Abstract
Objective
Human Pygopus 2 (Pygo2) was recently discovered to be a component of the Wnt signaling pathway required for β-catenin/Tcf-mediated transcription. But the role of Pygo2 in malignant cell proliferation and invasion has not yet been determined.
Methods
Lentivirus-mediated small interfering RNA (siRNA) and vector-based overexpression were used to study the function of Pygo2 in OS-RC-2 cells. The resulted cells were subject to Western blotting assay, MTT assay, colony formation and cell invasion assays. Furthermore, renal cell carcinoma (RCC) models were established in BALB/c nude mice inoculated with OS-RC-2 cells. Immunohistochemistry (IHC) staining of matrix metalloproteinase-7 (MMP-7), matrix metalloproteinase-9 (MMP-9) and vascular endothelial growth factor (VEGF) was performed in tumor tissue.
Results
Pygo2 gene was successful knocked down and overexpressed in RCC OS-RC-2 cells by using an shRNA and overexpressing vector, respectively. Overexpression of Pygo2 effectively promoted cell proliferation, colony formation and invasion in vitro. Knockdown of Pygo2 obviously inhibited xenograft tumor growth in nude mice. In addition, overexpression of Pygo2 increased the levels of MMP-7, MMP-9 and VEGF in the xenograft tumors.
Conclusion
Pygo2 has a role in promoting cell proliferation, invasion and metastasis, and may regulate angiogenesis via the Wnt/β-catenin signaling pathway.
Keywords: Pygo2, Renal cell carcinoma, Matrix metalloproteinase-7, Matrix metalloproteinase-9, Vascular endothelial growth factor
1. Introduction
Wnt signaling controls important aspects of animals development, and its deregulation has been casually linked to cancer [1]. Aberrant regulation of the Wnt signaling pathway is a prevalent theme in cancer biology [2]. A key effector of the canonical Wnt pathway is β-catenin, or Armadillo in Drosophila, a highly unstable cytoplasmic protein that is constitutively phosphorylated and targeted for proteasomal degradation [3]. Once established, β-catenin accumulates in the nucleus and associates with DNA-bound TCF/LEF factors, thus providing a platform for the recruitment of various general transcriptional co-activators to Wnt target genes, including chromatin modifiers and remodeling factors [4].
Pygopus (Pygo), a new component of Wnt signaling pathway, contains a single zinc-coordinated PHD finger in its C-terminus, by which it binds directly to a short Lgs domain (called homology domain 1 (HD1)); Lgs in turn uses its homology domain 2 to bind to Armadillo, thus forming a “chain-of-adaptors” [5], [6].
The Zn-coordinated PHD fingers of Pygopus proteins are critical for β-catenin-dependent transcriptional switches in normal and malignant tissues [4]. Pygo and Lgs interact with β-catenin during the formation of the canonical transcriptional complex and are required for accumulation of β-catenin in the nucleus [8]. Additionally, a putative nuclear localization signal (NLS) was identified within the N-terminal domain of Pygo, suggesting a possible role of nuclear importation of β-catenin [9], [10].
Pygo has also been implicated in Wnt-independent roles both in cancer [11] and in development [12], [13]. Human genome carries two orthologs of Drosophila Pygo, Pygo1 and Pygo2[14]. Previous studies have provided evidence that the human Pygo is expressed in the majority of breast and epithelial ovarian primary carcinomas [15]. Additionally, Pygo2 was highly expressed in glioma tissues and required for the growth of glioblastoma cells [16].
In the present study, we investigated the potential effects of Pygo2 on the growth of renal cell carcinoma (RCC) cells in vivo and in vitro. However, little or no information is currently available about the invasiveness of renal malignant tumors by which Pygo 2 proteins increased, and the expression of its related proteins (matrix metalloproteinase-7 (MMP-7), matrix metalloproteinase-9 (MMP-9), vascular endothelial growth factor (VEGF)) is still unknown. We also explore VEGF expression level in nude mice which involved by pygo2 how to exert its effects.
