Abstract
Background/Objective
Platelet-rich plasma (PRP) and hyaluronic acid (HA) injection are both therapeutic options for osteoarthritis and chronic tendinopathy. Although several comparative studies on the two have been published, the effects of mixing PRP and HA are not fully understood. The purpose of this study is to investigate the influence of HA on platelets in PRP by measuring releasing growth factors.
Methods
PRP was produced from nine healthy adult volunteers (mean age, 32.8 ± 2.9 years; range, 29–37) with a commercial separation system. HA of weight-average molecular weight of 50–120 kDa was used. PRP group (PRP 1 mL + phosphate buffered saline 0.2 mL) and PRP + HA group (PRP 1 mL + HA 0.2 mL) were incubated at 37°C for 2 hours. The amounts of transforming growth factor β1 (TGF-β1) and platelet-derived growth factor (PDGF-AA) released from the PRP and PRP + HA samples were measured on Day 0, Day 3, and Day 5. In addition, the same growth factors on Day 5 were measured for PRP + high HA group (PRP 1 mL + HA 0.6 mL) with five donors. After collecting all of the samples on Day 5, the remaining gels were observed with Giemsa stain. Statistical analyses were performed using paired t tests to compare the PRP and HA groups at each time point, and a one-way analysis of variance (one-way ANOVA) with Tukey post hoc tests was used to compare the PRP, PRP + HA, and PRP + high HA groups.
Results
The TGF-β1 concentrations in the PRP and PRP + HA were 24.3 ± 7.2 μg/mL and 22.4 ± 1.8 μg/mL (p = 0.689) on Day 0, 17.2 ± 13.9 μg/mL and 25.4 ± 7.1 μg/mL (p = 0.331) on Day 3, and 12.7 ± 10.5 μg/mL and 33.7 ± 8.3 μg/mL (p = 0.034) on Day 5. The TGF-β1 concentrations on Day 5 were 24.1 ± 5.2 μg/mL (PRP group), 28.3 ± 2.4 μg/mL (PRP + HA), and 31.9 ± 4.8 μg/mL (PRP + high HA; one-way ANOVA: p = 0.003; post hoc PRP vs. PRP + HA: p = 0.016). The PDGF-AA concentrations in the PRP and PRP + HA groups were 2.30 ± 1.21 μg/mL and 2.32 ± 0.79 μg/mL (p = 0.931) on Day 0, 2.03 ± 0.53 μg/mL and 2.13 ± 0.73 μg/mL (p = 0.500) on Day 3, and 1.51 ± 0.40 μg/mL and 2.00 ± 0.52 μg/mL (p = 0.003) on Day 5. The PDGF-AA concentrations were 1.48 ± 0.46 μg/mL (PRP group), 1.94 ± 0.57 μg/mL (PRP + HA), and 2.69 ± 0.70 μg/mL (PRP + high HA; one-way ANOVA: p = 0.0002; PRP vs. PRP + high HA: p = 0.002; PRP + HA vs. PRP + high HA: p = 0.011) on Day 5. The PRP showed larger coagulated masses than the PRP + HA. The high concentration HA group had the smallest coagulated mass of all of the group.
Conclusion
The levels of growth factors released by PRP on Day 5 were increased by the addition of HA. A mixture of PRP and HA may be a more effective therapy than PRP or HA alone for osteoarthritis and tendinopathy.
Keywords: growth factor, hyaluronic acid, platelet-derived growth factor, platelet-rich plasma, transforming growth factor-β1
Introduction
The use of platelet-rich plasma (PRP) to treat musculoskeletal soft tissue injuries,1 bone grafts,2 osteoarthritis (OA),3, 4 and even skin ulcers5 is increasing. Although the long-term effects of PRP remain controversial, the high concentration of autologous growth factors in PRP is expected to reduce the time needed for healing based on the accumulated basic and clinical research. Therefore, assessment of the levels of growth factors released from PRP is important.
Hyaluronic acid (HA) is widely used to treat OA of the knee.6 The beneficial effects of HA are attributed to its function as a viscosupplement and its anti-inflammatory activity. HA injection is also used to treat tendon and ligament injuries and after surgery.7, 8
Several reports that compare the clinical outcomes achieved with HA and PRP for OA have been published.3, 4 However, the clinical results of simultaneous HA and PRP injections have not yet been reported.
