Abstract
Background
Suitable analgesic drugs and techniques are needed for the acute care of the approximately 18 200—18 400 seriously injured patients in Germany each year.
Methods
This systematic review and meta-analysis of analgesia in trauma patients was carried out on the basis of randomized, controlled trials and observational studies. A systematic search of the literature over the 10-year period ending in February 2016 was carried out in the PubMed, Google Scholar, and Springer Link Library databases. Some of the considered trials and studies were included in a meta-analysis. Mean differences (MD) of pain reduction or pain outcome as measured on the Numeric Rating Scale were taken as a summarizing measure of treatment efficacy.
Results
Out of 685 studies, 41 studies were considered and 10 studies were included in the meta-analysis. Among the drugs and drug combinations studied, none was clearly superior to another with respect to pain relief. Neither fentanyl versus morphine (MD –0.10 with a 95% confidence interval of [-0.58; 0.39], p = 0.70) nor ketamine versus morphine (MD -1.27 [-3.71; 1.16], p = 0.31), or the combination of ketamine and morphine versus morphine alone (MD -1.23 [-2.29; -0.18], p = 0.02) showed clear superiority regarding analgesia.
Conclusion
Ketamine, fentanyl, and morphine are suitable for analgesia in spontaneously breathing trauma patients. Fentanyl and ketamine have a rapid onset of action and a strong analgesic effect. Our quantitative meta-analysis revealed no evidence for the superiority of any of the three substances over the others. Suitable monitoring equipment, and expertise in emergency procedures are prerequisites for safe and effective analgesia by healthcare professionals..
Each year 18 200–18 400 patients require treatment for severe injuries in Germany (1). In 2016, ca. 396 700 persons were injured in road traffic accidents (2). Adequate analgesia following trauma is a central aspect of emergency medical treatment before and after hospital admission (3– 5). Although relief of pain is a fundamental human right (6), studies suggest that many trauma patients are undertreated in this respect (7). One of the tasks of the group convened to revise the German S3 guideline on treatment of multiple trauma and severe injuries in 2016 was to take steps towards the development of national recommendations on the treatment of pain in trauma patients (8).
The goals of this systematic analysis of the published literature were to compare the effects of various analgesics, alone and in combination, in (severely) injured but spontaneously breathing patients with no need for airway management; to review safety and adverse effects; and to formulate recommendations. To this end, we performed qualitative and quantitative analysis of the data from randomized controlled trials (RCTs) and observational studies.
Method
This study adhered to the principles of the PRISMA statement for systematic reviews and followed the PICO scheme (population, interventions, comparison, outcome). The protocol, search strategy, and search terms were entered in the PROSPERO registry of systematic reviews and have been published (www.crd.york.ac.uk/PROSPERO, ID: CRD42016046110). Details of the study’s methods can be found in eBox 1. The endpoints of the meta-analysis were pain reduction (difference in pain rating before and after administration of analgesics) and the post-treatment score on the numeric rating scale (NRS).
eBOX 1. Material and methods.
Search
A systematic survey of the literature in the PubMed database was carried out with the following search terms: (prehospital OR pre-hospital OR out-of-hospital OR emergenc*) AND (injury OR injuries OR trauma) AND (analgesia OR analgesic* OR pain management) AND (controlled clinical trial OR randomized controlled trial OR clinical trial OR meta-analysis OR systematic review). Publications over a 10-year period up to February 2016 were searched in order to make the survey as up-to-date as possible. The search was extended by scrutinizing the reference lists of the identified reviews and original articles. Furthermore, Google Scholar und the SpringerLink Library were searched.
Inclusion criteria
Published studies on analgesia without invasive airway management in trauma patients were included. Various analgesics were assessed, alone and in combination, with regard to safe pain reduction. The endpoints were pain reduction or post-treatment Numeric Rating Scale (NRS) score for emergency medical treatment, onset of action, and adverse effects profile, if reported, Only publications in German or English were included. Two authors independently decided on inclusion or exclusion. If they did not agree, they could consult an additional author.
Study selection and evaluation
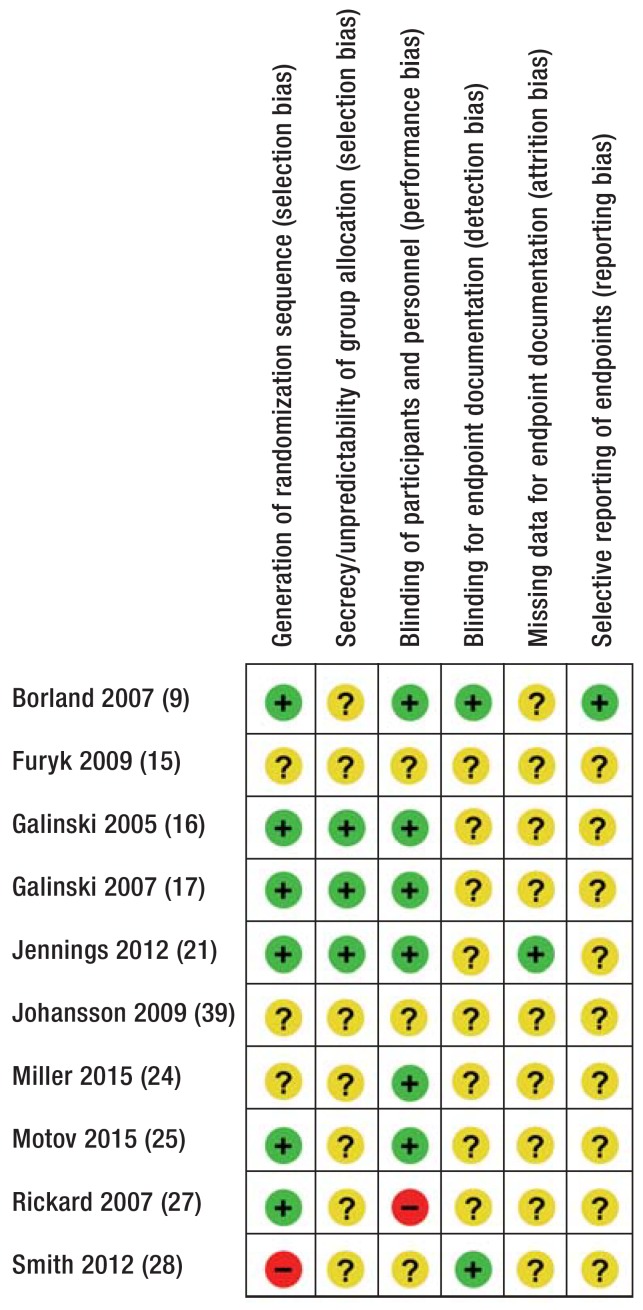
After exclusion of duplicates, all titles and abstracts were checked by two authors and it was decided whether to access the full text. The numbers of indexed titles and abstracts were documented. Full texts were checked for relevance and included or excluded accordingly. In the case of disagreement, a further author could be consulted. Two reviewers working independently estimated the risk of bias of each study using Cochrane’s Revman 5. The risk of bias was rated as low, unclear, or high (efigure).
Data analysis
Because our review was not limited to the meta-analysis of medications, we included not only randomized controlled trials (RCTs) and cohort studies, but also, for example, observational studies, particularly for research questions concerning routes of administration, safety and monitoring, undertreatment with analgesics, pain assessment, nonpharmacological pain treatment, and undesired events. The corresponding findings were described qualitatively.
Statistical method
The meta-analysis compared the analgesia achieved by two different analgesics, with post-treatment NRS score and pain reduction as endpoints. The results for continuous data were expressed as mean difference with 95% confidence interval. Heterogeneity was tested by means of the chi-square test. Because the power of the test was expected to be low (given the small number of studies with predominantly low case numbers), the level of significance for heterogeneity was set at 0.2. The meta-analysis was performed on pooled endpoints using Review Manager (RevMan, version 5.3; Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). Conversions of measures of dispersion or effect size were carried out according to the recommendations of the Cochrane Handbook (http://handbook.cochrane.org). Some controlled studies could not be included in the meta-analysis because the endpoint parameters were not comparable or relevant data were missing (11, 18, 20, 26, 29, 38, 40, e8). Details of the study populations can be found in eTable 1.
