Abstract
Objective
Recent reports on high-intensity focused ultrasound (HIFU) treatment of localized prostate cancer suggest that preoperative risk groups of tumor recurrence are strong predictors of oncological outcomes. The purpose of this study is to determine the prognostic significance of treatment-related factors in relation to patient characteristics for biochemical outcomes after HIFU.
Methods
This retrospective single-center study included patients treated from December 2002 to December 2010 for localized prostate cancer with two generations of Ablatherm® HIFU devices (A1 and A2). All the patients underwent single HIFU treatment session under the concept of whole-gland therapy. Prostate surgery was performed before HIFU to downsize enlarged glands. Androgen deprivation therapy (ADT) was discontinued before HIFU. Biochemical failure (BCF) was defined as prostate specific antigen (PSA) nadir + 1.2 ng/mL (Stuttgart definition). Predictors of BCF were determined using Cox regression models. As covariates, patient-related factors (age, tumor characteristics, ADT) were compared with treatment-related factors (prostate volume, HIFU device generation, conduct of therapy, prostate edema, patient movement, anesthetic modalities).
Results
Three hundred and twenty-three (98.8%) out of 327 consecutive patients were evaluable for BCF. Median (interquartile range) follow-up was 51.2 (36.6–80.4) months. The overall BCF-rate was 23.8%. In multivariate analyses, higher initial PSA-values (Hazard ratio [HR]: 1.03; p < 0.001) and higher D'Amico risk stages (HR: 3.45; p < 0.001) were patient-related predictors of BCF. Regarding treatment-related factors, the A2 HIFU device was associated with a decreased risk of BCF (HR: 0.51; p = 0.007), while prostate edema had an adverse effect (HR: 1.8; p = 0.027). Short follow-up and retrospective study design are the primary limitations.
Conclusion
Success in a single HIFU session depends not merely on tumor characteristics, but also on treatment-related factors. Ablation is more efficacious with the technically advanced A2 HIFU device. Heat-induced prostate edema might adversely affect the outcome.
Keywords: Prostate cancer, High-intensity focused ultrasound, Efficacy, Biochemical failure, Prostate edema, Patient movement
1. Introduction
High-intensity focused ultrasound (HIFU) is a non-surgical, minimal invasive procedure that enables ablation of the prostate in situ. Extensive experience in treating clinically localized prostate cancer (PCa) has been gained using Ablatherm® HIFU devices (EDAP-TMS, Vaulx-en-Velin, France). The curative potential was already recognized in 1996 with the use of a prototype [1]. Since then, the oncologic efficacy has been evaluated with two commercially available devices. Recently, oncologic outcomes have been reported from large studies [2], [3], [4]. All reports demonstrate that the efficacy of HIFU treatment is associated with the risk stages of tumor recurrence [5], which illustrates the strong influence of preoperative tumor characteristics.
By contrast, the impact of treatment-related factors on cancer control is not well documented.
The principles of a complete prostate ablation (whole-gland therapy) as a prerequisite of complete tumor eradication have been delineated recently [6], [7]. Whether the evolving HIFU technology is associated with improving outcomes in patients treated under these principles has still to be determined [2]. Moreover, variations in planning and conducting whole-gland therapy might affect the outcomes. In addition, intraoperative prostate edema or unintentional patient movements might interfere with the treatment plan and influence the results of therapy.
The present retrospective single-center study reports on biochemical outcomes after whole-gland treatment of localized PCa involving two generations of Ablatherm® HIFU devices. We focused on the efficacy of a single HIFU application and determined whether treatment-related factors (prostate volume, HIFU device generation, conduct of treatment, prostate edema, patient movement, anesthetic modalities) have prognostic significance as outcome predictors independent of preoperative patient characteristics.
2. Methods
2.1. Patients
The records of all patients with clinically localized PCa who underwent a single session of whole-gland HIFU treatment as a first-line therapy with curative intent between December 2002 and December 2010 were assessed retrospectively. All men were unsuitable candidates for radical prostatectomy because of age or comorbidity and were unwilling to undergo radiotherapy. Extracapsular tumor extension and lymph node status was assessed with pelvic CT or MRI. Staging included a bone scan in patients with prostate specific antigen (PSA) ≥10 ng/mL, and laparoscopic lymphadenectomy was recommended in patients with PSA >20 ng/mL. Androgen deprivation therapy (ADT) was discontinued at the time of HIFU therapy.