Recently, Chen et al9 published an in vitro study of the synergistic anabolic actions of HA and PRP on cartilage regeneration in OA. In that report, the combination of HA and PRP reduced the levels of proinflammatory cytokines and increased articular chondrocyte proliferation and chondrogenic differentiation. The authors concluded that the observed synergistic effects were the result of different molecular mechanisms: the HA-dependent Erk1/2 pathway and the PRP-dependent Smad2/3 pathway. However, the direct influence of HA on the platelets in PRP was not discussed. In the present study, we tested the hypothesis that the addition of HA increases the levels of growth factors released by PRP.
Materials and methods
The protocol for this study was approved by the Ethics Committee of Hirosaki University Graduate School of Medicine, Aomori, Japan.
Preparation of PRP
Nine healthy adult volunteers (2 women and 7 men) with an average age of 32.8 ± 2.9 years (range, 29–37 years) were included in this study. Only one patient was taking medication of any kind, and that person was taking purgative medicine. No impairment of liver or kidney functions was detected in the patient blood samples.
Forty-five mL of peripheral blood for PRP preparation and an additional 1 mL of blood for the whole blood cell count were collected from the median cubital veins of each donor using a 21-gauge needle. No anticoagulant or activation materials, such as calcium chloride, were used. The PRP was produced using a commercial PRP separation system (Arthrex ACP; Arthrex, Naples, FL, USA) using a double syringe system according to the manufacturer's instructions. From each donor, 10–12 mL of PRP was prepared. Blood counts for the PRP preparations were measured using 1 mL of PRP.
PRP culture and harvest of released growth factors
ARTZ-Dispo HA (Seikagaku, Tokyo, Japan) with a weight-average molecular weight of 50–120 kDa was used as the HA. Three replicate wells of 1 mL of PRP and 0.2 mL of phosphate buffered saline (PBS; PRP group), three replicate wells of 1 mL of PRP and 0.2 mL of HA (PRP + HA group), and one well of 1 mL of PRP and 0.6 mL of HA (PRP + high HA group) were incubated on noncoated six-well dishes (Nunc, Shanghai, China) in a cell culture incubator at 37°C with 5% of CO2 immediately after PRP preparation. After 2 hours of incubation (defined as Day 0), all the specimens had formed gels. At that time, 8.8 mL of PBS was added to one well from the PRP and PRP + HA groups to a 10-fold dilution, and all the liquid was collected 1 hour later. Any remaining platelets were removed with gentle centrifugation for 15 minutes at 200g and then another centrifugation for 15 minutes at 10,000g. The samples were immediately frozen with liquid nitrogen and stored at −80°C until the growth factors were assessed. In the same way, samples from the PRP and PRP + HA groups were obtained on Day 3 and Day 5 after PRP preparation.
For five of the donor PRPs (n = 5 donors), the PRP + high HA group samples obtained on Day 5 were diluted with 8.4 mL of PBS because of the higher dose of 0.6 mL of HA. In addition, to confirm that the growth factors were continuously released from the PRP, 0.2 mL of PBS for the PRP group and 0.2 mL of HA for the HA group was added to the remaining gels (n = 5 per group) after sample collection on Day 0 and Day 3. The released growth factors were collected on Day 3 (Days 0–3) and Day 5 (Days 3–5) in a similar way as was done for the PRP and PRP + HA groups.
Gross appearance on Day 5
After collecting all of the samples, the remaining gels were fixed with absolute methanol for 5 minutes and Giemsa stained for 5 minutes. Microscopic images (Olympus IMT-2-21 RFM; Olympus Corp., Tokyo, Japan) were taken using a digital camera (Canon DS 126181; Canon Inc., Tokyo, Japan).
Haematology
The platelet, white blood cell, neutrophil, lymphatic cell, and red blood cell counts in the peripheral blood and PRP were determined using an automated cell count analyser (Sysmex XE-5000; Sysmex Corp., Kobe, Japan).
