Abstract
Exercise-induced leg pain is a common condition in athletes and in people involved in recreational sports. The diagnosis is not always straightforward: many conditions may cause exercise-induced leg pain. The aim of the present review is to provide a complete discussion of the most common pathologies related to this condition. Particular attention is dedicated to the history and the physical examination, which are fundamental for requesting the correct diagnostic tests or imaging techniques necessary for a precise diagnosis.
Keywords: chronic exertional compartment syndrome, exercise-induced leg pain, medial tibial stress syndrome, popliteal artery entrapment syndrome
Introduction
Exercise-induced leg pain includes a broad range of conditions that involve different tissues. The conditions include the following (Table 1): (1) Bones: stress fractures, medial tibial stress syndrome, and neoplasm; (2) Muscles: chronic exertional compartment syndrome, herniae, exercise-induced rhabdomyolysis, delayed onset muscle soreness, and neoplasm; (3) Blood vessels: popliteal artery entrapment syndrome, endofibrotic disease, popliteal artery aneurysm, cystic adventitial disease, and peripheral arterial dissection; (4) Nerves: entrapment syndromes (e.g., saphenous, peroneal, and tibial nerves) and lumbar radiculopathy; (5) Tendons: tibialis anterior, tibialis posterior, peroneals, and Achilles.
Table 1.
Summary of the most common conditions causing exercise-induced leg pain.
| Tissue | Condition | Special features | Symptoms & signs | Diagnosis | Treatment |
|---|---|---|---|---|---|
| Bone | Stress fracture (SF) | More common at the posteromedial tibia; less common at the anterior tibia, medial malleolus or fibula. | Gradual onset of localized pain. The pain is elicited by activity & decreases with rest. With the progression of a fracture, pain occurs with walking or at rest; there is localized pain on palpation; pain occurs with sustained one leg hop & with percussion at a distant site. |
X-ray imaging. If X-ray imaging is negative but suspicion of a fracture is high, repeat X-ray imaging 2–3 wk later. Computed tomography (CT) scan or magnetic resonance imaging (MRI) can be obtained to expedite the diagnosis. |
Conservative treatment for low-risk fractures (e.g., posteromedial tibia & fibula): rest (for 4–8 wk), ice, & pain killers. For failed conservative treatment of high-risk fractures (e.g., anterior tibia), surgery is required: intramedullary nailing for complete shaft fractures, drilling with bone grafting in incomplete fractures. |
| Bone | Medial tibial stress syndrome (MTSS) | Also called “shin splints”; the incidence ranges 4–35% in athletic & military populations. | Pain at posteromedial tibia. The pain is cumulative with activity & persists for a long time (sometimes days) before it improves with rest (this differs from CECS in which pain subsides after minutes of rest). On physical examination, the patient has diffuse tenderness along the posteromedial tibia (this differs from localized pain in SF). |
History & physical examination are usually sufficient. X-ray imaging is usually negative; MRI usually shows medial & posteromedial periosteal oedema. The bone scan shows uptake along the posteromedial tibia on delayed-phase images; the uptake is longitudinally oriented, & one-third or more of the tibia is involved (this differs from localized uptake in SF) |
Initially conservative treatment is attempted such as rest, ice, modification of the training schedule & shoes, stretching, & strengthening. For recalcitrant cases, surgery is indicated (e.g., fasciotomy of the deep posterior compartment & release of the periosteum). |
| Muscle | Chronic exertional compartment syndrome (CECS) | CECS can involve four compartments of the leg: anterior, lateral, posterior & deep posterior. The anterior & lateral compartments are involved in 95% of patients; muscle herniae are present in 40–60% of patients, mostly at the level of superficial peroneal nerve's exit from the lateral compartment. |
History of leg pain & tightness at the same time, distance, or intensity of exercise; the pain increases with exercise & resolves after rest (in approximately 30 min). The patient can experience numbness or a “floppy foot”. Physical examination at rest is usually normal; after exercise; pain occurs with palpation of the involved muscles. Weakness of foot dorsiflexion, eversion, & plantarflexion are commonly associated with increased pressure at the level of anterior, lateral & posterior compartments, respectively. |
Intracompartmental pressure (ICP) measurements, MRI, & near-infrared spectroscopy (NIRS) are used to diagnose the disorder. An MRI is more sensitive postexercise. The pre-exertional ICP measurement (normal values 5–12 mmHg) & immediate postexertion ICP measurement (normal values 9–20 mmHg) should be obtained for all compartments in both legs. |
If the patient does not want to modify the activity level, fasciotomy is the only treatment. Open, subcutaneous, & endoscopic fasciotomies have been described. |
| Vessel | Popliteal artery entrapment syndrome (PAES) | There are six types of PAES that are based on anatomic variants; a 7th type is functional PAES. | Claudicatory symptoms in the anterior &/or posterior aspect of the leg; numbness & tingling of the foot may be present; the pain may be elicited by running (especially uphill) or by repetitive jumping. Functional PAES is often associated with paraesthesias in the tibial nerve distribution. In advanced PAES, the posterior tibial & dorsalis pedis pulses may be diminished or absent. The pulses may decrease with passive ankle dorsiflexion or active ankle plantarflexion with the knee extended |
The resting ankle brachial index (ABI) is usually normal, but the 1-minute postexercise ABI is frequently decreased. The ABI can be decreased during PAES provocative manoeuvres (e.g., passive ankle dorsiflexion or active ankle plantarflexion). Other diagnostic techniques include duplex ultrasonography, conventional angiography, magnetic resonance angiography, & computed tomography angiography. |
The treatment is usually surgical & depends on the PAES classification. The goal is to remove the compression & reconstruct the artery if it is chronically damaged. |
| Vessel | Arterial endofibrosis | The most common location is the external iliac artery (EIA) in 90% of patients. External iliac artery endofibrosis is characterized by isolated narrowing of the lumen; it is unilateral in 85% of patients. | EIA is associated with endurance sports that involve repetitive hip flexion (e.g., cycling); patients subjectively have a sensation of swollen thigh & loss of power during maximal exercise (submaximal exercise does not have any symptoms); symptoms improve as soon as the exercise is decreased or stopped (<5 min); a bruit may be present at auscultation. | The pre-exercise ABI is normal & the postexercise ABI is reduced (<0.66). In unilateral cases of EIA, a postexercise ABI difference > 0.18 is suggestive of the disorder. Further imaging may be obtained for additional anatomic information. |
Activity modification usually resolves symptoms. Symptoms worsen in 80% of patients with EIA endofibrosis who are noncompliant with activity modification. Surgery is the treatment for recalcitrant cases. |
| Nerve | Saphenous nerve neuropathy | Symptoms range from minimal sensory loss or pain (at the level of the medial knee, medial calf &/or foot) to severe neuropathic pain; the patient has no motor deficit & a positive Tinel sign. | In nerve conduction studies, there may be very small differences between the unaffected side & the affected side. Needle examination is generally normal & used to rule out femoral neuropathy, L4 radiculopathy, or a lumbar plexopathy. |
Conservative treatment with serial nerve blocks. Neurolysis or neurectomy is the treatment in the event of conservative treatment failure. |
|
| Nerve | Peroneal neuropathy | Peroneal neuropathy can involve the superficial peroneal nerve (SPN) or the deep peroneal nerve (DPN). | The SPN supplies the peroneus longus & peroneus brevis muscles & sensory innervation to the lower two-thirds of the lateral leg & the dorsum of the foot. The DPN supplies the anterior tibialis, extensor hallucis longus, peroneus tertius, & extensor digitorum brevis muscles, & sensory innervation to the first web space. Symptoms can vary from pain & sensory loss to weakness of the muscles. |