Results
Selection of studies
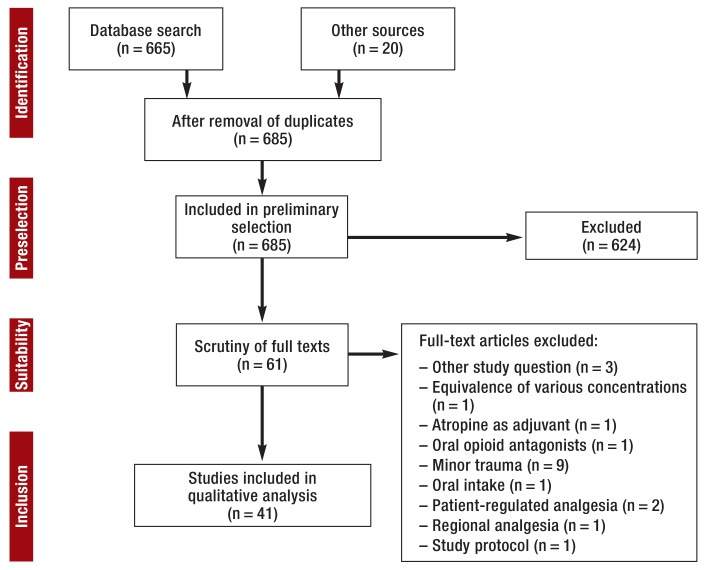
The initial literature survey turned up 665 relevant publications. A further 20 items were identified by an additional hand search. Of these 685 publications, 624 were excluded after perusal of titles and abstracts. Full-text inspection eliminated a further 20 publications, leaving 41 for analysis (figure 1).
Figure 1.
PRISMA flow chart of literature survey and study selection
Study characteristics
Twenty-three of the 41 studies were of the emergency rescue services, covering a total of 67 269 patients (10, 16, 21, 22, 27– 32, 37– 40, e1– e9), and the remaining 18 studies, comprising 1899 patients, were carried out in hospital emergency departments (9, 11– 15, 17– 20, 23– 26, 33– 36). Overall, the substances most commonly used for analgesia were the opiates fentanyl and morphine, the NMDA receptor antagonist ketamine, and combinations of ketamine with opiates, followed by methoxyflurane, nitrous oxide, paracetamol, pentazocine, and sufentanil or combinations thereof. An overview of the studies can be found in eTable 2. Ten studies were included in a meta-analysis of pain reduction but were highly heterogeneous.
eTable 2. Adverse effects.
| Fentanyl | Ketamine | Morphine | Ketamine + morphine | |||||
| n | % [95% CI] | n | % [95% CI] | n | % [95% CI] | n | % [95% CI] | |
| Total number of patients in all studies that reported adverse effects | 6373 | 2105 | 1098 | 119 | ||||
| Loss of vigilance | 31 | 0.49 [0.33–0.69 ] | 58 | 2.76 [2.10–3.55 ] | 36 | 3.28 [2.31–4.51 ] | 3 | 2.52 [0.52–7.19 ] |
| Dysphoria | 6 | 0.09 [0.03–0.20 ] | 41 | 1.95 [1.40–2.63 ] | 3 | 0.27 [0.06–0.80 ] | 13 | 10.08 [5.95–17.96 ] |
| Pruritus | 0 | 0.00 [0.00–0.05 ] | 0 | 0.00 [0.00–0.14 ] | 3 | 0.27 [0.06–0.80 ] | 0 | 0.00 [0.00–2.49 ] |
| Decrease in pulse oximetric oxygen saturation | 37 | 0.58 [0.41–0.80 ] | 8 | 0.58 [0.16–0.75 ] | 6 | 0.55 [0.20–1.19 ] | 0 | 0.00 [0.00–2.49 ] |
| Ventilation required | 1 | 0.02 [0.00–0.09 ] | 1 | 0.05 [0.00–0.26 ] | 0 | 0.00 [0.00–0.27 ] | 0 | 0.00 [0.00–2.49 ] |
| Salivation | 0 | 0.00 [0.00–0.05 ] | 4 | 0.19 [0.05–0.49 ] | 0 | 0.00 [0.00–0.27 ] | 0 | 0.00 [0.00–2.49 ] |
| Nausea | 23 | 0.36 [0.23–0.54 ] | 21 | 1.00 [0.62–1.52 ] | 26 | 2.37 [1.55–3.45 ] | 15 | 12.61 [7.23–19.94 ] |
| Vomiting | 97 | 1.52 [1.24–1.85 ] | 13 | 0.62 [0.33–1.05 ] | 53 | 4.83 [3.64–6.27 ] | 4 | 3.36 [0.92–8.32 ] |
| Hypotension | 100 | 1.57 [1.28–1.91 ] | 0 | 0.00 [0.00–0.14 ] | 5 | 0.46 [0.15–1.06 ] | 0 | 0.00 [0.00–2.49 ] |
The table lists the adverse effects for fentanyl, ketamine, and morphine (9, 10, 13, 15– 33, 35, 36, 38, 39, e1– e5, e9). Studies that reported no adverse effects or undesired outcomes (37, 40, e6, e8) were excluded, as were studies that (according to the manufacturer’s information) used a dosage designed to produce anesthesia (1 to 2 mg/kg bodyweight) and not analgesia (0.25 to 0.5 mg/kg bodyweight) (11, 33, 34).
For all studies that reported adverse effects, the number of patients, the reported events (n,%), and the 95% confidence interval (95% CI) are given.
Analgesics
Morphine
One RCT with 300 patients compared intravenous and inhaled morphine (10 or 20 mg). Inhalation of morphine was found to have efficacy comparable with that of intravenous administration, together with high safety (19). Observational studies showed that the intravenous administration of morphine can be safe and effective (30, 32).
Ketamine
Retrospective studies showed safe analgesia with ketamine (alone or in combination with midazolam) (33, e1, e2, e4, e9). Only in one of the included studies was S-ketamine used (e10). Administration of ketamine in a dosage <2 mg/kg was followed by a decrease in pulse-oximetric oxygen saturation (SpO2) in 0.7% of cases, but no ventilation was required (e9); assisted ventilation was needed, however, in 6% of cases after high-dose ketamine (2 mg/kg) (34). The adverse effects reported as being associated with ketamine included dysphoria (4%), hypersalivation (1%), and vomiting (5%) (e9). In one single case, laryngospasm lasting for 1 min was reported (34). Intranasal ketamine seems to be safe and effective in children (36).
Fentanyl
Intravenous administration of fentanyl by paramedics and emergency physicians was safe and effective with no significant adverse effects (31, 37, 38, e3, e5). Two observational studies showed that intranasal fentanyl in a dose of 50 to 100 µg or 2 µg/kg was safe, effective, and associated with no or only few adverse effects in adults and children (31, 35).
Fentanyl versus morphine
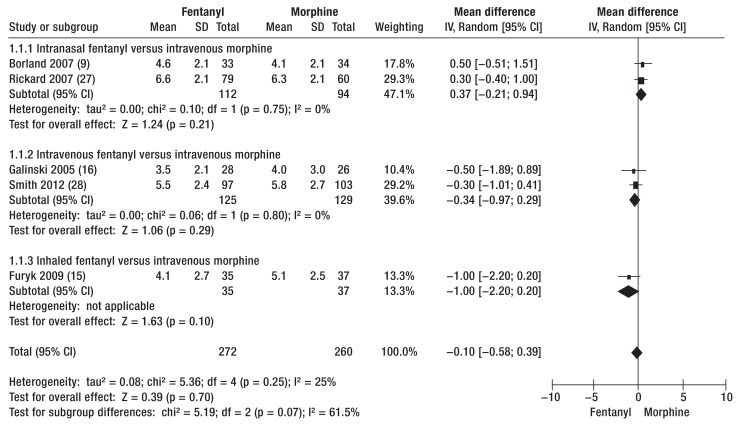
Data from four RCTs (9, 15, 16, 27) and one cohort study (28) permitted comparison of analgesia with fentanyl and with morphine in terms of post-treatment NRS score and pain reduction (RCTs: fentanyl pre-NRS 6.8 to 8.4/post-NRS 3.5 to 6.6, morphine pre-NRS 7.0 to 8.3/post-NRS 4.0 to 6.2; cohort study: fentanyl pre-NRS 8.0/post-NRS 5.5, morphine pre-NRS 8.0/post-NRS 5.8). The post-treatment NRS score showed no clear-cut advantage of fentanyl over morphine (mean difference: -0.10, 95% confidence interval [-0.58; 0.39], p = 0.70) (figure 2), but all medications investigated brought about a marked reduction in pain.
Figure 2.
Post-therapeutic pain status according to Numeric Rating Scale (NRS) score after analgesia with fentanyl or morphine. The data showed no clear-cut advantage of fentanyl over morphine.