Excluded from the study were patients with nerve-sparing HIFU ablation (preserving the neurovascular bundles by sparing the lateral prostate regions [8]), and patients with nodal extension or metastatic disease.
2.2. HIFU technology
Treatment involved two generations of Ablatherm® devices, the Ablatherm Maxis® and Ablatherm Integrated Imaging® (after February 2006), hereafter addressed respectively as device A1 and A2. Both devices comprised a 3 MHz therapeutic and a 7.5 MHz imaging transducer. The treatment transducer generates a focused ultrasound field and creates spindle-shaped elementary lesions of 1.7 mm in diameter by heat (85–100 °C) and cavitation. By variable focusing, the focal length is adjustable (19–26 mm) together with the rectum distance length (3–8 mm). The maximum penetration depth in prostatic tissue is limited to 30 mm [3]. The treatment-head moves computer-driven and larger target volumes can be ablated through repeated shots in juxtapositions. With the more advanced A2 device a new electronic probe with optimized treatment parameters was introduced which allows direct visual control of the procedure via transrectal ultrasound (TRUS) [9]. Local movements of the applicator system were reduced, thus providing a more accurate targeting of the prostate [10].
2.3. Standard planning and conduct of treatment
The intention of whole-gland therapy is destruction of the prostate with a safety margin of 6 mm from the apex to preserve the urethral sphincter. The ablation technique should avoid leaving gaps of untreated tissue at prostate margins and within the gland [6]. In patients at risk of extracapsular tumor extension, the treatment can be extended by millimeters beyond the lateral organ boundaries [7]. A safety margin of at least 3 mm is maintained around the rectum. In patients with enlarged prostate glands, prostate surgery is performed prior to HIFU in order to ensure that the anterior prostate margins are within the limited spatial span of the ultrasound focus [3].
In treatment planning, the prostate is divided into several cranio-caudal blocks which are planned and treated separately [7]. Within each block the apical safety margin and the treatment boundaries are defined using transverse and longitudinal ultrasound images. Planning proceeds stepwise from the apex to the bladder neck. Within each step the corresponding transverse ultrasound image is used to determine the number of target lesions, to adjust the focal length to the anterior-posterior prostate diameter and to define the distance from the rectal wall. The blocks are planned with overlapping target lesions. The conduct of the procedure is automated and follows the plan accurately [11].
2.4. Data acquisition
All statistical analysis was performed using StataSE v.13 (StataCorp LP, College Station, USA). For the purposes of this retrospective study, patient characteristics and treatment data were analyzed using prospectively collected data of an Access database (since 2002) and the individual treatment protocols. Patients were stratified into risk categories of tumor recurrence according to D'Amico et al. [5]. Criteria for the high-risk category were modified to include patients with pathological stages cT3A and cT3B (TNM 2002). Treatment protocols provided detailed information on the conduct of therapy with each procedure, including the block strategy (number of treated blocks) and the type of approach. We distinguished between a standard approach, following exactly the pre-plan, and an approach with adjustments of the plan to varying treatment conditions. Adjustments, made either along the anterior-posterior, cranio-caudal or latero-lateral prostate axis, included the targeting of additional areas and modifications in the length (reduction, extension) of the planned treatment blocks. The protocols were also assessed for records of prostate edema and patient movement. In patients with prostate edema the shift in prostate height and/or rectoprostatic distance was estimated by comparing ultrasound images taken at the beginning and the end of treatment near the mid-prostate. Measurements were made using ImageJ image processing software [12].
For this study all available postoperative PSA values were analyzed, which were either assessed in our clinic or by the referring urologists. Biochemical relapse was defined as PSA nadir +1.2 ng/mL (Stuttgart definition [13]). Follow-up period was defined as the interval between HIFU treatment and the last available PSA values or death.