Nerve conduction studies & electromyography | Conservative treatment such as activity modification, physical therapy, & orthotics. Surgical nerve decompression in resistant cases. For absent sensory nerve action potentials, the injured nerve segment can be removed & grafted. |
| Nerve | Tibial neuropathy | The tibial nerve contributes to the sural nerve (SuN) & supplies the muscles of the posterior compartment of the leg. The tibial nerve runs posterior to the medial malleolus through the tarsal tunnel. Sural nerve entrapment may present with pain or paraesthesias within the posterolateral aspect of the calf & foot. |
Electrodiagnostic evaluation is essential in determining the origin & the level of the lesion. For compression due to a mass, imaging techniques are required to identify & describe the neoformation. |
Conservative treatment. Surgical nerve release for resistant cases. |
|
| Nerve | Lumbar radiculopathy & spinal stenosis | Sciatica is generally caused by compression of the lower spinal nerve roots (L5 & S1). In cruralgia, higher nerve roots (e.g., L2, L3, & L4) are affected. |
Pain usually involves the thigh & sometimes the low back; leg pain can range from discomfort to debilitating pain; the pain may be described as aching, searing, throbbing, or burning. Other symptoms or signs are a pins-&-needles sensation, leg or foot numbness (with dermatome distribution), muscle group weakness, & the absence or reduction of reflexes. |
Static & dynamic X-ray imaging, MRI, & electrodiagnostic studies. | Treatment approaches range from conservative management (e.g., rest, activity modification, physical therapy, NSAIDs, corticosteroids, & opioids, & epidural corticosteroid injections) to surgery (e.g., discectomy, herniectomy, decompression, & fusion). |
| Tendon | Tendonitis | Tibialis anterior, posterior, flexor hallucis longus, & peroneal tendons, noninsertional Achilles tendinopathy. | Tenderness at palpation; pain with stretching & against resistance of the involved tendon is usually present; thickening of the tendon can be palpated in patients with chronic cases. | Usually based on physical examination. X-ray imaging, ultrasound, or MRI may be required. |
Treatment is usually conservative. In resistant cases, treatment may involve platelet-rich plasma, dry needling, or surgery. |
Chronic exertional compartment syndrome (CECS), medial tibial stress syndrome (MTSS), stress fractures, and popliteal artery entrapment syndrome (PAES) are more common than the other pathologies. However, the exact incidence of each condition is unknown. Studies show that MTSS accounts for 6–16% of all running injuries, and it can represent up to 50% of lower leg injuries in selected populations such as military personnel.1 Chronic exertional compartment syndrome may be difficult to diagnose and is often underestimated, but it is very common in runners—mostly in the anterior and lateral compartments.2 In a retrospective review of 150 athletes with exercise-induced leg pain, 33% of athletes were diagnosed as having CECS; 25% of athletes had stress fractures; 13% of athletes had MTSS; and 10% of athletes had nerve entrapment syndromes.3 The aim of the present review is to provide a complete discussion of the most common pathologies related to exercise-induced leg pain. Particular attention is paid to the history and physical examination, which are fundamental for requesting the correct diagnostic tests or imaging techniques necessary for a precise diagnosis.
Chronic exertional compartment syndrome
Chronic exertional compartment syndrome is widely discussed, but its pathophysiology seems to be largely unknown. It has traditionally been described as occurring mostly in young individuals (median age, 20 years4), recreational and elite runners, military recruits, and athletes who participate in ball and puck sports.5 No different incidence has been described for men and women.5 Chronic exertional compartment syndrome is bilateral in 70–80% of patients.5 However, CECS seems to be often misdiagnosed in older nonathletic individuals.6 Risk factors for the development of CECS include anabolic steroid and creatine use, overuse, eccentric exercise, previous traumas, and hyperpronation of the foot.5, 7 Diabetes is also an identified risk factor for CECS, which may result from vasomotor disturbances or soft tissue alterations.6
Four compartments have classically been described in the leg: anterior compartment, lateral compartment, posterior compartment, and deep posterior compartment. One or more leg compartments can be involved in CECS. In 95% of patients, CECS involves the anterior and lateral compartments of the leg.5 The origin of pain in CECS remains unclear. Different causes have been theorized such as (1) ischaemia, (2) disproportionate oxygen supply versus oxygen demand, and (3) stimulation of pressure fibres.5
In diagnosing CECS, the history is essential because the physical exam is often unrevealing. A history of pain in a compartment of the leg at the same time, distance, or intensity of exercise is common.5 The pain increases as the patient continues exercising. Pain and tightness resolve after a rest period (30–40 minutes). The patient can experience pain (described as “burning”, “aching”, or “pressure”), numbness, and drop foot (i.e., difficulty controlling foot movement or a complaint of a “slapping or floppy foot”). Physical examination at rest is usually normal, except for malalignment and gait abnormalities (e.g., hyperpronation), if present.
After exercise and the onset of the symptoms, pain with palpation and stretching of the involved muscles in association with firmness of the involved compartments may be present. Muscle herniae exist in 40–60% of patients,5 and most herniae are located at the level of the superficial peroneal nerve's exit from the lateral compartment. Arterial pulses are generally normal. Weakness of foot dorsiflexion, eversion, and plantarflexion are commonly associated with increased pressure at the level of anterior, lateral, and posterior compartments respectively.5
Intracompartmental pressure (ICP) monitoring, magnetic resonance imaging (MRI), and near-infrared spectroscopy (NIRS) are among the diagnostic methods used to diagnose CECS. Magnetic resonance imaging is more sensitive postexercise.8 An increase in T2-weighted signal intensity correlates well with increased ICP.5 Muscular oedema, muscular swelling, fascial thickening, and fatty infiltration of the muscle are also common in CECS. Near-infrared spectroscopy is able to detect the haemoglobin saturation of tissues. Patients with an elevated ICP show a larger decrease in saturation after exercise, compared to healthy controls.9 All of these methods seem to be useful in confirming CECS; however, the role of MRI and NIRS in clinical diagnosis is yet to be established, and the ICP measurement is the most commonly used technique (Fig. 1). Many controversies also exist regarding the ICP measurement. Pedowitz et al10 described the reference criteria and thresholds for the ICP measurement in CECS: (1) resting pressure, a pressure > 15 mmHg; (2) immediate (1 minute), a postexertion pressure > 30 mmHg; and (3) delayed (5 minutes), a pressure > 20 mmHg. However, these criteria have been questioned. In a recent systematic review,9 the mean resting pressure values ranged 7.4–50.8 mmHg for CECS patients and 5.7–12 mmHg for the controls, and the mean values measured during exercise ranged 42–150 mmHg for CECS patients and 28–141 mmHg for the controls. There was no overlap in mean ICP measurements between patients and the controls only at the 1-minute postexercise time interval: values ranged 34–55.4 mmHg for CECS patients and 9–19 mmHg for the controls. For this reason, the need for premeasurements and delayed measurements has been debated.9 In agreement with Hutchinson,11 we believe that pre-exertional and immediate postexertion ICP measurements should be obtained in all four compartments of both legs to precisely plan the surgery and reduce failure rates.
Fig. 1.
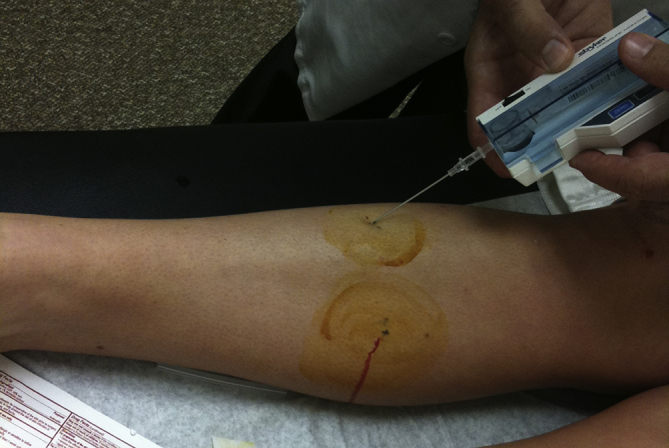
The intracompartmental pressure measurement. After administering a local anaesthetic, a skin puncture is used to test the deep and superficial posterior compartments. A second puncture is used to test the anterior and lateral compartments (shown in the picture). This is performed by redirecting the needle in the subcutaneous tissue.
