Abstract
Background
Over the past two decades, minimally invasive techniques for classic heart valve surgery and isolated bypass surgery have been developed that enable access to the heart via partial sternotomy for most aortic valve procedures and via sternotomy-free mini-thoracotomy for other procedures.
Methods
We review the current evidence on minimally invasive cardiac surgery on the basis of pertinent randomized studies and database studies retrieved by a selective search in the MEDLINE and PubMed Central databases, as well as by the Google Scholar search engine.
Results
A PubMed search employing the search term “minimally invasive cardiac surgery” yielded nearly 10 000 hits, among which there were 7 prospective, randomized, controlled trials (RCTs) on aortic valve replacement, with a total of 477 patients, and 3 RCTs on mitral valve surgery, with a total of 340 patients. Only limited reports of specified centers are currently available for multiple valvular procedures and multiple coronary artery bypass procedures. The RCTs reveal that the minimally invasive techniques are associated with fewer wound infections and faster mobilization, without any difference in survival. Minimally invasive procedures are technically demanding and have certain anatomical prerequisites, such as appropriate coronary morphology for multiple bypass operations and the position of the aorta in the chest for sternotomy-free aortic valve procedures. The articles reviewed here were presumably affected by selection bias, in that patients in the published studies were preselected, and there may have been negative studies that were not published at all.
Conclusion
Specialized surgeons and centers can now carry out many cardiac valvular and bypass operations via minithoracotomy rather than sternotomy. According to current evidence, these minimally invasive techniques yield results that are at least as good as classic open-heart surgery.
The origins of minimally invasive surgery date back to the 1950s (1). Laparoscopic and fully endoscopic procedures have in the meantime become the standard in visceral surgery and gynecology (2). In cardiac surgery, it was only in the mid-1990s that the sternum was only partially opened up (3) or to gain access to the heart through a minithoracotomy (4) (foregoing sternotomy altogether). A search using the term “minimally invasive cardiac surgery” identified almost 10 000 publications in PubMed. In spite of all this, safety and quality are still the subject of heated discussions. Furthermore, there is hardly any evidence from controlled studies (Table).
Table. Prospectively randomized controlled studies comparing minimally invasive techniques versus sternotomy in aortic or mitral valve operations.
|
Study First author and year of publication (reference) |
N (minimally invasive surgery/ sternotomy) |
Mortality (%) |
Time aorta was cross-clamped (min) |
Time on cardiopulmonary bypass (min) |
Converted into sternotomy n (%) |
Result | |||
|
Minimally invasive surgery |
Sternotomy |
Minimally invasive surgery |
Sternotomy |
Minimally invasive surgery |
Sternotomy | ||||
| Aortic valve | |||||||||
| Ahangar 2013 (e1)*1 | 30/30 | N/A | N/A | 68.0 ± 8.9 | 67.7 ± 13.4 | 122 ± 20.8 | 122 ± 18.6 | N/A | Difference did not reach significance. Minimally invasive surgery: better cosmetic result and ‧significantly less pain |
| Aris 1999 (e2)*2 | 20/20 | 10 | 10 | 70 ± 19 | 51 ± 13 | 95 ± 20 | 83 ± 19 | 2 (10) | Significantly longer operating times for minimally ‧invasive surgery, with the same result |
| Bonacchi 2002 (e3)*2 | 40/40 | 2.5 | 5 | 51.7 ± 12 | 52.4 ± 10 | N/A | N/A | 1 (2.5) | Significantly less pain and better ‧cosmetic result after minimally invasive surgery, with shorter inpatient stay |
| Calderon 2009 (e4)*2 | 38/39 | 0.0 | 2.6 | 55 ± 9.3 | 50.6 ± 11.9 | 77.1 ± 13.4 | 71.3 ± 20.4 | N/A | Respiratory function not significantly improved by using minimally invasive surgery, but significantly ‧fewer hemorrhages |
| Dogan 2003 (e5)*2 | 20/20 | 0.0 | 0.0 | 73 ± 6.5 | 72 ± 6.2 | 115 ± 6.5 | 107 ± 5.4 | 1 (5) | Operating times no longer for minimally invasive ‧surgery; significantly fewer hemorrhages and better‧cosmetic result |
| Maechler 1999 (e6)*2 | 60/60 | 1.67 | 0.0 | 60(35–116)*3 | 57 (34–110)*3 | 84 (51–179)*3 | 82 (55–300)*3 | 2 (3.3) | Fewer hemorrhages and less pain after minimally invasive surgery, results otherwise comparable |
| Moustafa 2007 (e7)*2 | 30/30 | N/A | N/A | 44.3 ± 3.1 | 45.5 ± 4.0 | 85.7 ± 6.8 | 90.0 ± 8.3 | N/A | Less pain, fewer hemorrhages after minimally ‧invasive surgery. Also greater stability of sternum |
| Total N | 477 | Difference minimally invasive surgery vs. sternotomy: | 3.69 (7 %) | Difference minimally invasive surgery vs. sternotomy: | 3.92 (4.62 %) | ||||
| Mitral valve | |||||||||
| Dogan 2005 (e8)*1 | 20/20 | 0.0 | 0.0 | 84.8 ± 24.4 | 88 ± 28.7 | 142 ± 32.1 | 133 ± 35.6 | 0 (0) | Survival equal, better cosmetic result after minimally invasive surgery |
| Nasso 2014 (e9)*1 | 80/80 | 3.75 | 3.75 | 91 ± 22 | 69 ± 19 | 137 ± 28 | 102 ± 23 | 0 (0) | Comparable results independent of access route |
| Speziale 2011 (e10)*1 | 70/70 | 4.29 | 1.3 | 92 ± 23.3 | 70 ± 18 | 139 ± 28 | 101 ± 24 | 1 (1.4) | Minimally invasive surgery yields comparable results; time on cardiopulmonary bypass significantly longer, inpatient stay shorter inpatient stay shorter |
| Total N | 340 | Difference minimally invasive surgery vs. sternotomy: | 13.6 (19.89 %) | Difference minimally invasive surgery vs. sternotomy: | 27.33 (26.23 %) | ||||
*1Study compares minithoracotomy vs. sternotomy; *2Study compares partial vs. median sternotomy; *3Data reported as means (ranges) N/A, not available; N/n, number of patients; vs., versus
It therefore cannot come as a surprise that most cardiac surgical procedures are still done by using classic median sternotomy (open-heart surgery). In Germany, this applies to 92% of all and 98% of isolated bypass operations. The use of sternotomy-free techniques currently seems center-specific or surgeon-specific, presumably because such procedures are of notably greater complexity (5).
In Germany, the most common cardiac operation that is done without sternotomy is mitral valve surgery, with almost 50% of all mitral valve operations (2928 of 6027 procedures in the year 2015). A quarter (3016 of a total of 11 307 procedures) of classic aortic valve replacement procedures use partial sternotomy (6). These rates have increased in particular over the past 10 years (5). Whether the development of classic cardiac surgery is associated with the introduction of catheter techniques is not the subject of this article and will therefore not be discussed. Furthermore, to date no prospectively randomized studies have compared interventional approaches with minimally invasive cardiac surgery.
