene analysis. The curated contigs were annotated using the RAST Server60–62.
Tetracycline treatment of ticks
Groups of 10 fully engorged females (average 250 mg/tick) were washed once in 70% ethanol and twice in sterile water. Tetracycline hydrochloride (Merck, Darmstadt, HE, DEU) was administered in sterile 137 mM NaCl, 2.7 mM KCl, 4.3 mM sodium phosphate, and 1.4 mM potassium phosphate, pH 7.0 (PBS) at concentrations of 0.75 µg/µl and 7.5 µg/µl, based on the treatment of Q-fever in humans (30 mg/kg/day)63. The injection (1 µl of tetracycline at concentrations described above or PBS in control group) was performed with a micro-syringe (Hamilton - 33-gauge needle) into the hemocoel by the ventral surface of females in the pre-oviposition period on the 2nd day after natural detachment. The needle was left for one minute inside the tick before removal, allowing the antibiotic to be distributed through the hemolymph.
Vertebrate host treatment
On the 19th and 20th days post-infestation with 1 gram of ten-day old larvae, the cattle (approximately 400 kg) received intramuscular applications of three 12 gram doses of a veterinary use solution of 100 mg/ml tetracycline hydrochloride - Solutetra (Ibasa, Porto Alegre, RS, BRA), administered at intervals of 12 hours. The concentration of tetracycline used in the cattle was based on the treatment of fully engorged females at a concentration of 7.5 µg/µl.
Tetracycline quantification in cattle blood and the gut of fully engorged females
In the treatment of cattle with tetracycline, the levels of the antibiotic in the cattle blood and in the gut of fully engorged females that were fed on the animal were quantified by tandem mass spectrometry. EDTA was added to the samples and the samples were extracted with acetonitrile. Two samples of cattle blood were collected: one 4 hours after the first administration of the antibiotic and the other at the end of the treatment. In addition, two guts were collected after the natural detachment of the fully engorged females at the end of the cattle treatment.
Sample collection for RNA isolation and cDNA synthesis
After tetracycline treatment, fully engorged females were kept in an incubator until oviposition completion. The eggs laid on the 1st day were weighed and collected to quantify the levels of CERM. After five days of embryonic development, the eggs were macerated in TRIzol reagent (Invitrogen, Carlsbad, CA, EUA) for RNA extraction. The eggs laid on the 2nd day were weighed and maintained at incubation until hatching. Larvae at the 10th day after hatching were further macerated to quantify the levels of CERM.
Fully engorged females (230–300 mg; average 250 mg/tick) and adult males were collected after natural detachment or were manually removed from the cattle, respectively. Tick female dissections were performed under a microscope in sterile PBS. After the removal of the dorsal cuticle with dissecting scissors, the specific organs were isolated with fine-tipped forceps. Ovary, Malpighian tubule, gut and the salivary gland of fully engorged females were macerated in TRIzol (Invitrogen, Carlsbad, CA, EUA). Each ovary, Malpighian tubule and gut sample contained two organs each, whereas salivary gland samples represented a pool of four organs. Each male sample contained a single individual.
RNA concentration and purity of all samples were determined on a NanoDrop spectrophotometer. The cDNA was synthesized from equal amounts (1 µg) of total RNA using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Waltham, MA, EUA).
Saliva collection for gDNA extraction
Saliva was collected from 30 fully engorged females after administration of 5 µl of 2% pilocarpine (Merck, Darmstadt, HE, DEU) in PBS on the ventral surface. The sample was collected in Tris-EDTA, pH 8.0 and loaded with 10 µg of salmon sperm DNA (Sigma-Aldrich, St. Louis, MO, EUA). gDNA extraction was performed using the phenol-chloroform method64 and its concentration and purity were determined using a NanoDrop spectrophotometer.