Discontinuing the activity that elicits pain or decreasing the intensity of training are generally sufficient to avoid symptoms and treat the problem. By contrast, if the patient does not want to modify his or her activity, fasciotomy is the only definitive treatment. Other conservative management options have been described and report high failure rates. These options include a resting period, followed by slowly increasing the exercise level; arch support orthotics to reduce pronation; avoiding running on hard surfaces; changing footwear; changing running techniques; deep tissue massage; ultrasound; stretching before exercise; and osteopathic manipulation techniques.5
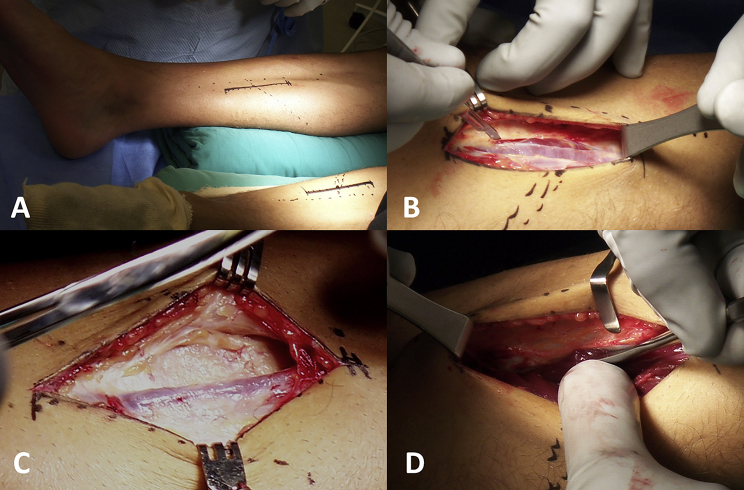
Open fasciotomy, subcutaneous fasciotomy,12 and endoscopic fasciotomy13, 14 have been previously described. The key point in this type of surgery is to achieve at least a 90% fascial release15 and to avoid the recurrence of symptoms. For this reason, the authors prefer an open approach with the three-incision technique (Fig. 2).
Fig. 2.
Superficial and deep posterior compartment release for chronic exertional compartment syndrome of the posterior compartments and for medial tibial stress syndrome. (A) A 12-cm anteromedial longitudinal incision is formed 1 cm posterior to the posteromedial border of tibia. It is centred at the level where the gastrocnemius curves anteriorly toward the Achilles tendon and tibia. (B) The superficial posterior compartment has a thin fascia, which is incised with the scalpel and scissors. (C) Branches of the saphenous vein and nerve are posterior to this incision. (D) To release the deep posterior compartment, the muscles and fascia are released from the posterior border of the tibia with a Cobb elevator.
Anterior and lateral fasciotomies have a success rate > 80%.16 Deep posterior fasciotomies have a lower success rate ranging 30–65%.17 In a case series in a military population,18 the results of fasciotomy seemed to be less encouraging: among 611 patients (representing 754 fasciotomies), 44.7% of patients reported symptom recurrence; 27.7% of patients were unable to return to full activity; 15.7% of patients had surgical complications; 5.9% of patients underwent surgical revision; and 17.3% of patients were referred for medical discharge because of CECS. Multivariate analysis of prognostic factors revealed that surgical failure was associated with perioperative complications, activity limitations, and persistence of preoperative symptoms.
Stress fracture of the leg
The tibial shaft is the most common site for lower extremity stress fractures and accounts for nearly 50% of all stress fractures in athletes.19, 20 Fibular stress fractures occur less frequently and represent 4.6–21% of all stress fractures in athletes.20, 21 Bone will fatigue and fail when it is under repetitive loads without sufficient time to remodel and repair. In the tibia, the resulting stress fracture usually occurs on the compression (i.e., concave) side and involves the posteromedial cortex.22 Stress fractures along the anterior cortex of the midtibia or medial malleolus are less frequent, are associated with slower healing, and may require surgical treatment. Fibular stress fractures typically occur on the distal shaft, 5–6 cm proximal to the lateral malleolus.20
Risk factors for tibial stress fractures include low bone mineral density, menstrual imbalance, and low-fat diet, high weekly training mileage, leg length discrepancy, high longitudinal arch of the foot, and excessive forefoot varus.20 Patients with this condition experience a gradual onset of localized pain at the inner aspect of the tibia or other areas involved by the stress fracture. The pain is often sharp or acute and increases with impact activity and decreases with rest; however, as the stress fracture progresses, the pain tends to persist with simple walking or at rest. Localized pain at palpation is typical. Pain with sustained one leg hop and pain with percussion at a distant site are helpful signs.23
X-ray imaging is commonly used to determine a stress fracture. However, radiographic findings may lag up to 2–4 weeks behind symptoms in people with stress fractures. When an immediate diagnosis is unnecessary, X-ray scans can be repeated after 2 weeks. Computed topography (CT) scan, bone scan, or MRI is otherwise necessary.
Low-risk stress fractures (e.g., posteromedial tibia and fibula) usually respond well to conservative treatment. Rest (4–8 weeks), ice, pain medications, and a gradual and progressive return to sports are the cornerstones of treatment. Other treatments that reportedly hasten healing include electromagnetic field therapy, extracorporeal shock wave therapy, pneumatic leg braces, and bisphosphonates.
