Abstract
Objective
To assess the clinicopathological features and overall survival between two groups of Chinese patients older or younger than 70 years after retropubic radical prostatectomy.
Methods
From January 2001 to February 2010, 390 patients receive dretropubic radical prostatectomy. After excluding 89 patients with adjuvant or neoadjuvant hormonal therapy or radiotherapy, a total of 301 patients were included in this study. We arbitrarily divided these patients into younger age group (<70 years, 140 cases, 46.5%) and older age group (≥70 years, 161 cases, 53.5%). The differences in serum prostate specific antigen (PSA), Gleason score, clinical tumor stage, and biochemical-free survival were analyzed between the two groups.
Results
There were not significant differences between the two groups in high Gleason score rate and clinical tumor stage. However, older patients had significantly lower biochemical recurrence rate than those of younger patients, and had significantly higher PSA levels. Multivariate analysis showed that older age, PSA level and clinical tumor stage were significantly associated with biochemical recurrence free survival.
Conclusion
In Chinese men, older age (≥70 years) is associated with better outcome. If the physical condition permits, older age alone should not exclude patients from radical prostatectomy.
Keywords: Prostate cancer, Age, Biochemical recurrence-free survival, Radical prostatectomy
1. Introduction
In 2013, prostate cancer was the most commonly diagnosed malignancy and the second leading cause of cancer-related death in men in America [1]. However, the incidence rate of prostate cancer in China is significantly lower. A study had reported a 26-fold higher incidence rate of prostate cancer in American than in Chinese men with an intermediate rate in Chinese-American men. Such differences may be due to genetic and/or environment factors [2]. An RNA-seq study using prostate cancer tissue from Chinese patients found two novel gene fusions which appear to be specifically associated with Chinese patients [3]. In this article, we will reveal an age-related Chinese people specific feature.
2. Patients and methods
From January 2001 to February 2010, a cohort of 390 men underwent retropubic radical prostatectomy in our institution for the treatment of clinically localized prostate cancer. Eighty-nine cases were excluded because of insufficient clinical data, adjuvant or neoadjuvant hormonal therapy, and radiotherapy. The remaining 301 cases were included in the study and divided into two groups based on age, with 161 (53.5%) men aged ≥70 years and 140 (46.5%) aged <70 years.
Two hundred and seventy-five patients were diagnosed by ultrasound guided prostate biopsy, and 26 patients were diagnosed by Transurethral Resection of Prostate (TURP). Ultrasound and pelvic MRI or CT were performed before biopsy. The indications for prostate biopsy were prostate peripheral zone nodule detected by digital rectal examination (DRE), ultrasound, MRI or CT with any prostate specific antigen (PSA) level, or PSA = 4–10 ng/mL and free PSA/total PSA < 0.16 without detectable nodule, or PSA > 10 ng/mL. In order to comparatively analyze the age related differences, we considered Gleason score, PSA and clinical stage (Table 1). Biochemical recurrence was defined as two consecutive increasing PSA values of >0.2 ng/mL.
Table 1.
Assignment of PSA, Gleason score and clinical stage.
| Process of variable assignment | |
|---|---|
| PSA (ng/mL) | Level 1: 0–4; Level 2: 4–10; Level 3: 10–20; Level 4: ≥20 |
| Gleason score | Level 1: 3/3; Level 2: 3/4–5; Level 3: 4–5/3–5 |
| Clinical stage | Level 1: T1a; Level 2: T1b; Level 3: T1c; Level 4: T2a; Level 5: T2b; Level 6: T2c; Level 7: T3a |
A Chi-square test and an independent test were used to perform a comparative analysis of clinical factors according to age. Univariate Cox regression model and Kaplan–Meier analysis were used for examining the effect of age on the outcomes of biochemical recurrence-free survival (BCRFS). Mean overall follow-up for the analysis was 57 months. SPSS19.0 for Windows software (IBM, NY, USA) was used, and p < 0.05 was considered statistically significant.
3. Results
Demographics and clinical characteristics are described in Table 2. Except for PSA, there were no significant differences in other parameters between the two groups. However, the low Gleason score (3/3) rate in older group (46.6%) was higher than that in younger group (41.4%).
Table 2.