CERM relative abundance analysis
The CERM relative abundance was measured in samples with a primer pair (forward-5′TTCGGTGGGAAAGAAAGTTTC3′; reverse-5′TAGGGCTTTCACATTCGACTTAAAT3′) specific for the 16S rRNA gene sequence (KT726373) of the CERM that was identified in 16S rRNA survey from eggs. R. microplus 40 S ribosomal gene (EW679928) was used as a reference gene for data normalization after amplification with specific primers (forward-5′GGACGACCGATGGCTACCT3′; reverse 5′TGAGTTGATTGGCGCACTTCT3′)65. The identity of the amplicons was confirmed by Sanger sequencing. qPCR was carried out on a 7500 Real-Time PCR System (Applied Biosystems, Waltham, MA, EUA) with 40 cycles of 95 °C (15 s) and 60 °C (45 s) following an initial denaturation of 95 °C (10 min). A melting curve was generated to confirm the identity of the amplicons. Each 15 µl reaction mixture contained 7.5 µl of 1x Power SYBR Green PCR Master Mix (Applied Biosystems, Waltham, MA, EUA), 3.5 µM of each primer, and 5 µl of cDNA 10x diluted in sterile water. Relative abundance was analyzed by the comparative Ct method66.
Analysis of the presence of CERM
The detection of the CERM in the saliva of fully engorged females was performed by PCR. Each 15 µl reaction mixture contained 7.5 µl of PCR Master Mix (Fermentas, Waltham, MA, EUA), 10 µM of the CERM 16S rRNA gene primers and 1 µl of gDNA in sterile water. The gDNA of salivary glands of fully engorged females was used as a positive control. The reaction was carried out in a 96-well thermal cycler (Applied Biosystems, Waltham, MA, EUA) with 35 cycles of 95 °C (30 s), 60 °C (30 s) and 72 °C (30 s) with an initial denaturation of 95 °C (5 min) and a final incubation of 70 °C (10 min). The amplicon (178 bp) was visualized using 1.5% agarose gel electrophoresis.
Evaluation of the effect of antibiotic on reproductive fitness
The ratio between the weight of the pre-oviposition fully engorged females and the total mass of the eggs laid after tetracycline treatment was used as an oviposition index. To determine the percentage of egg hatching, 25 eggs from each fully engorged female were randomly selected following oviposition completion. After egg hatching, the larvae and infertile eggs were frozen and counted under a microscope.
Effect of antibiotic on larvae viability
Ten-day-old larvae hatched from eggs of tetracycline-treated and non-treated tick females were used to simultaneously infest a head of cattle through two cloth bags glued to animal skin on the lumbar region67. Ticks were collected at the 5th, 10th, 14th, 17th, 19 th, 21st, 22nd, 23rd and 24th days post-infestation and fixed with 4% paraformaldehyde in 100 mM cacodylate buffer, pH 7.4. Tick life stages were determined using a microscope, and images were acquired with an Olympus DP72 digital camera.
Ten-day-old larvae hatched from one gram of eggs were used to infest one tetracycline-treated and one untreated head of cattle, and fully engorged females were collected after natural detachment. Subsequently, the larvae obtained from the eggs laid by these females were used to infest new, untreated cattle. On the 21st day post-infestation, ticks from both cattle were collected, fixed and examined as described above.
Statistical analysis
All the data were tested for normality using Shapiro-Wallis test. The differences among three or more groups were determined using Kruskal-Wallis test. Other comparisons were performed using Mann Whitney test or two-tailed t-test depending on gaussian distribution. The values were statistically significant when p < 0.05. All statistical calculations were performed using GraphPad Prism software (version 7) except the effect size, which was calculated as the Cohen’s d68.
Data Availability
The partial 16S rRNA gene sequences from larvae were deposited in GenBank under the accession codes SRR2681092, SRR2681676, SRR2681677, SRR2681678, SRR2681680, SRR2681682, SRR2681683, SRR2681684 and SRR2681685. The partial CERM 16S rRNA gene sequence from eggs was deposited in GenBank under the accession number KT726373. The genome of CERM were deposited in GenBank under the accession code NSHJ01000000.