IV, Inverse variance; Random, random effect; SD, standard deviation; 95% CI, 95% confidence interval
One cohort study that was not included in the meta-analysis showed greater pain reduction with fentanyl i.v. than with morphine i.v. (fentanyl pre-NRS 8.5/post-NRS 4.4, morphine pre-NRS 8.2/post-NRS 5.9) (38). Both RCTs and retrospective studies compared intranasal/inhaled fentanyl with morphine i.v. The RCTs found that intranasal/inhaled fentanyl was equivalent to morphine i.v. for pain reduction (fentanyl pre-NRS 6.8 to 8.4/post-NRS 3.0 to 6.6, morphine pre-NRS 7.0 to 8.7/post-NRS 3.0 to 6.2) (9, 13, 15, 27, 40). Retrospective analysis also showed that morphine i.v. was comparable in efficacy with intranasal fentanyl (pre-NRS: fentanyl 8.4, morphine 8.3; pain difference: fentanyl -4.5, morphine -4.5) (e8).
Ketamine versus morphine
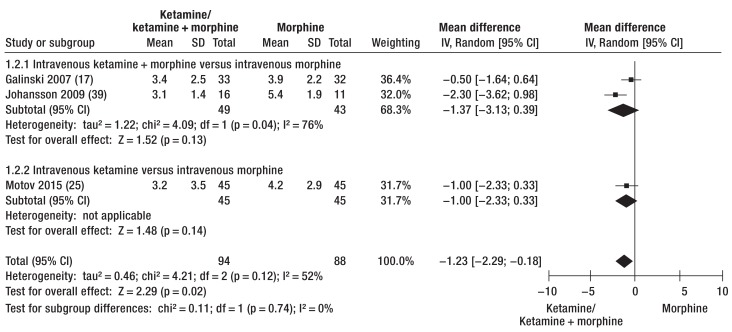
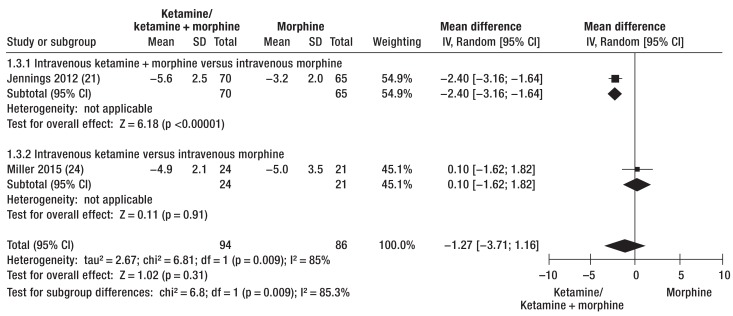
Analgesia with ketamine or ketamine/morphine and analgesia with morphine could be compared in terms of post-treatment NRS score and pain reduction using the data from four RCTs (ketamine: pre-NRS 7.1 to 8.6/post-NRS 3.2 to 3.4, pain reduction 4.9 to 5.6; morphine: pre-NRS 7.0 to 8.5/post-NRS 3.9 to 4.2, pain reduction 3.2 to 5.0) (17, 21, 24, 25) and one cohort study (39). Ketamine and ketamine combinations were more effective analgesics than morphine alone (post-NRS: mean difference -1.23 [-2.29; -0.18], p = 0.02 [Figure 3]; pain reduction: mean difference -1.27 [-3.71; 1.16], p = 0.31 [Figure 4]). Analgesia with morphine and ketamine took effect significantly more rapidly than morphine alone (17, 21, 39). However, two of the RCTs showed that pain reduction was comparable after 30 min (24, 25).
Figure 3.
Post-therapeutic pain status after administration of ketamine or ketamine/morphine versus analgesia with morphine alone.
Scores on the Numeric Rating Scale (NRS) show advantages for the ketamine combinations.
IV, Inverse variance; Random, random effect; SD, standard deviation; 95% CI, 95% confidence interval
Figure 4.
Pain reduction expressed as difference in scores on the Numeric Rating Scale (NRS) before and after analgesia with ketamine/morphine or ketamine alone and with morphine
IV, Inverse variance; Random, random effect; SD, standard deviation; 95% CI, 95% confidence interval
One cluster-randomized study that was not included in the meta-analysis showed comparable pain reduction with morphine i.v. (3.1) and with ketamine i.v. (3.5); however, airway problems and vomiting were reported more frequently in the morphine group (29).
Fentanyl versus ketamine
Two RCTs compared ketamine/midazolam i. v. with fentanyl/midazolam i. v. In one study the results were comparable (20), while the other found swifter pain reduction with a lower risk of hypoxia in the ketamine group (11) (fentanyl: pre-NRS 7 to 8/post-NRS 1 to 2; ketamine: pre-NRS 7 to 9/post-NRS 1 to 3). Owing to high heterogeneity, however, no meta-analysis could be performed. One RCT compared ketamine/propofol i.v. with fentanyl/midazolam i. v. for short anesthesia in the emergency department (post-NRS: median = 0, interquartile ratio [IQR] 0 to 1 versus median = 3, IQR 1 to 6; p <0.001). In the course of treatment, better pain reduction was described in the ketamine group and a higher incidence of SpO2 decrease in the fentanyl group (26). One RCT in children compared intranasal fentanyl with intranasal ketamine and found comparable pain reduction (fentanyl: pre-NRS 8, post-NRS 3; ketamine: pre-NRS 8, post-NRS 3) (18).
Sufentanil versus morphine
One RCT compared sufentanil i.v. with morphine i.v. in trauma-related pain (10). Sufentanil acted more rapidly than morphine, but was not superior with regard to pain reduction after 15 min (sufentanil: pre-NRS = 6, post-NRS 3.0; morphine pre-NRS = 6, post-NRS 4.0).
Onset and course of pain reduction by fentanyl, ketamine, and morphine
One cohort study reported that fentanyl and morphine were equally effective (28), but it seems that analgesia can usually be achieved more quickly with fentanyl than with morphine (16, 38). With regard to the onset of pain reduction, inhaled fentanyl and morphine i.v. were described as equivalent (9, 27, 40), but sometimes fentanyl was faster acting (13, 15). One RCT reported that morphine and ketamine were equally effective, while in other studies ketamine, alone or in combination with other substances, was more effective or quicker-acting than morphine alone (17, 21, 24, 25). Compared with fentanyl, ketamine was faster (11) or equally fast (18, 20) to take effect. The duration of effect that can be expected was given as 10 to 15 min for ketamine, 20 to 40 min for fentanyl, and up to 4 h for morphine (4, e11).
Other analgesics
Two RCTs compared the effect of N2O with that of ketamine or fentanyl (22, 23) and described equivalent analgesia. Similar results were reported for pentazocine (e6). A prehospital observational study in which paracetamol i.v. was administered to patients who predominantly had trauma of the extremities found pain reduction of NRS <5 in 50% of cases. The analgesia was described as mostly insufficient to manage severe pain (e7). One RCT compared paracetamol i.v. and morphine i.v.: the pain reduction was comparable, but morphine was quicker to take effect than paracetamol (12). Another RCT comparing paracetamol with ibuprofen described similar pain reduction in the two groups (14).
Adverse effects of the most commonly used analgesics
The documented adverse effects of fentanyl, ketamine, and morphine, the most commonly used analgesics, are summarized in eTable 2. Ketamine or combinations including ketamine led to (desired) reduced vigilance in 1.5 to 18% of cases (17, 18, 21, 36). Agitation may occur with ketamine. Decreases in SpO2 were found in all studies for fentanyl (mean 0.6%, maximum 16.1% [26]), ketamine (mean 0.4%, maximum 11.5% [33]), and morphine (mean 0.6%, maximum 4.8% [24]). Overall, assisted ventilation was necessary for 0.05% of patients with ketamine, 0.02% with fentanyl, and 0% with morphine (etable 2). Hypersalivation was reported in 0.5 to 3% of cases, predominantly in children, but was clinically irrelevant and required no intervention (e9, 29, 33). Nausea and vomiting were the principal adverse effects of morphine (4.8%), fentanyl (1.5%), and ketamine (0.5%). Hypotension was described in 1.6% of cases for fentanyl and in 0.5% of cases for morphine (etable 2).