In our experience, however, the fact is that patients’ perceptions and expectations have changed. Patients increasingly ask for a therapeutic approach that leaves the sternum intact. Those doctors who want to meet this new challenge therefore need to realize that minimal incisions in cardiac surgery require greater technical skills. For this reason, doctors should become familiar with current study data.
Minimally invasive cardiac surgical procedures: evidence from studies
In order to ascertain current study data, we conducted an extensive literature search in MEDLINE, PubMed Central, and by using the Google Scholar search engine. We used the following search terms: “prospective randomized trial”, “aortic valve surgery”, “mitral valve surgery”, “minimally invasive versus sternotomy”, “port access”, and “minithoracotomy versus median sternotomy”.
From 10 000 search results, we were able to identify only 10 prospectively randomized controlled trials, which included a total of 477 patients in 7 aortic valve studies and 340 patients in 3 mitral valve studies (Table). Among the many non-randomized studies, we identified 24 including a minimum of 200 patients each (eTable 1 [aortic valve] and eTable 2 [mitral valve]), all of which used multivariate analysis for their statistical evaluation; 14 additionally used propensity matching to adjust risk. All studies compared procedures using minimally invasive access versus those using median sternotomy.
eTable 1. Non-controlled registry studies including at least 200 patients comparing the results of aortic valve operations with minimally invasive access versus sternotomy.
|
Study First author and year of publication (reference) |
Compared access routes (n/n) | Mortality (%) | Complications of minimally invasive surgery vs. sternotomy |
Converted into sternotomy (n (%)) |
|||
|
Strokes (%) |
Revisions (%) |
Wound infections (%) |
Blood transfusion required (units or % of patients) |
||||
| Attia 2016 (e13) | Partial vs median sternotomy (307/307)* |
1.3 vs. 2.0 (n.s.) | N/A | N/A | N/A | N/A | |
| Bakir 2006 (e14) | Partial vs median sternotomy (232/274) |
2.6 vs. 4.4 (n.s.) | 2.1 vs. 0.7 (n.s.) | 7.8 vs. 6.2 (n.s.) | 0.9 vs. 0.7 (n.s.) | N/A | 8 (2.9) |
| Bowdish 2016 (e15) | Minithoracotomy vs. median sternotomy (294/198)* |
1.0 vs. 2.5 (n.s.) | 1.4 vs. 2.5 (n.s.) | 3.7 vs. 3.5 (n.s.) |
1.0 vs. 6.6 (p = 0.001) |
1.2 vs. 1.9 (p <0.001) |
3 (1.0) |
| Brinkman 2010 (e16) | Minithoracotomy vs. median sternotomy (90/360)* |
0.0 vs. 3.1 (n.s.) | 1.2 vs. 1.5 (n.s.) | 8.1 vs. 4.6 (n.s.) | 0.0 vs. 0.2 (n.s.) | 64.4 % vs. 57.3 % (n. s.) |
1 (1.1) |
| Doll 2002 (e17) | Partial vs median sternotomy (176/258) |
3.0 vs. 9.0 (p = 0.008) |
6.0 vs. 5.0 (n.s.) | 7.0 vs. 9.0 (n.s.) | 2.0 vs. 2.0 (n.s.) |
2.0 vs. 3.5 (p <0.05) |
8 (2.0) |
| Furukawa 2014 (e18) | Partial vs median sternotomy (404/404)* |
1.0 vs. 1.0 (n. s.) | 1.0 vs. 1.0 (n. s.) | 6.0 vs. 6.0 (n. s.) | N/A | N/A | N/A |
| Ghanta 2015 (e19) | Partial sternotomy or minithoracotomy vs. median sternotomy (289/289)* |
0.3 vs. 2.1 (n. s.) | 1.0 vs. 2.1 (n. s.) | 5.2 vs. 2.1 (n. s.) | N/A |
24.6 % vs. 31.8 % (p = 0.05) |
N/A |
| Gilmanov 2015 (23) | Minithoracotomy vs. median sternotomy (100/100)* |
2.0 vs. 4.0 (n. s.) |
0.0 vs. 4.0 (p <0.05) |
6.0 vs. 4.0 (n. s.) | 1.0 vs. 1.0 (n. s.) | N/A | 2 (2.0) |
| Glauber 2013 (e20) | Minithoracotomy vs. median sternotomy (138/138)* |
0.7 vs. 0.7 (n. s.) | 0.7 vs.1.5 (n. s.) | 6.5 vs. 4.3 (n. s.) | 0.0 vs. 0.7 (n. s.) |
18.8 % vs. 34.1 % (p <0.001) |
2 (1.5) |
| Glower 2014 (e21) | Minithoracotomy vs. median sternotomy (452/672)* |
1.5 vs. 3.3 (n. s.) | 1.5 vs. 1.2 (n. s.) |
0.4 vs. 4.0 (p <0.0001) |
0.0 vs. 0.7 (n. s.) |
1.4 vs. 3.4 (p = 0.0003) |
15 (3.3) |
| Johnston 2012 (e22) | Partial vs median sternotomy (832/832)* |
0.96 vs. 0.96 (n. s.) | 1.3 vs. 1.3 (n. s.) | 5.5 vs. 4.3 (n. s.) | 0.6 vs. 0.8 (n. s.) |
24 % vs. 34 % (p <0.0001) |
34 (4.0) |
| Klokocovnik 2012 (e23) | Partial sternotomy or minithoracotomy vs. median sternotomy (217/236) |
1.8 vs. 1.2 (n. s.) | 0.9 vs. 1.7 (n. s.) | 6.0 vs. 9.0 (n. s.) | 0.9 vs. 0.4 (n. s.) | N/A | 5 (2.0) |
| Merk 2015 (9) | Partial vs median sternotomy (477/477)* |
0.4 vs. 2.3 (p = 0.013) |
0.7 vs. 2.0 (n. s.) |
4.2 vs. 1.5 (p = 0.009) |
0.0 vs. 0.0 (n. s.) |
28.1 % vs. 19.7 % (p = 0.002) |
4 (0.8) |
| Miceli 2014 (18) | Minithoracotomy vs. median sternotomy (251/155) |
1.2 vs. 1.3 (n. s.) | 1.2 vs. 1.3 (n. s.) | 4.8 vs. 3.2 (n. s.) | N/A | 20.3 % vs. 25.8 % (n. s.) |
4 (1.6) |
| Neely 2015 (e24) | Partial vs median sternotomy (552/552)* |
2.5 vs. 3.4 (n. s.) | 2.2 vs. 2.9 (n. s.) | 1.6 vs. 1.8 (n. s.) | N/A |
20.0 % vs. 27.9 % (p <0.003) |
N/A |
| Semsroth 2017 (16) | Partial sternotomy vs. minithoracotomy vs. median sternotomy (118/118/118)* |
1.0 vs. 3.0 vs. 5.0 (n. s.) |
0.8 vs. 0.8 vs. 0.8 (n. s.) |
3.4 vs. 14.4 vs. 8.5 (n. s.) |
0.8 vs. 0.8 vs. 2.5 (n. s.) |
48.3 vs. 36.4 vs. 36.4 (p = 0.043) |
2 (1.7) (partial sternotomy)17 (14.4) (Minithoracotomy) |
| Stamou 2003 (e25) | Partial vs median sternotomy (56/455) |
5.0 vs. 5.0 (n. s.) | 4.0 vs. 4.0 (n. s.) | 0.0 vs. 5.0 (n. s.) | N/A |
29.0 % vs. 49.0 % (p <0.01) |
0 (0.0) |
| Stolinski 2016 (27) | Minithoracotomy vs. median sternotomy (211/211) |
1.0 vs. 1.4 (n. s.) | 0.5 vs. 1.4 (n. s.) | 3.8 vs. 5.2 (n. s.) |
0.0 vs. 2.8 (p = 0.04) |
48.8 % vs. 67.3 % (p <0.001) |
6 (2.8) |
* Risk adjusted (multivariate analysis ± propensity matching); bold type = difference reaches significance; N/A, not available; n. s., non-significant; vs.. versus
eTable 2. Non-controlled registry studies including at least 200 patients comparing the results of mitral valve surgery using minimally invasive techniques versus sternotomy.