Electronic supplementary material
Acknowledgements
We thank Fabiano Barreto from the Laboratório de Análise de Resíduos de Pesticidas e Medicamentos Veterinários do Ministério da Agricultura, Pecuária e Abastecimento for tetracycline measurements in cattle blood and tick samples. We are grateful to C. Cosme, N. Hahn, P. Camargo and S. R Cássia for technical assistance. Sequencing services using MiSeq for CERM genome were performed at INTA, Consorcio Argentino de Tecnología Genómica (CATG) funded by grant PPL Genómica, MINCYT; AECI A1/041041/11. CERM genome assembly used HPC resources from BioCAD-INTA, CATG-MINCYT, PNBIO 1131043, Unidad de Bioinformática, Instituto de Biotecnología, CICVyA, INTA. This work was supported by INCT-Molecular Entomology, CNPq, CAPES, FAPERJ and FAPERGS.
Author Contributions
M.G. conducted the experiments, analyzed the results and wrote the main manuscript text; L.F.P., L.T. and D.P.O. conducted the experiment of cattle treatment with tetracycline; R.D.N. and R.M.A. conducted the experiments of 16S rRNA survey of larvae; R.S. analyzed the results of 16S rRNA survey of larvae; R.P.V. conducted the experiment and analyzed the results of 16S rRNA survey of eggs, W.H.C.O. and M.S.L. conducted the experiment of 16S rRNA survey of eggs; M.F. and S.A.G. conducted the experiment and analyzed the results of genome sequencing of CERM; O.B.M. conceived the experiments of 16S rRNA survey of eggs and analyzed the results; I.S.V. and P.L.O. conceived the experiments, analyzed the results and wrote the main manuscript text. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-17309-x.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Brownlie JC, Johnson KN. Symbiont-mediated protection in insect hosts. Trends in Microbiology. 2009;17:348–354. doi: 10.1016/j.tim.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Hosokawa T, Koga R, Kikuchi Y, Meng X-Y, Fukatsu T. Wolbachia as a bacteriocyte-associated nutritional mutualist. Proc. Natl. Acad. Sci. 2009;107:769–774. doi: 10.1073/pnas.0911476107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaenike J, Unckless R, Cockburn SN, Boelio LM, Perlman SJ. Adaptation via Symbiosis: Recent Spread of a Drosophila Defensive Symbiont. Science (80-.). 2010;329:212–215. doi: 10.1126/science.1188235. [DOI] [PubMed] [Google Scholar]
- 4.Kikuchi Y, et al. Symbiont-mediated insecticide resistance. Proc. Natl. Acad. Sci. 2012;109:8618–8622. doi: 10.1073/pnas.1200231109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ricci I, et al. Symbiotic control of mosquito borne disease. Pathog. Glob. Health. 2012;106:380–5. doi: 10.1179/2047773212Y.0000000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sassera D, Epis S, Pajoro M, Bandi C. Microbial symbiosis and the control of vector-borne pathogens in tsetse flies, human lice, and triatomine bugs. Pathog. Glob. Health. 2013;107:285–92. doi: 10.1179/2047773213Y.0000000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parola P, Raoult D. Tick-borne bacterial diseases emerging in Europe. Clin. Microbiol. Infect. 2001;7:80–83. doi: 10.1046/j.1469-0691.2001.00200.x. [DOI] [PubMed] [Google Scholar]
- 8.Narasimhan S, Fikrig E. Tick microbiome: The force within. Trends in Parasitology. 2015;31:315–323. doi: 10.1016/j.pt.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grisi L, et al. Reassessment of the potential economic impact of cattle parasites in Brazil. Braz. J. Vet. Parasitol., Jaboticabal. 2014;23:150–156. doi: 10.1590/S1984-29612014042. [DOI] [PubMed] [Google Scholar]
- 10.George JE, Pound JM, Davey RB. Chemical control of ticks on cattle and the resistance of these parasites to acaricides. Parasitology. 2004;129(Suppl):S353–S366. doi: 10.1017/S0031182003004682. [DOI] [PubMed] [Google Scholar]