Discussion
Analgesics
Despite the variously defined endpoints, all the analgesics used in the identified studies and the meta-analyses seem to be similar in efficacy; nevertheless, fentanyl, ketamine, and combinations of fentanyl or ketamine with other substances take effect more rapidly than morphine (1 to 3 min versus 5 to 15 min after i.v. administration). Morphine is the oldest of the analgesics investigated in this systematic review. It has a very wide field of application, and its adverse effects are nausea, vomiting, decreased SpO2, and reduced vigilance (e12). Fentanyl is described as very effective with a swift onset of action (e12) and a low risk of adverse effects (e.g., hypotension and hypoxemia) (e13). International guidelines recommend morphine, fentanyl, and ketamine, administered by trained personnel, for pre-hospital analgesia (e14, e15). There are no data on the pre-hospital use of piritramide.
Numerous studies in Germany and other countries have shown that ketamine, alone or in combination with an opioid, is safe and effective when used not just by physicians but also by appropriately trained paramedics and nurses (20, 21, 24, 25, 29, 33, 36, 39, e1, e2, e4, e9, e16, e17). Analgosedation with ketamine can lead to dysphoria and vivid hallucinations or even to agitation (e18). For this reason, accompanying administration of a low-dose benzodiazepine is recommended (e19). Ketamine has the advantage that the patient is sufficiently protected from pain and shielded from external stimuli during the rescue process or invasive procedures (e.g., repositioning or splinting). Moreover, ketamine is particularly well-suited for analgesia of hemodynamically unstable patients (e20– e22). Several reviews have shown that ketamine does not differ from other substances in respect of intracranial pressure (ICP), cerebral perfusion pressure (CPP), neurological outcome, mortality, or length of stay in the intensive care unit (e23, e24); in fact, it is especially suitable for use in patients with head injuries (e25). In ventilated patients with elevated ICP, ketamine is effective in lowering the ICP and prevents undesired increases in ICP with stable blood pressure and CPP (e26). Steps must be taken to avoid hypercapnia. Nonpharmacological pain treatment and the embryotoxological aspects of the analgesics reviewed here are discussed in eBoxes 2 and 3.
eBOX 2. Nonpharmacological pain treatment.
The management of injuries of the extremities (e.g., repositioning or splinting) should prevent further damage without prolonging the time before the patient reaches the hospital (e54). Repositioning and splinting go a long way towards relieving pain and are intended to prevent soft tissue necrosis, neurovascular damage, and compartment syndrome, as well as maintaining perfusion. In the case of musculoskeletal trauma, the injured limb should be positioned flat and immobilized above and below the site of injury. Regular reassessment of the neurovascular status before and after manipulations is mandatory (e55– e57). However, repositioning should be performed only by properly trained personnel with the patient under adequate analgesia. The principal goal is restoration and maintenance of local perfusion by continuous longitudinal tension and manual correction in a position as close as possible to neutral (e58– e61). If the neurovascular status is unaffected and the user has no experience of repositioning, the injured extremity can be fixed in the position as found and the patient swiftly transported to an appropriate hospital, particularly in life-threatening situations (e55, e60).
eBOX 3. Embryotoxicological evaluation.
In pregnant women, not only does the appropriate drug have to be chosen with care but the altered physiological circumstances have to be taken into account. Particular attention should be paid to nausea and vomiting, aspiration, and vena cava compression syndrome in advanced pregnancy. Also during pregnancy, the analgetic potency of paracetamol is often inadequate for severe injuries. The Pharmacovigilance and Information Center for Embryonal Toxicology, Charité—Universitätsmedizin Berlin has stated that opioids do not always constitute a teratogenic risk factor. Single doses in the context of initial emergency care are unproblematic. In the case of extended treatment or repeated doses before delivery, the newborn may present with respiratory depression, withdrawal symptoms, and adjustment disorders.
Neither morphine nor fentanyl has been systematically investigated, but there are no indications that either substance presents a serious risk when used in pregnancy. There are no data on short-term use, particularly in the initial emergency care scenario. Morphine has a half-life of 1.7 to 4.5 h, in newborns up to 13.9 h. The relative dose (child’s dose via breast milk per kg divided by mother’s dose per kg) ranges from 9.09 to 35% (e62, e63, e64), depending on the route of administration. To date, no abnormal findings have been reported in breast-fed infants. The half-life of fentanyl is ca. 2 to 12 h, depending on how it is given, and up to 17 h in neonates. The relative dose is 2.9 to 5%, depending on the route of administration. Again, no abnormalities have been reported in breast-fed infants. After a single intravenous dose (short half-life) the amount of drug transferred to the child will generally be low, because the swift distribution means that the serum concentration decreases rapidly (e65, e66, e67).
The use of ketamine in breast-feeding women has not been systematically investigated, and there are no data on crossover into breast milk. Because of the fast distribution, no great amount of drug can be expected to be transferred to the infant, particularly with low oral bioavailability (20 to 30%). In pregnancy, ketamine exerts a dose-dependent stimulatory action on the tone and contraction frequency of the uterus (e68, e69), which may lead to undersupply of the fetus. For this reason, in severely injured pregnant women it should be decided case by case whether the risk of hypotension is higher or lower than that of elevated uterine activity. In the initial emergency care scenario, the mother’s life takes precedence. Thus ketamine can be used in the initial treatment of severely injured pregnant women, but should if possible be avoided in monotrauma due to the danger of increased uterine activity. Further information can be found (in German) at: www.embryotox.de.
Alternative routes of administration
Analgesics should be administered intravenously in the context of emergency medicine (5). All analgesics approved for i.v. administration can also be given by the intraosseous (i.o.) route (e27). Intranasal administration is an alternative in both children and adults. Most analgesics have not been approved for intranasal use, but clinical experience with ketamine and fentanyl has been reported (18, 31, e10, e28, e29).
Safety and monitoring
The prerequisites for safe analgesia are knowledge of the pharmacological characteristics of the substances involved, training in their administration, and presence of emergency equipment for treatment of any complications, independent of the user (e.g., nurse, paramedic, or emergency physician) or the situation (prehospital or in the hospital). The monitoring measures and the emergency equipment needed at hand depend on the expected complications and adverse effects. Monitoring of a spontaneously breathing patient under analgesia comprises ECG, blood pressure, breathing rate, heart rate, and SpO2, together with capnography if required (e30– e32). Patients under analgesia should regularly receive oxygen. The equipment for mask ventilation and suction must be available, and every user must be in the position to keep the airway free and perform ventilation. An intravenous access is recommended for treatment of hypotension or administration of naloxone as an opioid antagonist (e22, e23). Titrated administration is advised to avoid respiratory depression.
Undertreatment
Pain has direct physiological effects (blood pressure, breathing rate, heart rate, oxygen consumption, inflammatory reaction) and is a risk factor for post-traumatic stress disorder (e34). From the patient’s point of view, adequate analgesia is an important goal of emergency medical care (5, e35). However, only half of trauma patients receive analgesics at all, and in most of those cases the analgesia achieved is insufficient (4, 7, e36– e40); this is independent of the professional role of the person administering the analgesic (e41– e44). The principal reasons for inadequate analgesia are concern about adverse effects and uncertainty regarding dosage. Proper training in analgesia is therefore essential and the corresponding steps must be taken (5).
Pain assessment
The perception of pain is subjective, and pain is rated differently by patients and professionals (e45). Not all patients want analgesia, so every patient should be asked (e46). The NRS cannot be used in all patients; an alternative is to ask patients whether they are suffering severe or unbearable pain (e47).
In most cases, however, the NRS is useful in assessing the success of analgesia. The aim is to achieve an NRS score = 4 (e19, e48, e49). The vital signs (e.g., breathing rate) may also serve to indicate whether adults are in pain (e50), and for children one can use pain evaluation scales adapted to the pediatric age group (e51– e53). Geriatric patients often have more and worse comorbidities, are frequently accustomed to pain, and are less liable to complain of pain; they must therefore be questioned in greater depth (e19).
Limitations
This review and meta-analysis focuses on the analgesics most commonly used in Germany. The selected study design, with several endpoints (e.g., safety, efficacy, and adverse effects), led to a wide-ranging survey of the literature. However, the high degree of heterogeneity among the studies with regard to endpoints, study quality, and study characteristics represents a crucial limitation. For example, not all controlled studies recorded the time of onset of pain reduction. Many of the studies included did not report adverse effects uniformly. These limitations have to be borne in mind when interpreting the results. Only a small number of studies assessed the trauma by means of the Injury Severity Score (ISS), and very few of them investigated analgesia in seriously injured patients (ISS >15).