Comparison of different age groups in clinical features.
| Feature | Total | ≥70 years old | <70 years old | p-Value |
|---|---|---|---|---|
| n = 301 | n = 161 | n = 140 | ||
| PSA (ng/mL) | ||||
| <4, n (%) | 8 (2.7) | 2 (1.2) | 6 (4.3) | 0.2 |
| 4–10, n (%) | 88 (29.2) | 48 (29.8) | 40 (28.6) | 0.9 |
| 10–20, n (%) | 108 (35.9) | 64 (39.8) | 44 (31.4) | 0.2 |
| ≥20, n (%) | 97 (32.2) | 47 (29.2) | 50 (35.7) | 0.3 |
| Mean PSA (ng/mL) | 19.9 | 18.6 | 21.4 | 0.003 |
| Gleason score | ||||
| 3/3, n (%) | 133 (44.2) | 75 (46.6) | 58 (41.4) | 0.6 |
| 3/4–5, n (%) | 76 (25.2) | 40 (24.8) | 36 (25.7) | 0.9 |
| 4–5/3–5, n (%) | 92 (30.6) | 46 (28.6) | 46 (32.9) | 0.5 |
| Clinical stage | ||||
| T1a, n (%) | 22 (7.3) | 10 (6.2) | 12 (8.6) | 0.6 |
| T1b, n (%) | 4 (1.3) | 0 (0) | 4 (2.9) | 0.09 |
| T1c, n (%) | 91 (30.2) | 49 (30.4) | 42 (30.0) | 1.0 |
| T2a, n (%) | 64 (21.3) | 34 (21.1) | 30 (21.4) | 0.9 |
| T2b, n (%) | 100 (33.2) | 56 (34.8) | 44 (31.4) | 0.6 |
| T2c, n (%) | 12 (4.0) | 8 (5.0) | 4 (2.9) | 0.5 |
| T3a, n (%) | 8 (2.7) | 4 (2.5) | 4 (2.9) | 0.9 |
| Mean RPBR | 0.34 | 0.31 | 0.36 | 0.9 |
PSA, prostate specific antigen; RPBR, recent positive biopsy rate.
Mean operation time was 200 min (range: 150–300 min). Average blood loss was 230 (100–1100) mL. Among the 301 patients, rectum injury rate was 1.0% (3 cases), and lymphatic leakage rate was 1.99% (6 cases). The 1 year, 2 years, 3 years and 5 years BCRFS rate were 95.0% (286 cases), 92.0% (277 cases), 91.0% (274 cases) and 89.4% (269 cases). The 5 year BCRFS rate of the old group was 92.5% (149 in 161 cases), and it 85.7% (120 in 140 cases) in young group.
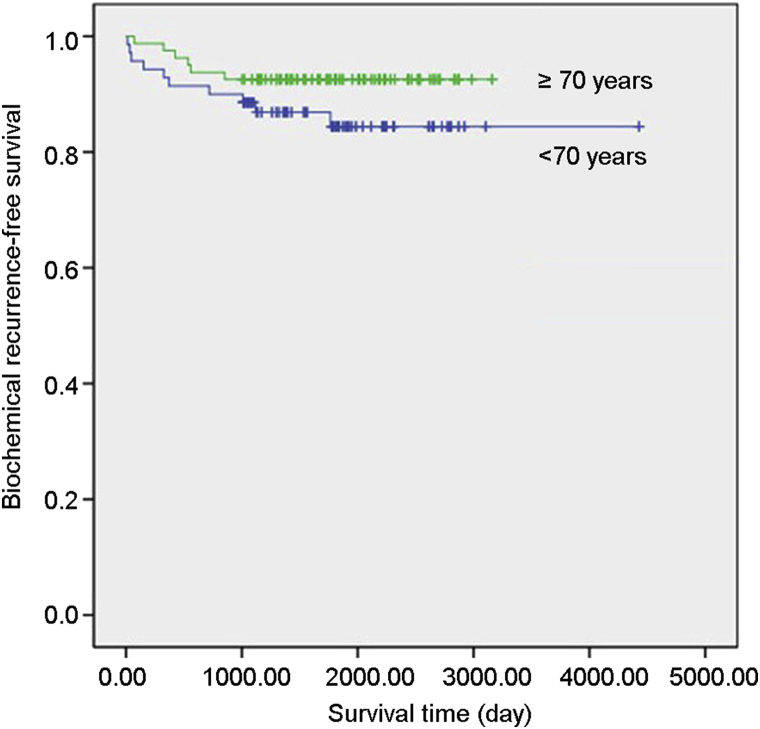
We analyzed the relationship between the four factors and 5 years BCRFS rate. The PSA and clinical stage were positive risk factors, and age was a negative risk factor. There was no relation between Gleason score and 5 years BCRFS rate (Table 3). The survival analysis of different age groups showed that the older patients had higher BCRFS rate than that of the younger patients (Fig. 1).