2.5. Statistical analysis
Patient characteristics and treatment variables (prostate measurements, conduct of therapy) were summarized in frequency tables, cross-tabulated with subgroups, by Ablatherm® HIFU devices, occurrence of prostate edema, and patient movement. Categorical variables were expressed as percentages and compared using Fisher's exact test. Quantitative variables were reported as median and interquartile range (IQR) and compared with the Mann–Whitney U-test. The Cox regression model was used in the univariate and multivariate analysis of the prognostic relevance of preoperative and intraoperative factors of biochemical progression. Backward elimination was used and only the final model is given. The Kaplan–Meier method was used to construct survival curves, which were compared using the log-rank test. A p-value <0.05 was considered statistically significant.
3. Results
Totally 327 consecutive patients underwent a single session of whole-gland HIFU therapy between December 2002 and December 2010. Median (IQR) age was 70 (66.5–74.0) years. Baseline patient characteristics are summarized in Table 1. The number of patients treated with the HIFU devices A1 and A2 were 139 (42.5%) and 188 (57.5%), respectively. In all, 112 (34.2%) men received ADT prior to HIFU for a median (interquartile range, IQR) duration of 2.4 (1.3–5.6) months. Two hundred and sixty-four (80.7%) patients with enlarged prostate glands (anterior–posterior diameter >3 cm [TRUS]) underwent procedures to reduce the prostate size (TURP, n = 258; adenomectomy, n = 6). In the post-surgery group, the median (IQR) prostate volume at the time of HIFU treatment was lower (17 [13–21] mL) than in the group without surgery (21 [16–24] mL) (p = 0.001).
Table 1.
Baseline characteristics in 327 patients and in subgroups by Ablatherm® HIFU devices A1 and A2.
| Characteristics | Total (n = 327) | Ablatherm® HIFU devices |
p-value | |
|---|---|---|---|---|
| Device A1a (n = 139) | Device A2a (n = 188) | |||
| Age (years) | 70 (66.5–74.0) | 71 (66.5–75.0) | 70 (66.7–74.0) | 0.46 |
| Prostate volume at visit (mL) | 28.0 (20.0–36.8) | 23.0 (17.0–31.0) | 31.1 (23.3–40.0) | <0.001 |
| PSA at diagnosis (ng/mL) | 7.1 (5.0–11.0) | 7.2 (4.8–13.2) | 7.1 (5.1–10.5) | 0.58 |
| Clinical tumor stagesb | 0.72 | |||
| T1 | 185 (56.6) | 77 (55.4) | 108 (57.4) | |
| T2 | 120 (36.7) | 54 (38.8) | 66 (35.1) | |
| T3 | 22 (6.7) | 8 (5.8) | 14 (7.4) | |
| Gleason sum | 0.066 | |||
| ≤6 | 202 (61.8) | 90 (64.7) | 112 (59.6) | |
| 7 | 90 (27.5) | 30 (21.6) | 60 (31.9) | |
| 8–10 | 35 (10.7) | 19 (13.7) | 16 (8.5) | |
| Risk groupsc | 0.115 | |||
| Low | 130 (39.8) | 51 (36.7) | 79 (42.0) | |
| Intermediate | 115 (35.2) | 45 (32.4) | 70 (37.2) | |
| High | 82 (25.1) | 43 (30.9) | 39 (20.7) | |
| ADTd | 112 (34.2) | 67 (48.2) | 45 (23.9) | <0.001 |
Values are median (interquartile range) or n (%).
A1 = Ablatherm Maxis®, A2 = Ablatherm Integrated Imaging®.
TNM-classification 2002.
Risk groups according to D'Amico et al. [5].
Androgen deprivation therapy (preoperative).
Table 2 summarizes the treatment data of the whole study population and of subgroups, by generations of HIFU devices, occurrence of prostate edema, and patient movement, respectively. In general, prostate volumes had been reduced sufficiently by previous surgery to ensure that the prostate height (pre-plan) corresponded to the limited length of the ultrasound focus (26 mm) and anterior prostate margins were exposed to HIFU. With most patients a 2-block-strategy and standard approach was used. In 93 (28.4%) men, the operator decided for adjustments during the procedure, either ablation of additional areas (n = 51) or modifications in the length of the treatment blocks (n = 42).
Table 2.