Conclusion
Healthcare professionals must be in the position to carry out safe and effective analgesia. The basis is formed by physical measures for pain relief. The preferred means of administration of analgesic drugs is the intravenous route; however, other routes are possible. The recommended analgesics are fentanyl, ketamine, and morphine, with comparable efficacy. Our quantitative meta-analysis shows that there are very few comparable studies of acceptable quality. The current state of knowledge permits no evidence-based statement of superiority of any one of these substances over the others for analgesia in trauma patients in emergency medicine. Analgesia must be carried out only by properly trained persons, the patient must be monitored without interruption, and emergency equipment for treatment of complications must be at hand.
The Clinical Perspective.
The most familiar opioid or ketamine should be used for analgesia.
Opioids and ketamine should be given intravenously. In exceptional cases the intranasal (atomizer) or intraosseous routes can be used instead.
Monitoring of analgesia should comprise: three-channel ECG, breathing rate, heart rate, optional capnography, pulse oximetric oxygen saturation, and blood pressure. Emergency equipment for airway management, ventilation, suction, and resuscitation must be available.
In the prehospital setting and after reaching the hospital, trauma patients should receive pain treatment comprising repositioning and pharmacotherapy adapted to the patients’ pain.
-
Together with assessment of vital signs and body language, the following questions should be asked:
Are you in pain? If so, where is the pain?”
Would you like a painkiller?”
How strong is your pain (on a scale of 0 = no pain to 10 = worst imaginable pain)?”
Nonpharmacological pain treatment should avoid further damage but not prolong the total rescue time in the case of life-threatening injuries.
Severely displaced fractures and joint injuries should be repositioned, particularly in the presence of ischemic or neurovascular deficits and if a long delay before reaching the hospital is expected.
Key Messages.
The analgesics fentanyl, ketamine and morphine possess comparably efficacy.
The quality of the studies included was mostly low and the heterogeneity (I2) high. The lack of consistent reporting limits the comparability of the studies. For this reason, no one substance can be stated as superior to the others.
Many studies point out that only half of trauma patients receive analgesics at all, and in most of those cases the analgesia achieved is insufficient. The principal reasons are concern about adverse effects and uncertainty regarding dosage.
Analgesia is safe when administered by trained personnel.
eFigure.
Risk of bias table according to the Cochrane Collaboration’s risk of bias tool.
+, Low risk; ?, unclear risk; –, high risk.
Acknowledgments
Acknowledgment
We extend heartfelt thanks to Stefanie Hultzsch (Pharmacovigilance and Information Center for Embryonal Toxicology, Charité University Medicine, Berlin) for her opinions and advice on embryonal toxicology.
Footnotes
Conflict of interest statement
Prof. Böttiger has received lecture fees and/or reimbursement of travel costs from medupdate, Baxalta Deutschland, Bayer Vital, Boehringer Ingelheim Pharma, ZOLL Medical Deutschland, C.R. Bard, and Forum für medizinische Fortbildung.
The remaining authors declare that no conflict of interest exists.
Translated from the original German by David Roseveare
References
- 1.Debus F, Lefering R, Frink M, et al. Numbers of severely injured patients in Germany—a retrospective analysis from the DGU (German Society for Trauma Surgery) Trauma Registry. Dtsch Arztebl Int. 2015;112:823–829. doi: 10.3238/arztebl.2015.0823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Statistisches Bundesamt 7,1 % weniger Verkehrstote im Jahr 2016. Pressemitteilung Nr. 065 2017 [Google Scholar]
- 3.Matthes G, Trentzsch H, Wölfl CG, et al. Essential measures for prehospital treatment of severely injured patients: The trauma care bundle. Unfallchirurg. 2015;118:652–656. doi: 10.1007/s00113-015-0042-7. [DOI] [PubMed] [Google Scholar]
- 4.Stork B, Hofmann-Kiefer K. Analgesie in der Notfallmedizin. Anaesthesist. 2009;58 doi: 10.1007/s00101-009-1585-1. [DOI] [PubMed] [Google Scholar]
- 5.Kumle B, Wilke P, Koppert W, Kumle K, Gries A. Schmerztherapie in der Notfallmedizin. Fokus Notaufnahme. Anaesthesist. 2013;62 doi: 10.1007/s00101-013-2247-x. [DOI] [PubMed] [Google Scholar]
- 6.Brennan F, Carr DB, Cousins M. Pain management: a fundamental human right. Anesth Analg. 2007;105:205–221. doi: 10.1213/01.ane.0000268145.52345.55. [DOI] [PubMed] [Google Scholar]
- 7.Albrecht E, Taffe P, Yersin B, Schoettker P, Decosterd I, Hugli O. Undertreatment of acute pain (oligoanalgesia) and medical practice variation in prehospital analgesia of adult trauma patients: a 10 yr retrospective study. Br J Anaesth. 2013;110:96–106. doi: 10.1093/bja/aes355. [DOI] [PubMed] [Google Scholar]
- 8.DGU. S3-Leitlinie Polytrauma/Schwerverletzten-Behandlung: AWMF Register-Nr. 012/19. www.awmf.org/leitlinien/detail/ll/012-019.html (last accessed on 9 March 2017) [Google Scholar]
- 9.Borland M, Jacobs I, King B, O’Brien D. A randomized controlled trial comparing intranasal fentanyl to intravenous morphine for managing acute pain in children in the emergency department. Ann Emerg Med. 2007;49:335–340. doi: 10.1016/j.annemergmed.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 10.Bounes V, Barthelemy R, Diez O, Charpentier S, Montastruc JL, Ducasse JL. Sufentanil is not superior to morphine for the treatment of acute traumatic pain in an emergency setting: a randomized, double-blind, out-of-hospital trial. Ann Emerg Med. 2010;56:509–516. doi: 10.1016/j.annemergmed.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 11.Cevik E, Bilgic S, Kilic E, et al. Comparison of ketamine-low-dose midozolam with midazolam-fentanyl for orthopedic emergencies: a double-blind randomized trial. Am J Emerg Med. 2013;31:108–113. doi: 10.1016/j.ajem.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 12.Craig M, Jeavons R, Probert J, Benger J. Randomised comparison of intravenous paracetamol and intravenous morphine for acute traumatic limb pain in the emergency department. Emerg Med J. 2012;29:37–39. doi: 10.1136/emj.2010.104687. [DOI] [PubMed] [Google Scholar]
- 13.Farahmand S, Shiralizadeh S, Talebian M-T, et al. Nebulized fentanyl vs intravenous morphine for ED patients with acute limb pain: a randomized clinical trial. Am J Emerg Med. 2014;32:1011–1015. doi: 10.1016/j.ajem.2014.05.051. [DOI] [PubMed] [Google Scholar]
- 14.Friday JH, Kanegaye JT, McCaslin I, Zheng A, Harley JR. Ibuprofen provides analgesia equivalent to acetaminophen-codeine in the treatment of acute pain in children with extremity injuries: a randomized clinical trial. Acad Emerg Med. 2009;16:711–716. doi: 10.1111/j.1553-2712.2009.00471.x. [DOI] [PubMed] [Google Scholar]
- 15.Furyk JS, Grabowski WJ, Black LH. Nebulized fentanyl versus intravenous morphine in children with suspected limb fractures in the emergency department: a randomized controlled trial. Emerg Med Australas. 2009;21:203–209. doi: 10.1111/j.1742-6723.2009.01183.x. [DOI] [PubMed] [Google Scholar]