|
Study First author and year of publication (reference) |
Access routes under comparison (n/n) | Mortality (%) | Complications of minimally invasive techniques vs. sternotomy |
Converted into sternotomy (n (%)) |
|||
|
Strokes (%) |
Revisions (%) |
Wound infections (%) |
Blood transfusion required (units or % patients) |
||||
| Goldstone 2013 (26) | Minithoracotomy vs median sternotomy (201/201)* |
0.0 vs. 0.5 (n. s.) | 0.0 vs. 0.5 (n. s.) | 2.5 vs. 0.5 (n. s.) | 0.0 vs. 0.0 (n. s.) |
14.0 vs. 22.9 (p = 0.03) |
N/A |
| Grossi 2001 (11) | Minithoracotomy vs median sternotomy (100/100) |
0.0 vs. 1.0 (n. s.) | 2.0 vs. 1.0 (n. s.) | 3.2 vs. 5.6 (n. s.) | N/A | N/A | N/A |
| Iribarne 2010 (e26) | Minithoracotomy vs median sternotomy (382/382)* |
1.8 vs. 1.8 (n. s.) | 1.0 vs. 2.6 (n. s.) | 3.7 vs. 2.9 (n. s.) | 0.0 vs. 0.79 (N/A) | N/A | 0 (0.0) |
| Suri 2009 (13) | Minithoracotomy vs median sternotomy (350/365)* |
0.5 vs. 0.0 (N/A) | 3.0 vs. 1.0 (n. s.) | 6.0 vs. 3.0 (n. s.) | 1.0 vs. 0.3 (n. s.) |
29 % vs. 22 % (p = 0.023) |
N/A |
| Tang 2015 (e27) | Minithoracotomy vs median sternotomy (215/215)* |
2.0 vs. 4.0 (n. s.) | 1.0 vs. 3.0 (n. s.) | N/A | 0.0 vs. 1.0 (n. s.) |
2 vs. 3 (p = 0.001) |
N/A |
| Zhai 2017 (e28) | Minithoracotomy vs median sternotomy(112/112)* |
2.7 vs. 1.8 (n. s.) | N/A | 2.0 vs. 0.9 (n. s.) | 1.8 vs. 0.0 (n. s.) |
33.9 % vs. 67.0 % (p <0.001) |
N/A |
* Risk adjusted (multivariate analysis ± propensity matching); bold type, difference reaches significance; N/A, not available; n. s., non-significant; vs., versus
None of the prospectively randomized controlled trials found a difference in perioperative mortality (in a small total number of patients, as described). Especially in the aortic valve studies, the trend was one of fewer blood transfusions (significant in 2/7 studies), less pain, and faster mobilization (significant in 3/7 studies). In mitral valve surgery, the results were neutral except for the smaller incision and longer periods on the heart-lung machine (cardiopulmonary bypass) (Table). The large database and registry analyses did not show any difference in perioperative mortality or better results for minimally invasive aortic valve replacement either (7– 9).
With survival being practically the same, our focus is on secondary endpoints and complication rates. A point of substantial criticism of minimally invasive procedures is the commonly used femoral arterial cannulation. Because of descending aortic flow reversal, an increased risk of stroke is the subject of controversial discussion (10). While individual studies underline this worry (11– 13), the overall view does not actually identify any increased risk of stroke for minimally invasive aortic valve surgery nor for minimally invasive mitral valve surgery (Table, eTables 1 and 2) (14).
The situation is similar for other results and complications. Individual studies have reported increased rates of hemorrhages and revision thoracotomies (eTables 1 and 2), complications after femoral vessel cannulation (15), or a seemingly very high rate of conversion into median sternotomy (14.4% [16] and 15% [17]). Other groups, however, have described positive effects of minimally invasive procedures (Table, Boxes 1 and 2). Especially for sternotomy-free techniques, their results showed earlier extubation (-1 h) and reintegration into working life (7 days earlier) as well as less pain compared with complete or partial sternotomy (18, 19). These results are consistent with our own experiences (Färber et al., 2017 annual meeting of the German Society for Thoracic and Cardiovascular Surgery [Deutsche Gesellschaft für Thorax-, Herz- und Gefäßchirurgie, DGTHG]. Additionally, we showed in a recent analysis including more than 450 patients that percutaneous femoral vessel cannulation reduces local complication rates significantly (20).
BOX 1. Examples for broadening the spectrum of procedures by using minimally invasive cardiac surgery*1.
Tricuspid valve—Reconstruction or valve replacement without sternotomy or cross-clamping the aorta. As reoperation transpericardial access without substantial risk of hemorrhage*2
Mitral valve—After prior operations repair or replacement with beating/fibrillating heart, with a minimal amount of tissue dissection and without having to cross clamp the aorta (33)*3
Reoperations with prior wound infections (especially in the absence of a sternum)*4
Procedures in patients with morbid obesity, to prevent sternum instability and wound infections (e11)
Procedures in patients with severely impaired lung function (8)
Procedures in patients with pulmonary hypertension (e12)
Procedures in patients with severe osteoporosis*4
Procedures in patients with a tendency to hemorrhage, dual platelet inhibition (e2)
Procedures in patients with renal failure or severe diabetes mellitus (e2)
*1 Situations in which minimally invasive surgical techniques have broadened the spectrum of cardiac surgical therapies and have made their undertaking easier or possible; *2 Congress contribution Färber et al., annual meeting of the European Association for Cardio-Thoracic Surgery (EACTS) 2016;
*3 Congress contribution Färber et al., annual meeting of the German Society for Thoracic and Cardiovascular Surgery (DGTHG) 2017; *4 Authors’ own observations, unpublished.
BOX 2. Limitations of cardiac surgery through minithoracotomy.
