Abstract
Objective
Clear cell renal cell carcinoma (ccRCC) is the most common subtype of renal cell carcinoma (RCC) and is characterized by biallelic inactivation of the von Hippel-Lindau (VHL) tumor suppressor gene. One effect of VHL inactivation is hypoxia inducible factor alpha (HIFα)-independent constitutive activation of nuclear factor kappa B (NF-κB) and c-jun N-terminal kinase (JNK). Both NF-κB and JNK drive ccRCC growth and epithelial to mesenchymal transition (EMT). The purpose of this study was to determine the biochemical effects of pomegranate juice extracts (PE) on RCC cell lines.
Methods
The pre-clinical effects of PE on NF-κB, JNK, and the EMT phenotype were assayed, including its effect on proliferation, anchorage-independent growth, and invasion of pVHL-deficient RCCs.
Results
PE inhibits the NF-κB and JNK pathways and consequently inhibits the EMT phenotype of pVHL-deficient ccRCCs. The effects of PE are concentration-dependent and affect not only biochemical markers of EMT (i.e., cadherin expression) but also functional manifestations of EMT, such as invasion. These effects are manifested within days of exposure to PE when diluted 2000-fold. Highly dilute concentrations of PE (106 dilution), which do not impact these pathways in the short term, were found to have NF-κB and JNK inhibitory effects and ability to reverse the EMT phenotype following prolonged exposure.
Conclusion
These findings suggest that PE may mediate inhibition growth of pVHL-deficient ccRCCs and raises the possibility of its use as a dietary adjunct to managing patients with active surveillance for small, localized, incidentally identified renal tumors so as to avoid more invasive procedures such as nephrectomy.
Keywords: Pomegranate extract, von Hippel-Lindau (VHL) tumor suppressor, Clear cell renal cell carcinoma, c-Jun N-terminal kinase, Epithelial to mesenchymal transition, Nuclear factor kappa B
1. Introduction
Almost 65,000 new cases of kidney cancer are expected in the United States in 2014, of which 90% are renal cell carcinomas (RCC) [1]. RCCs consist of several subtypes, the most common of which is clear cell RCC (ccRCC), accounting for 80%–85% of all renal epithelial malignancies [1], [2]. The genetic hallmark of both hereditary and sporadic forms of ccRCC is biallelic inactivation of the von Hippel-Lindau (VHL) tumor suppressor gene, the protein product of which, pVHL, encodes a component of an E3 ubiquitin ligase, the most well characterized target of which is hypoxia inducible factor alpha (HIFα). In the context of biallelic VHL gene loss, mutation, or inactivation that occurs in upwards of 90% of sporadic ccRCCs, HIFα accumulates and induces a transcriptional program, including upregulation of cell cycle proteins (cyclin D, cyclin G2), angiogenesis factors (VEGF), and growth factors (TGFα), that drives ccRCC oncogenesis [3], [4].
Unfortunately, our ability to exploit the pVHL-HIFα interaction for therapeutic benefit of patients is hampered by the fact that efficient and selective restoration of VHL gene expression in tumor cells of actual patients is not achievable with current gene therapy technologies, and transcription factors such as HIFα are not readily amenable to drug development. Moreover, small molecule therapies that have come to the clinic over the last several years, including the tyrosine kinase inhibitors and rapamycin analogs that target select downstream targets of HIFα, have relatively modest impact on overall survival, which is not unexpected given that the HIFα-induced gene expression profile dozens of genes, many of which can drive tumor growth but are not inhibited by the aforementioned drug classes. As such, HIFα-independent effects of pVHL have been investigated and identified, and may represent druggable targets for therapeutic intervention. Nuclear factor kappa B (NF-κB) is constitutively activated in ccRCC patient specimens and drives pVHL-deficient ccRCC growth and EMT [5], [6], [7], [8], [9]. Recently, we have shown that the c-jun N-terminal kinase (JNK)/activator protein 1 (AP1) pathway is constitutively activated in pVHL-deficient RCCs in a HIFα-independent fashion, and can also promote EMT and tumorigenesis of ccRCCs [10].
Pomegranate extract (PE) has been shown to inhibit NF-κB in normal human cells, including chondrocytes, epidermal keratinocytes, and vascular endothelial cells [11], [12], [13]. We have also shown that PE blocks NF-κB activity in prostate cancer models both in vitro and in vivo [14]. Inhibition of JNK by PE in immune cells has been demonstrated as well [15]. PE also manifests JNK modulatory effects in prostate cancer [16]. Given the role of the NF-κB and JNK pathways in the EMT and tumorigenesis of ccRCC and the potential of PE to inhibit these pathways, we investigated the ability of PE to inhibit constitutive NF-κB and JNK activity and EMT in ccRCC models.