- 16.Galinski M, Dolveck F, Borron SW, et al. A randomized, double-blind study comparing morphine with fentanyl in prehospital analgesia. Am J Emerg Med. 2005;23:114–119. doi: 10.1016/j.ajem.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 17.Galinski M, Dolveck F, Combes X, et al. Management of severe acute pain in emergency settings: ketamine reduces morphine consumption. Am J Emerg Med. 2007;25:385–390. doi: 10.1016/j.ajem.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 18.Graudins A, Meek R, Egerton-Warburton D, Oakley E, Seith R. The PICHFORK (Pain in Children Fentanyl or Ketamine) trial: a randomized controlled trial comparing intranasal ketamine and fentanyl for the relief of moderate to severe pain in children with limb injuries. Ann Emerg Med. 2015;65 doi: 10.1016/j.annemergmed.2014.09.024. [DOI] [PubMed] [Google Scholar]
- 19.Grissa MH, Boubaker H, Zorgati A, et al. Efficacy and safety of nebulized morphine given at 2 different doses compared to IV titrated morphine in trauma pain. Am J Emerg Med. 2015;33:1557–1561. doi: 10.1016/j.ajem.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 20.Jamal SM, Fathil SM, Nidzwani MM, Ismail AK, Yatim FM. Intravenous ketamine is as effective as midazolam/fentanyl for procedural sedation and analgesia in the emergency department. Med J Malaysia. 2011;66:231–233. [PubMed] [Google Scholar]
- 21.Jennings PA, Cameron P, Bernard S, et al. Morphine and ketamine is superior to morphine alone for out-of-hospital trauma analgesia: A randomized controlled trial. Ann Emerg Med. 2012;59:497–503. doi: 10.1016/j.annemergmed.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 22.Kariman H, Majidi A, Amini A, et al. Nitrous oxide/oxygen compared with fentanyl in reducing pain among adults with isolated extremity trauma: a randomized trial. Emerg Med Australas. 2011;23:761–768. doi: 10.1111/j.1742-6723.2011.01447.x. [DOI] [PubMed] [Google Scholar]
- 23.Lee JH, Kim K, Kim TY, et al. A randomized comparison of nitrous oxide versus intravenous ketamine for laceration repair in children. Pediatr Emerg Care. 2012;28:1297–1301. doi: 10.1097/PEC.0b013e3182768a86. [DOI] [PubMed] [Google Scholar]
- 24.Miller JP, Schauer SG, Ganem VJ, Bebarta VS. Low-dose ketamine vs morphine for acute pain in the ED: a randomized controlled trial. Am J Emerg Med. 2015;33:402–408. doi: 10.1016/j.ajem.2014.12.058. [DOI] [PubMed] [Google Scholar]
- 25.Motov S, Rockoff B, Cohen V, et al. Intravenous subdissociative-dose ketamine versus morphine for analgesia in the emergency department: A randomized controlled trial. Ann Emerg Med. 2015;66 doi: 10.1016/j.annemergmed.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Nejati A, Moharari RS, Ashraf H, Labaf A, Golshani K. Ketamine/propofol versus midazolam/fentanyl for procedural sedation and analgesia in the emergency department: a randomized, prospective, double-blind trial. Acad Emerg Med. 2011;18:800–806. doi: 10.1111/j.1553-2712.2011.01133.x. [DOI] [PubMed] [Google Scholar]
- 27.Rickard C, O’Meara P, McGrail M, Garner D, McLean A, Le Lievre P. A randomized controlled trial of intranasal fentanyl vs intravenous morphine for analgesia in the prehospital setting. Am J Emerg Med. 2007;25:911–917. doi: 10.1016/j.ajem.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 28.Smith MD, Wang Y, Cudnik M, Smith DA, Pakiela J, Emerman CL. The effectiveness and adverse events of morphine versus fentanyl on a physician-staffed helicopter. J Emerg Med. 2012;43:69–75. doi: 10.1016/j.jemermed.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 29.Tran KP, Nguyen Q, Truong XN, et al. A comparison of ketamine and morphine analgesia in prehospital trauma care: a cluster randomized clinical trial in rural Quang Tri province, Vietnam. Prehosp Emerg Care. 2014;18:257–264. doi: 10.3109/10903127.2013.851307. [DOI] [PubMed] [Google Scholar]
- 30.Greb I, Wranze E, Hartmann H, Wulf H, Kill C. Analgesie beim Extremitätentrauma durch Rettungsfachpersonal. Notfall Rettungsmed. 2011;14:135–142. [Google Scholar]
- 31.Karlsen AP, Pedersen DM, Trautner S, Dahl JB, Hansen MS. Safety of intranasal fentanyl in the out-of-hospital setting: a prospective observational study. Ann Emerg Med. 2014;63:699–703. doi: 10.1016/j.annemergmed.2013.10.025. [DOI] [PubMed] [Google Scholar]
- 32.Kill C, Greb I, Wranze E, et al. Kompetenzentwicklung im Rettungsdienst. Ein Pilotprojekt zur erweiterten Notfalltherapie durch Rettungsassistenten. Notfall Rettungsmed. 2007;10 [Google Scholar]
- 33.Bisanzo M, Nichols K, Hammerstedt H, et al. Nurse-administered ketamine sedation in an emergency department in rural Uganda. Ann Emerg Med. 2012;59:268–275. doi: 10.1016/j.annemergmed.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 34.Chudnofsky CR, Weber JE, Stoyanoff PJ, et al. A combination of midazolam and ketamine for procedural sedation and analgesia in adult emergency department patients. Acad Emerg Med. 2000;7:228–235. doi: 10.1111/j.1553-2712.2000.tb01064.x. [DOI] [PubMed] [Google Scholar]
- 35.Saunders M, Adelgais K, Nelson D. Use of intranasal fentanyl for the relief of pediatric orthopedic trauma pain. Acad Emerg Med. 2010;17:1155–1161. doi: 10.1111/j.1553-2712.2010.00905.x. [DOI] [PubMed] [Google Scholar]
- 36.Yeaman F, Oakley E, Meek R, Graudins A. Sub-dissociative dose intranasal ketamine for limb injury pain in children in the emergency department: a pilot study. Emerg Med Australas. 2013;25:161–167. doi: 10.1111/1742-6723.12059. [DOI] [PubMed] [Google Scholar]
- 37.Soriya GC, McVaney KE, Liao MM, et al. Safety of prehospital intravenous fentanyl for adult trauma patients. J Trauma Acute Care Surg. 2012;72:755–759. doi: 10.1097/TA.0b013e31823c4444. [DOI] [PubMed] [Google Scholar]
- 38.Garrick JF, Kidane S, Pointer JE, Sugiyama W, van Luen C, Clark R. Analysis of the paramedic administration of fentanyl. J Opioid Manag. 2011;7:229–234. [PubMed] [Google Scholar]
- 39.Johansson P, Kongstad P, Johansson A. The effect of combined treatment with morphine sulphate and low-dose ketamine in a prehospital setting. Scand J Trauma Resusc Emerg Med. 2009;17 doi: 10.1186/1757-7241-17-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bendall JC, Simpson PM, Middleton PM. Effectiveness of prehospital morphine, fentanyl, and methoxyflurane in pediatric patients. Prehosp Emerg Care. 2011;15:158–165. doi: 10.3109/10903127.2010.541980. [DOI] [PubMed] [Google Scholar]
- E1.Bredmose PP, Grier G, Davies GE, Lockey DJ. Pre-hospital use of ketamine in paediatric trauma. Acta Anaesthesiol Scand. 2009;53:543–545. doi: 10.1111/j.1399-6576.2008.01852.x. [DOI] [PubMed] [Google Scholar]
- E2.Bredmose PP, Lockey DJ, Grier G, Watts B, Davies G. Pre-hospital use of ketamine for analgesia and procedural sedation. Emerg Med J. 2009;26:62–64. doi: 10.1136/emj.2007.052753. [DOI] [PubMed] [Google Scholar]
- E3.Friesgaard KD, Nikolajsen L, Giebner M, et al. Efficacy and safety of intravenous fentanyl administered by ambulance personnel. Acta Anaesthesiol Scand. 2016;60:537–543. doi: 10.1111/aas.12662. [DOI] [PubMed] [Google Scholar]
- E4.Häske D, Schempf B, Gaier G, Niederberger C. Prähospitale Analgosedierung durch Rettungsassistenten: Effektivität und Prozessqualität unter ärztlicher Supervision [Prehospital analgesia performed by paramedics: quality in processes and effects under medical supervision] Anaesthesist. 2014;63:209–216. doi: 10.1007/s00101-014-2301-3. [DOI] [PubMed] [Google Scholar]