2. Materials and methods
2.1. Cell culture
The human RCC cell lines, OS-RC-2, was purchased from the Cell Center of Chinese Academy of Sciences and maintained in RPMI-1640 supplemented with 10% fetal bovine serum (FBS) (Invitrogen Corp, USA) and at 37°C in a humidified atmosphere containing 95% air and 5% CO2. Cells were trypsinized upon confluency and propagated twice before being subcultured into 6- or 96-well plates for further experiments.
2.2. Lentivirus packaging, cell infection, and generation of stable cell lines
To determine the role of Pygo2 gene in the growth of OS-RC-2 cells, we used a lentivirus vector to mediate Pygo2 overexpression or silencing according to the manufacturer's instructions. Briefly, 293FT cells were co-transfected with the constructs containing Pbobi-HA-Pygo2-full-IRES-Puro, Pbobi-GFP, Pll3.7-neo-Pygo2 (Pygo2-shRNA), or Pll3.7-control (Pygo2-scrRNA), and packaging mixture. The viral supernatant was harvested after 48–72 h and titers of the resulted virus were determined by infecting 293T cells with serial dilutions of concentrated lentivirus. After 48 h of incubation, the cells were analyzed by Western blotting to determine silent efficiency or overexpression of the Pygo2 gene. For the generation of stable cell lines, OS-RC-2 cells were infected with viral supernatant plus 2000× polybrene (Sigma, San Francisco, USA) for 24 h. After antibiotic selection for 2 weeks, stable clones were obtained.
2.3. Western blot analysis
At 48 h after infection, cells were washed twice with cold Phosphate buffer saline (PBS), then harvested using cell scrapers. By centrifuging (3000 rpm, 4 min), cell debris were removed. Total protein was extracted using a protein extraction solution containing lysis buffer, 100× Phenylmethanesulfonyl fluoride (PMSF), and 100× protease inhibitor cocktail. Proteins (80 μg) were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and electrotransferred to nitrocellulose membranes (Amersham Pharmacia Biotech, Montreal, Canada). Rabbit polyclonal antibodies against human Pygo2 were presented from Xiamen University, College of Life Science, China.
2.4. Cell proliferation assay
At 48 h after infection, cells were seeded at 5 × 103 cells per well in 96-well plates and incubated in medium containing 10% FBS and allowed to attach for 24 h. MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) was added to each well and incubated for 4 h at 37°C. Finally, the medium was discarded carefully, and 150 μL dimethylsulfoxide (DMSO) was added to each well to dissolve MTT. Absorbance at 590 nm with reference filter of 620 nm was measured using a spectrophotometer. The experiment was independently repeated three times.
2.5. Cell invasion assay
At 48 h after infection, The cell was diverted and maintained in dulbecco's modified eagle medium (DMEM) with no FBS and cultured at 37°C for 24 h. Five hundred microlitre DMEM with no FBS was added into the wells of a Tanswell and cultured at 37°C for 1 h. Tanswell were diverted to 24-well plates and added to 200 μL cell suspension (1.5 × 105/mL) in upper well and 200 μL DMEM in lower well. After cultured in a 37°C for 24 h, Tanswells were washed thrice with PBS, fixed in 4% paraformaldehyde (PFA) for 30 min, stained with 0.1% viola crystalline for 20 min. Photographs were taken at a magnification of 200×. The number of invading cells was quantified by counting the stained cells in randomly selected fields of the membrane. All experiments were repeated there times.
2.6. Colony formation assay
At 48 h after infection, 1000 cells per well were seeded in 6-well plates and cultured. After 12 days, the plates were stained with Giemsa (0.4 g Giemsa, 25 mL methanol, 25 mL glycerol), followed by washing with water to remove excess dye. Colonies were quantified by counting. Each experiment was repeated in triplicates. The results are presented as the average of three independent experiments.