2. Materials and methods
2.1. Reagents
PE is a standardized extract (POMX™, provided by POM Wonderful, Inc., Los Angeles, CA, USA) of pomegranate fruit grown in California, USA (Punica granatum L., Wonderful variety, Paramount Farms, Lost Hills, CA, USA). PE is made from fruit skins standardized to ellagitannins (ETs), as punicalagins (37%–40%), and free ellagic acid (3.4%), as determined by high performance liquid chromatography (HPLC) using previously described methods [17]. 1000 mg of POMX powder includes up to 600 mg of polyphenol from extract, which delivers pomegranate polyphenols in an amount equivalent to about 8 oz of pomegranate juice. The original PE liquid represents the “1×” dilution. Further dilutions were made in culture medium to obtain the final concentrations employed for all experiments. PE dilutions that were employed for most experiments (≤48 h) were generally between 4 × 10−4 and 5 × 10−2. The IKKβ (IKK inhibitor IV) was purchased from Calbiochem (Gibbstown, NJ, USA; catalog 401480).
2.2. Cell lines
The RCC cell lines, ACHN and SN12C, which endogenously express wild-type pVHL, were transduced with a lentivirus that encodes VHL-specific shRNA or a scrambled control, and were a kind gift of Dr. George Thomas (Oregon Health Sciences University). These cell lines and the VHL shRNA sequences have been previously described [18].
2.3. Transient transfections and reporter gene assays
Cells were plated at 105 cells/well in 24-well plates the day prior to transfection. NF-κB or AP1 driven reporter constructs (pκB-luc and pAP1-luc, BD Sciences, Clontech, Mountain View, CA, USA; 1 μg per well), which express Firefly luciferase, were co-transfected with the pRL-SV40 plasmid (Promega, Madison, WI, USA; 1 ng/well), the latter to normalize for transfection efficiency. The plasmids were transfected with Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. Protein was extracted 48 h after transfection, and firefly and Renilla luciferase were measured on a TD20/20 tube luminometer using a Dual Luciferase Assay kit (Promega, Madison, WI, USA) according to the manufacturer's instructions.
2.4. Electrophoretic mobility shift assays (EMSAs)
Wild-type and mutant κB and AP1 double-stranded oligonucleotide probes were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Fifteen μg of nuclear protein were combined with end-labeled, double-stranded oligonucleotide probe, 1 μg of poly-dIdC (Amersham Pharmacia Biotech, Piscataway, NJ, USA), 1 μg of BSA, and 5 mmol/L spermidine in a final reaction volume of 20 μL for 20 min at room temperature. The DNA protein complex was run on a 4% non-denaturing polyacrylamide gel with 0.4× TBE running buffer prior to subsequent autoradiography. Cold-competition experiments were performed with a 100-fold molar excess of cold wild-type or cold mutant κB or AP1 oligonucleotides. For supershift assays, nuclear protein was pre-incubated with specific or control antibodies (6 μg; purchased from Santa Cruz) for 20 min at room temperature. An identical strategy was employed for EMSAs with Oct-1 probes as a specificity control for the effects of PEs.
2.5. Cell growth assay
Cells were seeded in 96-well plates at 1.5 × 104 cells per well in 100 μL of culture medium. Cell viability was assessed by the MTT (3,[4,5-dimethylthiazol-2-yl-] diphenyltatrazolium bromide) assay. Twenty-five μL of MTT (5 mg/mL) was added to each well for 3 h at 37 °C. Subsequently, 100 μL of 10% sodium dodecyl sulfate/0.01 mol/L HCl was added overnight at 37 °C. Absorbance was measured at 570 nm on a microplate reader. All experiments were performed in quadruplicate.
2.6. In vitro kinase assay
An in vitro kinase assay kit (Cell Signaling Technology, Danvers, MA, USA) was performed to measure the activity of JNK according to the manufacturer's instructions. An IKKβ in vitro kinase assay was modified as described [19]. Briefly, 250 μg of whole cell lysates was immunoprecipitated with an anti-IKKβ antibody (1:50) and then mixed with 1 μL recombinant GST-IκB (Cell Signalling Technology) in kinase buffer with 5 μCi of γ-32P-ATP for 30 min at 30 °C. The reaction was analyzed on a Tris-Glycine gel and subjected to autoradiography.
2.7. Matrigel invasion assay
The matrigel invasion assay was performed according to the manufacturer's instructions (BD Biosciences, San Diego, CA, USA). Briefly, 2.5 × 104 cells in 0.5 mL of medium containing 1% fetal bovine serum (FBS) were to the transwell insert, which was seated in 750 μL of complete medium (10% FBS) with or without the IKKβ inhibitor (10 μmol/L). After an overnight (12 h) incubation at 37 °C in a 5% CO2 humidified atmosphere, non-invading cells were mechanically removed. Cells that had migrated through the matrigel were stained with the Diff-Quick staining kit (Dad Behring, Inc., Newark, DE, USA) according to the manufacturer's instructions. Cells were counted in five representative microscopic fields (200× magnification) and photographed.