For failed conservative treatment, primarily for high-risk stress fractures, the surgical treatment of choice is intramedullary nailing.24 In addition, drilling with bone grafting has shown favourable results in incomplete fractures, and patients thus treated have a faster return to sports and minimal morbidity.25
Medial tibial stress syndrome
A very common cause of overuse leg injuries is medial tibial stress syndrome (MTSS; also known as “shin splints”), which has an incidence ranging 4–35% in athletic and military populations.3, 26, 27 The aetiology of MTSS remains unknown and many theories have been proposed such as (1) underlying periostitis of the tibia resulting from tibial strain when loaded; (2) tendinopathy of the tibialis posterior, tibialis anterior, and soleus muscles; (3) periosteal remodelling; (4) stress reactions (e.g., repetitive loads that cause abnormal strain and bending of the tibia); and (5) decreased bone density.28, 29 Some risk factors that have been associated with MTSS include female sex, previous history of MTSS, fewer years of running experience, orthotic use, increased body mass index, increased navicular drop, and increased external rotation hip range of motion.30 No significant relationship between lower extremity alignment and MTSS has been described.31 No current evidence supports any prevention method for MTSS.32
Patients typically experience pain at the level of the posteromedial aspect of the tibia. The pain is cumulative with activity and persists longer (sometimes for days) before it improves with rest. This finding is useful for the differential diagnosis from CECS in which pain subsides within minutes after the cessation of exercise. At the physical examination, pain and tenderness along the posteromedial aspect of the tibia is diffuse, as opposed to a tibial stress fracture in which pain is localized.23 No neurological signs are present. The history and physical examination are usually sufficient to diagnose MTSS. If the diagnosis is unclear, imaging can be obtained. X-ray images are usually negative. The MRI usually shows medial and posteromedial periosteal oedema, whereas the bone scan shows a distinct appearance with uptake along the posteromedial tibia on delayed-phase images. The uptake is longitudinally oriented and one-third or more of the tibia is involved, as opposed to localized uptake in stress fractures.23
The treatment of MTSS is initially conservative and the modalities include (1) rest and ice during the acute phase; (2) modifying the training program (e.g., decreasing the intensity, frequency, and duration of training); (3) using low-impact and cross-training exercises; (4) gradually returning to sports with pain-free activity; (5) stretching and strengthening exercises; (6) using proper-fitting shoes with good shock absorption; (7) changing shoes every 250–500 miles; (8) use of orthotics, if indicated; (9) treating key dysfunctions of the entire kinetic chain; (10) manual therapy; and (11) the possible addition of complementary techniques such as extracorporeal shock wave therapy, injections, and acupuncture.29
Surgery is indicated for recalcitrant cases of MTSS. The authors' preferred surgical treatment consists of performing a fasciotomy of the deep posterior compartment with the release of the painful portion of the periosteum23 (Fig. 2). The technique is comparable to what has been described for the CECS with regard to the posterior compartment (i.e., medial approach).29 Cauterization of the posteromedial ridge of the tibia can be performed, based on the surgeon's preference. Variable rates of failure have been described, although improvement in pain and function is generally reported for the surgical treatment.33, 34
Entrapment neuropathies
Saphenous nerve neuropathy
The saphenous nerve (SN) is the longest sensory branch of the femoral nerve. It is prone to injury and/or entrapment within the adductor canal at the distal thigh. The SN originates from the femoral nerve distal to the inguinal ligament, travels through the femoral triangle with the femoral artery, and then enters the adductor canal (i.e., Hunter's canal). The SN exits the canal to supply sensation to the medial leg, ankle, and arch of the foot.35 Saphenous nerve neuropathy can be associated with valgus knee, internal tibial rotation, vascular procedures at the level of the thigh, and knee surgery.36
Clinical presentation may range from minimal sensory loss or pain (at the level of the medial knee, medial calf, and/or foot) to severe neuropathic pain. No motor deficit exists. The Tinel sign at the site of entrapment may be present. Nerve conduction studies may show very small differences between the affected side and the unaffected side. Needle examination is generally normal and is used to rule out femoral neuropathy, L4 radiculopathy, or a lumbar plexopathy.36
Most patients with SN entrapment respond to conservative treatment. Serial nerve blocks have been described with an 80% success rate.37 Neurolysis or neurectomy of the SN can be considered in the event of conservative treatment failure. Neurectomy is associated with more consistent results, but usually results in anaesthesia in the SN distribution.35, 38
Peroneal neuropathy
Fibres from the L4-S1 nerve roots form the common peroneal nerve (CPN), which runs with the tibial nerve as part of the sciatic nerve in the posterior thigh. The CPN diverges at the level of the popliteal fossa, and turns around the fibular neck where it is very vulnerable to compression and trauma. It then divides into its terminal branches: the superficial peroneal nerve (SPN) and the deep peroneal nerve (DPN). The SPN supplies muscular branches to the peroneus longus and peroneus brevis muscles and sensory innervation to the lower two-thirds of the lateral leg and the dorsum of the foot. The DPN supplies the anterior tibialis, extensor hallucis longus, peroneus tertius, and extensor digitorum brevis muscles, and sensory innervation to the first web space.36
Peroneal neuropathy is the most common mononeuropathy in the lower extremity. At the level of the femoral head, the CPN can be injured by trauma (e.g., lateral knee contusion, knee dislocation, fibular head fractures), external compression (e.g., habitual knee crossers, prolonged squatting or kneeling, bedridden patients, casts, braces, prolonged lateral decubitus, meniscal cysts, neuromas, and tumours), and surgery (e.g., direct damage or nerve traction after valgus knee correction). Severe inversion ankle sprains can cause traction injuries because of damage to the vasa nervorum.36
Based on the level of the lesion or compression, patients may complain about weakness of the muscle supplied and numbness or neuropathic pain at the level of the innervated skin (see above). Tinel's sign can be positive at the level of the nerve lesion. Damage to the CPN causes partial or complete drop foot.
Lesions to the SPN are most common where the nerve exits the deep fascia (approximately 11 cm proximal to the tip of the lateral malleolus) and are generally a consequence of recurrent ankle sprains, hernias, or increased lateral compartment pressure. No muscular deficits occur for SPN lesions at this level because the innervation of the peroneal muscles is proximal to the site of compression. Pain can be elicited by palpation or percussion at the nerve exit point while the patient actively dorsiflexes and everts against resistance or when the foot is passively plantarflexed.23
Isolated DPN injuries can occur with anterior CECS. Compartment pressure measurements can be useful to differentiate peroneal nerve neuropathies from anterior or lateral CECS, which may have a similar presentation.23
Electrophysiological tests are important in differentiating peroneal entrapment from L5 radiculopathy or a more proximal CPN lesion. Sensory nerve action potentials of the SPN may be reduced or absent, but should be spared in L5 radiculopathy. Another electrophysiological technique is to record from the extensor digitorum brevis muscle or tibialis anterior muscle while stimulating the CPN above and below the fibular neck.35 Electromyography should be performed on peroneal-innervated muscles and on nonperoneal L5-innervated muscles such as the tibialis posterior and the flexor digitorum longus. The short head of the biceps should also be studied because it is the only peroneal-innervated muscle proximal to the peroneal tunnel; an electrical abnormality suggests a lesion proximal to the popliteal fossa.35 In the event of compression, X-ray imaging, CT scan, or MRI of the area can identify a mass (e.g., meniscal cysts, neuromas, tumours, haematomas, and hypertrophic reparative bone callus).
Conservative therapy is effective for most cases of CPN entrapment. This mostly consists of activity modification, physical therapy, and orthotics (e.g., ankle braces for recurrent ankle sprains or drop foot braces). Surgical nerve decompression is often recommended in resistant cases of CPN entrapment, and has an 88% success rate.39 If sensory nerve action potentials are absent, the injured nerve segment can be removed and grafted. Functional outcomes are better in patients who require a shorter graft (i.e., > 6 cm).39 Tibialis posterior tendon transfer can be considered for persistent drop foot, primarily in patients younger than 30 years.35, 40
Tibial neuropathy
The tibial division of the sciatic nerve is formed by the L5–S2 nerve roots. Within the popliteal fossa, the tibial nerve (TN) contributes to the sural nerve (with the peroneal communicating nerve) and supplies the muscles of the posterior compartment of the leg (i.e., gastrocnemius, soleus, tibialis posterior, flexor digitorum longus, and flexor hallucis longus). The TN then runs posterior to the medial malleolus through the tarsal tunnel and then divides into its terminal branches (i.e., calcaneal, medial plantar, and lateral plantar branches).36 The sural nerve (SuN) runs adjacent to the Achilles tendon with the short saphenous vein and terminates in the posterolateral aspect of the foot. Sural nerve entrapment is uncommon and patients may present with pain or paraesthesias within the posterolateral aspect of the calf and foot.
The TN is rarely affected by trauma. Injury at the level of the knee may occur because of space-occupying lesions in the popliteal fossa (e.g., Baker cyst, tumour, haematoma). Distal compression at the level of the ankle can occur at the flexor retinaculum and is commonly referred to as tarsal tunnel syndrome.36 Tarsal tunnel syndrome entails symptoms primarily to the foot (i.e., pain and paraesthesias along the medial aspect of the heel and plantar aspect of the foot and toes); it is therefore beyond the scope of this review.
Lesions to the TN proximal to the popliteal fossa can cause weakness in plantarflexion and inversion of the ankle, loss of toe flexion strength, and calf atrophy. Sensory loss can occur in the sole of the foot and the posterolateral lower leg and foot in the sural distribution. Loss of SuN sensation may be variable because of the aforementioned peroneal contribution. The Achilles reflex is also depressed or absent. In distal (i.e., midleg) TN lesions, ankle strength can be preserved. Differential diagnosis of TN neuropathy includes S1 radiculopathy, sciatic neuropathy, and lumbar plexopathy.36 Electrodiagnostic evaluation is essential in determining the origin and level of the lesion. In the event of compression by a mass, imaging techniques are required to identify and describe the neoformation. Surgical nerve release should be considered in the patients that are resistant to conservative treatment.