Transforming growth factor-β1 and platelet-derived growth factor-AA levels
After thawing the stored samples, quantitative determinations of the transforming growth factor-β1 (TGF-β1) and platelet-derived growth factor-AA (PDGF-AA) levels were performed using a commercially available enzyme-linked immunosorbent assay kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions. The colour intensity of each well was measured using a spectrophotometer (Multiskan FC; Thermo Fisher Scientific, Yokohama, Japan) at 450 nm with a wavelength correction of 570 nm. The final calculations were made using 10-fold sample dilutions.
Statistical analysis
All data are expressed as mean ± standard deviation. The statistical analyses were performed using paired t tests to compare the PRP and HA groups at each time point, and a one-way analysis of variance (one-way ANOVA) with Tukey posthoc tests was used to compare the PRP, PRP + HA, and PRP + high HA groups. A p value < 0.05 was considered statistically significant. All statistical analyses were performed in GraphPad Prism 6.0 (GraphPad Software, San Diego, CA, USA).
Results
Blood cell counts
The concentrations of platelets and WBCs in the PRP preparations were 2.35 ± 0.30 times and 0.305 ± 0.152 times those found in the peripheral blood. The percentage of lymphocytes was higher in the PRP preparations (Table 1).
Table 1.
Cell counts from the platelet-rich plasma and peripheral blood.
| Platelets (× 1012/L) |
WBC (× 109 cells/L) |
Neutrophils (% of WBC) |
Lymphocytes (% of WBC) |
Monocytes (% of WBC) |
Eosinophils (% of WBC) |
Basophils (% of WBC) |
RBC (× 1012/L) |
|
|---|---|---|---|---|---|---|---|---|
| PRP | 4.68 ± 0.95 | 1.53 ± 0.64 | 15.2 ± 7.8 | 77.3 ± 8.2 | 6.8 ± 2.7 | 0.1 ± 0.4 | 0.5 ± 0.4 | 0.31 ± 0.12 |
| PB | 2.13 ± 0.53 | 5.15 ± 0.97 | 55.6 ± 22.1 | 27.1 ± 12.9 | 4.2 ± 1.0 | 2.4 ± 1.7 | 0.4 ± 0.2 | 56.1 ± 10.2 |
Hb = hemoglobin; PB = peripheral blood; PRP = platelet-rich plasma; RBC = red blood cells; WBC = white blood cells.
Gross appearance at Day 5
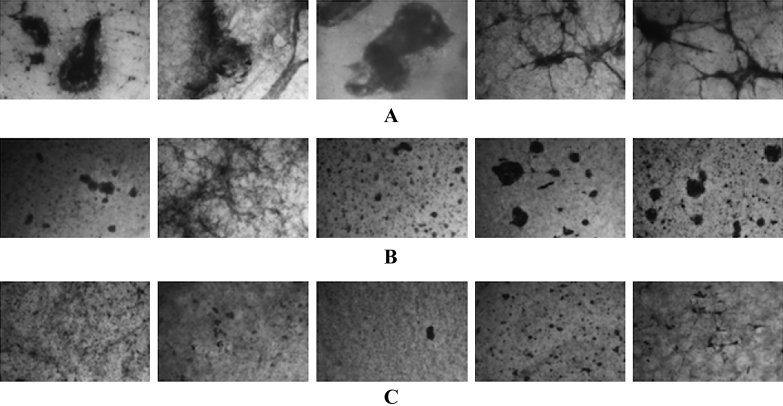
The PRP group showed larger coagulated masses than the PRP + HA group. The PRP + high HA group had the smallest coagulated mass of all of the groups (Figure 1).
Figure 1.
Morphology of the platelet-rich plasma (PRP) gels on Day 5. The gels placed in wells were stained after all measurements with Giemsa stain (n = 5). (A) PRP group; (B) PRP + hyaluronic acid (HA) group; and (C) PRP + high HA group. The size of the stained clots inside the gels was decreased in the samples with higher HA concentrations.