-
Bypass surgery
Diffuse coronary heart disease with a poor periphery
-
Valve surgery (general)
Presence of mitral annular calcification (MAC)
Substantially impaired ventricular function
Pulmonary adhesions
Abscess formation in endocarditis
Porcelain aorta
-
Valve surgery (especially of the aortic valve and combinations with the aortic valve)
Vertically ascending aorta
Very narrow aorta (<25 mm diameter)
The listed points describe situations that may in some cases represent a true contraindication for minimally invasive access and in other cases do not impair such access or even necessitate it. Individual fine-tuning is therefore required.
The results seem to depend on the center where the procedure was performed, but also, and primarily, on the operating surgeon. Holzhey et al. have published remarkable differences in the learning curves of different surgeons at the same center in terms of conducting minimally invasive mitral valve surgery (21). The authors described how some surgeons delivered the expected results or better than expected results from the off, without any learning curve, whereas others always achieved worse results than expected and therefore remained below any expected learning curve (21).
Given that using a minimally invasive procedure yields the same result in situ as would a sternotomy, it might be expected that durability and prognosis are also identical (22– 24). These assumptions are supported by Ariyaratnam et al. (22) and Glauber et al. (24), who showed results that were identical to those after sternotomy, 10 years after a minimally invasive procedure.
However, it should be pointed out that in this area, the pre-selection of patients (selection bias) impairs the ability to interpret the results in two different ways:
A surgeon will obviously select only a suitable patient for minimally invasive surgery, which probably means pre-selection (details in Box 2).
In most of the non-randomized studies and a very small number of randomized patient groups, the possibility exists that less positive results are never published. For this reason, the current recommendation is that such specialized procedures should be undertaken only at specified reference centers (5, 25).
Anesthesia and postoperative care in minimally invasive procedures
Although special anesthesiologic aspects have thus far not been studied in isolation, secondary endpoints—such as the length of stay in intensive care, the need for transfusion, and the rate of wound infections (5, 26, 27)—indicate differences in the administration of the anesthesia and in postoperative care. In our experience, for example, patients treated by using minimally invasive techniques are more suitable for early postoperative extubation. Wound infections usually take a milder course after sternotomy-free methods and do not entail the risk of permanent instability of the sternum (unpublished observations).
These potential advantages are often countered by longer periods on cardiopulmonary bypass and longer aortic cross-clamping times (Table). Furthermore, reports exist of mostly unilateral pulmonary edema after access through right-sided minithoracotomy. Keyl et al. described—in a study of 485 patients treated by using minimally invasive surgery—a rate of 7.85% (28). The causes of pulmonary edema, which in practice has been described only for minimally invasive valve surgery, are not known. What is striking, however, is that all publications on this subject used one-lung ventilation through a double-lumen endotracheal tube. The studies described the following risk factors:
Diabetes mellitus
Chronic obstructive pulmonary disease
Pulmonary hypertension
The use of dexamethasone during cardiopulmonary bypass had a protective effect (28), which lends support to the possibility of an inflammatory trigger. However, unilateral pulmonary edema after minimally invasive surgery is rarely clinically apparent (28, 30). In our experience, this problem is rare (in our subjective perception/observation <1%). However, we undertake double-lumen endotracheal intubation and one-lung ventilation only in exceptional circumstances (20). From an anesthesiologic perspective, minimally invasive access to the heart allows a greater degree of flexibility in administering anesthesia and in postoperative care in the intensive care ward.
The spectrum of minimally invasive techniques
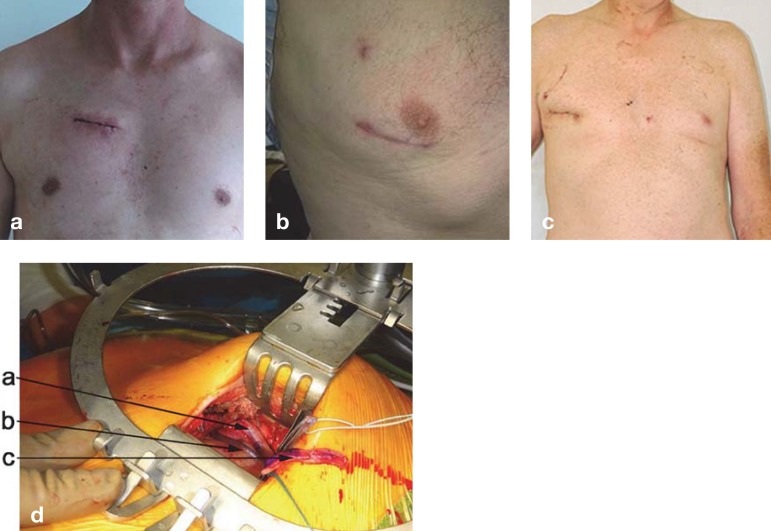
The Figure shows a collection of postoperative photographs after different minimally invasive cardiac procedures, for which to date sternotomy would have been the only access route. Figure 1a shows the access route for aortic valve surgery. A skin incision of approximately 5 cm was used to open up the second intercostal space on the right side of the sternum. The patient furthermore underwent aortic valve reconstruction of a bicuspid valve. Normally, the aortic valve is replaced in this setting. Figure 1b shows the usual access route for minimally invasive mitral and tricuspid valve surgery, through the fourth intercostal space anterolaterally. The patient underwent reconstruction of the mitral valve. Figure 1c shows the access route for multiple valve procedures, including the aortic valve through to the tricuspid valve. We adapted the access route individually to the patient by using preoperative computed tomography analysis. Usually the access route will be through the second or third intercostal space anterolaterally. The patient received an aortic valve replacement for stenosis and a mitral valve reconstruction for anterior leaflet prolapse. It should be borne in mind, however, that especially these multiple valve procedures entail a high degree of complexity. We can only repeat our recommendation to attend specialized centers (5, 25). In such centers, these procedures are even now part of the standard healthcare repertoire (20, 31, 32).
Figure.
Postoperative result to illustrate surgical access in different minimally invasive procedures
Patient, 5 days after aortic valve reconstruction. Access through the second intercostal space is particularly suitable for isolated aortic valve replacement if the aorta takes a convex right course
Patient, 6 weeks after mitral valve reconstruction for posterior leaflet prolapse. Anterolateral access through the fourth intercostal space is an option for most mitral or tricuspid valve procedures.
Patient, 6 days after aortic valve replacement for stenosis and mitral valve reconstruction in for anterior leaflet prolapse. Anterolateral access through the second or third intercostal space provides access to all valves (except the pulmonary valve) if the aorta takes a convex right course.
Intraoperative photograph of a surgical site in minimally invasive triple bypass surgery through a left anterolateral minithoracotomy. Deep down the aorta is visible with the already placed anastomoses of a radial artery (a) and a leg vein (b). The microclamp that is secured with a rubber band in the right margin of the photo sits on the left internal mammary artery (LIMA) (c). The patient underwent a triple bypass operation, with the LIMA grafted to the left anterior descending (LAD) artery, the radial artery to the circumflex artery, and the vein to the interventricular posterior branch of the right coronary artery.
