Abstract
Objective
To test the diagnostic performance of percent free prostate-specific antigen (%fPSA) in predicting any prostate cancer (PCa) and high-grade prostate cancer (HGPCa) in a retrospective multi-center biopsy cohort with a PSA level of 4.0–10.0 ng/mL in China.
Methods
Consecutive patients with a PSA of 4.0–10.0 ng/mL who underwent transrectal ultrasound-guided biopsy were enrolled at 16 Chinese medical centers from January 1st, 2010 to December 31st, 2013. Total and free serum PSA determinations were performed using three types of electro-chemiluminescence immunoassays recalibrated to the World Health Organization (WHO) standard. The diagnostic accuracy of PSA, %fPSA, and %fPSA in combination with PSA (%fPSA + PSA) was determined using the area under the receiver operating characteristic (ROC) curve (AUC).
Results
A total of 2310 consecutive men with PSA levels between 4.0 and 10.0 ng/mL were included, and the detection rate of PCa was 25.1%. The AUC of %fPSA and %fPSA + PSA in predicting any PCa was superior to PSA alone in men aged ≥60 years (0.623 vs. 0.534, p < 0.0001) but not in men aged 40–59 years (0.517 vs. 0.518, p = 0.939). Similar result was yield in predicting HGPCa.
Conclusion
In a clinical setting of Chinese men with 4.0–10.0 ng/mL PSA undergoing initial prostate biopsy, adding %fPSA to PSA can moderately improve the diagnostic accuracy for any PCa and HGPCa compared with PSA alone in patients ≥60 but not in patients aged 40–59 years.
Keywords: Prostate cancer, Diagnosis, Prostate-specific antigen, Percent free PSA, Chinese population, ROC curve
1. Introduction
Prostate cancer (PCa) is the second-most frequently diagnosed malignancy in men globally [1]. Although the incidence of PCa in China is much lower than in Western countries [2], PCa ranks as the fastest growing malignancy with respect to incidence in recent years [3], [4] due to changing lifestyles and increasing health awareness. Although new biomarkers are emerging, prostate-specific antigen (PSA) and its derivatives remain the most widely used and practical test to detect PCa. Percent free PSA (%fPSA) has been demonstrated to improve positive test results during prostate biopsy and to reduce the number of unnecessary biopsies in men with a moderately elevated serum PSA level (4.0–10.0 ng/mL) in white and black populations [5], [6]. The diagnostic accuracy (area under the receiver operating characteristic curve, AUC) has been reported to be approximately 0.7 in patients with a PSA of 4.0–10.0 ng/mL [7], [8]. However, PCa is believed to differ epidemiologically and biologically between Western and East Asian populations. First, the age-standardized PCa incidence rate in China was reported to be 5.3/100,000 in 2012 by the World Health Organization (WHO), which was only 1/12 of the rate in European populations and 1/18 of the rate in North Americans [9]. In addition, PSA reference ranges have been reported to be much lower in Chinese and Japanese populations [10], [11]. Thus there may also be some other differences in the application of %fPSA in East Asian population.
Although several studies of %fPSA in China indicated an improvement in diagnostic accuracy over PSA alone [12], [13], these studies were mostly published in Chinese journals with limitations with respect to sample size. In this study, we attempted to examine the effectiveness of %fPSA in a retrospective multi-center Chinese cohort.
2. Methods and materials
2.1. Patients
This study was approved by the Institutional Review Board of each of the participating hospitals. This study involved 2310 consecutive patients who underwent transrectal ultrasound (TRUS)-guided prostate biopsy in 16 participating hospitals between January 1st, 2010 and December 31st, 2013. Patients visiting the outpatient department of urology for health checkups and urinary symptoms with a PSA of 4.0–10.0 ng/mL were included in this study, regardless of digital rectal examination (DRE) results. Patients with prior biopsy, urinary tract infections, urinary retention or instrumentation or catheterization of the urethra within 2 weeks and those received finasteride or hormonal treatment were excluded. TRUS-guided systematic 8-, 10- and 12-core biopsies were performed in 490, 483 and 1337 patients, respectively.