- 11.Abbas RZ, Zaman MA, Colwell DD, Gilleard J, Iqbal Z. Acaricide resistance in cattle ticks and approaches to its management: The state of play. Veterinary Parasitology. 2014;203:6–20. doi: 10.1016/j.vetpar.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Parizi LF, et al. Multi-antigenic vaccine against the cattle tick Rhipicephalus (Boophilus) microplus: A field evaluation. Vaccine. 2012;30:6912–6917. doi: 10.1016/j.vaccine.2012.08.078. [DOI] [PubMed] [Google Scholar]
- 13.Roberts JA. Resistance of Cattle to the Tick Boophilus microplus (Canestrini). I. Development of Ticks on Bos taurus. Allen Press Am. Soc. Parasitol. 1968;54:663–666. [PubMed] [Google Scholar]
- 14.Bernasconi MV, Casati S, Péter O, Piffaretti JC. Rhipicephalus ticks infected with Rickettsia and Coxiella in Southern Switzerland (Canton Ticino) Infect. Genet. Evol. 2002;2:111–120. doi: 10.1016/S1567-1348(02)00092-8. [DOI] [PubMed] [Google Scholar]
- 15.Mediannikov O, et al. Molecular Evidence of Coxiella -like Microorganism Harbored by Haemaphysalis concinnae Ticks in the Russian Far East. Ann. N.Y. Acad. Sci. 2003;990:226–228. doi: 10.1111/j.1749-6632.2003.tb07367.x. [DOI] [PubMed] [Google Scholar]
- 16.Reeves WK, et al. Molecular and biological characterization of a novel Coxiella-like agent from Carios capensis. Ann. N. Y. Acad. Sci. 2005;1063:343–345. doi: 10.1196/annals.1355.055. [DOI] [PubMed] [Google Scholar]
- 17.Jasinskas A, Zhong J, Barbour AG. Highly prevalent Coxiella sp. bacterium in the tick vector Amblyomma americanum. Appl. Environ. Microbiol. 2007;73:334–336. doi: 10.1128/AEM.02009-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reeves WK. Molecular evidence for a novel Coxiella from Argas monolakensis (Acari: Argasidae) from Mono Lake, California, USA. Exp. Appl. Acarol. 2008;44:57–60. doi: 10.1007/s10493-008-9128-z. [DOI] [PubMed] [Google Scholar]
- 19.Andreotti R, et al. Assessment of bacterial diversity in the cattle tick Rhipicephalus (Boophilus) microplus through tag-encoded pyrosequencing. BMC Microbiol. 2011;11:6. doi: 10.1186/1471-2180-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Almeida AP, et al. Coxiella symbiont in the tick Ornithodoros rostratus (Acari: Argasidae) Ticks Tick. Borne. Dis. 2012;3:203–206. doi: 10.1016/j.ttbdis.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Duron O, et al. Evolutionary changes in symbiont community structure in ticks. Mol. Ecol. 2017;26:2905–2921. doi: 10.1111/mec.14094. [DOI] [PubMed] [Google Scholar]
- 22.Zhong, J., Jasinskas, A. & Barbour, A. G. Antibiotic treatment of the tick vector Amblyomma americanum reduced reproductive fitness. PLoS One2 (2007). [DOI] [PMC free article] [PubMed]
- 23.Duron, O. et al. The Recent Evolution of a Maternally-Inherited Endosymbiont of Ticks Led to the Emergence of the Q Fever Pathogen, Coxiella burnetii. PLoS Pathog. 11 (2015). [DOI] [PMC free article] [PubMed]
- 24.Smith TA, Driscoll T, Gillespie JJ, Raghavan R. A Coxiella-like endosymbiontis a potential vitamin source for the lone star tick. Genome Biol. Evol. 2015;7:831–838. doi: 10.1093/gbe/evv016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duron O, et al. The diversity of reproductive parasites among arthropods: Wolbachia do not walk alone. BMC Biol. 2008;6:27. doi: 10.1186/1741-7007-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clay K, et al. Microbial communities and interactions in the lone star tick, Amblyomma americanum. Mol. Ecol. 2008;17:4371–4381. doi: 10.1111/j.1365-294X.2008.03914.x. [DOI] [PubMed] [Google Scholar]
- 27.Lalzar I, Friedmann Y, Gottlieb Y. Tissue tropism and vertical transmission of Coxiella in Rhipicephalus sanguineus and Rhipicephalus turanicus ticks. Environ. Microbiol. 2014;16:3657–3668. doi: 10.1111/1462-2920.12455. [DOI] [PubMed] [Google Scholar]
- 28.Machado-Ferreira E, et al. Coxiella Symbionts in the Cayenne Tick Amblyomma cajennense. Microb. Ecol. 2011;62:134–142. doi: 10.1007/s00248-011-9868-x. [DOI] [PubMed] [Google Scholar]