2.8. Western blotting
Western blotting was performed as previously described [19], [20].
2.9. Anchorage-independent growth assay
This assay was performed as described by us [19].
3. Results
3.1. PE inhibits NF-κB and JNK activity in RCC cells
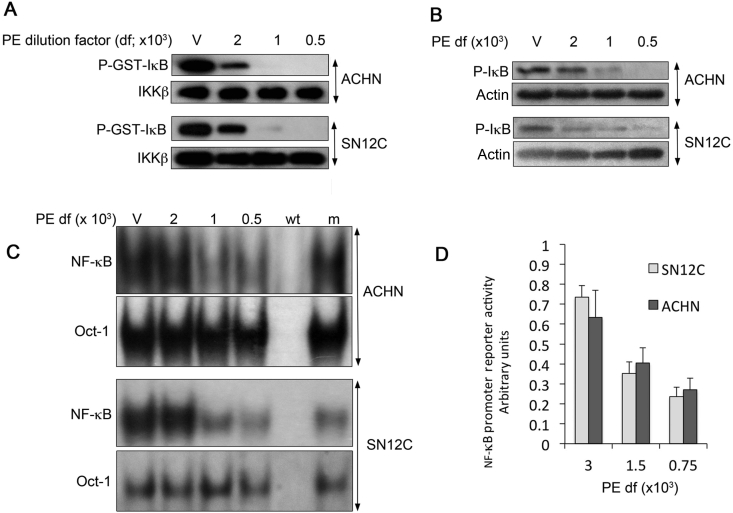
NF-κB activation that is driven by VHL loss induces expression of transcription factors such as Twist and Slug that suppress E-cadherin expression, increase N-cadherin expression, and heighten cellular invasiveness and anchorage-independent growth. We have previously shown that the SN12C-VHLlow and ACHN-VHLlow cell lines, which are isogenic partners of SN12C and ACHN stably transduced with VHL-specific shRNA, acquire an EMT phenotype consistent with their constitutive activation of NF-κB [18], [20]. NF-κB activation is mediated through the IKK complex, which phosphorylates the NF-κB inhibitor, IκB, thereby marking it for ubiquitination and proteasome-dependent degradation. We and others have previously described that pVHL-deficient RCCs manifest increased NF-κB activity compared to isogenic cells that express wild-type pVHL [8], [21]. Given the previously described NF-κB inhibitory effects of PE in other model systems [14], we investigated the effects of PE on constitutive NF-κB activity in pVHL-deficient RCC cells. Indeed, IKK activity as measured by in vitro kinase assays was inhibited in a concentration dependent fashion (Fig. 1A). The phosphorylation of IκB, the substrate of the IKK complex, was also reduced as demonstrated by Western blotting (Fig. 1B).
Figure 1.
PE inhibits constitutive NF-κB activity in pVHL-deficient RCCs. (A) IKKβ in vitro kinase assays. After overnight exposure of pVHL deficient RCCs (ACHN-VHLlow and SN12C-VHLlow) to the indicated dilutions of PE, IKKβ was immunoprecipitated and phosphorylation of a recombinant substrate, GST-IκB, was assessed by immunoblotting. The dilutions of PE are shown, with “V” indicating vehicle control. (B) Western blots for phosphorylated IκB after overnight exposure to the indicated dilutions of PE. (C) EMSAs for NF-κB after overnight exposure to PE. Cold competition experiments illustrate the specificity of the gel shifted bands: m, mutant κB oligonucleotide probe; wt, wild-type κB oligonucleotide probe. (D) NF-κB driven reporter assays after 48 h of PE exposure. Results are means ± SD of triplicate experiments. Results were normalized to that of vehicle treated cells.
Upon degradation of IκB, NF-κB transcription factors, which reside in the cytoplasm in the quiescent state, are disinhibited and translocate to the nucleus, where they bind to κB DNA binding sites in the regulatory regions of target genes. Inhibition of NF-κB binding to consensus κB response elements by PE was demonstrated by electrophoretic mobility shift assays (EMSAs) as shown in Fig. 1C. Lastly, the functional effects of PE on the NF-κB pathway were illustrated by the concentration-dependent inhibition of NF-κB driven reporter gene activity (Fig. 1D).