2.7. In vivo tumor formation
OS-RC-2 cells were harvested with trypsin-EDTA, washed and resuspended in PBS. Nude mice (female 3–4 weeks old) were injected in the axillary subcutaneous with 4 × 106 OS-RC-2 cells. Tumor sizes were measured using a caliper when tumor nodules were visible. The animals were maintained in laminar flow cabinets under specific pathogen-free conditions. The 16 xenograft model mice were randomly divided into four groups: Pygo2shRNA group, Pygo2scrRNA group, Pygo2-overexpression (Pygo2OE) group, and green fluorescent protein (GFP) group. The size of the tumors was measured every 3 days after inoculation. Tumor volume was calculated using the formula: L × S2/2, where L is the longest tumor diameter and S is the shortest tumor diameter. The tumor-bearing mice were sacrificed 4 weeks after inoculation and the tumors were incised and weighed. The tumors were fixed in 10% formalin solution for immunohistochemistry staining.
2.8. Histological analysis
Formalin-fixed tissue samples were embedded in paraffin and sectioned. The resulted sections were stained with Hematoxylin and Eeosin (H&E).
2.9. Immunohistochemistry
Sections were subject to the Super Vision (SV) method using the following primary monoclonal rabbit anti-human anti-bodies: Pygo2 (1: 200, obtained from Xiamen University, College of Life Science, China), MMP-7 (1: 100), MMP-9 (1: 100), VEGF (1:100) (Pierce, NJ, USA). These antibodies were incubated overnight at 4 °C, the reactions were amplified with peroxidase-labeled secondary antibody and visualized with DAB (3, 3′ N-Diaminobenzidine Tertrahydrochloride) (Maixin Biotechnology, Fuzhou, China). Hematoxylin was used to counterstain.
2.10. Statistical methods
Statistical analyses of the data were performed using the SPSS (version 13.0) statistical software (IBM, NY, USA). One-Way ANOVA analyses followed by Fisher's Exact Test were performed to compare differences among treatment groups. p value < 0.05 was considered statistically significant.
3. Results
3.1. Knockdown and overexpression of Pygo2 in OS-RC-2 cells
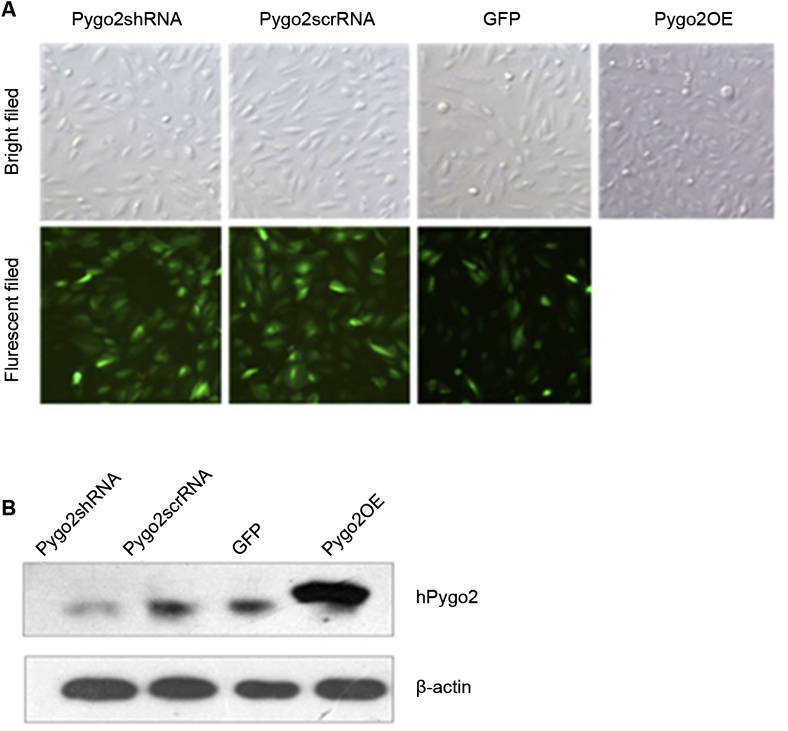
To study the function of Pygo2 in OS-RC-2 cells, we used lentivirus vector mediated Pygo2 knockdown (Pygo2shRNA) and overexpression (Pygo2OE). Lentivirus was packaged and used to infect OS-RC-2 cells. Lentivirus expressing a scrambled shRNA (Pygo2scrRNA) was used as a control for Pygo2shRNA, and GFP as a control for Pygo2OE. The lentivirus infection rate was quite high as measured by the percent of cells expressing GFP fluorescence 48 h following infection (Fig. 1A). To determine the expression of Pygo2 in the infected cells, we performed Western blotting assay. As shown in Fig. 1B, Pygo2 protein was successfully knocked down by its shRNA (comparing Pygo2shRNA to PygoscrRNA). Overexpression of Pygo2 protein was also successfully achieved in OS-RC-2 cells by Pygo2OE (Fig. 1B).