Table 3.
The relationship between risk factors and 5 years biochemical recurrence survival (result of Cox regression analysis).
| Regression coefficients | Standard error | Wald | p-Value | Relative risk | |
|---|---|---|---|---|---|
| PSA | 0.019 | 0.006 | 9.282 | 0.002 | 1.02 |
| Age | −0.084 | 0.024 | 12.06 | 0.001 | 0.919 |
| Gleason score | 0.259 | 0.174 | 2.202 | 0.138 | 1.295 |
| Clinical stage | 0.316 | 0.143 | 4.872 | 0.027 | 1.371 |
PSA, prostate specific antigen.
Figure 1.
Biochemical recurrence-free survival: the Kaplan–Meier curve for biochemical recurrence-free survival according to the two age groups (<70 and ≥70 years). The log rank test showed significant difference (p < 0.05).
4. Discussion
As radical prostatectomy is traditionally recommended for patients with longer than 10 years life expectancy, many surgeons intend to offer radical surgery to patients aged ≥70 years [4]. However, with the rapidly increasing elderly population and prolonged life expectancy, more and more old (≥70 years) patients with localized prostate cancer will have longer than 10 years life expectancy [5], [6]. How to manage these patients become an important issue. It is important to know the outcomes of radical prostatectomy for those patients.
Several articles had focused on this question, however, the result seemed controversial. In 2008, an US study of 4035 patients demonstrate that even though the older patients had higher clinical stage, pathology Gleason sums and a lower frequency of organ-confined disease, there was no difference in cancers specific survival or biochemical progression-free probability between the younger and older patents [7], and similar opinion appeared in another US study [8]. With the advent of new robotic assisted RP technique, the result seemed to be confirmed. In a RARP study, Greco observed that except for a higher pathological Gleason grade and longer time to return to driving, outcomes of older patients are largely comparable to that in younger patients [9]. However, Pfitzenmaier et al. [10] analyzed 626 RP patients in Germany, and showed that the 10-year PSA-free survival was 51.8% for the younger and 57.4% for the older patients respectively. Another American and British study showed the similar result [11], [12]. In Asia, biochemical recurrence are more likely in older Korean and Japanese patients [13], [14]. Although these results are controversial, it appeares that older patients have no better outcome than the young patients.
Our present study showed that older patients have better outcomes than young patients. When we reviewed the previous studies, we found that those studies were based on the population of developed countries in which PSA screening is universal. Compared to these patients, Chinese patients have two unique characteristics. First, cancer in most of the Chinese patients was not detected by PSA screening. Because most patients with localized prostate cancers have few symptoms, the detection of prostate cancer is mostly depended on self medical awareness. As a result, poor medical awareness can delay the detection of prostate cancer in China. Because young Chinese patients are relatively healthier and busier than older patients who usually have more free time and more likely to go to hospital because of other diseases. Thus the prostate cancer diagnoses of older patients can be confirmed timely due to better medical awareness. The detections of prostate cancer of young patients are relatively delayed because of the relatively poor medical awareness. Second, environmental and genetic factors may also contribute to the differences.
5. Conclusion
To conclude, the Chinese prostate cancer patients have unique characteristics compared with patients in developed countries'. Elderly patients (≥70 years) have better outcome than younger patients (<70 years). If the physical condition permits, old age should not exclude patients from radical prostatectomy.
Conflicts of interest
The authors declare no conflict of interest.
Footnotes
Peer review under responsibility of Shanghai Medical Association and SMMU.