2.2. PSA measurement and biopsy techniques
Peripheral blood samples were obtained within 2 weeks prior to DRE and prostate biopsies. Three types of PSA/fPSA electro-chemiluminescence immunoassays were used in the participating hospitals: Abbott AxSYM, Beckman Coulter Access, and Roche Elecsys 2010 with recalibration to the WHO standard (PSA-WHO 96/670) using an appropriate correction factor. Prostate volume (PV) was calculated using the equation D1 × D2 × D3 × (π/6) and the three dimensions of the prostate as measured by TRUS.
2.3. Statistical analysis
The Mann–Whitney U test was used to analyze the total PSA, %fPSA, PV, and age because of the non-normal distribution of these parameters. Univariate logistic regression analyses were used to assess the correlations between clinical parameters and biopsy results. A multivariate logistic regression model was used to predict PCa risk. The demographic and clinical variables used in the model included age, logarithm of PSA, logarithm of %fPSA and logarithm of PV because the logarithm of these parameters fit the model better than the raw data. We combined PSA and %fPSA using logistic regression (%fPSA + PSA) to estimate the effectiveness of the combination of these two parameters. Receiver operating characteristic (ROC) curves were calculated for PSA, %fPSA and %fPSA + PSA by plotting the sensitivity versus 1-specificity for predicting any PCa and for predicting high-grade prostate cancer (HGPCa, Gleason score ≥7). The areas under the ROC curves (AUC) were used to measure the diagnostic accuracy of PSA, %fPSA, and %fPSA + PSA. The statistical significance of any difference was calculated using a z test. All of the statistical analyses were performed using the SPSS v.17.0 (SPSS Inc., Chicago, IL, USA) and MedCalc v.10.4.7.0 (MedCalc Software bvba, Mariakerke, Belgium). All of the p-values were two-sided, and p < 0.05 was considered statistically significant.
3. Results
The PCa detection rate for the whole cohort was 25.1%. The median %fPSA was 14.0% in the PCa group and 15.8% in the negative biopsy group (p < 0.0001, Mann–Whitney U test). The median age of PCa patients was 70 years, significantly higher than the median age of men with a negative biopsy (median 66 years, p < 0.0001). The median total PSA was significantly higher in the PCa group compared with the negative biopsy group (p = 0.010). The median PV was smaller in PCa patients than in patients with a negative biopsy (36.45 vs. 45.97 mL, p < 0.0001). The number of biopsy cores was higher in the PCa group compared with the negative biopsy group (mean 11.31 vs. 10.95, p < 0.0001) (Table 1). The rate of positive DRE averaged 13.1% in the cohorts but it ranged from 8.1% to 32.0% at different hospitals. Considering the subjective nature of DRE, we decided not to include DRE results in the predictive model.
Table 1.
Clinical variable in prostate cancer and negative biopsy subjects.
| Prostate cancer | Negative biopsy | p Value | |
|---|---|---|---|
| n | 579 | 1731 | |
| Age | |||
| Mean (SD) | 69.41 (7.73) | 65.85 (8.87) | |
| Median (IQR) | 70.00 (11.00) | 66.00 (11.00) | <0.0001a |
| PSA | |||
| Mean (SD) | 7.31 (1.61) | 7.11 (1.66) | |
| Median (IQR) | 7.40 (2.60) | 7.10 (2.80) | 0.0102a |
| Percent free PSA | |||
| Mean (SD) | 0.151 (0.078) | 0.170 (0.082) | |
| Median (IQR) | 0.140 (0.091) | 0.158 (0.102) | <0.0001a |
| Prostate volume | |||
| Mean (SD) | 42.29 (22.78) | 52.03 (27.61) | |
| Median (IQR) | 36.45 (21.75) | 45.97 (32.10) | <0.0001a |
| No. of biopsy cores | |||
| Mean (SD) | 11.31 (1.87) | 10.95 (1.98) | |
| Median (IQR) | 12.00 (2.00) | 12.00 (2.00) | <0.0001a |
SD, standard deviation; IQR, interquartile range; PSA, prostate specific antigen.
Mann-Whitney U test.
Univariate logistic regression analyses indicated that older age, higher PSA, lower %fPSA, and larger PV correlated with a positive biopsy (p < 0.0001, p = 0.0097, p < 0.0001, p < 0.0001, respectively). Multivariate logistic regression analyses indicated that lower %fPSA, older age, higher PSA, and smaller PV were independent predictors of prostate cancer in all patients (Supplementary Table 1, p = 0.0002, p < 0.0001, p = 0.0073, p < 0.0001, respectively).