Figure 1.
Knockdown and overexpression of Pygo2 by Pygo2shRNA and an overexpressing vector respectively. (A) OS-RC-2 cells were infected with the indicated lentivirus including Pygo2-targeting shRNA (Pygo2shRNA), control shRNA (Pygo2scrRNA), Pygo2-overexpression (Pygo2OE), and GFP control. Bright field and fluorescence cell images were taken 2 days following transduction (100× magnification). (B) Protein extracted from the cells transduced as in (A) was subject to Western blotting assay. Protein levels of Pygo2 were detected by using an anti-Pygo2 antibody, and β-actin was detected to serve as a control for protein loading.
3.2. Effect of Pygo2 on cell proliferation in vitro
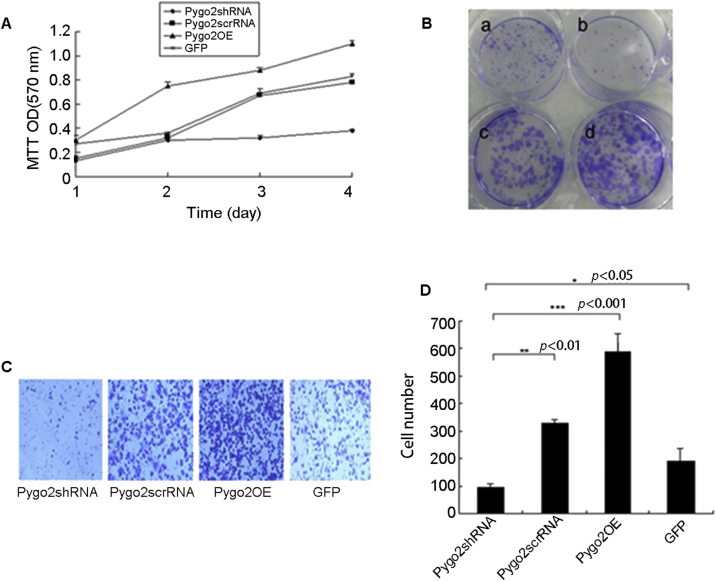
To determine the effect of Pygo2 knockdown on OS-RC-2 proliferation, we first determined cell proliferation by MTT assay. Forty-eight hours after virus infection, cells infected with Pygo2shRNA showed significantly slower growth compared those infected with Pygo2OE, Pygo2scrRNA and GFP (Fig. 2A).
Figure 2.
Effect of Pygo2 on OS-RC-2 cells growth in vitro. (A) Growth rates of OS-RC-2 cells as determined by MTT assay. (B) The effects of the Pygo2shRNA and overexpression of Pygo2 on colony formation of OS-RC-2 cells: a, Pygo2scrRNA; b, Pygo2shRNA; c, GFP; and d, Pygo2OE. (C) Invasion assay using BD BioCoat Matrigel Invasion Chambers. The stained cells were invasive cells in the lower chamber (200× magnification). (D) The number of migrating cells in (C) was quantified by photography. A 6-fold increase in the number of migrating cells in Pygo2OE was noted compared to Pygo2 knockdown in OS-RC-2 cell lines (p < 0.001).
We next investigated the effects of the Pygo2shRNA and overexpression of Pygo2 on cell colony formation of the RCC cells. Our results showed that Pygo2shRNA apparently prevented colony formation of RCC cells. Compared with other treatments, cells infected with Pygo2shRNA exhibited significantly less colonies (Fig. 2B).
3.3. Effect of Pygo2 on tumor cell invasion
To investigate the role of Pygo2 in tumor invasion, we performed matrigel migration assay. Suppression of Pygo2 by its shRNA significantly decreased the invasion ability of OS-RC-2 cell, whereas overexpression of Pygo2 increased cell invasion (Fig. 2C and D). This result thus suggests that Pygo2 gene has a role in promoting cells invasion in OS-RC-2 cells.