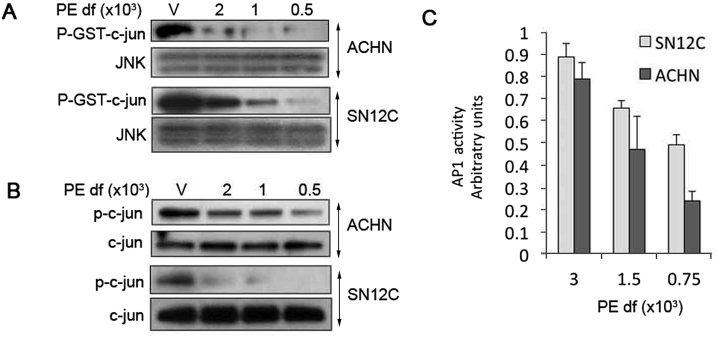
We recently reported that pVHL-deficiency was causally linked to constitutive activation of JNK, which in turn phosphorylates c-jun, leading to subsequent heterodimerization of phospho-c-jun to c-fos to form transcriptionally competent AP1 complexes [10]. AP1 induces expression of Twist, a key mediator of EMT that suppresses E-cadherin and induces N-cadherin expression [10]. The net effect of AP1 activation is heightened growth and tumorigenesis of pVHL-deficient ccRCCs [10]. In light of these AP1-mediated effects, we determined whether PE could inhibit JNK activity analogously to its effects on the NF-κB pathway. PE inhibited the functional activity of JNK in a concentration-dependent fashion as measured by in vitro kinase assays (Fig. 2A). Moreover, phosphorylation of c-jun at serine 73, a phosphorylation site of JNK, was reduced by PE in a similar manner (Fig. 2B). As further evidence of JNK blockade, a concentration dependent inhibition of AP1-driven reporter gene expression by PE was demonstrated (Fig. 2C).
Figure 2.
PE inhibits constitutive JNK activity in pVHL-deficient RCCs. (A) JNK in vitro kinase assays. After overnight exposure to PE, total JNK was immunoprecipitated, and JNK activity was measured by phosphorylation of recombinant GST-c-jun by immunoblotting. (B) Western blots for phosphorylated c-jun and total c-jun after overnight exposure to PE. (C) AP1 driven reporter assays performed 48 h of PE exposure. Results are means ± SD of triplicate experiments. Results were normalized to that of vehicle treated cells.
3.2. PE inhibits growth and EMT of pVHL-deficient RCCs
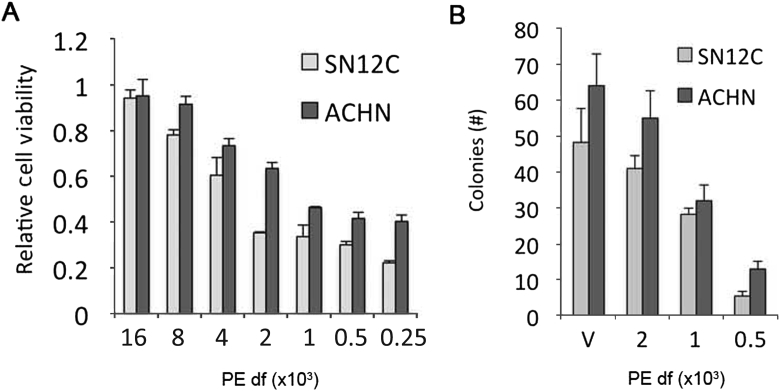
Given the NF-κB and JNK inhibitory properties of PE and the role of these signaling pathways in promoting proliferation, growth and EMT, we assessed the functional impact of PE on pVHL-deficient RCCs. PE inhibited the in vitro growth of both SN12C-VHLlow and ACHN-VHLlow cells in a concentration dependent manner as measured in MTT assays over 120 h (Fig. 3A). Next, we tested the effect of PE on anchorage-independent growth. PE reduced not only the size of individual colonies (not shown), an indicator of proliferation, but also the total number of colonies formed in soft agar (Fig. 3B), an in vitro marker of metastatic potential [22].
Figure 3.
Effects of PE on in vitro growth on plastic and anchorage-independent growth in soft agar. (A) ACHN-VHLlow or SN12C-VHLlow cells were treated with the indicated dilutions of PE for 120 h. Total cell number was measured in MTT assays. Results are means ± SD of quadruplicate experiments. (B) Anchorage-independent growth was assessed by growth of ACHN-VHLlow or SN12C-VHLlow in soft agar with the indicated dilutions of PE for 14 days. Results are means ± SD of number of colonies per microscopic field (final magnification, 200×).