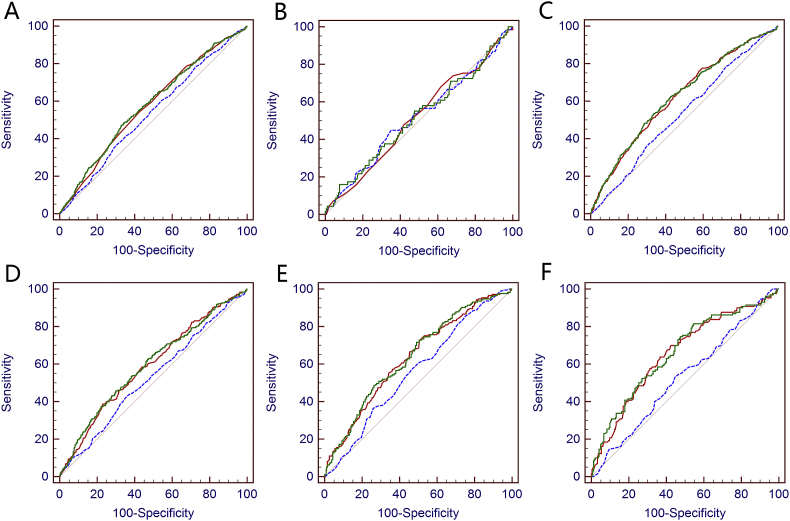
In patients aged 40–54 years, both %fPSA + PSA failed to improve the diagnostic accuracy compared with PSA alone (Table 2, AUC = 0.610 and 0.580, respectively, p = 0.595). In patients aged 55–69, 70–75 and over 75 years, %fPSA + PSA improved the diagnostic accuracy of any PCa compared with PSA alone (Table 2, p = 0.003, p = 0.004, p = 0.001, respectively, z-test). The ROC curve predicting any PCa for patients aged 55–69, 70–75 and over 75 years were illustrated in Figure 1, Figure 2.
Table 2.
Diagnostic accuracy of total PSA and %fPSA stratified by age, prostate volume and biopsy techniques.
| n | AUC |
AUC improvement | p valueb | |||
|---|---|---|---|---|---|---|
| PSA (95% CI) | %fPSA (95% CI) | %fPSA + PSA (95%CI) | ||||
| Age (year) | ||||||
| 40–54 | 142 | 0.580 (0.404–0.756) | 0.575 (0.432–0.718) | 0.610 (0.445–0.776) | 0.030 | 0.595 |
| 55–69 | 1168 | 0.529 (0.500–0.558) | 0.590 (0.562–0.619) | 0.596 (0.555–0.636) | 0.067 | 0.003 |
| 70–75 | 515 | 0.562 (0.518–0.606) | 0.635 (0.591–0.676) | 0.641 (0.591–0.691) | 0.079 | 0.004 |
| >75 | 370 | 0.530 (0.478–0.582) | 0.657 (0.607–0.706) | 0.663 (0.604–0.722) | 0.133 | 0.001 |
| 40–59 | 411 | 0.518 (0.440–0.597) | 0.516 (0.441–0.592) | 0.517 (0.439–0.596) | 0.001 | 0.939 |
| ≥60 | 1709 | 0.534 (0.504–0.564) | 0.620 (0.591–0.650) | 0.623 (0.594–0.653) | 0.089 | <0.0001 |
| PV (mL)a | ||||||
| < 30 | 447 | 0.606 (0.552–0.660) | 0.532 (0.478–0.587) | 0.611 (0.558–0.665) | 0.006 | 0.532 |
| 30–39.9 | 437 | 0.533 (0.476–0.590) | 0.509 (0.452–0.567) | 0.532 (0.475–0.590) | 0.001 | 0.920 |
| 40–49.9 | 334 | 0.528 (0.454–0.602) | 0.503 (0.432–0.574) | 0.529 (0.454–0.604) | 0.001 | 0.750 |
| ≥50 | 764 | 0.543 (0.488–0.598) | 0.563 (0.508–0.618) | 0.569 (0.514–0.624) | 0.024 | 0.281 |
| Biopsy technique | ||||||
| 8-cores | 490 | 0.545 (0.486–0.604) | 0.604 (0.544–0.664) | 0.613 (0.551–0.675) | 0.068 | 0.016 |
| 10-cores | 483 | 0.557 (0.491–0.624) | 0.543 (0.475–0.610) | 0.566 (0.495–0.636) | 0.008 | 0.616 |
| 12-cores | 1337 | 0.531 (0.497–0.565) | 0.575 (0.542–0.609) | 0.577 (0.544–0.611) | 0.047 | 0.013 |
| Total | 2310 | 0.536 (0.515–0.556) | 0.575 (0.555–0.595) | 0.580 (0.554–0.607) | 0.045 | 0.001 |
PSA, prostate specific antigen; %fPSA, percent free PSA; AUC, areas under the receivers operating characteristic (ROC) curve; PV, prostate volume.