3.4. Effect of Pygo2 on tumor growth in a nude mice xenograft model
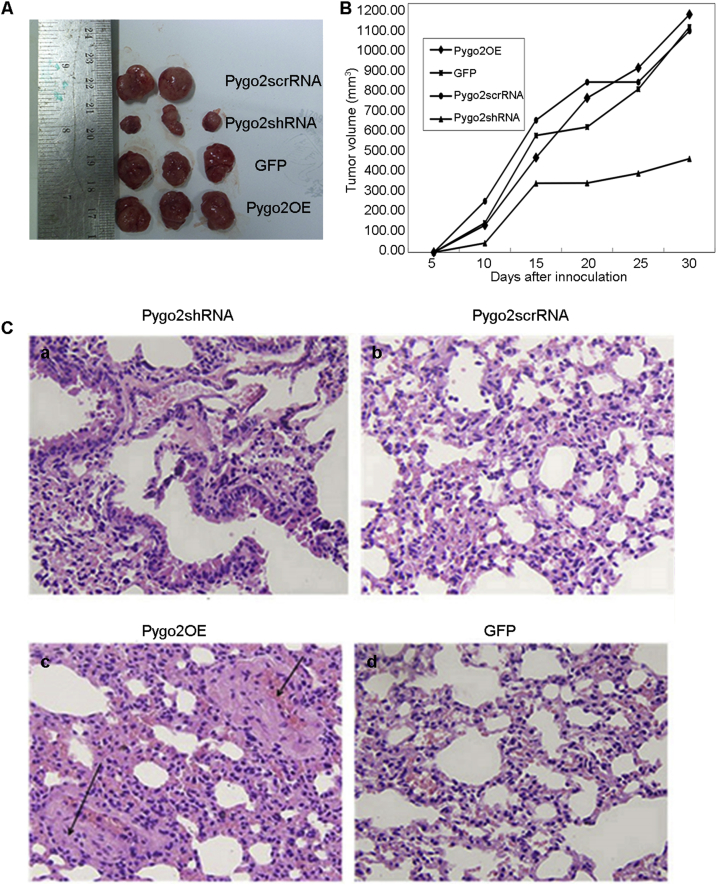
To further investigate the role of Pygo2 in RCC in vivo, we established xenografts by subcutaneous injection of 4 × 106 OS-RC-2 cells including Pygo2shRNA, Pygo2scrRNA, GFP and Pygo2OE. As shown in Fig. 3A and B, Pygo2shRNA strikingly decreased the growth of OS-RC-2 cells xenografts in nude mice, whereas in mice treated with overexpression of Pygo2 tumors grew promptly on the third day, the other animals had large tumors after 1. Notably, in Pygo2shRNA group, tumor growth was delayed compared to the other three groups. After 4 weeks, we observed that the volume of tumor derived from Pygo2 knockdown cells was significantly smaller than that derived from control cells (Fig. 3B). When the nude mice were dissected, we found the lung surface in mice treated with Pygo2 overexpression has scattered nodules. As shown in Fig. 3C, H&E staining confirmed that scattered nodules were metastatic tumors. These results indicated that Pygo2 can participate in the regulation of cell migration and invasion.
Figure 3.
The effect of Pygo2 knockdown and overexpression on tumor growth. (A) Nude mice inoculated with Pygo2shRNA, Pygo2scrRNA, GFP and Pygo2OE cells were sacrificed 5 weeks after inoculation. Note one mouse inoculated with Pygo2shRNA did not form tumor. (B) Tumor growth curves. Average volume of tumors in each group is plotted at different time points (p < 0.05). (C) Lung metastatic tumor was observed by light microscopy (Hematoxylin and Eeosin (H&E) staining, 200× magnification). (a) Pygo2shRNA group showing the effect of Pygo2 suppression on OS-RC-2 cells exhibit no lung metastatic tumor; (b) Control group; (c) Pygo2OE group showing the overexpression effect of Pygo2 on OS-RC-2 cells exhibit lung metastatic tumor (as shown by the arrow); (d) GFP group.