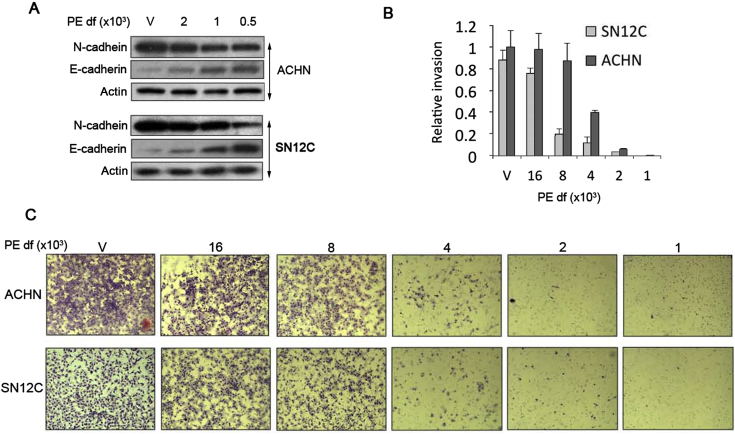
At baseline, pVHL-deficient ACHN and SN12C cells exhibit high N-cadherin and low E-cadherin expression characteristic of a mesenchymal phenotype (Fig. 4A). Upon exposure to PE, cells underwent a “cadherin switch” in which the heightened N-cadherin/low E-cadherin pattern reverted to a high E-cadherin/low N-cadherin pattern typified by epithelial cells (Fig. 4A). Importantly, PE also inhibited invasiveness of pVHL-deficient cells in a matrigel invasion chamber in a concentration-dependent manner, a hallmark of EMT (Fig. 4B and C); cell numbers were not significantly affected in pVHL-deficient cells during the overnight time course required for the matrigel invasion assay, indicating that the effects of PE on proliferation did not influence the results of the invasion assays. Taken together, our results indicate that PE inhibits growth and EMT of pVHL-deficient cells by inducing a reversion of the mesenchymal phenotype that is characteristic of pVHL-deficient RCCs to a phenotype that more closely resembles that of epithelial cells. These findings are consistent with previous reports demonstrating the plasticity of EMT in ccRCCs, which can undergo a reversion to an epithelial phenotype (i.e., a mesenchymal to epithelial transition or MET) upon inhibition of the JNK or NF-κB pathways [10], [20].
Figure 4.
Inhibition of EMT phenotype by PE. (A) PE induces a “cadherin switch”. Western blots for the indicated proteins after 48 h of exposure to the indicated dilutions of PE. (B) PE inhibits invasion of pVHL-deficient RCCs. Invasion was measured in a Matrigel invasion assay after overnight exposure to the indicated dilutions of PE. PE did not affect the overall number of cells during overnight exposure, so that the invasion results were not influenced by differences in cell number. Results are the means ± SD of the number of cells counted per microscopic field (final magnification, 200×). Results were normalized to that of vehicle treated cells. (C) Images of cell invasion assays.
3.3. Prolonged exposure to low concentrations of PE inhibits constitutive NF-κB and JNK activation and induces a “cadherin switch”
In the aforementioned experiments, we exposed SN12C-VHLlow and ACHN-VHLlow to concentrations of PE that were sufficient to inhibit molecular targets of interest over 24–48 h. In the following experiments, we determined whether substantially more dilute concentrations of PE, which perhaps may not exhibit NF-κB and/or JNK inhibitory activity over relatively short time periods, may in fact effectively inhibit these signaling pathways when cells are exposed to PE for prolonged durations (i.e. weeks).
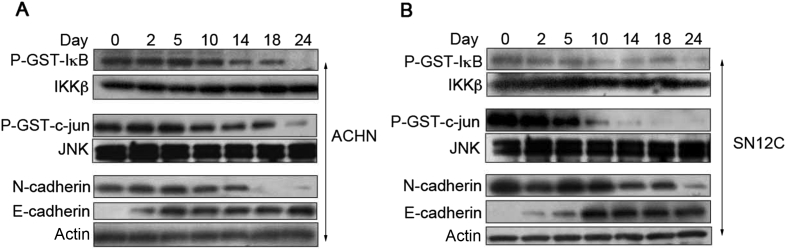
We used PE at a 106 dilution of the stock solution and tested the effects of dilute PE on NF-κB and JNK activity as well as the expression of E-cadherin and N-cadherin. Effects of dilute PE on JNK and NF-κB were not observed until approximately 10 days of exposure; continued treatment led to further inhibition with robust blockade of both IKK and JNK activity observed after 24 days (Fig. 5). The reversal of cadherin expression manifested different kinetics for N-cadherin and E-cadherin. N-cadherin expression remained largely unaffected until Day 14, after which time its expression was reduced through Day 24 (Fig. 5). Conversely, E-cadherin expression was induced at earlier time points (i.e. Day 2) and reached maximal expression levels by Day 5 (ACHN) or Day 10 (SN12C). The relatively early change to E-cadherin expression in the absence of demonstrable inhibition of NF-κB or JNK suggests that biochemical signaling pathways independent of NF-κB and JNK may be operative in regulating E-cadherin expression in response to PE. This phenomenon does not exclude the role of NF-κB and JNK perhaps at later time points, especially given the abundance of previous reports causally linking these pathways to EMT and cadherin expression in pVHL-deficient models. Nonetheless, prolonged exposure to extremely dilute concentrations of PE led to a time dependent inhibition of constitutive NF-κB and JNK activity and induced a “cadherin switch” characterized by increasing E-cadherin and decreasing N-cadherin expression consistent with reversion to an epithelial phenotype.