Growth factor concentrations
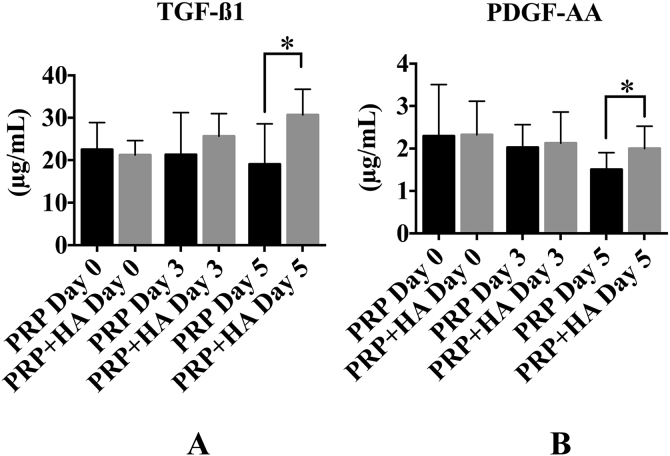
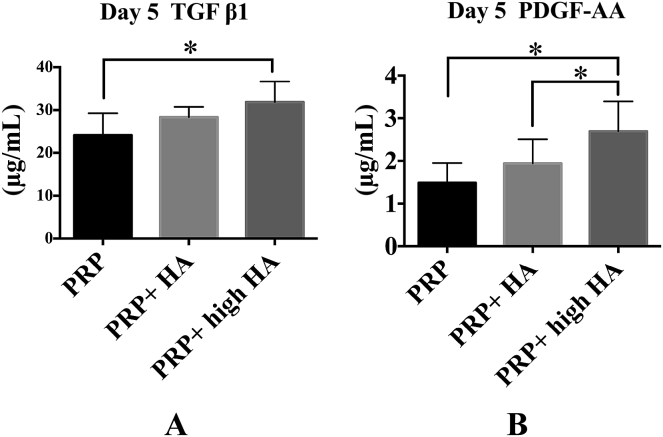
The TGF-β1 concentrations in the PRP and PRP + HA were 24.3 ± 7.2 μg/mL and 22.4 ± 1.8 μg/mL (p = 0.689) on Day 0, 17.2 ± 13.9 μg/mL and 25.4 ± 7.1 μg/mL (p = 0.331) on Day 3, and 12.7 ± 10.5 μg/mL and 33.7 ± 8.3 μg/mL (p = 0.034) on Day 5 (Figure 2A). The TGF-β1 concentrations on Day 5 were 24.1 ± 5.2 μg/mL (PRP group), 28.3 ± 2.4 μg/mL (PRP + HA), and 31.9 ± 4.8 μg/mL (PRP + high HA; one-way ANOVA: p = 0.003; posthoc PRP vs. PRP + HA: p = 0.016; Figure 3A).
Figure 2.
Comparison of the concentration of growth factors between the platelet-rich plasma (PRP) alone and hyaluronic acid (HA) groups (n = 9). (A) The transforming growth factor β1 (TGF-β1) level in the PRP + HA group was higher than that in the PRP group on Day 5. (B) The platelet-derived growth factor (PDGF-AA) level in the PRP + HA group was higher than that in the PRP group on Day 5. * p < 0.005.
Figure 3.
Comparison of the concentration of growth factors with hyaluronic acid (HA) concentration on Day 5 (n = 5). (A) The transforming growth factor β1 (TGF-β1) level in the platelet-rich plasma (PRP) + high HA group was higher than that in the PRP group. (B) The platelet-derived growth factor (PDGF-AA) level in the PRP + high HA group was higher than that in the PRP and PRP + HA groups. * p < 0.005.
The PDGF-AA concentrations in the PRP and PRP + HA groups were 2.30 ± 1.21 μg/mL and 2.32 ± 0.79 μg/mL (p = 0.931) on Day 0, 2.03 ± 0.53 μg/mL and 2.13 ± 0.73 μg/mL (p = 0.500) on Day 3, and 1.51 ± 0.40 μg/mL and 2.00 ± 0.52 μg/mL (p = 0.003) on Day 5 (Figure 2B). The PDGF-AA concentrations were 1.48 ± 0.46 μg/mL (PRP group), 1.94 ± 0.57 μg/mL (PRP + HA), and 2.69 ± 0.70 μg/mL (PRP + high HA; one-way ANOVA: p = 0.0002; PRP vs. PRP + high HA: p = 0.002; PRP + HA vs. PRP + high HA: p = 0.011) on Day 5 (Figure 3B).