3.5. The expression of MMP-7, MMP-9, VEGF, as determined by immunohistochemistry
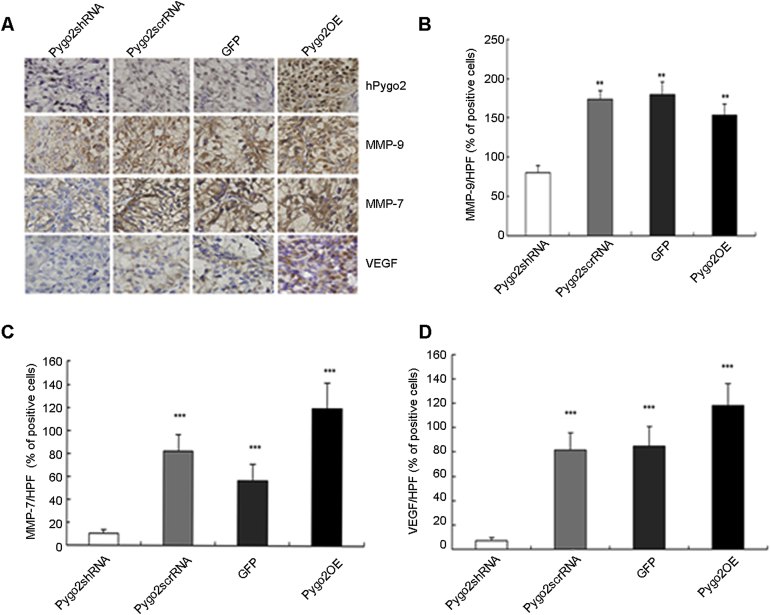
To examine whether Pygo2 expression in vivo was associated with invasion and angiogenesis, we detected the expression of MMP-7, MMP-9 and VEGF in tumor tissue. As shown in Fig. 4A, we verified the expression of Pygo2 in tumor tissue by immunohistochemistry (IHC). We noticed that in Pygo2 knockdown tumors, no signals seemed to diffuse in the cytoplasm of cancer cells, the other three groups were prominently augmented, especially in tumor tissue treated with overexpression of Pygo2. However, as shown in Fig. 4A, Pygo2 depletion could not obviously reduce the expression of MMP-9, suggesting that Pygo2 protein may not affect MMP-9 by regulating the levels of β-catenin.
Figure 4.
Immunohistochemical staining for Pygo2, MMP-9, MMP-7 and VEGF in tumor tissue. (A) Representative images of immunohistochemical staining of Pygo2, MMP-9, MMP-7 and VEGF expression (400× magnification). (B) Quantification of MMP-9-expressing cells (% of positive cells). The staining index was decreased in Pygo2shRNA-treated tumor tissues compared with that in control groups (**p < 0.01). (C) Statistical summary of MMP-7 positive cells, Pygo2shRNA protein expression was at a much lower percentage, a significant dose-dependent reduction in tumor tissues compared with control tumor tissues (***p < 0.001). (D) The rate of VEGF expression in Pygo2OE group is higher than that in Pygo2shRNA group (***p < 0.001).
We then explored the cause–effect relationship between the expression of Pygo2 and VEGF, and found that VEGF expression was strongly upregulated in a background of Pygo2 deficiency (Fig. 4). Taken together, these results suggest that Pygo2 may be involved in RCC progression by regulating cell invasion and angiogenesis.
4. Discussion
To date, RCC, the most common form of adult neoplastic renal disease, has only been marginally investigated for aberrations in the Wnt/beta-catenin signaling pathway [17]. Pygo2 is an important component of Wnt/β-catenin transcriptional complex [18]. Pygo and Lgs interact with β-catenin during the formation of the canonical transcriptional complex and are required for accumulation of β-catenin in the nucleus [19]. In this study, we demonstrated that Pygo2 knockdown inhibited cell proliferation, colony formation and cell invasion by in vitro assays. Notably, Pygo2 is overexpressed and required in OS-RC-2 cell lines. Therefore, Pygo2 may serve a prognostic molecular marker in association with RCC.