Lumbar radiculopathy and spinal stenosis
Radicular pain often occurs because of compression or inflammation of a spinal nerve. Root compression most commonly is the consequence of a herniated disc, foraminal stenosis, nerve root injury, or scar tissue from a previous spinal surgery. Sciatica is a pain that radiates down the back of the leg to the calf or foot, whereas cruralgia involves the front of the thigh and the shin. Sciatica is generally caused by compression of the lower spinal nerve roots (L5 and S1). By contrast, higher nerve roots are affected (L2, L3, and L4) in cruralgia. In cruralgia and sciatica, the pain usually involves the thigh, and this finding helps in the differential diagnosis from other causes of leg pain. Patient-related factors (e.g., age, weight, work, and sports), history, and physical examination are paramount to establish a correct diagnosis.
Some positions can elicit or relieve the pain. For example, bending over may relieve pain from spinal stenosis, whereas twisting can increase groin, hip, and leg ache because of facet joint syndrome. The pain can be persistent or elicited by specific activities such as walking, running, sitting, or standing. The pain to the leg can be isolated or accompanied by a history of low back pain. Leg pain can range from discomfort to debilitating pain. The pain can be described as aching, searing, throbbing, or burning. Other symptoms or signs can be a pins-and-needles sensation, leg or foot numbness (with dermatome distribution), muscle group weakness, and absence or reduction of reflexes.23
Static and dynamic X-ray imaging of the lumbar spine (in maximum flexion and extension) with more advanced techniques (e.g., MRI and electrodiagnostic studies) are useful to establish a precise diagnosis. The treatment ranges from conservative management (e.g., rest, activity modification, physical therapy, nonsteroidal anti-inflammatory drugs, corticosteroids and opioids, and epidural corticosteroid injections) to surgery such as discectomy, herniectomy, decompression, and fusion.23
Vascular diseases
Popliteal artery entrapment syndrome
Popliteal artery entrapment syndrome has an incidence of 0.165% in young males.41 Patients with anatomic causes of PAES are usually male (72%), have a mean age of 43 years, and are sedentary (86%).42 Patients with functional PAES have a mean age of 24 years, are more commonly female (66%), physically active (90%), and participate in sports (e.g., football, basketball, hockey, and dance).42, 43 The popliteal artery and vein normally course deep into the soleus and gastrocnemius muscles, and course superficially to the popliteus muscle as these vessels exit the popliteal fossa. Popliteal artery entrapment syndrome can be divided into seven types: Type I, the popliteal artery runs medial to the medial head of gastrocnemius; Type II, the medial head of the gastrocnemius muscle is laterally attached; Type III, the accessory slip of the gastrocnemius muscle or the fibrous bands are around the artery; Type IV, the popliteal artery passes below the popliteus muscle or fibrous bands arising from the popliteus; Type V, the popliteal artery and vein are both entrapped; Type VI, other variants; and Type F, functional entrapment.44
Patients with PAES report claudicatory symptoms in the anterior and/or posterior aspect of the leg. Numbness and tingling of the foot may also be present. The pain may be elicited by running, especially uphill, or by repetitive jumping. Functional PAES is often associated with paraesthesias in the TN distribution. In advanced PAES, the posterior tibial and/or dorsalis pedis pulses may be diminished or absent. The pulses may decrease with passive ankle dorsiflexion or active ankle plantarflexion with the knee extended.41
The resting ankle brachial index (ABI) is usually normal, but the 1-minute postexercise ABI is frequently decreased, as described later in the “Arterial endofibrosis” section. In addition, the ABI can be decreased in PAES provocative manoeuvres (e.g., passive ankle dorsiflexion or active ankle plantarflexion). Other imaging techniques that are useful in making the diagnosis and classification of PAES include duplex ultrasonography (Fig. 3), conventional angiography, magnetic resonance angiography (Fig. 3), and computed tomography angiography (Fig. 4). These techniques must be performed at rest and during provocative manoeuvres.
Fig. 3.
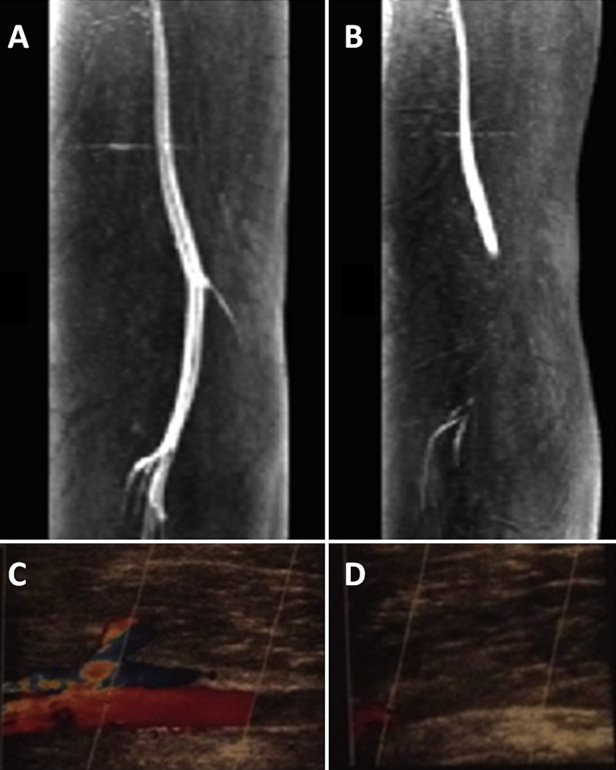
Popliteal artery entrapment syndrome (PAES). (A) Magnetic resonance angiography of the left leg with PAES before muscle contraction. The popliteal artery has a regular blood flow. (B) Magnetic resonance angiography of the same leg after muscle contraction. The blood flow is obstructed at the level of the popliteal fossa. (C) Duplex ultrasonography of a patient with PAES before muscle contraction. (D) Duplex ultrasonography of a patient with PAES after muscle contraction. The blood flow is obstructed.
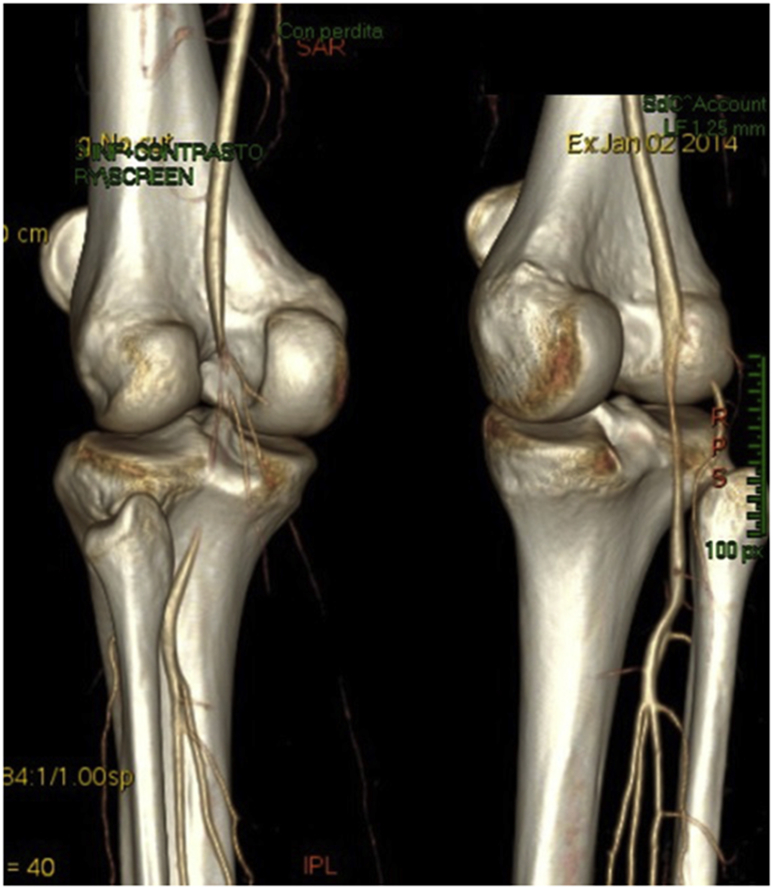
Fig. 4.