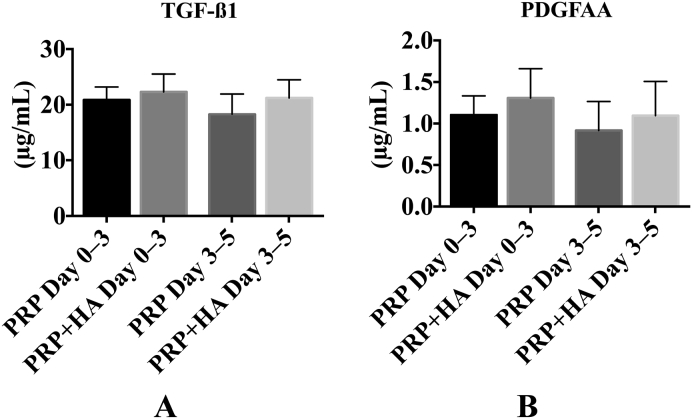
The TGF-β1 and PDGF-AA levels on Day 3 after the wells were washed on Day 0 (PRP Days 0–3, PRP + HA Days 0–3) were more than half of that found on Day 3 without washing. Those on Day 5 after being washed on Day 3 (PRP Days 3–5, PRP + HA Days 3–5) were also more than half of those on Day 5 without washing out (Figure 4), which suggests that the TGF-β1 and PDGF-AA were continuously released from the PRP gels until at least 5 days after incubation.
Figure 4.
Growth factor release between the observation time points (n = 5). Day 0–3 samples were assessed on Day 3 after being washed on Day 0, and Day 3–5 samples were assessed on Day 5 after being washed on Day 3. (A) Transforming growth factor β1 (TGF-β1) and (B) platelet-derived growth factor (PDGF-AA) levels were assayed, and all the values were more than half of that of the same condition when assessed without being washed out.
Discussion
PRP can stimulate the healing process of different tissues by delivering various growth factors and cytokines that are released by platelets. In the present study, we hypothesized that adding HA to the PRP would increase the concentration of growth factors released. This hypothesis was correct on Day 5, but not on Day 0 or Day 3. These findings suggest that stimulatory effect of HA on growth factor release seems to appear slowly. Frelinger et al10 suggested that pulse electric field may cause a selective permeabilization of specific granules or populations of α granules. In this study, after removing growth factors in the supernatant, both TGF-β1 and PDGF-AA were released from platelets and the concentrations were elevated close to Day 0 levels. Platelets may detect the surrounding growth factor concentrate and release growth factors depending on the concentrate. Surrounding HA possibly affects the selective permeabilization and population of α granules. Activated platelets also form CD41 microparticles, which function as a transport and delivery system for bioactive molecules, participating in haemostasis and thrombosis, inflammation, malignancy infection transfer, angiogenesis, and immunity.11 Hu et al12 showed that the expression of P-selectin dramatically increased after PRP interacted with biomacromolecule complex film (HA–collagen (I)/chitosan). HA engagement of CD44 leads to MAP kinase-dependent increased trafficking of TGF-β receptors to lipid raft-associated pools, which facilitates increased receptor turnover and attenuation of TGF-β1-dependent alteration in proximal tubular cell function. Further investigation is need to elucidate the mechanism on growth factor delivery.
Fibrin networks are formed by the conversion of fibrinogen. Different fibre diameters, mass/length ratios, densities, porosities, and permeabilities of the fibrin networks can alter cell adhesion and migration.13 Perez et al14 found that different PRP preparations made different fibrin networks. In the present study, smaller fibrin clots were observed in the HA group than in the other groups. Srinivasan et al15 reported that heparin sulfate proteoglycan (Perlecan/HSPG2) protects bone morphogenetic protein 2 (BMP2) from proteolytic cleavage through storing and controlling the release kinetics of BMP2, which reduced knee OA in mice. Viscosupplementation with HA may inhibit the aggregation of platelets and may affect the delivery of growth factors.