We further demonstrated that Pygo2 participated in the regulation of angiogenesis and invasive ability of OS-RC-2 cells by in vivo assays. Our results indicated that Pygo2 knockdown effectively induced growth inhibition of OS-RC-2 cell lines. However, overexpression of Pygo2 did not significantly increase tumor size and weight. This result may be explained that the overexpression of Pygo2 cannot stimulate the expression of β-catenin. In addition, Schwab et al. [14] reported that the proteins encoded by mammalian Pygopus genes were often mere modulators of canonical Wnt signaling intensity, and not essential components. The important finding from our study was the effect of Pygo2OE on pulmonary metastasis in a nude mice model, suggesting its potential involvement in regulating cell migration and invasion.
Angiogenesis is a key process in the growth and metastasis of solid tumors [8]. VEGF regulates endothelial cell proliferation and the formation of blood vessels, in addition to promoting angiogenesis. Matrix metalloproteinases (MMPs), a large group of secreted proteinases that require divalent cations for their catalytic activities, play important roles in a variety of physiological and pathological processes such as tumor invasion, as well as distant metastasis [7]. In the present study, we have shown that down-regulated Pygo2 is associated with decreased expression of MMP-7 and VEGF. Barnes [20] found that expression of β-catenin enhanced expression of MMP-7 protein, suggesting that expression of Pygo2 could activate Wnt signal transduction pathway leading to the expression of MMP-7. Interestingly, we noted, however, that tumor invasion was not completely eliminated by suppressing the expression of Pygo2 protein. We found that the lack of Pygo2 could not obviously reduce the expression of MMP-9, suggesting Pygo2 accumulation in tumor tissues may not activate the expression of MMP-9 by the canonical Wnt pathway.
In summary, we demonstrate that a reduced expression of Pygo2 inhibits RCC growth in vitro and in vivo. Additionally, Pygo2 is involved tumor invasion, metastasis and blood-vessel growth (high expression of VEGF), implying that Pygo2 may have a key function in the carcinogenesis of RCC. Thus, we speculate its requirement for growth in RCC, which definitely needs further experiment data to support the hypothesis.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgments
We thank Xiaohong Ma for statistical analysis and technical assistance. We also thank Professor Boan Li of college of Life Science of Xiamen University for contributing research reagents. This study was supported by grants (No. 2011J01254) from Natural Science Foundation of Fujian Province.
Footnotes
Peer review under responsibility of Shanghai Medical Association and SMMU.
References
- 1.Hoffmans R., Städeli R., Basler K. Pygopus and legless provide essential transcriptional coactivator functions to armadillo/beta-catenin. Curr Biol. 2005;15:1207–1211. doi: 10.1016/j.cub.2005.05.054. [DOI] [PubMed] [Google Scholar]
- 2.Polakis P. Wnt signaling in cancer. Cold Spring Harb Perspect Biol. 2012;4(5) doi: 10.1101/cshperspect.a008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mieszczanek J., de la Roche M., Bienz M. A role of Pygopus as an anti-repressor in facilitating Wnt-dependent transcription. Proc Natl Acad Sci U S A. 2008;105:19324–19329. doi: 10.1073/pnas.0806098105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller T.C., Rutherford T.J., Johnson C.M., Fiedler M., Bienz M. Allosteric remodelling of the histone H3 binding pocket in the Pygo2 PHD finger triggered by its binding to the B9L/BCL9 co-factor. J Mol Biol. 2010;401:969–984. doi: 10.1016/j.jmb.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kramps T., Peter O., Brunner E., Nellen D., Froesch B., Chatterjee S. Wnt/wingless signaling requires BCL9/legless-mediated recruitment of pygopus to the nuclear β-catenin-TCF complex. Cell. 2002;109:47–60. doi: 10.1016/s0092-8674(02)00679-7. [DOI] [PubMed] [Google Scholar]