The increasing expertise in minimally invasive techniques has in some centers led to an expansion of surgical treatment options (5). Box 1 lists situations where minimally invasive access has either simplified a surgical procedure or was actually the crucial factor in making it at all possible. To give an example, this would include patients with isolated tricuspid valve regurgitation, who have often had previous cardiac surgery. In our experience, many such patients are treated conservatively because the risk for surgery is estimated to be prohibitively high. However, we showed in more than 100 patients with isolated tricuspid valve regurgitation that sternotomy was associated with higher perioperative mortality compared with a minimally invasive, beating-heart, transpericardial technique, and is actually an independent risk factor (from 27% to 7%, hazard ratio 2.67; 95% confidence interval 1.18 to 6.03). In these patients, the mean NYHA score (classification of the New York Heart Association) was improved by more than 1 (Färber et al., 2016 annual meeting of the European Association for Cardio-Thoracic Surgery). Similarly, in reoperations, the mitral valve can be minimally invasively replaced or reconstructed on the beating or fibrillating heart ([33] and authors’ own unpublished observation 2017).
The situation is different in patients who because of morbid obesity or severe osteoporosis are at increased risk of impaired wound healing after sternotomy (5). The only cardiac valve that cannot be tackled by means of right-sided minithoracotomy is the pulmonary valve, which rarely requires surgical treatment in adults.
Minimally invasive coronary surgery
Developments have also taken an interesting course in coronary surgery. The minimally invasive procedures known to date were restricted to grafting the left internal mammary artery (LIMA) to the left anterior descending artery (LAD) (34). This so called MIDCAB (minimally invasive direct coronary artery bypass grafting) procedure was then combined with interventions in the area of the circumflex artery and the right coronary artery (so called hybrid procedure) (35).
The latest developments, especially in the area of thoracic retractors and minimally invasive instruments, allow complete revascularization in a wide range of techniques by using a left-anterolateral (MIDCAB) access ([35] ad Diab et al., DGTHG annual meeting 2017). Figure 1d shows an intraoperative image from our operating theater of such a minimally invasive procedure, using the left internal mammary artery, a radial artery, and a vein, with the proximal anastomoses conventionally connected to the aorta. Y graft and T graft techniques are normally used in this setting.
Ruel et al. used such approaches in 91 consecutive, prospectively observed patients (not controlled or randomized) and after 6 months used CT angiography to check for the patency of the bypass graft as a primary endpoint (35). They found a patency rate from LIMA to LAD of 100% as well as 92% patency of the additional bypass graft, and thus showed that these procedures are technically feasible. These techniques do, however, present particular challenges to the surgeon and have to date become notably less well established than the minimally invasive valve operations.
Conditions for and limitations of cardiac surgery by using minithoracotomy
As partly mentioned earlier, undertaking cardiac surgery through left or right anterior minithoracotomy—especially aortic valve surgery—requires certain anatomical conditions. For coronary surgery, the quality of the target vessels is of primary importance. If a patient’s coronary morphology is complex—for example, if they have small distal target vessels covered in plaques—minimally invasive access seems disadvantageous, as left lateral access provides easier access to the peripheral vessels but not to the more proximal parts.
Especially for aortic valve surgery, the anatomical conditions are more important. Box 2 lists several conditions that make surgery through minithoracotomy more difficult and can therefore be regarded as a contraindication. However, as is usually the way, the number of contraindications falls with the increase in experience (21)—so most limitations can be considered a relative contraindication in case the surgeon has the relevant expertise. For example, if the aorta ascends vertically straight directly behind the sternum, this makes access to the aortic valve from the right more difficult, and partial or complete sternotomy may be the more suitable procedure (18). Sternotomy-free access is still often possible, however (5).
The driver behind developments in cardiac surgery
It is interesting to observe that minimally invasive techniques in cardiac surgery developed initially slowly, but how, in the past few years, their development has accelerated (5). The reasons might include as a long and steep learning curve, late skills acquisition in the course of a long surgical career, and increased technical skills, with “only” comparably good results (5, 21). We believe, however, that these developments are happening independently of the existing evidence (5), and that patients’ and doctors’ individual perceptions have a crucial role in all this.
Traditional cardiac surgery, for which, so far, no alternatives have existed, is now in competition with continually increasing options for intervention. Patients can choose—and their perception often is—that a smaller incision or percutaneous groin vessel cannulation is associated with a lower risk or a gentler procedure. This perception, which is also observed in doctors, is often not supported by the actual evidence, however. Most studies that compared a “large incision” with a “small incision” or with femoral percutaneous groin vessel cannulation showed identical in-hospital mortality or mortality at 30 days, independently of whether the procedure was interventional aortic valve implantation versus surgical replacement or a cardiopulmonary bypass procedure versus a percutaneous intervention (36– 38).
A valuable new development in terms of minimizing or avoiding invasiveness is the option of imaging the coronary arteries by using CT scanning (39). This means that there is no longer a need for prior diagnostic cardiac catheterization. This development will lead to further reaching changes.
The deliberations above therefore allow the plausible conclusion that patients as well as many doctors have an enormous amount of respect for open-heart surgery using sternotomy. This insight, which is not primarily based on prospective randomized controlled trials, is in our opinion what is behind the recent crucial developments in conventional heart surgery. It also means, however, that defining the indication responsibly in terms of the achievable surgical result should crucially influence the decision-making process in favor of minimally invasive access or sternotomy.
Conclusions
Conventional heart surgery has undergone extensive changes over the past decade. Specialist surgeons and centers are now equipped to undertake many isolated and combined cardiac valve procedures as well as isolated single and multiple bypass operations without using sternotomy, through minithoracotomy. Although evidence from prospective randomized trials is poor, the available information, paired with a multitude of additional database and registry analyses, implies that minimally invasive procedures are in many indications able to yield at least equal results to conventional heart surgery. Without sternotomy or by using partial sternotomy, surgical results can be achieved that are supported by decades of documentation and follow-up.
Such proof will still have to be brought for new procedures and products, whether these are interventional or surgical. In our experience, and with short term and long term results being equal, informed patients practically always decide in favor of the less invasive procedure.
Key Messages.
With the relevant specialization it is possible to undertake many heart operations as minimally invasive procedures. This can be done not only through partial sternotomy, but also completely without sternotomy, through right or left minithoracotomy (sternotomy-free).
There is no evidence to date that allows concrete conclusions about the superiority or inferiority of such sternotomy-free techniques. Registry studies imply that minimally invasive procedures can also yield good results.
Nationwide in Germany, half of all mitral valve procedures are being done without using sternotomy, and a quarter of conventionally operated aortic valves are being replaced by using partial sternotomy.
Some highly specialized centers also undertake combined valve procedures (aortic and/or mitral valve and/or tricuspid valve operations) or multiple bypass operations through a minithoracotomy, sternotomy-free.
Pulmonary valve disorders and combined procedures, consisting of valve surgery and bypass, are not suitable for a minithoracotomy.
Acknowledgments
Acknowledgment
We thank Benjamin Gloy for his help in composing the manuscript.