References
- 1.Siegel R., Naishadham D., Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Yu H., Harris R.E., Gao Y.T., Gao R., Wynder E.L. Comparative epidemiology of cancers of the colon, rectum, prostate and breast in Shanghai, China versus the United States. Int J Epidemiol. 1991;20:76–81. doi: 10.1093/ije/20.1.76. [DOI] [PubMed] [Google Scholar]
- 3.Ren S., Peng Z., Mao J.H., Yu Y., Yin C., Gao X. RNA-seq analysis of prostate cancer in the Chinese population identifies recurrent gene fusions, cancer-associated long noncoding RNAs and aberrant alternative splicings. Cell Res. 2012;22:806–821. doi: 10.1038/cr.2012.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fowler F.J., Jr., McNaughton C.M., Albertsen P.C., Zietman A., Elliott D.B., Barry M.J. Comparison of recommendations by urologists and radiation oncologists for treatment of clinically localized prostate cancer. JAMA. 2000;283:3217–3222. doi: 10.1001/jama.283.24.3217. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC) Trends in aging–United States and worldwide. MMWR Morb Mortal Wkly Rep. 2003;52:101–104. 106. [PubMed] [Google Scholar]
- 6.Minino A.M., Smith B.L. Deaths: preliminary data for 2000. Natl Vital Stat Rep. 2001;49:1–40. [PubMed] [Google Scholar]
- 7.Richstone L., Bianco F.J., Shah H.H., Kattan M.W., Eastham J.A., Scardino P.T. Radical prostatectomy in men aged > or =70 years: effect of age on upgrading, upstaging, and the accuracy of a preoperative nomogram. BJU Int. 2008;101:541–546. doi: 10.1111/j.1464-410X.2007.07410.x. [DOI] [PubMed] [Google Scholar]
- 8.Siddiqui S.A., Sengupta S., Slezak J.M., Bergstralh E.J., Leibovich B.C., Myers R.P. Impact of patient age at treatment on outcome following radical retropubic prostatectomy for prostate cancer. J Urol. 2006;175:952–957. doi: 10.1016/S0022-5347(05)00339-3. [DOI] [PubMed] [Google Scholar]
- 9.Greco K.A., Meeks J.J., Wu S., Nadler R.B. Robot-assisted radical prostatectomy in men aged > or =70 years. BJU Int. 2009;104:1492–1495. doi: 10.1111/j.1464-410X.2009.08718.x. [DOI] [PubMed] [Google Scholar]
- 10.Pfitzenmaier J., Pahernik S., Buse S., Haferkamp A., Djakovic N., Hohenfellner M. Survival in prostate cancer patients > or =70 years after radical prostatectomy and comparison to younger patients. World J Urol. 2009;27:637–642. doi: 10.1007/s00345-009-0414-0. [DOI] [PubMed] [Google Scholar]
- 11.Brassell S.A., Rice K.R., Parker P.M., Chen Y., Farrell J.S., Cullen J. Prostate cancer in men 70 years old or older, indolent or aggressive: clinicopathological analysis and outcomes. J Urol. 2011;185:132–137. doi: 10.1016/j.juro.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 12.Xu D.D., Sun S.D., Wang F., Sun L., Stackhouse D., Polascik T. Effect of age and pathologic Gleason score on PSA recurrence: analysis of 2911 patients undergoing radical prostatectomy. Urology. 2009;74:654–658. doi: 10.1016/j.urology.2008.12.063. [DOI] [PubMed] [Google Scholar]
- 13.Kim J.K., Cho S.Y., Jeong C.W., Lee S.B., Ku J.H., Hong S.K. Patients aged more than 70 had higher risk of locally advanced prostate cancers and biochemical recurrence in Korea. BJU Int. 2012;110:505–509. doi: 10.1111/j.1464-410X.2011.10927.x. [DOI] [PubMed] [Google Scholar]
- 14.Masuda H., Fukushima H., Kawakami S., Numao N., Fujii Y., Saito K. Impact of advanced age on biochemical recurrence after radical prostatectomy in Japanese men according to pathological stage. Jpn J Clin Oncol. 2013;43:410–416. doi: 10.1093/jjco/hyt017. [DOI] [PubMed] [Google Scholar]