Figure 5.
Highly diluted PE inhibits NF-κB and JNK activity and induces a “cadherin switch” over prolonged exposure times. (A)ACHN-VHLlow and (B) SN12C-VHLlow were exposed to highly dilute concentrations of PE (diluted 10−6 in vehicle). Cells were then exposed to dilute PE for the indicated number of days, and IKKβ in vitro kinase assays (top panels), JNK in vitro kinase assays (middle panels), and Western blots for cadherins (bottom panels) were performed.
4. Discussion
Approximately 65,000 cases of and 13,500 deaths attributable to kidney cancer are expected in the United States in 2012 [2]. Most kidney cancers are RCCs, and deaths are caused by metastatic disease and its complications. Novel treatments, which include rapamycin analogs (e.g., everolimus and temsirolimus) and tyrosine kinase inhibitors (e.g., pazopanib, sunitinib, etc.) that target various plasma membrane (e.g., VEGFR) and intracellular kinases (e.g., Raf), are modestly and only transiently effective and do not eradicate the disease [23]. Thus, despite the advent of several agents to treat metastatic RCC, the disease remains essentially incurable.
Efforts to identify signaling pathways that are hyperactivated in tumor cells as a result of “driver” genetic lesions such as biallelic inactivation of the von Hippel-Lindau tumor suppressor gene (VHL), can provide opportunities for therapeutic intervention. For example, the NF-κB pathway is constitutively activated in response to VHL loss and induces RCC growth and EMT [5], [6], [7], [8], [9], [21]. Recently, we identified JNK activation in VHL-inactivated tumors; in this context, JNK induces EMT and heightened tumorigenesis [10]. Thus, both NF-κB and JNK represent pathways that appear to be primed for therapeutic intervention of ccRCC.
Here, we have shown that PE inhibits the NF-κB and JNK pathways and consequently inhibits the EMT phenotype of pVHL-deficient ccRCCs. The effects of PE are concentration-dependent and affect not only biochemical markers of EMT (i.e., cadherin expression) but also functional manifestations of EMT, such as invasion. Anchorage-independent growth was also inhibited. Whereas the antiproliferative effects of PE may account for the reduced colony size in anchorage-independent growth assays, the number of colonies is not affected by proliferation. Importantly, highly diluted concentrations of PE when maintained over a prolonged duration were also able to reverse EMT, a finding that may have clinical and translational implications in that low dose daily ingestion of PE may have anti-tumor effects. Additional in vivo studies will be warranted to further pre-clinically validate PE as a potentially active clinical approach to RCC. One particular scenario in which clinical usage of PE in this fashion may be particularly germane is in the management of incidentally identified small renal tumors. The incidence of RCCs has dramatically increased over the last 10–15 years, with the marked rise in incidence attributable to detection of early stage but not advanced stage tumors [24], [25]. The increased usage of radiographic studies, including CT and ultrasonography are capturing incidental, small renal tumors. Many of these tumors have slow growth kinetics and fairly low risk for progression to more advanced stage [26], [27], [28], [29], [30]. The recognition that many small renal tumors demonstrate low malignant potential and that treatment may pose a greater risk than following patients over time has led to the concept of active surveillance. Initial active surveillance with delayed treatment for patients who progress has become an accepted practice in elderly patients or those with significant co-morbidities in whom the risk of treatment is deemed high [26], [27], [28], [29], [30]. It is in this setting that PE consumption may have a clinical role in preventing the progression of small, incidentally detected RCCs from disease progression. Such an approach could obviate the need for invasive procedures, such as partial or radical nephrectomy or other techniques such as cryoablation and radiofrequency ablation. Of course, the use of PE for the long-term management of small renal tumors would require formal clinical investigation, but the results presented herein along with previous reports relating NF-κB and JNK activation to RCC progression provide the pre-clinical foundation for clinical trials aimed at demonstrating that PE can effectively prevent the progression or perhaps induce regression of small renal tumors, an increasing clinical problem arising from the heightened use of radiographic modalities for abdominal imaging.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgment
We thank George W. Thomas (Oregon Health Sciences University) for the SN12C and ACHN cell lines with stable expression of VHL-specific shRNA. This work was supported by a Merit Review grant from The Department of Veterans Affairs (M.B. Rettig) and Pom Wonderful (A.J. Pantuck).