HA has been widely used to treat OA, especially in Japan.6 HA provides viscoelastic properties to the synovial fluid and contributes to boundary lubrication.16 HA demonstrates several pleiotropic signalling properties, including anti-inflammatory, antiapoptotic, antiangiogenic, and antifibrotic effects on animal models of OA.17 HA also has analgesic properties with a specific activity on opioid receptors.18, 19 Chen et al9 showed, in an in vitro study, the synergistic anabolic actions of HA and PRP on cartilage regeneration in OA. In that report, a combination of HA and PRP reduced the proinflammatory cytokines and increased articular chondrocyte proliferation and chondrogenic differentiation via the HA-dependent Erk1/2 pathway and the PRP-dependent Smad2/3 pathway. Together, those reports and our results suggest that the clinical application of a PRP and HA mixture may be more effective than either PRP or HA alone for certain tissues.
There were several limitations to the present study. First, we only examined two of the many potential growth factors, such as epidermal growth factor, fibroblast growth factor, vascular endothelial growth factor, or insulin-like growth factor. We selected TGF-β1 and PDGF for analysis because those are two of the most widely studied growth factors that play central roles in tissue regeneration. However, the effects of HA on the release of other growth factors may be different than the results obtained for those two. Second, only two female donors were included in this study. Sex differences may have influenced the results, although Weibrich et al20 found no effect of sex on growth factor concentration. Third, we showed only five donors data of high HA group. This group was added after first four donors sample data examined. Finally, the PRP samples were produced at different times, and circadian rhythms may have influenced the data. However, Aoto et al21 reported that there were no significant diurnal variations in the release of TGF-β1 and PDGF-BB.
In conclusion, HA increased the release of TGF-β1 and PDGF-AA from PRP on Day 5. Thus, a mixture of PRP and HA may result in an enhanced the healing effect on certain tissues.
Conflicts of interest
Arthrex Japan G. K. (Tokyo, Japan) provided special syringes for the PRP production (the ACP double syringe system). The authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers' bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Funding/support
No financial support was received for the work described in this article.
Acknowledgements
The authors thank the volunteers for their enthusiastic participation in this study. The authors also thank the members of the Department of Laboratory Medicine, Hirosaki University Graduate School of Medicine for helping with the blood count analysis. K.I. thanks Professor Manabu Murakami for his generous advice and support throughout this work.
References
- 1.Moraes V.Y., Lenza M., Tamaoki M.J., Faloppa F., Belloti J.C. Platelet-rich therapies for musculoskeletal soft tissue injuries. Cochrane Database Syst Rev. 2014;29 doi: 10.1002/14651858.CD010071.pub3. 4:CD010071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roffi A., Filardo G., Kon E., Marcacci M. Does PRP enhance bone integration with grafts, graft substitutes, or implants? A systematic review. BMC Musculoskelet Disord. 2013;14:330. doi: 10.1186/1471-2474-14-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raeissadat S.A., Rayegani S.M., Hassanabadi H. Knee osteoarthritis injection choices: platelet- rich plasma (PRP) versus hyaluronic acid (a one-year randomized clinical trial) Clin Med Insights Arthritis Musculoskelet Disord. 2015;8:1–8. doi: 10.4137/CMAMD.S17894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kon E., Mandelbaum B., Buda R. Platelet-rich plasma intra-articular injection versus hyaluronic acid viscosupplementation as treatments for cartilage pathology: from early degeneration to osteoarthritis. Arthroscopy. 2011;27:1490–1501. doi: 10.1016/j.arthro.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Ramos-Torrecillas J., García-Martínez O., De Luna-Bertos E., Ocaña-Peinado F.M., Ruiz C. Effectiveness of platelet-rich plasma and hyaluronic acid for the treatment and care of pressure ulcers. Biol Res Nurs. 2015;17:152–158. doi: 10.1177/1099800414535840. [DOI] [PubMed] [Google Scholar]