- 29.Sterkel M, Oliveira JHM, Bottino-Rojas V, Paiva-Silva GO, Oliveira PL. The Dose Makes the Poison: Nutritional Overload Determines the Life Traits of Blood-Feeding Arthropods. Trends in Parasitology. 2017 doi: 10.1016/j.pt.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 30.Engel P, Moran NA. The gut microbiota of insects - diversity in structure and function. FEMS Microbiology Reviews. 2013;37:699–735. doi: 10.1111/1574-6976.12025. [DOI] [PubMed] [Google Scholar]
- 31.Akman L, et al. Genome sequence of the endocellular obligate symbiont of tsetse flies, Wigglesworthia glossinidia. Nat. Genet. 2002;32:402–7. doi: 10.1038/ng986. [DOI] [PubMed] [Google Scholar]
- 32.Gottlieb Y, Lalzar I, Klasson L. Distinctive genome reduction rates revealed by genomic analyses of two Coxiella- like endosymbionts in ticks. Genome Biol. Evol. 2015;7:1779–1796. doi: 10.1093/gbe/evv108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Costa IR, Thompson JD, Ortega JM, Prosdocimi F. Metazoan remaining genes for essential amino acid biosynthesis: Sequence conservation and evolutionary analyses. Nutrients. 2014;7:1–16. doi: 10.3390/nu7010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Upadhyay, S. K. et al. Whitefly genome expression reveals host-symbiont interaction in amino acid biosynthesis. PLoS One10 (2015). [DOI] [PMC free article] [PubMed]
- 35.Lalzar I, Harrus S, Mumcuoglu KY, Gottlieb Y. Composition and seasonal variation of Rhipicephalus turanicus and Rhipicephalus sanguineus bacterial communities. Appl. Environ. Microbiol. 2012;78:4110–4116. doi: 10.1128/AEM.00323-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klyachko O, Stein BD, Grindle N, Clay K, Fuqua C. Localization and visualization of a Coxiella-type symbiont within the lone star tick, Amblyomma americanum. Appl. Environ. Microbiol. 2007;73:6584–6594. doi: 10.1128/AEM.00537-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qiu, Y., Nakao, R., Ohnuma, A., Kawamori, F. & Sugimoto, C. Microbial population analysis of the salivary glands of ticks; a possible strategy for the surveillance of bacterial pathogens. PLoS One9 (2014). [DOI] [PMC free article] [PubMed]
- 38.Mariconti M, et al. Humans parasitized by the hard tick Ixodes ricinus are seropositive to Midichloria mitochondrii: is Midichloria a novel pathogen, or just a marker of tick bite? Pathog. Glob. Health. 2012;106:391–6. doi: 10.1179/2047773212Y.0000000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Francischetti IMB, Sa-Nunes A, Mans BJ, Santos IM, Ribeiro JMC. The role of saliva in tick feeding. Front. Biosci. Landmark Ed. 2009;14:2051–88. doi: 10.2741/3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Douglas AE. Symbiotic microorganisms: untapped resources for insect pest control. Trends Biotechnol. 2007;25:338–342. doi: 10.1016/j.tibtech.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 41.Ali A, et al. Immunoprotective potential of a Rhipicephalus (Boophilus) microplus metalloprotease. Vet. Parasitol. 2015;207:107–114. doi: 10.1016/j.vetpar.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 42.de La Fuente J, Kocan KM, Contreras M. Prevention and control strategies for ticks and pathogen transmission. Rev. Sci. Tech. 2015;34:249–64. doi: 10.20506/rst.34.1.2357. [DOI] [PubMed] [Google Scholar]
- 43.Reck J, et al. Systemic alterations of bovine hemostasis due to Rhipicephalus (Boophilus) microplus infestation. Res. Vet. Sci. 2009;86:56–62. doi: 10.1016/j.rvsc.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 44.Pohl PC, et al. ABC transporters as a multidrug detoxification mechanism in Rhipicephalus (Boophilus) microplus. Parasitol. Res. 2012;111:2345–2351. doi: 10.1007/s00436-012-3089-1. [DOI] [PubMed] [Google Scholar]
- 45.Vieira RP, et al. Relationships between bacterial diversity and environmental variables in a tropical marine environment, Rio de Janeiro. Environ. Microbiol. 2008;10:189–199. doi: 10.1111/j.1462-2920.2007.01443.x. [DOI] [PubMed] [Google Scholar]