- E5.Kanowitz A, Dunn TM, Kanowitz EM, Dunn WW, Vanbuskirk K. Safety and effectiveness of fentanyl administration for prehospital pain management. Prehosp Emerg Care. 2006;10:1–7. doi: 10.1080/10903120500373264. [DOI] [PubMed] [Google Scholar]
- E6.Losvik OK, Murad MK, Skjerve E, Husum H. Ketamine for prehospital trauma analgesia in a low-resource rural trauma system: a retrospective comparative study of ketamine and opioid analgesia in a ten-year cohort in Iraq. Scand J Trauma Resusc Emerg Med. 2015;23 doi: 10.1186/s13049-015-0176-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E7.Luiz T, Scherer G, Wickenkamp A, et al. Prähospitale Analgesie durch Rettungsassistenten in Rheinland-Pfalz: Praktikabilität, analgetische Wirkung und Sicherheit bei i v.-verabreichtem Paracetamol [Prehospital analgesia by paramedics in Rhineland-Palatinate: Feasability, analgesic effectiveness and safety of intravenous paracetamol] Anaesthesist. 2015;64:927–936. doi: 10.1007/s00101-015-0089-4. [DOI] [PubMed] [Google Scholar]
- E8.Middleton PM, Simpson PM, Sinclair G, Dobbins TA, Math B, Bendall JC. Effectiveness of morphine, fentanyl, and methoxyflurane in the prehospital setting. Prehosp Emerg Care. 2010;14:439–447. doi: 10.3109/10903127.2010.497896. [DOI] [PubMed] [Google Scholar]
- E9.Porter K. Ketamine in prehospital care. Emerg Med J. 2004;21:351–354. doi: 10.1136/emj.2003.010843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E10.Johansson J, Sjöberg J, Nordgren M, Sandström E, Sjöberg F, Zetterström H. Prehospital analgesia using nasal administration of S-ketamine—a case series. Scand J Trauma Resusc Emerg Med. 2013;21 doi: 10.1186/1757-7241-21-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E11.Adams HA, Flemming A. Analgesie, Sedierung und Anästhesie in der Notfallmedizin. Anästh Intensivmed. 2015;56:75–90. [Google Scholar]
- E12.Thomas SH, Shewakramani S. Prehospital trauma analgesia. J Emerg Med. 2008;35:47–57. doi: 10.1016/j.jemermed.2007.05.041. [DOI] [PubMed] [Google Scholar]
- E13.Krauss WC, Shah S, Shah S, Thomas SH. Fentanyl in the out-of-hospital setting: variables associated with hypotension and hypoxemia. J Emerg Med. 2011;40:182–187. doi: 10.1016/j.jemermed.2009.02.009. [DOI] [PubMed] [Google Scholar]
- E14.American College of Emergency Physicians ACEP. Out-of-hospital use of analgesia and sedation: approved by the Board of Directors June 2015. www.acep.org/Clinical–-Practice-Management/Out-of-Hospital-Use-of-Analgesia-and-Sedation/ (last accessed on 24 May 2016) [Google Scholar]
- E15.Gausche-Hill M, Brown KM, Oliver ZJ, et al. An evidence-based guideline for prehospital analgesia in trauma. Prehosp Emerg Care. 2014;18 Suppl 1:25–34. doi: 10.3109/10903127.2013.844873. [DOI] [PubMed] [Google Scholar]
- E16.Höll M. Präklinische Analgosedierung mit Ketamin S und Midazolam durch Notfallsanitäter. Anästh Intensivmed. 2017;58 [Google Scholar]
- E17.Schempf B, Casu S, Häske D. Prähospitale Analgosedierung durch Notärzte und Rettungsassistenten. Anaesthesist. 2017;66:325–332. doi: 10.1007/s00101-017-0288-2. [DOI] [PubMed] [Google Scholar]
- E18.Moy RJ, Le Clerc S. Ketamine in prehospital analgesia and anaesthesia. Trends in Anaesthesia and Critical Care. 2011;1:243–245. [Google Scholar]
- E19.Hossfeld B, Holsträter S, Bernhard M, Lampl L, Helm M, Kulla M. Prähospitale Analgesie beim Erwachsenen. Notf.med. up2date. 2015;10:269–284. doi: 10.1055/s-0042-101466. [DOI] [PubMed] [Google Scholar]
- E20.Morris C, Perris A, Klein J, Mahoney P. Anaesthesia in haemodynamically compromised emergency patients: does ketamine represent the best choice of induction agent? Anaesthesia. 2009;64:532–539. doi: 10.1111/j.1365-2044.2008.05835.x. [DOI] [PubMed] [Google Scholar]
- E21.Paal P, Herff H, Mitterlechner T, et al. Anaesthesia in prehospital emergencies and in the emergency room. Resuscitation. 2010;81:148–154. doi: 10.1016/j.resuscitation.2009.10.023. [DOI] [PubMed] [Google Scholar]
- E22.German Trauma Society (DGU) S3 - Guideline on Treatment of Patients with Severe and Multiple Injuries: AWMF-Registry No. 012/019. www.awmf.org/leitlinien/detail/ll/012-019.html. [Google Scholar]
- E23.Cohen L, Athaide V, Wickham ME, Doyle-Waters MM, Rose NG, Hohl CM. The effect of ketamine on intracranial and cerebral perfusion pressure and health outcomes: a systematic review. Ann Emerg Med. 2015;65 doi: 10.1016/j.annemergmed.2014.06.018. [DOI] [PubMed] [Google Scholar]
- E24.Roberts DJ, Hall RI, Kramer AH, Robertson HL, Gallagher CN, Zygun DA. Sedation for critically ill adults with severe traumatic brain injury: a systematic review of randomized controlled trials. Crit. Care Med. 2011;39:2743–2751. doi: 10.1097/CCM.0b013e318228236f. [DOI] [PubMed] [Google Scholar]
- E25.Filanovsky Y, Miller P, Kao J. Myth: Ketamine should not be used as an induction agent for intubation in patients with head injury. CJEM. 2010;12:154–157. doi: 10.1017/s1481803500012197. [DOI] [PubMed] [Google Scholar]
- E26.Bar-Joseph G, Guilburd Y, Tamir A, Guilburd JN. Effectiveness of ketamine in decreasing intracranial pressure in children with intracranial hypertension. J Neurosurg Pediatr. 2009;4:40–46. doi: 10.3171/2009.1.PEDS08319. [DOI] [PubMed] [Google Scholar]
- E27.Bernhard M, Gräsner J, Gries A, et al. Die intraossäre Infusion in der Notfallmedizin. Anästh Intensivmed. 2010;51:s615–s620. [Google Scholar]
- E28.Riediger C, Haschke M, Bitter C, et al. The analgesic effect of combined treatment with intranasal S-ketamine and intranasal midazolam compared with morphine patient-controlled analgesia in spinal surgery patients: a pilot study. J Pain Res. 2015;8:87–94. doi: 10.2147/JPR.S75928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E29.Samuel N, Steiner IP, Shavit I. Prehospital pain management of injured children: a systematic review of current evidence. Am J Emerg Med. 2015;33:451–454. doi: 10.1016/j.ajem.2014.12.012. [DOI] [PubMed] [Google Scholar]
- E30.Deutsche Gesellschaft für Anästhesiologie und Intensivmedizin und Berufsverband Deutscher Anästhesisten. Analgosedierung bei Erwachsenen. Anästh Intensivmed. 2010;51:598–602. [Google Scholar]
- E31.Godwin SA, Caro DA, Wolf SJ, et al. Clinical policy: procedural sedation and analgesia in the emergency department. Ann Emerg Med. 2005;45:177–196. doi: 10.1016/j.annemergmed.2004.11.002. [DOI] [PubMed] [Google Scholar]
- E32.Dewdney C, MacDougall M, Blackburn R, Lloyd G, Gray A. Capnography for procedural sedation in the ED: a systematic review. Emerg Med J. 2017;34:476–484. doi: 10.1136/emermed-2015-204944. [DOI] [PubMed] [Google Scholar]
- E33.National Association of Emergency Medical Technicians (NAEMT) PHTLS: Prehospital trauma life support. Burlington, MA: Jones & Bartlett Learning. 2016 [Google Scholar]
- E34.Norman SB, Stein MB, Dimsdale JE, Hoyt DB. Pain in the aftermath of trauma is a risk factor for post-traumatic stress disorder. Psychol. Med. 2008 38:533–542. doi: 10.1017/S0033291707001389. [DOI] [PubMed] [Google Scholar]
- E35.Cepeda MS, Africano JM, Polo R, Alcala R, Carr DB. What decline in pain intensity is meaningful to patients with acute pain? Pain. 2003;105:151–157. doi: 10.1016/s0304-3959(03)00176-3. [DOI] [PubMed] [Google Scholar]
- E36.Bakkelund KE, Sundland E, Moen S, Vangberg G, Mellesmo S, Klepstad P. Undertreatment of pain in the prehospital setting: a comparison between trauma patients and patients with chest pain. Eur J Emerg Med. 2013;20:428–430. doi: 10.1097/MEJ.0b013e32835c9fa3. [DOI] [PubMed] [Google Scholar]