- 6.Städeli R., Basler K. Dissecting nuclear wingless signalling: recruitment of the transcriptional co-activator Pygopus by a chain of adaptor proteins. Mech Dev. 2005;122:1171–1182. doi: 10.1016/j.mod.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Nelson A.R., Fingleton B., Rothenberg M.L., Matrisian L.M. Matrix metalloproteinases: biologic activity and clinical implications. J Clin Oncol. 2000;18:1135–1149. doi: 10.1200/JCO.2000.18.5.1135. [DOI] [PubMed] [Google Scholar]
- 8.Townsley F.M., Cliffe A., Bienz M. Pygopus and legless target Armadillo/beta-catenin to the nucleus to enable its transcriptional co-activator function. Nat Cell Biol. 2004;6:626–633. doi: 10.1038/ncb1141. [DOI] [PubMed] [Google Scholar]
- 9.Belenkaya T.Y., Han C., Standley H.J., Lin X., Houston D.W., Heasman J. Pygopus encodes a nuclear protein essential for wing-less/Wnt signaling. Development. 2002;129:4089–4101. doi: 10.1242/dev.129.17.4089. [DOI] [PubMed] [Google Scholar]
- 10.Thompson B., Townsley F., Rosin-Arbesfeld R., Musisi H., Bienz M. A new nuclear component of the Wnt signalling pathway. Nat Cell Biol. 2002;4:367–373. doi: 10.1038/ncb786. [DOI] [PubMed] [Google Scholar]
- 11.Andrews P.G., Kennedy M.W., Popadiuk C.M., Kao K.R. Oncogenic activation of the human Pygopus2 promoter by E74-like factor-1. Mol Cancer Res. 2008;6:259–266. doi: 10.1158/1541-7786.MCR-07-0068. [DOI] [PubMed] [Google Scholar]
- 12.Lake B.B., Kao K.R. Pygopus is required for embryonic brain patterning in Xenopus. Dev Biol. 2003;261:132–148. doi: 10.1016/s0012-1606(03)00305-1. [DOI] [PubMed] [Google Scholar]
- 13.Song N., Schwab K.R., Patterson L.T., Yamaguchi T., Lin X., Potter S.S. Pygopus 2 has a crucial, Wnt pathway-independent function in lens induction. Development. 2007;134:1873–1885. doi: 10.1242/dev.001495. [DOI] [PubMed] [Google Scholar]
- 14.Schwab K.R., Patterson L.T., Hartman H.A., Song N., Lang R.A., Lin X. Pygo1 and Pygo2 roles in Wnt signaling in mammalian kidney development. Dev Cell. 2007;5:15. doi: 10.1186/1741-7007-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Popadiuk C.M., Xiong J., Wells M.G., Andrews P.G., Dankwa K., Hirasawa K. Antisense suppression of pygopus2 results in growth arrest of epithelial ovarian cancer. Clin Cancer Res. 2006;12:2216–2223. doi: 10.1158/1078-0432.CCR-05-2433. [DOI] [PubMed] [Google Scholar]
- 16.Wang Z.X., Chen Y.Y., Li B.A., Tan G.W., Liu X.Y., Shen S.H. Decreased Pygopus 2 expression suppresses glioblastoma U251 cell growth. J Neuroomcol. 2010;100:31–41. doi: 10.1007/s11060-010-0144-6. [DOI] [PubMed] [Google Scholar]
- 17.Tycko B., Li C.M., Buttyan R. The Wnt/beta-catenin pathway in Wilms tumors and prostate cancers. Curr Mol Med. 2007;7:479–489. doi: 10.2174/156652407781387118. [DOI] [PubMed] [Google Scholar]
- 18.De D., Chen A., Wu Z., Lv S., He G., Qi Y. Overexpression of Pygopus2 protects HeLa cells from vinblastine-induced apoptosis. Biol Chem. 2009;390:157–165. doi: 10.1515/BC.2009.014. [DOI] [PubMed] [Google Scholar]
- 19.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 20.Barnes E.A., Kenerson H.L., Mak B.C., Yeung R.S. The loss of tuberin promotes cell invasion through the β-catenin pathway. Am J Respir Cell Mol Biol. 2010;43:617–627. doi: 10.1165/rcmb.2008-0335OC. [DOI] [PMC free article] [PubMed] [Google Scholar]