- 46.Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the miseq illumina sequencing platform. Appl. Environ. Microbiol. 2013;79:5112–5120. doi: 10.1128/AEM.01043-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cole, J. R. et al. Ribosomal Database Project: Data and tools for high throughput rRNA analysis. Nucleic Acids Res. 42 (2014). [DOI] [PMC free article] [PubMed]
- 48.Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27:2194–2200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schloss PD, et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009;75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quast, C. et al. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 41 (2013). [DOI] [PMC free article] [PubMed]
- 51.Schloss, P. D., Gevers, D. & Westcott, S. L. Reducing the effects of PCR amplification and sequencing Artifacts on 16s rRNA-based studies. PLoS One6 (2011). [DOI] [PMC free article] [PubMed]
- 52.Bolger AM, Lohse M, Usadel B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Magoč T, Salzberg SL. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27:2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Myers EW, et al. A whole-genome assembly of Drosophila. Science (80-.). 2000;287:2196–204. doi: 10.1126/science.287.5461.2196. [DOI] [PubMed] [Google Scholar]
- 56.Bankevich A, et al. SPAdes: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. J. Comput. Biol. 2012;19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsai IJ, Otto TD, Berriman M. Improving draft assemblies by iterative mapping and assembly of short reads to eliminate gaps. Genome Biol. 2010;11:R41. doi: 10.1186/gb-2010-11-4-r41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–10. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 59.Albertsen M, et al. Genome sequences of rare, uncultured bacteria obtained by differential coverage binning of multiple metagenomes. Nat. Biotechnol. 2013;31:533–8. doi: 10.1038/nbt.2579. [DOI] [PubMed] [Google Scholar]
- 60.Aziz RK, et al. The RAST Server: rapid annotations using subsystems technology. BMC Genomics. 2008;9:75. doi: 10.1186/1471-2164-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Overbeek, R. et al. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res. 42 (2014). [DOI] [PMC free article] [PubMed]
- 62.Brettin T, et al. RASTtk: a modular and extensible implementation of the RAST algorithm for building custom annotation pipelines and annotating batches of genomes. Sci. Rep. 2015;5:8365. doi: 10.1038/srep08365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Raoult D. Minireview: Treatment of Q Fever. Antimicrob. Agents Chemother. 1993;37:1733–1736. doi: 10.1128/AAC.37.9.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Halos L, et al. Determination of an efficient and reliable method for DNA extraction from ticks. Vet. Res. 2004;35:709–713. doi: 10.1051/vetres:2004038. [DOI] [PubMed] [Google Scholar]
- 65.Pohl PC, et al. An extraovarian aspartic protease accumulated in tick oocytes with vitellin-degradation activity. Comp. Biochem. Physiol. - B Biochem. Mol. Biol. 2008;151:392–399. doi: 10.1016/j.cbpb.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 66.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barriga OO, da Silva SS, Azevedo JS. Inhibition and recovery of tick functions in cattle repeatedly infested with Boophilus microplus. The Journal of parasitology. 1993;79:710–5. doi: 10.2307/3283609. [DOI] [PubMed] [Google Scholar]
- 68.Maher JM, Markey JC, Ebert-May D. The other half of the story: Effect size analysis in quantitative research. CBE Life Sci. Educ. 2013;12:345–351. doi: 10.1187/cbe.13-04-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The partial 16S rRNA gene sequences from larvae were deposited in GenBank under the accession codes SRR2681092, SRR2681676, SRR2681677, SRR2681678, SRR2681680, SRR2681682, SRR2681683, SRR2681684 and SRR2681685. The partial CERM 16S rRNA gene sequence from eggs was deposited in GenBank under the accession number KT726373. The genome of CERM were deposited in GenBank under the accession code NSHJ01000000.