Bilateral popliteal artery entrapment syndrome. Computed tomography angiography of both legs after muscle contraction. There is complete blood flow obstruction in the left leg and blood flow reduction in the right leg.
The treatment is usually surgical and depends on the PAES classification. Various surgical techniques have been described with the goal of removing the compression and reconstructing the artery, if it is chronically damaged. Bypass is advocated over thromboendarterectomy. The success rate of the surgery is reportedly 70–100% in anatomic PAES, 48–57% in popliteal vein entrapment syndrome, and 100% in functional PAES.45 Little role exists for endovascular procedures.41
Arterial endofibrosis
The incidence of lower extremity arterial endofibrosis is difficult to determine because of limited research regarding this topic. The most common location is the external iliac artery (EIA) (90%).41, 46 External iliac artery endofibrosis commonly occurs in individuals involved in endurance sports that require repetitive hip flexion (e.g., cycling, cross-country skiing, speed skating, and running). It is characterized by an isolated narrowing (<20% diameter reduction) of the arterial lumen. In 85% of arterial endofibrosis cases are unilateral, primarily on the left side.41, 47
The EIA travels along the anterior portion of the psoas muscle and then anterior to the hip joint. During hip flexion, the EIA takes on a serpentine course and may have kinking.47 It has been theorized that prolonged exercise with repeated hip flexion causes increased turbulence in the EIA, and results in intimal hyperplasia and arterial stenosis.41, 46
Symptoms are generally characterized by a subjective sensation of a swollen thigh and loss of power on the affected limb during maximal exercise, whereas submaximal exercise can be performed without any symptoms. Symptoms improve as soon as the level of exercise is decreased or exercise is stopped (<5 minutes).47 A bruit may be present at auscultation over the anterior hip at rest.41
Pre- and postexercise ABI is an important tool in EIA endofibrosis. In a patient with vascular stenosis, the ABI is normal at rest (0.9–1.2) and is reduced after exercise. An ABI of ≤ 0.66 at 1 minute after exercise is 90% sensitive and 87% specific for exercise-induced leg pain due to a vascular condition. In patients with unilateral pain, a postexercise ABI difference > 0.18 is suggestive of EIA endofibrosis.41 Additional anatomic information may be further obtained by imaging techniques such as duplex ultrasonography, magnetic resonance angiography, and computed tomography angiography.
Activity modification usually resolves the symptoms. Symptoms worsen in 80% of patients with EIA endofibrosis who are noncompliant with activity modification. Multiple surgical options are available such as surgical release of the external iliac and common iliac arteries, shortening vessels through segmental excision with or without the release of adhesions, and lesion excision with revascularization. Percutaneous transluminal angioplasty and vascular stenting have been described with poor results.41, 44
Other vascular conditions
Popliteal artery aneurisms
Popliteal artery aneurisms (PAAs) are the most common peripheral aneurysm, and they account for > 80% of all cases with a prevalence of 1% in men older than 65 years. Popliteal artery aneurisms are bilateral in 50% of patients and are associated with an abdominal aortic aneurysm in 50% of patients. Eighty percent of patients are asymptomatic at the time of diagnosis, although they become symptomatic at a rate of 14% per year. Symptoms include acute limb ischaemia (e.g., acute thrombosis), chronic ischaemia, and intermittent claudication (e.g., repeated microemboli or stenosis), knee discomfort, leg swelling (with or without deep vein thrombosis secondary to compression of the popliteal vein), nerve compression, and rupture.48 In nearly all patients, symptomatic PAA requires urgent intervention, whereas the surgical timing in asymptomatic patients continues to generate vigorous debate. A PAA rupture is unusual with a reported incidence of 2–4%. Diagnosing a ruptured PAA can be challenging. It is often misdiagnosed as deep venous thrombosis, a ruptured Baker's cyst, peroneal nerve palsy, or other causes of acute limb ischaemia. Delayed diagnosis increases the risk of limb loss and death. A ruptured PAA should be considered in any elderly man with popliteal fossa pain or swelling. Elective methods to repair a PAA can be employed in a rupture setting, although endovascular repair may provide the quickest and less invasive method in an unstable and high-risk patient.48
Exercise-induced vasculitis is common, although it is usually misdiagnosed and ignored in the literature. It occurs mostly in long-distance runners and in females after long walks, especially in hot weather. Erythematous, urticarial, or purpuric plaques arise on the lower legs, but these plaques do not arise on skin compressed by socks. Symptoms included itching, pain, and a burning sensation. Lesions resolve after some days. Relapses are frequent with further muscular exercise, and could be prevented in some patients by compression hosiery, manual lymphatic drainage, and the intake of oedema protective agents or steroids (local or systemic).49
Effort thrombosis
Effort thrombosis is a well-described rare cause of deep vein thrombosis in the upper limb after strenuous activity (e.g., Paget–Schroetter syndrome). Effort thrombosis has rarely been described in the lower limb of athletes (e.g., joggers, runners, skiers, soccer players, and after performing martial arts). In all cases, patients presented with unilateral swelling in the lower limb after exertion. At physical examination, pain was present with flexion or extension of the knee and hip, and a positive Homan sign was present. Imaging techniques used in diagnosing effort thrombosis (e.g., venography, duplex ultrasonography, and impedance plethysmography) are similar to those used for the diagnosis of deep venous thrombosis. An underlying hypercoagulable state should be investigated. The treatment is based on intravenous and oral anticoagulation, compression garment therapy (with pressures of at least 20–30 mmHg), and catheter-directed venous thrombolysis (particularly for ileofemoral clots and if the thrombosis is less than 14 days old).41
Tendinopathies
Tibialis anterior tendonitis
Patients with tibialis anterior tendonitis experience pain at the front of the shin, ankle, or foot during activities that involve stress on the tibialis anterior tendon. These activities include walking or running (mostly up or down hills or on uneven surfaces), kicking (e.g., soccer and football), wearing tight shoes, and repetitive kneeling. The pain can also be present at rest, especially upon waking in the morning. The pain associated with this condition tends to have a gradual onset that progressively worsens.
Noninsertional Achilles tendinopathy
Noninsertional Achilles tendinopathy is a common cause of posterior leg pain. Factors influencing Achilles tendinopathy are intrinsic and mechanical. Intrinsic factors (e.g., regional hypovascularity, endocrine or metabolic diseases, and genetic makeup) can predispose the tendon to degeneration. Mechanical factors (e.g., overuse and lack of flexibility) increase tendon strain that surpasses the energy-absorbing ability and leads to microtears. Training errors (e.g., technique, inappropriate footwear, and inconsistent surfaces) are also associated with tendon degeneration.20
Tibialis posterior tendon dysfunction
Tibialis posterior tendon dysfunction (TPTD) is a sudden or progressive loss of strength of the tibialis posterior tendon. The tibialis posterior is a phasic muscle that eccentrically contracts at heel strike to resist pronation and internal tibial rotation, and at heel rise to lock the bones of the arch and rearfoot. This action thus converts the foot into a rigid lever. The tibialis posterior muscle is the main dynamic stabiliser of the medial longitudinal arch.50 There is much debate regarding the aetiologies of TPTD. These aetiologies are broadly divided into (1) acute traumatic injury; (2) inflammatory synovitis secondary to mechanical overuse or systemic disease; and (3) chronic tendon degeneration. A slow, pathological tendon rupture could occur as degeneration progresses. Geidemann and Johnson51 identified a strong correlation between TPTD and acquired adult flatfoot deformity.