Prostate measurements (pre-plan) and conduct of treatment in the whole study population and in subgroups, by different Ablatherm® HIFU devices, occurrence of prostate edema, or patient movement.
| Measurements | Total (n = 327) | Ablatherm® HIFU devices |
Prostate edema |
Patient movement |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Device A1a(n = 139) | Device A2a(n = 188) | p-value | Yes (n = 43) | No (n = 284) | p-value | Yes (n = 63) | No (n = 264) | p-value | ||
| TRUS-measurements (pre-plan) | ||||||||||
| Prostate volume (mL) | 18 (14.0–22.0) | 19 (13.5–24.0) | 18 (14.0–21.0) | 0.033 | 21 (18.0–24.5) | 17 (13.0–21.0) | 0.001 | 18 (13.0–21.6) | 18 (14.0–22.0) | 0.985 |
| Prostate height (mm) | 22 (19.0–25.0) | 23 (20.0–25.0) | 20 (18.0–24.0) | <0.001 | 24 (20.5–25.8) | 21 (18.0–24.0) | 0.006 | 21 (18.0–24.0) | 21 (18.0–24.0) | 0.877 |
| Block strategy | <0.001 | <0.001 | 0.480 | |||||||
| 2 blocks | 194 (59.3) | 63 (45.3) | 131 (69.7) | 17 (39.5) | 177 (62.3) | 35 (55.6) | 159 (60.2) | |||
| 3 blocks | 128 (39.1) | 71 (51.1) | 57 (30.3) | 22 (51.2) | 106 (37.3) | 28 (44.4) | 100 (37.9) | |||
| 4 blocks | 5 (1.5) | 5 (3.6) | 0 | 4 (9.3) | 1 (0.4) | 0 | 5 (1.9) | |||
| Approach | <0.001 | 0.046 | 0.031 | |||||||
| Standard | 234 (71.6) | 83 (59.7) | 151 (80.3) | 25 (58.1) | 209 (73.6) | 38 (60.3) | 196 (74.2) | |||
| With adjustments | 93 (28.4) | 56 (40.3) | 37 (19.7) | 18 (41.9) | 75 (26.4) | 25 (39.7) | 68 (25.8) | |||
| Metric treatment data | ||||||||||
| Duration (min) | 145 (125–175) | 165 (135–200) | 135 (120–155) | <0.001 | 160 (135–190) | 145 (121–170) | 0.006 | 150 (130–180) | 145 (120–170) | 0.090 |
| Lesions (No.) | 589 (505–676) | 629 (505–759) | 571 (504–644) | 0.008 | 635 (550–697) | 580 (497–668) | 0.024 | 598 (516–701) | 586 (500–670) | 0.463 |
| Tx-volume (mL)b | 37.9 (31.0–44.0) | 40.5 (32.0–50.2) | 36 (30.0–41.0) | <0.001 | 42 (36.5–46.5) | 37 (31.0–43.0) | 0.003 | 38 (31.0–45.5) | 37 (31.0–44.0) | 0.659 |
| Tx-ratioc | 2.1 (1.8–2.6) | 2.1 (1.7–2.8) | 2.1 (1.8–2.4) | 0.864 | 2 (1.7–2.5) | 2.1 (1.8–2.6) | 0.264 | 2.1 (1.7–2.7) | 2.1 (1.7–2.5) | 0.594 |
| Anesthesia | 0.039 | 0.590 | 1.000 | |||||||
| Spinal | 295 (90.2) | 131 (94.2) | 164 (87.2) | 38 (88.4) | 257 (90.5) | 57 (90.5) | 239 (90.2) | |||
| General | 32 (9.8) | 8 (5.8) | 24 (12.8) | 5 (11.6) | 27 (9.5) | 6 (9.5) | 26 (9.8) | |||
Values are median (interquartile range) or n (%).
A1 = Ablatherm Maxis®, A2 = Ablatherm Integrated Imaging®.
Treatment-volume (computed sum of single lesion volumes).
Treatment ratio (treatment volume/calculated volume [pre-plan]).
With the A1 device, HIFU was more often delivered using a 3-to-4-block-strategy and the operator decided more often for adjustments, which explains the higher number of elementary lesions, the higher treatment volume (computed sum of single lesion volumes) and the longer treatment duration compared with that seen for the A2 device.