This work was supported by a Merit Review grant (M.B.R.) from the Department of Veterans Affairs and by an unrestricted research grant Pom Wonderful (A.P.).
Footnotes
Peer review under responsibility of Chinese Urological Association and SMMU.
Contributor Information
Allan J. Pantuck, Email: apantuck@mednet.ucla.edu.
Matthew B. Rettig, Email: mrettig@mednet.ucla.edu.
References
- 1.DeSantis C.E., Lin C.C., Mariotto A.B., Siegel R.L., Stein K.D., Kramer J.L. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R., DeSantis C., Virgo K., Stein K., Mariotto A., Smith T. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 3.Baba M., Hirai S., Yamada-Okabe H., Hamada K., Tabuchi H., Kobayashi K. Loss of von Hippel-Lindau protein causes cell density dependent deregulation of CyclinD1 expression through hypoxia-inducible factor. Oncogene. 2003;22:2728–2738. doi: 10.1038/sj.onc.1206373. [DOI] [PubMed] [Google Scholar]
- 4.Semenza G.L. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 5.Oya M., Takayanagi A., Horiguchi A., Mizuno R., Ohtsubo M., Marumo K. Increased nuclear factor-kappa B activation is related to the tumor development of renal cell carcinoma. Carcinogenesis. 2003;24:377–384. doi: 10.1093/carcin/24.3.377. [DOI] [PubMed] [Google Scholar]
- 6.Yang H., Minamishima Y.A., Yan Q., Schlisio S., Ebert B.L., Zhang X. pVHL acts as an adaptor to promote the inhibitory phosphorylation of the NF-kappaB agonist Card9 by CK2. Mol Cell. 2007;28:15–27. doi: 10.1016/j.molcel.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.An J., Fisher M., Rettig M.B. VHL expression in renal cell carcinoma sensitizes to bortezomib (PS-341) through an NF-kappaB-dependent mechanism. Oncogene. 2005;24:1563–1570. doi: 10.1038/sj.onc.1208348. [DOI] [PubMed] [Google Scholar]
- 8.An J., Rettig M.B. Mechanism of von Hippel-Lindau Protein-mediated suppression of nuclear factor kappa B (NF-kB) activity. Mol Cell Biol. 2005;25:7546–7556. doi: 10.1128/MCB.25.17.7546-7556.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.An J., Sun Y., Fisher M., Rettig M.B. Maximal apoptosis of renal cell carcinoma by the proteasome inhibitor bortezomib is nuclear factor-kappaB dependent. Mol Cancer Ther. 2004;3:727–736. [PubMed] [Google Scholar]
- 10.An J., Liu H., Magyar C.E., Guo Y., Veena M.S., Srivatsan E.S. Hyperactivated JNK is a therapeutic target in pVHL-deficient renal cell carcinoma. Cancer Res. 2013;73:1374–1385. doi: 10.1158/0008-5472.CAN-12-2362. [DOI] [PubMed] [Google Scholar]
- 11.Afaq F., Malik A., Syed D., Maes D., Matsui M.S., Mukhtar H. Pomegranate fruit extract modulates UV-B-mediated phosphorylation of mitogen-activated protein kinases and activation of nuclear factor kappa B in normal human epidermal keratinocytes paragraph sign. Photochem Photobiol. 2005;81:38–45. doi: 10.1562/2004-08-06-RA-264. [DOI] [PubMed] [Google Scholar]
- 12.Ahmed S., Wang N., Hafeez B.B., Cheruvu V.K., Haqqi T.M. Punica granatum L. extract inhibits IL-1beta-induced expression of matrix metalloproteinases by inhibiting the activation of MAP kinases and NF-kappaB in human chondrocytes in vitro. J Nutr. 2005;135:2096–2102. doi: 10.1093/jn/135.9.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schubert S.Y., Neeman I., Resnick N. A novel mechanism for the inhibition of NF-kappaB activation in vascular endothelial cells by natural antioxidants. FASEB J Off Publ Fed Am Soc Exp Biol. 2002;16:1931–1933. doi: 10.1096/fj.02-0147fje. [DOI] [PubMed] [Google Scholar]