- E37.Stalnikowicz R, Mahamid R, Kaspi S, Brezis M. Undertreatment of acute pain in the emergency department: a challenge. Int J Qual Health Care. 2005;17:173–176. doi: 10.1093/intqhc/mzi022. [DOI] [PubMed] [Google Scholar]
- E38.Bounes V, Barniol C, Minville V, Houze-Cerfon C-H, Ducassé JL. Predictors of pain relief and adverse events in patients receiving opioids in a prehospital setting. Am J Emerg Med. 2011;29:512–517. doi: 10.1016/j.ajem.2009.12.005. [DOI] [PubMed] [Google Scholar]
- E39.Schauer SG, Robinson JB, Mabry RL, Howard JT. Battlefield analgesia: TCCC guidelines are not being followed. J Spec Oper Med. 2015;15:85–89. [PubMed] [Google Scholar]
- E40.Pierik, Jorien G J, IJzerman MJ, Gaakeer MI, et al. Pain management in the emergency chain: the use and effectiveness of pain management in patients with acute musculoskeletal pain. Pain Med. 2015;16:970–984. doi: 10.1111/pme.12668. [DOI] [PubMed] [Google Scholar]
- E41.Ricard-Hibon A, Chollet C, Saada S, Loridant B, Marty J. A quality control program for acute pain management in out-of-hospital critical care medicine. Ann Emerg Med. 1999;34:738–744. doi: 10.1016/s0196-0644(99)70099-5. [DOI] [PubMed] [Google Scholar]
- E42.Moller JC, Ballnus S, Kohl M, et al. Evaluation of the performance of general emergency physicians in pediatric emergencies: Obstructive airway diseases, seizures, and trauma. Pediatr Emerg Care. 2002;18:424–428. doi: 10.1097/00006565-200212000-00005. [DOI] [PubMed] [Google Scholar]
- E43.Stelle zur trägerübergreifenden Qualitätssicherung im Rettungsdienst Baden-Württemberg (SQR-BW) Qualitätsbericht: Rettungsdienst Baden-Württemberg, Berichtsjahr 2014. www.sqrbw.de/de/sqr-bw/qualitaetsberichte (last accessed on 31 January 2016) [Google Scholar]
- E44.Vassiliadis J, Hitos K, Hill CT. Factors influencing prehospital and emergency department analgesia administration to patients with femoral neck fractures. Emerg Med (Fremantle) 2002;14:261–266. doi: 10.1046/j.1442-2026.2002.00341.x. [DOI] [PubMed] [Google Scholar]
- E45.van Dijk, Jacqueline F M, van Wijck, et al. Postoperative pain assessment based on numeric ratings is not the same for patients and professionals: a cross-sectional study. Int J Nurs Stud. 2012;49:65–71. doi: 10.1016/j.ijnurstu.2011.07.009. [DOI] [PubMed] [Google Scholar]
- E46.Singer AJ, Garra G, Chohan JK, Dalmedo C, Thode HC. Triage pain scores and the desire for and use of analgesics. Ann Emerg Med. 2008;52:689–695. doi: 10.1016/j.annemergmed.2008.04.017. [DOI] [PubMed] [Google Scholar]
- E47.Green SM, Krauss BS. The Numeric Scoring of Pain: This Practice Rates a Zero Out of Ten. Ann Emerg Med. 2016;675:73–75. doi: 10.1016/j.annemergmed.2015.06.002. [DOI] [PubMed] [Google Scholar]
- E48.Becker M, Pogatzki-Zahn EM. Postoperative Schmerztherapie: Pathophysiologie, Pharmakologie und Therapie; 99 Tabellen. Stuttgart [u.a.]: Thieme. 2008 [Google Scholar]
- E49.Maier C, Nestler N, Richter H, et al. The quality of pain management in German hospitals. Dtsch Arztebl Int. 2010;107:607–614. doi: 10.3238/arztebl.2010.0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E50.Bendall JC, Simpson PM, Middleton PM. Prehospital vital signs can predict pain severity: analysis using ordinal logistic regression. Eur J Emerg Med. 2011;18:334–339. doi: 10.1097/MEJ.0b013e328344fdf2. [DOI] [PubMed] [Google Scholar]
- E51.Atkinson P, Chesters A, Heinz P. Pain management and sedation for children in the emergency department. BMJ. 2009;339 doi: 10.1136/bmj.b4234. [DOI] [PubMed] [Google Scholar]
- E52.Jennings PA, Cameron P, Bernard S. Measuring acute pain in the prehospital setting. Emerg Med J. 2009;26:552–555. doi: 10.1136/emj.2008.062539. [DOI] [PubMed] [Google Scholar]
- E53.McConahay T, Bryson M, Bulloch B. Defining mild, moderate, and severe pain by using the color analogue scale with children presenting to a pediatric emergency department. Acad Emerg Med. 2006;13:341–344. doi: 10.1197/j.aem.2005.09.010. [DOI] [PubMed] [Google Scholar]
- E54.Melamed E, Blumenfeld A, Kalmovich B, et al. Prehospital Care of Orthopedic Injuries. Prehosp Disaster Med. 2007;22:22–25. [PubMed] [Google Scholar]
- E55.Worsing RA. Principles of prehospital care of musculoskeletal injuries. Emerg Med Clin North Am. 1984;2:205–217. [PubMed] [Google Scholar]
- E56.Cuske J. The Lost Art of Splinting: How to properly immobilize extremities & manage pain. JEMS. 2008;33(7):50–59. doi: 10.1016/S0197-2510(08)70253-5. [DOI] [PubMed] [Google Scholar]
- E57.Perkins TJ. Fracture management. Effective prehospital splinting techniques. Emerg Med Serv. 2007;36:35–39. [PubMed] [Google Scholar]
- E58.Beck A, Gebhard F, Kinzl L, Strecker W. Principles and techniques of primary trauma surgery management at the site [Prinzipien und Techniken der unfallchirurgischen Erstversorgung am Einsatzort] Unfallchirurg. 2001;104:1082–1096. doi: 10.1007/s001130170024. quiz 1097, 1099. [DOI] [PubMed] [Google Scholar]
- E59.Beck A, Gebhard F, Kinzl L. Notärztliche Versorgung des Traumapatienten. Notfall Rettungsmed. 2002;5:57–71. [Google Scholar]
- E60.Lee C, Porter KM. Prehospital management of lower limb fractures. Emerg Med J. 2005;22:660–663. doi: 10.1136/emj.2005.024489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E61.Probst C, Hildebrand F, Frink M, Mommsen P, Krettek C. Prehospital treatment of severely injured patients in the field: an update [Erstversorgung Schwerstverletzter am Unfallort: Ein Update] Chirurg. 2007;78:875–884. doi: 10.1007/s00104-007-1410-9. [DOI] [PubMed] [Google Scholar]
- E62.Feilberg VL, Rosenborg D, Broen Christensen C, Mogensen JV. Excretion of morphine in human breast milk. Acta Anaesthesiol Scand. 1989;33:426–428. doi: 10.1111/j.1399-6576.1989.tb02938.x. [DOI] [PubMed] [Google Scholar]
- E63.Robieux I, Koren G, Vandenbergh H, Schneiderman J. Morphine excretion in breast milk and resultant exposure of a nursing infant. J Toxicol Clin Toxicol. 1990;28:365–370. doi: 10.3109/15563659008994437. [DOI] [PubMed] [Google Scholar]
- E64.Wittels B, Scott DT, Sinatra RS. Exogenous opioids in human breast milk and acute neonatal neurobehavior: a preliminary study. Anesthesiology. 1990;73:864–869. doi: 10.1097/00000542-199011000-00012. [DOI] [PubMed] [Google Scholar]
- E65.Goma HM, Said RN, El-Ela AM. Study of the newborn feeding behaviors and fentanyl concentration in colostrum after an analgesic dose of epidural and intravenous fentanyl in cesarean section. Saudi Med J. 2008;29:678–682. [PubMed] [Google Scholar]
- E66.Nitsun M, Szokol JW, Saleh HJ, et al. Pharmacokinetics of midazolam, propofol, and fentanyl transfer to human breast milk. Clin Pharmacol Ther. 2006;79:549–557. doi: 10.1016/j.clpt.2006.02.010. [DOI] [PubMed] [Google Scholar]
- E67.Steer PL, Biddle CJ, Marley WS, Lantz RK, Sulik PL. Concentration of fentanyl in colostrum after an analgesic dose. Can J Anaesth. 1992;39:231–235. doi: 10.1007/BF03008782. [DOI] [PubMed] [Google Scholar]
- E68.Baraka A, Louis F, Dalleh R. Maternal awareness and neonatal outcome after ketamine induction of anaesthesia for Caesarean section. Can J Anaesth. 1990;37:641–644. doi: 10.1007/BF03006482. [DOI] [PubMed] [Google Scholar]
- E69.Reich DL, Silvay G. Ketamine: an update on the first twenty-five years of clinical experience. Can J Anaesth. 1989;36:186–197. doi: 10.1007/BF03011442. [DOI] [PubMed] [Google Scholar]