Flexor hallucis longus tendinopathy
Flexor hallucis longus (FHL) tendinopathy is common in dancers or athletes who use repetitive push-off manoeuvres. Os trigonum or hypertrophic posterolateral process of the talus are predisposing factors. Pain and swelling over the posteromedial aspect of the ankle and with resistive flexion of the great toe is common.
Peroneal tendinopathy
Peroneal tendinopathy can cause pain and swelling posterior to the lateral malleolus; the pain is elicited by active eversion and dorsiflexion against resistance. A history of chronic lateral ankle pain and instability is common.
A thorough physical examination is usually sufficient to diagnose all tendonitis/tendinopathies. Tenderness on palpation and pain with stretching and against resistance of the involved tendon are usually present. Thickening of the tendon can be palpated in chronic cases. Alignment of the leg and foot should be carefully evaluated to rule out predisposing factors. Imaging techniques include X-ray, ultrasound, or MRI, and may be required to assist with diagnosis and assess the severity of the condition. The treatment for tendonitis is usually conservative: rest, ice, nonsteroidal anti-inflammatory drugs, activity modification, and appropriate physical therapy. In resistant cases the following treatments should be considered: platelet-rich plasma, dry needling, and orthotics. Surgery is reserved to treat recalcitrant tendonitis. Surgical options include tendon release (open or endoscopic), debridement of the degenerated tissue, and scarification with or without augmentation. Every malalignment predisposing tendinopathy should be corrected when present. Posterior ankle arthroscopy with FHL debridement can be considered in recalcitrant cases of FHL tendonitis.
Conclusion
Exercise-induced leg pain is a common condition that results from numerous pathologies. A precise knowledge of all of these pathologies and their symptoms is fundamental in guiding the diagnosis and the correct treatment. In exercise-induced leg pain, a 360° examination of the leg (e.g., bone, blood vessels, muscles, nerves, and tendons) is mandatory. When the clinical presentation is unclear, noninvasive diagnostic tests should be performed first.
Conflicts of interest
All authors declare no conflicts of interest.
References
- 1.Hargrove R., Maclean C. Incidence and risk factors in the development of medial tibial stress syndrome among naval recruits. Am J Sports Med. 2005;33:463–464. doi: 10.1177/036354650503300319. author reply 464. [DOI] [PubMed] [Google Scholar]
- 2.Brennan F.H., Jr., Kane S.F. Diagnosis, treatment options, and rehabilitation of chronic lower leg exertional compartment syndrome. Curr Sports Med Rep. 2003;2:247–250. doi: 10.1249/00149619-200310000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Clanton T.O., Solcher B.W. Chronic leg pain in the athlete. Clin Sports Med. 1994;13:743–759. [PubMed] [Google Scholar]
- 4.Shah S.N., Miller B.S., Kuhn J.E. Chronic exertional compartment syndrome. Am J Orthop (Belle Mead NJ) 2004;33:335–341. [PubMed] [Google Scholar]
- 5.Tucker A.K. Chronic exertional compartment syndrome of the leg. Curr Rev Musculoskelet Med. 2010;3:32–37. doi: 10.1007/s12178-010-9065-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edmundsson D., Toolanen G., Sojka P. Chronic compartment syndrome also affects nonathletic subjects: a prospective study of 63 cases with exercise-induced lower leg pain. Acta Orthop. 2007;78:136–142. doi: 10.1080/17453670610013547. [DOI] [PubMed] [Google Scholar]
- 7.Tubb C.C., Vermillion D. Chronic exertional compartment syndrome after minor injury to the lower extremity. Mil Med. 2001;166:366–368. [PubMed] [Google Scholar]
- 8.Brown R.R., Rosenberg Z.S. MR imaging of exercise-induced lower leg pain. Magn Reson Imaging Clin N Am. 2001;9:533–552. [PubMed] [Google Scholar]
- 9.Aweid O., Del Buono A., Malliaras P. Systematic review and recommendations for intracompartmental pressure monitoring in diagnosing chronic exertional compartment syndrome of the leg. Clin J Sport Med. 2012;22:356–370. doi: 10.1097/JSM.0b013e3182580e1d. [DOI] [PubMed] [Google Scholar]
- 10.Pedowitz R.A., Hargens A.R., Mubarak S.J., Gershuni D.H. Modified criteria for the objective diagnosis of chronic compartment syndrome of the leg. Am J Sports Med. 1990;18:35–40. doi: 10.1177/036354659001800106. [DOI] [PubMed] [Google Scholar]
- 11.Hutchinson M. Chronic exertional compartment syndrome. Br J Sports Med. 2011;45:952–953. doi: 10.1136/bjsports-2011-090046. [DOI] [PubMed] [Google Scholar]
- 12.Finestone A.S., Noff M., Nassar Y., Moshe S., Agar G., Tamir E. Management of chronic exertional compartment syndrome and fascial hernias in the anterior lower leg with the forefoot rise test and limited fasciotomy. Foot Ankle Int. 2014;35:285–292. doi: 10.1177/1071100713514390. [DOI] [PubMed] [Google Scholar]
- 13.Wittstein J., Moorman C.T., 3rd, Levin L.S. Endoscopic compartment release for chronic exertional compartment syndrome: surgical technique and results. Am J Sports Med. 2010;38:1661–1666. doi: 10.1177/0363546510363415. [DOI] [PubMed] [Google Scholar]
- 14.Knight J.R., Daniels M., Robertson W. Endoscopic compartment release for chronic exertional compartment syndrome. Arthrosc Tech. 2013;2:e187–e190. doi: 10.1016/j.eats.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathis J.E., Schwartz B.E., Lester J.D., Kim W.J., Watson J.N., Hutchinson M.R. Effect of lower extremity fasciotomy length on intracompartmental pressure in an animal model of compartment syndrome: the importance of achieving a minimum of 90% fascial release. Am J Sports Med. 2015;43:75–78. doi: 10.1177/0363546514554601. [DOI] [PubMed] [Google Scholar]
- 16.Verleisdonk E.J., Schmitz R.F., van der Werken C. Long-term results of fasciotomy of the anterior compartment in patients with exercise-induced pain in the lower leg. Int J Sports Med. 2004;25:224–229. doi: 10.1055/s-2003-45255. [DOI] [PubMed] [Google Scholar]
- 17.Winkes M.B., Hoogeveen A.R., Scheltinga M.R. Is surgery effective for deep posterior compartment syndrome of the leg? A systematic review. Br J Sports Med. 2014;48:1592–1598. doi: 10.1136/bjsports-2013-092518. [DOI] [PubMed] [Google Scholar]
- 18.Waterman B.R., Laughlin M., Kilcoyne K., Cameron K.L., Owens B.D. Surgical treatment of chronic exertional compartment syndrome of the leg: failure rates and postoperative disability in an active patient population. J Bone Joint Surg Am. 2013;95:592–596. doi: 10.2106/JBJS.L.00481. [DOI] [PubMed] [Google Scholar]