Similarly, in 43 (13.1%) patients with prostate edema, the therapy conducted was more extensive than that in the remainder (Table 2). The measured shift in prostate height was a median (IQR) of 5.2 (3.6–7.3) mm and the prostatorectal shift was 3.6 (0.4–5.3) mm, indicating both prostatic and periprostatic swelling. At midprostate, the maximum diameter between the anterior prostate margin and the rectal wall was a median (IQR) of 36.9 (33.3–38.5) mm and the anterior gap not treatable by HIFU due to limited penetration depth of HIFU was 6.6 (4.3–8.6) mm. These patients exhibited a significantly higher prostate volume and height at pre-plan (Table 2). Moreover, 18.7% (21/112) of patients with previous ADT and 10.2% (22/215) without ADT exhibited prostate edema (p = 0.038). Conversely, edema occured in 9.5% (25/264) of patients with previous prostate surgery and in 28.6% (18/63) without surgery (p < 0.001).
Unintentional movement ocurred in 63 (19.3%) patients and required intraoperative adjustments of the procedure more frequently (Table 2). Fifty-seven men exhibited movements under spinal anesthesia and i.v. sedation due to agitation or cough and six men moved under shallow general anesthesia. The procedure was discontinued in four men treated under spinal anesthesia and interrupted in the remainder for the purpose of re-planning the procedure.
With only four patients lost during follow-up, biochemical outcome data were available for 323 (98.8%) men. Median (IQR) follow-up was 51.2 (36.6–80.4) months with significant differences between the two generations of HIFU devices (84.8 [67.0–92.8] vs. 42.3 [32.4–51.2] months; p < 0.001) according to the later introduction of the A2 device.
Overall, 77 (23.8%) men exhibited biochemical relapse. Median (IQR) time to failure was 18.2 (6.5–32.7) months.
Higher preoperative risk categories, the PSA value (at diagnosis), the A2 HIFU device and prostate edema were predictive factors of biochemical failure (Table 3), while multivariate analysis eliminated patient age, TNM-stages, Gleason sum, ADT, prostate volume (pre-plan), conduct of therapy (block strategies, type of approach), and spinal anesthesia as independent predictors (backward elimination). Notably, patient movements were neither a predictor in univariate analysis nor in multivariate analysis.
Table 3.
Univariate and multivariate analysis (final model) of preoperative and intraoperative biochemical failure predictors.
| Variable | Univariate analysis |
Multivariate analysis (final model) |
||||
|---|---|---|---|---|---|---|
| HR | 95%CI | p-value | HR | 95%CI | p-value | |
| Preoperative | ||||||
| Patients' age | 1.01 | 0.97–1.06 | 0.468 | – | ||
| PSA at diagnosis | 1.04 | 1.03–1.05 | <0.001 | 1.03 | 1.01–1.04 | <0.001 |
| Clinical tumor stagea | ||||||
| T1-2A | 1 | |||||
| ≥T2B | 1.93 | 1.23–3.05 | <0.001 | – | ||
| Gleason sum | 1.42 | 1.17–1.72 | <0.001 | – | ||
| Risk group (D'Amico) | ||||||
| Low | 1 | 1 | ||||
| Intermediate/high | 4.62 | 2.50–8.54 | <0.001 | 3.45 | 1.82–6.52 | <0.001 |
| ADTb | 0.38 | 0.24–0.59 | <0.001 | – | ||
| Intraoperative | ||||||
| Prostate volumec | 0.99 | 0.97–1.03 | 0.952 | – | ||
| HIFU deviced | ||||||
| A1 | 1 | 1 | ||||
| A2 | 0.43 | 0.27–0.70 | <0.001 | 0.51 | 0.32–0.83 | 0.007 |
| Block strategy | ||||||
| 2 blocks | 1 | |||||
| >2 blocks | 1.26 | 0.81–1.95 | 0.302 | – | ||
| Approach | ||||||
| With adjustments | 1 | |||||
| Standard | 0.57 | 0.36–0.89 | 0.014 | – | ||
| Prostate edema | 2.24 | 1.33–3.75 | <0.001 | 1.80 | 1.07–3.04 | 0.027 |
| Patients movements | 1.13 | 0.65–1.95 | 0.661 | – | ||
| Anesthesia | ||||||
| General | 1 | |||||
| Spinal | 4.03 | 0.99–16.44 | 0.052 | – | ||
HR, hazard ratio; CI, confidential interval.