Prostate volume available for 1991 patients.
z-test comparing %fPSA + PSA vs. PSA alone.
Figure 1.
ROC curves of %fPSA (red), PSA (blue), and PSA + %fPSA (green) for the detection of PCa in different age groups. (A) Whole cohort; (B) 40–59 years; (C) over 60 years; (D) 55–69 years; (E) 70–75 years; and (F) over 75 years.
Figure 2.
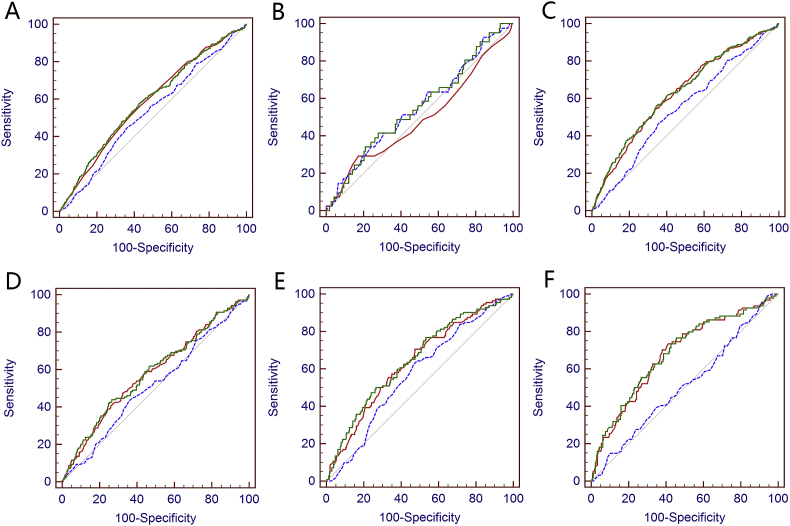
ROC curves of %fPSA (red), PSA (blue), and PSA + %fPSA (green) for the detection of HGPCa in different age groups. (A) Whole cohort; (B) 40–59 years; (C) over 60 years; (D) 55–69 years; (E) 70–75 years; and (F) over 75 years.
We further tested performance of %fPSA + PSA in several age ranges and found out that %fPSA + PSA was not more effective than PSA in predicting any PCa or HGPCa in patients aged 40–59 years (p = 0.939 and 0.847, Table 2, Supplementary Table 2, Figure 1, Figure 2). However, for patients aged ≥60 years, %fPSA + PSA outperformed PSA in the ROC curve analysis to predict any PCa and HGPCa (p < 0.0001 and p < 0.0001, Table 2, Supplementary Table 2, Figure 1, Figure 2C).
Since other factors may have influence on the diagnostic accuracy, we stratified the patients into different group by age, PV, and biopsy schemes (Table 2, Supplementary Table 2). We found that %fPSA + PSA failed to outperform PSA alone to predict any PCa and HGPCa in patients with different PVs. Additionally, %fPSA + PSA outperformed PSA alone to predict any PCa and HGPCa in patients who underwent 8- and 12-core biopsies (the difference for 12-core scheme for HGPCa failed to reach the commonly recognized level of significance with p = 0.072) but not in patients who underwent 10-core biopsies.