- 6.Ishijima M., Nakamura T., Shimizu K. Intra-articular hyaluronic acid injection versus oral non-steroidal anti-inflammatory drug for the treatment of knee osteoarthritis: a multi-center, randomized, open-label, non-inferiority trial. Arthritis Res Ther. 2014;16:R18. doi: 10.1186/ar4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abate M., Schiavone C., Salini V. The use of hyaluronic acid after tendon surgery and in tendinopathies. Biomed Res Int. 2014;2014:783632. doi: 10.1155/2014/783632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petrella R.J., Petrella M.J., Cogliano A. Periarticular hyaluronic acid in acute ankle sprain. Clin J Sport Med. 2007;17:251–257. doi: 10.1097/JSM.0b013e3180f6169f. [DOI] [PubMed] [Google Scholar]
- 9.Chen W.H., Lo W.C., Hsu W.C. Synergistic anabolic actions of hyaluronic acid and platelet-rich plasma on cartilage regeneration in osteoarthritis therapy. Biomaterials. 2014;35:9599–9607. doi: 10.1016/j.biomaterials.2014.07.058. [DOI] [PubMed] [Google Scholar]
- 10.Frelinger A.L., III, Torres A.S., Caiafa A. Platelet-rich plasma stimulated by pulse electric fields: platelet activation, procoagulant markers, growth factor release and cell proliferation. Platelets. 2015 Aug;19:1–8. doi: 10.3109/09537104.2015.1048214. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Italiano J.E., Jr., Mairuhu A.T., Flaumenhaft R. Clinical relevance of microparticles from platelets and megakaryocytes. Curr Opin Hematol. 2010;17:578–584. doi: 10.1097/MOH.0b013e32833e77ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu Y., Wu Y., Cai J., Ma S., Wang X. The procoagulant properties of hyaluronic acid-collagen (I)/chitosan complex film. J Biomater Sci Plym Ed. 2009;20:1111–1118. doi: 10.1163/156856209X444457. [DOI] [PubMed] [Google Scholar]
- 13.Davis H.E., Miller S.L., Case E.M., Leach J.K. Supplementation of fibrin gels with sodium chloride enhances physical properties and ensuing osteogenic response. Acta Biomater. 2011;7:691–699. doi: 10.1016/j.actbio.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Perez A.G., Rodrigues A.A., Luzo A.C., Lana J.F. Fibrin network architectures in pure platelet-rich plasma as characterized by fiber radius and correlated with clotting time. J Mater Sci Mater Med. 2014;25:1967–1977. doi: 10.1007/s10856-014-5235-z. [DOI] [PubMed] [Google Scholar]
- 15.Srinivasan P.P., McCoy S.Y., Jha A.K. Injectable perlecan domain 1-hyaluronan microgels potentiate the cartilage repair effect of BMP2 in a murine model of early osteoarthritis. Biomed Mater. 2012;7:024109. doi: 10.1088/1748-6041/7/2/024109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Musumeci G., Loreto C., Carnazza M.L., Cardile V., Leonardi R. Acute injury affects lubricin expression in knee menisci: an immunohistochemical study. Ann Anat. 2013;195:151–158. doi: 10.1016/j.aanat.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 17.Abate M., Pulcini D., Di Iorio A., Schiavone C. Viscosupplementation with intra-articular hyaluronic acid for treatment of osteoarthritis in the elderly. Curr Pharm Des. 2010;16:631–640. doi: 10.2174/138161210790883859. [DOI] [PubMed] [Google Scholar]
- 18.Baker J.F., Solayar G.N., Byrne D.P., Moran R., Mulhall K.J. Analgesic control and functional outcome after knee arthroscopy: results of a randomized double-blinded trial comparing a hyaluronic acid supplement with bupivacaine. Clin J Sport Med. 2012;22:109–115. doi: 10.1097/JSM.0b013e318240e123. [DOI] [PubMed] [Google Scholar]
- 19.Zavan B., Ferroni L., Giorgi C. Hyaluronic acid induces activation of the kappa-opioid receptor. PloS ONE. 2013;8:e55510. doi: 10.1371/journal.pone.0055510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weibrich G., Kleis W.K., Kunz-Kostomanolakis M., Loos A.H., Wagner W. Correlation of platelet concentration in platelet-rich plasma to the extraction method, age, sex, and platelet count of the donor. Int J Oral Maxillofac Implants. 2001;16:693–699. [PubMed] [Google Scholar]
- 21.Aoto K., Kanamori A., Yoshioka T., Uemura K., Sakane M., Yamazaki M. Circadian variation of growth factor levels in platelet-rich plasma. Clin J Sport Med. 2014;24:509–512. doi: 10.1097/JSM.0000000000000080. [DOI] [PubMed] [Google Scholar]