TNM classification 2002.
Androgen deprivation therapy (preoperative).
Prostate volume at HIFU treatment (pre-plan).
A1 = Ablatherm Maxis®, A2 = Ablatherm Integrated Imaging®.
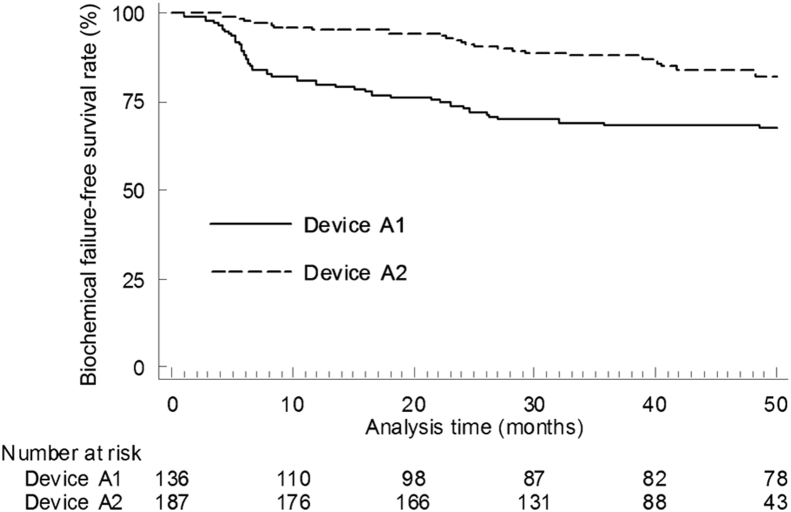
The outcomes with both HIFU devices were further analyzed with a follow-up truncated to 50 months in order to adjust for different monitoring periods. The A2 device was associated with a decreased risk of biochemical failure (HR [95%CI]: 0.415 [0.253–0.672]). The biochemical failure-free survival rates with devices A1 and A2 were 70.3% and 82.3%, respectively (p < 0.001) (Fig. 1).
Figure 1.
Kaplan–Meier estimates of biochemical failure-free survival at 50 months in 323 patients following single-session HIFU treatment (whole-gland therapy) of localized prostate cancer, by different generations of Ablatherm device. Device A1, Ablatherm Maxis®; Device A2, Ablatherm Integrated Imaging®. A1 vs. A2, p < 0.001.
4. Discussion
This study found that biochemical relapse is not excluded even if whole-gland treatment is performed to ensure complete eradication of localized PCa. The overall biochemical failure rate was 23.8%. Higher D'Amico risk stages proved to be strong predictors of adverse biochemical outcomes, as in previous reports [2], [3], [4]. However, HIFU efficacy was not merely dependent on tumor characteristics, but also on treatment-related factors.
In our hands, single-session HIFU treatment proved to be more efficacious with the latest HIFU device than with the older model, as indicated by a decreased risk of biochemical recurrence. The differing results with both devices were not biased by different distributions of preoperative D'Amico risk stages or differing PSA levels at diagnosis. Although differences in the conduct of therapy (block strategies, type of approach) and administration of short-term preoperative ADT were associated with the use of both devices, the changing practice in treating patients over time was not responsible for the decreasing biochemical failure rate, since these factors were not related to the outcomes on multivariate analysis. More likely, the increasing efficacy of HIFU treatment originated in technical advances.
By contrast to our results, the latest Ablatherm® device did not prove to be more efficacious in a recent study by Crouzet et al. [2]. However, their study included patients who received repeat HIFU treatments with retreatment rates differing between both devices. In addition, the Phoenix definition (PSA nadir + 2 ng/mL) of PSA relapse was used, originally designed to monitor biochemical response following radiotherapy [14]. We decided to focus on the efficacy of single-session HIFU treatment as in an earlier report [15]. Moreover, the Stuttgart definition of PSA failure (PSA nadir + 1.2 ng/mL) was selected as it provides a more specific evaluation of HIFU outcome [13], which is meaningful for comparisons between HIFU devices.