Footnotes
Conflict of interest statement
Prof Doenst, Dr Diab, and Dr Färber themselves conduct the minimally invasive procedures described in this article.
The remaining authors declare that no conflict of interest exists.
Translated from the original German by Birte Twisselmann, PhD.
References
- 1.Kalk H. Bemerkungen zur Technik der Laparoskopie und Beschreibung neuer laparoskopischer Instrumente. Med Klin. 1955;50:696–699. [PubMed] [Google Scholar]
- 2.Antoniou SA, Antoniou GA, Antoniou AI, Granderath FA. Past, present, and future of minimally invasive abdominal surgery. JSLS. 2015;19 doi: 10.4293/JSLS.2015.00052. e2015.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohn LH, Adams DH, Couper GS, et al. Minimally invasive cardiac valve surgery improves patient satisfaction while reducing costs of cardiac valve replacement and repair. Ann Surg. 1997;226:421–426. doi: 10.1097/00000658-199710000-00003. discussion 7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carpentier A, Loulmet D, Carpentier A, et al. Open heart operation under videosurgery and minithoracotomy First case (mitral valvuloplasty) operated with success. C R Acad Sci III. 1996;319:219–223. [PubMed] [Google Scholar]
- 5.Doenst T, Lamelas J. Do we have enough evidence for minimally-invasive cardiac surgery? A critical review of scientific and non-scientific information. J Cardiovasc Surg (Torino) 2017;58:613–623. doi: 10.23736/S0021-9509.16.09446-5. [DOI] [PubMed] [Google Scholar]
- 6.Beckmann A, Funkat AK, Lewandowski J, et al. German Heart Surgery Report 2015: The annual updated registry of the German Society for Thoracic and Cardiovascular Surgery. Thorac Cardiovasc Surg. 2016;64:462–474. doi: 10.1055/s-0036-1592124. [DOI] [PubMed] [Google Scholar]
- 7.Doll N, Borger MA, Hain J, et al. Minimal access aortic valve replacement: effects on morbidity and resource utilization. Ann Thorac Surg. 2002;74:S1318–S1322. doi: 10.1016/s0003-4975(02)03911-5. [DOI] [PubMed] [Google Scholar]
- 8.Johnston DR, Roselli EE. Minimally invasive aortic valve surgery: Cleveland Clinic experience. Ann Cardiothorac Surg. 2015;4:140–147. doi: 10.3978/j.issn.2225-319X.2014.10.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merk DR, Lehmann S, Holzhey DM, et al. Minimal invasive aortic valve replacement surgery is associated with improved survival: a propensity-matched comparison. Eur J Cardiothorac Surg. 2015;47:11–17. doi: 10.1093/ejcts/ezu068. discussion 7. [DOI] [PubMed] [Google Scholar]
- 10.Murzi M, Cerillo AG, Miceli A, et al. Antegrade and retrograde arterial perfusion strategy in minimally invasive mitral-valve surgery: a propensity score analysis on 1280 patients. Eur J Cardiothorac Surg. 2013;43:e167–e172. doi: 10.1093/ejcts/ezt043. [DOI] [PubMed] [Google Scholar]
- 11.Grossi EA, LaPietra A, Ribakove GH, et al. Minimally invasive versus sternotomy approaches for mitral reconstruction: comparison of intermediate-term results. J Thorac Cardiovasc Surg. 2001;121:708–713. doi: 10.1067/mtc.2001.112626. [DOI] [PubMed] [Google Scholar]
- 12.Ryan WH, Brinkman WT, Dewey TM, Mack MJ, Prince SL, Herbert MA. Mitral valve surgery: comparison of outcomes in matched sternotomy and port access groups. J Heart Valve Dis. 2010;19:51–58. discussion 9. [PubMed] [Google Scholar]
- 13.Suri RM, Schaff HV, Meyer SR, Hargrove WC. 3rdThoracoscopic versus open mitral valve repair: a propensity score analysis of early outcomes. Ann Thorac Surg. 2009;88:1185–1190. doi: 10.1016/j.athoracsur.2009.04.076. [DOI] [PubMed] [Google Scholar]
- 14.Sundermann SH, Czerny M, Falk V. Open vs minimally invasive mitral valve surgery: surgical technique, indications and results. Cardiovasc Eng Technol. 2015;6:160–166. doi: 10.1007/s13239-015-0210-5. [DOI] [PubMed] [Google Scholar]
- 15.Lamelas J, Williams RF, Mawad M, LaPietra A. Complications associated with femoral cannulation during minimally invasive cardiac surgery. Ann Thorac Surg. 2017;103:1927–1932. doi: 10.1016/j.athoracsur.2016.09.098. [DOI] [PubMed] [Google Scholar]
- 16.Semsroth S, Matteucci Gothe R, Raith YR, et al. Comparison of two minimally invasive techniques and median sternotomy in aortic valve replacement. Ann Thorac Surg. 2017;104:877–883. doi: 10.1016/j.athoracsur.2017.01.095. [DOI] [PubMed] [Google Scholar]
- 17.Foghsgaard S, Schmidt TA, Kjaergard HK. Minimally invasive aortic valve replacement: late conversion to full sternotomy doubles operative time. Tex Heart Inst J. 2009;36:293–297. [PMC free article] [PubMed] [Google Scholar]
- 18.Miceli A, Murzi M, Gilmanov D, et al. Minimally invasive aortic valve replacement using right minithoracotomy is associated with better outcomes than ministernotomy. J Thorac Cardiovasc Surg. 2014;148:133–137. doi: 10.1016/j.jtcvs.2013.07.060. [DOI] [PubMed] [Google Scholar]
- 19.Mihaljevic T, Koprivanac M, Kelava M, et al. Value of robotically assisted surgery for mitral valve disease. JAMA Surg. 2014;149:679–686. doi: 10.1001/jamasurg.2013.5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moschovas A, Amorim PA, Nold M, et al. Percutaneous cannulation for cardiopulmonary bypass in minimally invasive surgery is associated with reduced groin complications. Interact Cardiovasc Thorac Surg. 2017;25:377–383. doi: 10.1093/icvts/ivx140. [DOI] [PubMed] [Google Scholar]
- 21.Holzhey DM, Seeburger J, Misfeld M, Borger MA, Mohr FW. Learning minimally invasive mitral valve surgery: a cumulative sum sequential probability analysis of 3895 operations from a single high-volume center. Circulation. 2013;128:483–491. doi: 10.1161/CIRCULATIONAHA.112.001402. [DOI] [PubMed] [Google Scholar]
- 22.Ariyaratnam P, Loubani M, Griffin SC. Minimally invasive aortic valve replacement: comparison of long-term outcomes. Asian Cardiovasc Thorac Ann. 2015;23:814–821. doi: 10.1177/0218492315587606. [DOI] [PubMed] [Google Scholar]
- 23.Gilmanov D, Farneti PA, Ferrarini M, et al. Full sternotomy versus right anterior minithoracotomy for isolated aortic valve replacement in octogenarians: a propensity-matched study dagger. Interact Cardiovasc Thorac Surg. 2015;20:732–741. doi: 10.1093/icvts/ivv030. discussion 41. [DOI] [PubMed] [Google Scholar]