- 19.Harrast M.A., Colonno D. Stress fractures in runners. Clin Sports Med. 2010;29:399–416. doi: 10.1016/j.csm.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Gallo R.A., Plakke M., Silvis M.L. Common leg injuries of long-distance runners: anatomical and biomechanical approach. Sports Health. 2012;4:485–495. doi: 10.1177/1941738112445871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moretti B., Notarnicola A., Garofalo R. Shock waves in the treatment of stress fractures. Ultrasound Med Biol. 2009;35:1042–1049. doi: 10.1016/j.ultrasmedbio.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Boden B.P., Osbahr D.C., Jimenez C. Low-risk stress fractures. Am J Sports Med. 2001;29:100–111. doi: 10.1177/03635465010290010201. [DOI] [PubMed] [Google Scholar]
- 23.Korkola M., Amendola A. Exercise-induced leg pain: sifting through a broad differential. Phys Sportsmed. 2001;29:35–50. doi: 10.3810/psm.2001.06.825. [DOI] [PubMed] [Google Scholar]
- 24.Chang P.S., Harris R.M. Intramedullary nailing for chronic tibial stress fractures. A review of five cases. Am J Sports Med. 1996;24:688–692. doi: 10.1177/036354659602400522. [DOI] [PubMed] [Google Scholar]
- 25.Miyamoto R.G., Dhotar H.S., Rose D.J., Egol K. Surgical treatment of refractory tibial stress fractures in elite dancers: a case series. Am J Sports Med. 2009;37:1150–1154. doi: 10.1177/0363546508330973. [DOI] [PubMed] [Google Scholar]
- 26.Andrish J.T., Bergfeld J.A., Walheim J. A prospective study on the management of shin splints. J Bone Joint Surg Am. 1974;56:1697–1700. [PubMed] [Google Scholar]
- 27.Bennett J.E., Reinking M.F., Pluemer B., Pentel A., Seaton M., Killian C. Factors contributing to the development of medial tibial stress syndrome in high school runners. J Orthop Sports Phys Ther. 2001;31:504–510. doi: 10.2519/jospt.2001.31.9.504. [DOI] [PubMed] [Google Scholar]
- 28.Moen M.H., Holtslag L., Bakker E. The treatment of medial tibial stress syndrome in athletes; a randomized clinical trial. Sports Med Arthrosc Rehabil Ther Technol. 2012;4:12. doi: 10.1186/1758-2555-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galbraith R.M., Lavallee M.E. Medial tibial stress syndrome: conservative treatment options. Curr Rev Musculoskelet Med. 2009;2:127–133. doi: 10.1007/s12178-009-9055-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newman P., Witchalls J., Waddington G., Adams R. Risk factors associated with medial tibial stress syndrome in runners: a systematic review and meta-analysis. Open Access J Sports Med. 2013;4:229–241. doi: 10.2147/OAJSM.S39331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raissi G.R., Cherati A.D., Mansoori K.D., Razi M.D. The relationship between lower extremity alignment and medial tibial stress syndrome among non-professional athletes. Sports Med Arthrosc Rehabil Ther Technol. 2009;1:11. doi: 10.1186/1758-2555-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Craig D.I. Medial tibial stress syndrome: evidence-based prevention. J Athl Train. 2008;43:316–318. doi: 10.4085/1062-6050-43.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yates B., Allen M.J., Barnes M.R. Outcome of surgical treatment of medial tibial stress syndrome. J Bone Joint Surg Am. 2003;85-A:1974–1980. doi: 10.2106/00004623-200310000-00017. [DOI] [PubMed] [Google Scholar]
- 34.Abramowitz A.J., Schepsis A., McArthur C. The medial tibial syndrome. The role of surgery. Orthop Rev. 1994;23:875–881. [PubMed] [Google Scholar]
- 35.Toussaint C.P., Perry E.C., 3rd, Pisansky M.T., Anderson D.E. What's new in the diagnosis and treatment of peripheral nerve entrapment neuropathies. Neurol Clin. 2010;28:979–1004. doi: 10.1016/j.ncl.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 36.Craig A. Entrapment neuropathies of the lower extremity. PM R. 2013;5:S31–S40. doi: 10.1016/j.pmrj.2013.03.029. [DOI] [PubMed] [Google Scholar]
- 37.Romanoff M.E., Cory P.C., Jr., Kalenak A., Keyser G.C., Marshall W.K. Saphenous nerve entrapment at the adductor canal. Am J Sports Med. 1989;17:478–481. doi: 10.1177/036354658901700405. [DOI] [PubMed] [Google Scholar]
- 38.Worth R.M., Kettelkamp D.B., Defalque R.J., Duane K.U. Saphenous nerve entrapment. A cause of medial knee pain. Am J Sports Med. 1984;12:80–81. doi: 10.1177/036354658401200114. [DOI] [PubMed] [Google Scholar]
- 39.Kim D.H., Murovic J.A., Tiel R.L., Kline D.G. Management and outcomes in 318 operative common peroneal nerve lesions at the Louisiana State University Health Sciences Center. Neurosurgery. 2004;54:1421–1428. doi: 10.1227/01.neu.0000124752.40412.03. discussion 1428–1429. [DOI] [PubMed] [Google Scholar]
- 40.Yeap J.S., Birch R., Singh D. Long-term results of tibialis posterior tendon transfer for drop-foot. Int Orthop. 2001;25:114–118. doi: 10.1007/s002640100229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rajasekaran S., Kvinlaug K., Finnoff J.T. Exertional leg pain in the athlete. PM R. 2012;4:985–1000. doi: 10.1016/j.pmrj.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 42.Turnipseed W.D. Popliteal entrapment syndrome. J Vasc Surg. 2002;35:910–915. doi: 10.1067/mva.2002.123752. [DOI] [PubMed] [Google Scholar]
- 43.Lane R., Nguyen T., Cuzzilla M., Oomens D., Mohabbat W., Hazelton S. Functional popliteal entrapment syndrome in the sportsperson. Eur J Vasc Endovasc Surg. 2012;43:81–87. doi: 10.1016/j.ejvs.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 44.di Marzo L., Cavallaro A. Popliteal vascular entrapment. World J Surg. 2005;29:S43–S45. doi: 10.1007/s00268-004-2058-y. [DOI] [PubMed] [Google Scholar]
- 45.Sinha S., Houghton J., Holt P.J., Thompson M.M., Loftus I.M., Hinchliffe R.J. Popliteal entrapment syndrome. J Vasc Surg. 2012;55:252–262. doi: 10.1016/j.jvs.2011.08.050. e230. [DOI] [PubMed] [Google Scholar]
- 46.Ehsan O., Darwish A., Edmundson C., Mills V., Al-Khaffaf H. Non-traumatic lower limb vascular complications in endurance athletes. Review of literature. Eur J Vasc Endovasc Surg. 2004;28:1–8. doi: 10.1016/j.ejvs.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 47.Bruneau A., Le Faucheur A., Mahe G., Vielle B., Leftheriotis G., Abraham P. Endofibrosis in athletes: is a simple bedside exercise helpful or sufficient for the diagnosis? Clin J Sport Med. 2009;19:282–286. doi: 10.1097/JSM.0b013e3181b20456. [DOI] [PubMed] [Google Scholar]
- 48.Dawson J., Fitridge R. Update on aneurysm disease: current insights and controversies: peripheral aneurysms: when to intervene—is rupture really a danger? Prog Cardiovasc Dis. 2013;56:26–35. doi: 10.1016/j.pcad.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 49.Ramelet A.A. Exercise-induced vasculitis. J Eur Acad Dermatol Venereol. 2006;20:423–427. doi: 10.1111/j.1468-3083.2006.01504.x. [DOI] [PubMed] [Google Scholar]
- 50.Bowring B., Chockalingam N. Conservative treatment of tibialis posterior tendon dysfunction—a review. Foot (Edinb) 2010;20:18–26. doi: 10.1016/j.foot.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 51.Geideman W.M., Johnson J.E. Posterior tibial tendon dysfunction. J Orthop Sports Phys Ther. 2000;30:68–77. doi: 10.2519/jospt.2000.30.2.68. [DOI] [PubMed] [Google Scholar]