Heat-induced prostate edema occurred in a small proportion of patients (13.1%) and was an independent predictor of HIFU outcomes. Prostate swelling was first described by Shoji et al. [16] in patients without previous ADT or TURP treated with the alternative Sonablate HIFU device (Focus surgery, Indianapolis, IN, USA). We demonstrate that edema may not only involve the prostate gland but also the periprostatic tissue, then widening the recto-prostatic space. Both effects contribute to an enlarged distance between the anterior prostate margin and the rectal wall which may result in an anterior gap of untreated tissue. In these cases, HIFU may fail due to the limited penetration depth of the ultrasound. In contrast to the findings of Shoji et al. [16], prostate glands with edema had a higher volume and AP-diameter on pre-plan. Moreover, previous ADT might have an adverse effect, while previous TURP reduces the risk of swelling. This can be explained consistently by an imbalance between release and drainage of intraprostatic fluids after tissue destruction, which is pronounced in larger glands and even more accentuated by an ADT-induced involution of the prostatic vasculature [17], [18]. TURP provides additional drainage via the prostate cavity instead.
This is the first report on unintentional patient movements during HIFU therapy. Movements occurred in 19% of the men and are a challenge to the operator. Most movements were noted under spinal anesthesia and supplementary i.v. sedation which was the predominant anesthetic modality, as also in other clinics [3], [19]. Accordingly, this regimen is not ideal to provide both analgesia and immobilization in all patients. As shown, movements may enforce a discontinuation of the procedure, although treatment could be completed in most patients after adjustments for prostate shifts. Most importantly, movements were not an independent predictor of biochemical outcome, suggesting that movements during HIFU procedures could be compensated sufficiently by the operator to avoid untreated gaps within the prostate tissue.
This study has limitations according to its retrospective nature. The follow-up period with the latest HIFU device was still too short to verify a definite advantage over the older model and more studies with a longer follow-up are needed in order to confirm the present results using the same criteria of PSA relapse. The biochemical outcomes were used as a surrogate parameter for treatment efficacy whereas local control was not analyzed.
5. Conclusion
HIFU efficacy is not only dependent on preoperative tumor characteristics, but also on treatment-related factors. Our data support the preliminary estimate that the efficacy increases with the technically advanced latest HIFU device. Prostate edema might adversely affect the outcome.
Conflicts of interest
Dietrich Pfeiffer acted as a trainer for EDAP-TMS. Juergen Berger and Andreas Gross declare no conflict of interest.
Footnotes
Peer review under responsibility of Chinese Urological Association and SMMU.
References
- 1.Gelet A., Chapelon J.Y., Bouvier R., Souchon R., Pangaud C., Abdelrahim A.F. Treatment of prostate cancer with transrectal focused ultrasound: early clinical experience. Eur Urol. 1996;29:174–183. [PubMed] [Google Scholar]
- 2.Crouzet S., Chapelon J.Y., Rouvière O., Mege-Lechevallier F., Colombel M., Tonoli-Catez H. Whole-gland ablation of localized prostate cancer with high-intensity focused ultrasound: oncologic outcomes and morbidity in 1002 patients. Eur Urol. 2014;65:907–914. doi: 10.1016/j.eururo.2013.04.039. [DOI] [PubMed] [Google Scholar]
- 3.Thüroff S., Chaussy C. Evolution and outcomes of 3 MHz high-intensity focused ultrasound for localized prostate cancer during 15 years. J Urol. 2013;190:702–710. doi: 10.1016/j.juro.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Ganzer R., Fritsche H.M., Brandtner A., Bründl J., Koch D., Wieland W.F. Fourteen-year oncological and functional outcomes of high-intensity focused ultrasound in localized prostate cancer. BJU Int. 2013;112:322–329. doi: 10.1111/j.1464-410X.2012.11715.x. [DOI] [PubMed] [Google Scholar]