- 24.Glauber M, Miceli A, Canarutto D, et al. Early and long-term outcomes of minimally invasive mitral valve surgery through right minithoracotomy: a 10-year experience in 1604 patients. J Cardiothorac Surg. 2015;10 doi: 10.1186/s13019-015-0390-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castillo JG, Anyanwu AC, Fuster V, Adams DH. A near 100% repair rate for mitral valve prolapse is achievable in a reference center: implications for future guidelines. J Thorac Cardiovasc Surg. 2012;144:308–312. doi: 10.1016/j.jtcvs.2011.12.054. [DOI] [PubMed] [Google Scholar]
- 26.Goldstone AB, Atluri P, Szeto WY, et al. Minimally invasive approach provides at least equivalent results for surgical correction of mitral regurgitation: a propensity-matched comparison. J Thorac Cardiovasc Surg. 2013;145:748–756. doi: 10.1016/j.jtcvs.2012.09.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stolinski J, Plicner D, Grudzien G, et al. A comparison of minimally invasive and standard aortic valve replacement. J Thorac Cardiovasc Surg. 2016;152:1030–1039. doi: 10.1016/j.jtcvs.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 28.Keyl C, Staier K, Pingpoh C, et al. Unilateral pulmonary oedema after minimally invasive cardiac surgery via right anterolateral minithoracotomy. Eur J Cardiothorac Surg. 2015;47:1097–1102. doi: 10.1093/ejcts/ezu312. [DOI] [PubMed] [Google Scholar]
- 29.Tutschka MP, Bainbridge D, Chu MW, Kiaii B, Jones PM. Unilateral postoperative pulmonary edema after minimally invasive cardiac surgical procedures: a case-control study. Ann Thorac Surg. 2015;99:115–122. doi: 10.1016/j.athoracsur.2014.07.067. [DOI] [PubMed] [Google Scholar]
- 30.Irisawa Y, Hiraoka A, Totsugawa T, et al. Re-expansion pulmonary oedema after minimally invasive cardiac surgery with right mini-thoracotomy. Eur J Cardiothorac Surg. 2016;49:500–505. doi: 10.1093/ejcts/ezv089. [DOI] [PubMed] [Google Scholar]
- 31.Lamelas J. Minimally invasive concomitant aortic and mitral valve surgery: the „Miami Method“. Ann Cardiothorac Surg. 2015;4:33–37. doi: 10.3978/j.issn.2225-319X.2014.08.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lio A, Murzi M, Solinas M, Glauber M. Minimally invasive triple valve surgery through a right minithoracotomy. J Thorac Cardiovasc Surg. 2014;148:2424–2427. doi: 10.1016/j.jtcvs.2014.06.094. [DOI] [PubMed] [Google Scholar]
- 33.Kitamura T, Stuklis RG, Edwards J. Redo mitral valve operation via right minithoracotomy—“no touch“ technique. Int Heart J. 2011;52:107–109. doi: 10.1536/ihj.52.107. [DOI] [PubMed] [Google Scholar]
- 34.Lichtenberg A, Klima U, Paeschke H, et al. Impact of multivessel coronary artery disease on outcome after isolated minimally invasive bypass grafting of the left anterior descending artery. Ann Thorac Surg. 2004;78:487–491. doi: 10.1016/j.athoracsur.2003.11.044. [DOI] [PubMed] [Google Scholar]
- 35.Ruel M, Shariff MA, Lapierre H, et al. Results of the minimally invasive coronary artery bypass grafting angiographic patency study. J Thorac Cardiovasc Surg. 2014;147:203–208. doi: 10.1016/j.jtcvs.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 36.Mack MJ, Leon MB, Smith CR, et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385:2477–2484. doi: 10.1016/S0140-6736(15)60308-7. [DOI] [PubMed] [Google Scholar]
- 37.Head SJ, Davierwala PM, Serruys PW, et al. Coronary artery bypass grafting vs percutaneous coronary intervention for patients with three-vessel disease: final five-year follow-up of the SYNTAX trial. Eur Heart J. 2014;35:2821–2830. doi: 10.1093/eurheartj/ehu213. [DOI] [PubMed] [Google Scholar]
- 38.Sipahi I, Akay MH, Dagdelen S, Blitz A, Alhan C. Coronary artery bypass grafting vs percutaneous coronary intervention and long-term mortality and morbidity in multivessel disease meta-analysis of randomized clinical trials of the arterial grafting and stenting era. JAMA Intern Med. 2014;174:223–230. doi: 10.1001/jamainternmed.2013.12844. [DOI] [PubMed] [Google Scholar]
- 39.Opolski MP, Staruch AD, Jakubczyk M, et al. CT angiography for the detection of coronary artery stenoses in patients referred for cardiac valve surgery: Systematic review and meta-analysis. JACC Cardiovasc Imaging. 2016;9:1059–1070. doi: 10.1016/j.jcmg.2015.09.028. [DOI] [PubMed] [Google Scholar]
- E1.Ahangar AG, Charag AH, Wani ML, et al. Comparing aortic valve replacement through right anterolateral thoracotomy with median sternotomy. Int Cardiovasc Res J. 2013;7:90–94. [PMC free article] [PubMed] [Google Scholar]
- E2.Aris A, Camara ML, Montiel J, Delgado LJ, Galan J, Litvan H. Ministernotomy versus median sternotomy for aortic valve replacement: a prospective, randomized study. Ann Thorac Surg. 1999;67:1583–1587. doi: 10.1016/s0003-4975(99)00362-8. discussion 7-8. [DOI] [PubMed] [Google Scholar]
- E3.Bonacchi M, Prifti E, Giunti G, Frati G, Sani G. Does ministernotomy improve postoperative outcome in aortic valve operation? A prospective randomized study. Ann Thorac Surg. 2002;73:460–465. doi: 10.1016/s0003-4975(01)03402-6. discussion 5-6. [DOI] [PubMed] [Google Scholar]
- E4.Calderon J, Richebe P, Guibaud JP, et al. Prospective randomized study of early pulmonary evaluation of patients scheduled for aortic valve surgery performed by ministernotomy or total median sternotomy. J Cardiothorac Vasc Anesth. 2009;23:795–801. doi: 10.1053/j.jvca.2009.03.011. [DOI] [PubMed] [Google Scholar]
- E5.Dogan S, Dzemali O, Wimmer-Greinecker G, et al. Minimally invasive versus conventional aortic valve replacement: a prospective randomized trial. J Heart Valve Dis. 2003;12:76–80. [PubMed] [Google Scholar]
- E6.Machler HE, Bergmann P, Anelli-Monti M, et al. Minimally invasive versus conventional aortic valve operations: a prospective study in 120 patients. Ann Thorac Surg. 1999;67:1001–1005. doi: 10.1016/s0003-4975(99)00072-7. [DOI] [PubMed] [Google Scholar]