- 14.Rettig M.B., Heber D., An J., Seeram N.P., Rao J.Y., Liu H. Pomegranate extract inhibits androgen-independent prostate cancer growth through a nuclear factor-kappaB-dependent mechanism. Mol Cancer Ther. 2008;7:2662–2671. doi: 10.1158/1535-7163.MCT-08-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rasheed Z., Akhtar N., Anbazhagan A.N., Ramamurthy S., Shukla M., Haqqi T.M. Polyphenol-rich pomegranate fruit extract (POMx) suppresses PMACI-induced expression of pro-inflammatory cytokines by inhibiting the activation of MAP Kinases and NF-kappaB in human KU812 cells. J Inflamm (London, England) 2009;6:1. doi: 10.1186/1476-9255-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koyama S., Cobb L.J., Mehta H.H., Seeram N.P., Heber D., Pantuck A.J. Pomegranate extract induces apoptosis in human prostate cancer cells by modulation of the IGF-IGFBP axis. Growth Horm IGF Res Off J Growth Horm Res Soc Int IGF Res Soc. 2010;20:55–62. doi: 10.1016/j.ghir.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seeram N.P., Henning S.M., Zhang Y., Suchard M., Li Z., Heber D. Pomegranate juice ellagitannin metabolites are present in human plasma and some persist in urine for up to 48 hours. J Nutr. 2006;136:2481–2485. doi: 10.1093/jn/136.10.2481. [DOI] [PubMed] [Google Scholar]
- 18.Thomas G.V., Tran C., Mellinghoff I.K., Welsbie D.S., Chan E., Fueger B. Hypoxia-inducible factor determines sensitivity to inhibitors of mTOR in kidney cancer. Nat Med. 2006;12:122–127. doi: 10.1038/nm1337. [DOI] [PubMed] [Google Scholar]
- 19.An J., Mo D., Liu H., Veena M.S., Srivatsan E.S., Massoumi R. Inactivation of the CYLD deubiquitinase by HPV E6 mediates hypoxia-induced NF-kappaB activation. Cancer Cell. 2008;14:394–407. doi: 10.1016/j.ccr.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pantuck AJ, An J, Liu H, Rettig MB. NF-kappaB-dependent plasticity of the epithelial to mesenchymal transition induced by Von Hippel-Lindau inactivation in renal cell carcinomas. Cancer Res 70:752–61. [DOI] [PubMed]
- 21.Oya M., Ohtsubo M., Takayanagi A., Tachibana M., Shimizu N., Murai M. Constitutive activation of nuclear factor-kappaB prevents TRAIL-induced apoptosis in renal cancer cells. Oncogene. 2001;20:3888–3896. doi: 10.1038/sj.onc.1204525. [DOI] [PubMed] [Google Scholar]
- 22.Mori S., Chang J.T., Andrechek E.R., Matsumura N., Baba T., Yao G. Anchorage-independent cell growth signature identifies tumors with metastatic potential. Oncogene. 2009;28:2796–2805. doi: 10.1038/onc.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Figlin R., Sternberg C., Wood C.G. Novel agents and approaches for advanced renal cell carcinoma. J Urol. 2012;188:707–715. doi: 10.1016/j.juro.2012.04.108. [DOI] [PubMed] [Google Scholar]
- 24.Smith S.J., Bosniak M.A., Megibow A.J., Hulnick D.H., Horii S.C., Raghavendra B.N. Renal cell carcinoma: earlier discovery and increased detection. Radiology. 1989;170:699–703. doi: 10.1148/radiology.170.3.2644658. [DOI] [PubMed] [Google Scholar]
- 25.Hollingsworth J.M., Miller D.C., Daignault S., Hollenbeck B.K. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst. 2006;98:1331–1334. doi: 10.1093/jnci/djj362. [DOI] [PubMed] [Google Scholar]
- 26.Lamb G.W., Bromwich E.J., Vasey P., Aitchison M. Management of renal masses in patients medically unsuitable for nephrectomy–natural history, complications, and outcome. Urology. 2004;64:909–913. doi: 10.1016/j.urology.2004.05.039. [DOI] [PubMed] [Google Scholar]
- 27.Bosniak M.A., Birnbaum B.A., Krinsky G.A., Waisman J. Small renal parenchymal neoplasms: further observations on growth. Radiology. 1995;197:589–597. doi: 10.1148/radiology.197.3.7480724. [DOI] [PubMed] [Google Scholar]
- 28.Volpe A., Panzarella T., Rendon R.A., Haider M.A., Kondylis F.I., Jewett M.A. The natural history of incidentally detected small renal masses. Cancer. 2004;100:738–745. doi: 10.1002/cncr.20025. [DOI] [PubMed] [Google Scholar]
- 29.Rendon R.A., Stanietzky N., Panzarella T., Robinette M., Klotz L.H., Thurston W. The natural history of small renal masses. J Urol. 2000;164:1143–1147. [PubMed] [Google Scholar]
- 30.Wehle M.J., Thiel D.D., Petrou S.P., Young P.R., Frank I., Karsteadt N. Conservative management of incidental contrast-enhancing renal masses as safe alternative to invasive therapy. Urology. 2004;64:49–52. doi: 10.1016/j.urology.2004.02.026. [DOI] [PubMed] [Google Scholar]