- 5.D'Amico A.V., Whittington R., Malkowicz S.B., Schultz D., Blank K., Broderick G.A. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 6.Blana A., Robertson C.N., Brown S.C., Chaussy C., Crouzet S., Gelet A. Complete high-intensity focused ultrasound in prostate cancer: outcome from the @-registry. Prostate Cancer Prostatic Dis. 2012;15:256–259. doi: 10.1038/pcan.2012.10. [DOI] [PubMed] [Google Scholar]
- 7.Ficarra V., Antoniolli S.Z., Novara G., Parisi A., Fracalanza S., Martignoni G. Short-term outcome after high-intensity focused ultrasound in the treatment of patients high-risk prostate cancer. BJU Int. 2006;98:1193–1198. doi: 10.1111/j.1464-410X.2006.06561.x. [DOI] [PubMed] [Google Scholar]
- 8.Ward J.F., Stephen Jones J. Classification system: organ preserving treatment for prostate cancer. Urology. 2010;75:1258–1260. doi: 10.1016/j.urology.2009.10.044. [DOI] [PubMed] [Google Scholar]
- 9.Pichardo S., Gelet A., Curiel L., Chesnais S., Chapelon J.Y. New integrated imaging high intensity focused ultrasound probe for transrectal prostate cancer treatment. Ultrasound Med Biol. 2008;34:1105–1116. doi: 10.1016/j.ultrasmedbio.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Cordeiro E.R., Cathelineau X., Thüroff, Marberger M., Crouzet S., de la Rosette J.J. High-intensity focused ultrasound (HIFU) for definitive treatment of prostate cancer. BJU Int. 2012;110:1228–1242. doi: 10.1111/j.1464-410X.2012.11262.x. [DOI] [PubMed] [Google Scholar]
- 11.Rewcastle J. High intensity focused ultrasound for prostate cancer: a review of the scientific foundation, technology and clinical outcomes. Technol Cancer Res Treat. 2006;5:619–625. doi: 10.1177/153303460600500610. [DOI] [PubMed] [Google Scholar]
- 12.Rasband W.S. U. S. National Institutes of Health, Bethesda; Maryland, USA: 1997-2012. ImageJ.http://imagej.nih.gov/ij/ [Google Scholar]
- 13.Blana A., Brown S.C., Chaussy C., Conti G.N., Eastham J.A., Ganzer R. High-intensity focused ultrasound for prostate cancer: comparative definitions of biochemical failure. BJU Int. 2009;104:1058–1062. doi: 10.1111/j.1464-410X.2009.08518.x. [DOI] [PubMed] [Google Scholar]
- 14.Roach M., 3rd, Hanks G., Thames H., Jr., Schellhammer P., Shipley W.U., Sokol G.H. Defining biochemical failure following radiation therapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65:965–974. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 15.Pfeiffer D., Berger J., Gross A.J. Single application of high-intensity focused ultrasound as a first-line therapy for clinically localized prostate cancer: 5-yr outcomes. BJU Int. 2012;110:1702–1707. doi: 10.1111/j.1464-410X.2012.11375.x. [DOI] [PubMed] [Google Scholar]
- 16.Shoji S., Uchida T., Nakamoto M., Kim H., de Castro Abreu A.L., Leslie S. Prostate swelling and shift during high intensity focused ultrasound: implication for targeted focal therapy. J Urol. 2013;190:1224–1232. doi: 10.1016/j.juro.2013.03.116. [DOI] [PubMed] [Google Scholar]
- 17.Shabsigh A., Chang D.T., Heitjan D.F., Kiss A., Olsson C.A., Puchner P.J. Rapid reduction in blood flow to the rat ventral prostate gland after castration: preliminary evidence that androgens influence prostate size by regulating blood flow of the prostate gland and prostatic endothelial cell survival. Prostate. 1998;36:201–206. doi: 10.1002/(sici)1097-0045(19980801)36:3<201::aid-pros9>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 18.Wiart M., Curiel L., Gelet A., Lyonnet D., Chapelon J.Y., Rouvière O. Influence of perfusion on high-intensity focused ultrasound prostate ablation: a first-pass MRI study. Magn Reson Med. 2007;58:119–127. doi: 10.1002/mrm.21271. [DOI] [PubMed] [Google Scholar]
- 19.Pinthus J.H., Farrokhyar F., Hassouna M.M., Woods E., Whelan K., Shayegan B. Single-session primary high-intensity focused ultrasonography treatment for localized prostate cancer: biochemical outcomes using the third generation-based technology. BJU Int. 2012;110:1142–1148. doi: 10.1111/j.1464-410X.2012.10945.x. [DOI] [PubMed] [Google Scholar]