- E7.Moustafa MA, Abdelsamad AA, Zakaria G, Omarah MM. Minimal vs median sternotomy for aortic valve replacement. Asian Cardiovasc Thorac Ann. 2007;15:472–475. doi: 10.1177/021849230701500605. [DOI] [PubMed] [Google Scholar]
- E8.Dogan S, Aybek T, Risteski PS, et al. Minimally invasive port access versus conventional mitral valve surgery: prospective randomized study. Ann Thorac Surg. 2005;79:492–498. doi: 10.1016/j.athoracsur.2004.08.066. [DOI] [PubMed] [Google Scholar]
- E9.Nasso G, Bonifazi R, Romano V, et al. Three-year results of repaired Barlow mitral valves via right minithoracotomy versus median sternotomy in a randomized trial. Cardiology. 2014;128:97–105. doi: 10.1159/000357263. [DOI] [PubMed] [Google Scholar]
- E10.Speziale G, Nasso G, Esposito G, et al. Results of mitral valve repair for Barlow disease (bileaflet prolapse) via right minithoracotomy versus conventional median sternotomy: a randomized trial. J Thorac Cardiovasc Surg. 2011;142:77–83. doi: 10.1016/j.jtcvs.2010.08.033. [DOI] [PubMed] [Google Scholar]
- E11.Santana O, Reyna J, Grana R, Buendia M, Lamas GA, Lamelas J. Outcomes of minimally invasive valve surgery versus standard sternotomy in obese patients undergoing isolated valve surgery. Ann Thorac Surg. 2011;91:406–410. doi: 10.1016/j.athoracsur.2010.09.039. [DOI] [PubMed] [Google Scholar]
- E12.Santana O, Reyna J, Benjo AM, Lamas GA, Lamelas J. Outcomes of minimally invasive valve surgery in patients with chronic obstructive pulmonary disease. Eur J Cardiothorac Surg. 2012;42:648–652. doi: 10.1093/ejcts/ezs098. [DOI] [PubMed] [Google Scholar]
- E13.Attia RQ, Hickey GL, Grant SW, et al. Minimally invasive versus conventional aortic valve replacement: a propensity-matched study from the UK National Data. Innovations (Phila) 2016;11:15–23. doi: 10.1097/IMI.0000000000000236. discussion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E14.Bakir I, Casselman FP, Wellens F, et al. Minimally invasive versus standard approach aortic valve replacement: a study in 506 patients. Ann Thorac Surg. 2006;81:1599–1604. doi: 10.1016/j.athoracsur.2005.12.011. [DOI] [PubMed] [Google Scholar]
- E15.Bowdish ME, Hui DS, Cleveland JD, et al. A comparison of aortic valve replacement via an anterior right minithoracotomy with standard sternotomy: a propensity score analysis of 492 patients. Eur J Cardiothorac Surg. 2016;49:456–463. doi: 10.1093/ejcts/ezv038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E16.Brinkman WT, Hoffman W, Dewey TM, et al. Aortic valve replacement surgery: comparison of outcomes in matched sternotomy and PORT ACCESS groups. Ann Thorac Surg. 2010;90:131–135. doi: 10.1016/j.athoracsur.2010.03.055. [DOI] [PubMed] [Google Scholar]
- E17.Doll N, Borger MA, Hain J, et al. Minimal access aortic valve replacement: effects on morbidity and resource utilization. Ann Thorac Surg. 2002;74:S1318–S1322. doi: 10.1016/s0003-4975(02)03911-5. [DOI] [PubMed] [Google Scholar]
- E18.Furukawa N, Kuss O, Aboud A, et al. Ministernotomy versus conventional sternotomy for aortic valve replacement: matched propensity score analysis of 808 patients. Eur J Cardiothorac Surg. 2014;46:221–226. doi: 10.1093/ejcts/ezt616. discussion 6-7. [DOI] [PubMed] [Google Scholar]
- E19.Ghanta RK, Lapar DJ, Kern JA, et al. Minimally invasive aortic valve replacement provides equivalent outcomes at reduced cost compared with conventional aortic valve replacement: a real-world multi-institutional analysis. J Thorac Cardiovasc Surg. 2015;149:1060–1065. doi: 10.1016/j.jtcvs.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E20.Glauber M, Miceli A, Gilmanov D, et al. Right anterior minithoracotomy versus conventional aortic valve replacement: a propensity score matched study. J Thorac Cardiovasc Surg. 2013;145:1222–1226. doi: 10.1016/j.jtcvs.2012.03.064. [DOI] [PubMed] [Google Scholar]
- E21.Glower DD, Desai BS, Hughes GC, Milano CA, Gaca JG. Aortic valve replacement via right minithoracotomy versus median sternotomy: a propensity score analysis. Innovations (Phila) 2014;9:75–81. doi: 10.1097/IMI.0000000000000062. discussion. [DOI] [PubMed] [Google Scholar]
- E22.Johnston DR, Atik FA, Rajeswaran J, et al. Outcomes of less invasive J-incision approach to aortic valve surgery. J Thorac Cardiovasc Surg. 2012;144:852–858. doi: 10.1016/j.jtcvs.2011.12.008. e3. [DOI] [PubMed] [Google Scholar]
- E23.Klokocovnik T, Kersnik Levart T, Bunc M. Double venous drainage through the superior vena cava in minimally invasive aortic valve replacement: a retrospective study. Croat Med J. 2012;53:11–16. doi: 10.3325/cmj.2012.53.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E24.Neely RC, Boskovski MT, Gosev I, et al. Minimally invasive aortic valve replacement versus aortic valve replacement through full sternotomy: the Brigham and Women‘s Hospital experience. Ann Cardiothorac Surg. 2015;4:38–48. doi: 10.3978/j.issn.2225-319X.2014.08.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E25.Stamou SC, Kapetanakis EI, Lowery R, Jablonski KA, Frankel TL, Corso PJ. Allogeneic blood transfusion requirements after minimally invasive versus conventional aortic valve replacement: a risk-adjusted analysis. Ann Thorac Surg. 2003;76:1101–1106. doi: 10.1016/s0003-4975(03)00885-3. [DOI] [PubMed] [Google Scholar]
- E26.Iribarne A, Russo MJ, Easterwood R, et al. Minimally invasive versus sternotomy approach for mitral valve surgery: a propensity analysis. Ann Thorac Surg. 2010;90:1471–1477. doi: 10.1016/j.athoracsur.2010.06.034. discussion 7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E27.Tang P, Onaitis M, Gaca JG, Milano CA, Stafford-Smith M, Glower D. Right minithoracotomy versus median sternotomy for mitral valve surgery: a propensity matched study. Ann Thorac Surg. 2015;100:575–581. doi: 10.1016/j.athoracsur.2015.04.027. [DOI] [PubMed] [Google Scholar]
- E28.Zhai J, Wei L, Huang B, Wang C, Zhang H, Yin K. Minimally invasive mitral valve replacement is a safe and effective surgery for patients with rheumatic valve disease: a retrospective study. Medicine (Baltimore) 2017;96 doi: 10.1097/MD.0000000000007193. [DOI] [PMC free article] [PubMed] [Google Scholar]