We further tested the performance of %fPSA + PSA and PSA in patients aged 40–59 and over 60 years with further stratification by PV and biopsy scheme. The results indicated that %fPSA + PSA was not superior to PSA alone in patients aged 40–59 years but was significantly better than PSA alone in patients aged over 60 years in different PV categories and with different biopsy schemes for predicting both any PCa or HGPCa.
4. Discussion
To the best of our knowledge, this is the first study to systematically evaluate the effectiveness of %fPSA in a large multi-center Chinese cohort. Our results suggest that in a clinical setting of Chinese men with 4.0–10.0 ng/mL PSA undergoing initial prostate biopsy, adding %fPSA to PSA can moderately improve the diagnostic accuracy for any PCa and HGPCa compared with PSA alone in patients ≥60 years but not in patients aged 40–59 years.
Although the Prostate, Lung, Colorectal and Ovarian (PLCO) Screening Trial investigators suggested that race and ethnicity had little effect on %fPSA [14], the PLCO study included few East Asians living in their country of origin. Actually, the efficacy of %fPSA in East Asians remains controversial. For example, %fPSA was identified as one of the most important PSA-derived parameters to predict the risk of PCa in Japanese patients [15]. However, a multi-center prospective study in a Korean population demonstrated that %fPSA failed to exhibit any improvement compared with PSA in patients aged 50–65 years [16]. In another pilot study from Malaysia, the author indicated that %fPSA was not effective for PCa detection, neither [17].
A meta-analysis of Chinese populations indicated a moderate diagnostic benefit of %fPSA over PSA [13]. Nevertheless, statistical heterogeneity was detected in many of the analyses, and detailed information that would help to explain this heterogeneity was not reported (population, PSA assay type and reference standards used, etc.). In light of these findings, a recent report from Guangzhou, China, indicated that the %fPSA failed to provide a diagnostic benefit over PSA in patients with a PSA of 2.5–10.0 ng/mL or 10.0–20.0 ng/mL [12]. The results of that study appear to contradict our results; however, there was no stratified analysis based on age in that study. In our data set, the AUC of %fPSA was also close to the AUC of PSA for the whole cohort, but %fPSA outperformed PSA in the older subgroup. Thus, their findings do not in contradict our findings. Furthermore, the authors studied limited cohorts of 274 and 284 patients with PSA values of 2.5–10.0 ng/mL or 10.1–20.0 ng/mL, respectively.
The impact of age on the diagnostic accuracy of %fPSA remains controversial in Western populations [18], [19]. Our results shed light on the influence of age on the diagnostic performance of %fPSA, which had a lower AUC compared with reports in Western population [8], [20]. In accordance with findings in Korea [16], [21], we provide evidence that %PSA is effective in older populations (≥60 years) but not in younger patients (<60 years).
There are several factors that may account for the ineffectiveness of %fPSA in younger patients in our study. First, the median %fPSA was only 11.8% in the 40–59 year-old group (Table 3); by contrast, the median values increased to 18.0% and 18.7% in patients aged 70–75 and over 75 years. The median %fPSA was also lower in younger patients than in older patients in the Korean report (PCa: 15.8% vs. 17.0%; benign: 16.7% vs. 20.4%). For instance, a cutoff of 10.0% was reported to lead to a detection rate of 56% in a Western population. However, the detection rate in patients <60 years with %fPSA less than 10.0% is only 19.1% in our data set. Second, the incidence of PCa is quite low in both Chinese and Korean patients [22] aged under 60 years (16.9% in our cohort). As reported in the Prostate Cancer Prevention Trial, the incidence of PCa is 18.6% in patients with a PSA <4.0 ng/mL and 30.0% in patients with a PSA of 3.1–4.0 ng/mL [23]. Although there is not a direct relationship between a lower incidence and a poorer diagnostic performance for %fPSA, there are reports that %fPSA is of better diagnostic accuracy in patients with a PSA of 4.0–10.0 ng/mL (detection rate 49.0%) [7] than with a PSA of 2.51–4.0 ng/mL (detection rate 24%) [24] in the Western population. In this data set, the PCa detection rate increased to 33.6% and 35.1% in patients aged 70–75 and over 75 years, respectively, and the AUCs of %fPSA also increased substantially (Fig. 1E, F). Third, reports on the diagnostic performance %fPSA have mostly been performed in the United States and in European countries with screening projects. However, the clinical setting is much more complex in China. Fourth, DRE result was not included in the analysis which may be a contributing factor for the ineffectiveness of %fPSA in younger age, however, %fPSA was still effective in the older patients regardless of DRE result.
Table 3.
Clinical variable of patients aged 40-59 years and ≥60 years.
| Age |
p value | ||
|---|---|---|---|
| 40-59 | ≥60 | ||
| n | 411 | 1784 | |
| Positive rate | 16.8% | 27.6% | <0.0001a |
| Age | |||
| Mean (SD) | 54.7 (4.4) | 69.8 (6.2) | |
| Median (IQR) | 56 (5) | 69 (10) | <0.0001b |
| PSA | |||
| Mean (SD) | 7.23 (1.61) | 7.16 (1.65) | |
| Median (IQR) | 7.20 (1.70) | 7.20 (1.80) | 0.476 |
| Percent Free PSA | |||
| Mean (SD) | 0.127 (0.070) | 0.175 (0.081) | |
| Median (IQR) | 0.118 (0.081) | 0.161 (0.102) | <0.0001b |
| Prostate volume | |||
| Mean (SD) | 43.5 (25.0) | 51.3 (27.2) | |
| Median (IQR) | 38.1 (22.1) | 45.1 (31.2) | <0.0001b |
| No. of biopsy cores | |||
| Mean (SD) | 11.2 (2.0) | 11.1 (2.0) | |
| Median (IQR) | 12 (2) | 12 (2) | <0.0001b |
SD, standard deviation; IQR, interquartile range; PSA, prostate specific antigen.
Chi-square test.
Mann-Whitney U test.
Several circumstances must be taken into consideration when drawing conclusions from this data set. First, 16 different institutes and three different assays were involved in PSA testing. However, the variability of the total and fPSA results among the commercial assays was decreased by calibration. Second, there is no national PSA-based PCa screening program in China. Significant clinical differences exist between this outpatient cohort and screening cohorts in Western countries. Nevertheless, the population in this study reflects a practical clinical scenario in China and East Asian countries without systematic screening projects. Third, there is an inherent limitation of multi-center studies because the biopsies were performed by different physicians and examined by different pathologists. Fourth, although there were more than 2000 cases in this data set with 411 cases aged 40–59 years old, this ineffectiveness may still be the result of a limited sample size. Despite of this caveat, our data represent the effectiveness of %fPSA in an actual practical setting for Chinese men. These findings should be validated in follow-up prospective studies.
5. Conclusion
In a clinical setting of Chinese men with 4.0–10.0 ng/mL PSA undergoing initial prostate biopsy, adding %fPSA to PSA can moderately improve the diagnostic accuracy for any PCa and for HGPCa specifically compared with PSA alone in patients ≥60 years but not in patients aged 40–59 years. These findings should be validated in further prospective studies.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported by the Chinese Prostate Cancer Consortium, Program for Changjiang Scholars, the University Innovative Research Team of the Ministry of Education of China (NO.IRT1111, Yinghao Sun) and the National Basic Research Program of China (2012CB518300, 2012CB518306, Yinghao Sun).
Footnotes
Peer review under responsibility of Chinese Urological Association and SMMU.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.ajur.2015.04.022.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Siegel R., Naishadham D., Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Center M.M., Jemal A., Lortet-Tieulent J., Ward E., Ferlay J., Brawley O. International variation in prostate cancer incidence and mortality rates. Eur Urol. 2012;61:1079–1092. doi: 10.1016/j.eururo.2012.02.054. [DOI] [PubMed] [Google Scholar]
- 3.Zhao P., Chen W. Peking Union Medical College Press; Beijing: 2008. Annual report of malignancy in China 2004. [Google Scholar]
- 4.Hao J., Chen W.Q. China Military Medical Science Press; Beijing: 2013. Chinese Cancer Registry Annual report 2012. [Google Scholar]
- 5.Christensson A., Bjork T., Nilsson O., Dahlen U., Matikainen M.T., Cockett A.T. Serum prostate specific antigen complexed to alpha 1-antichymotrypsin as an indicator of prostate cancer. J Urol. 1993;150:100–105. doi: 10.1016/s0022-5347(17)35408-3. [DOI] [PubMed] [Google Scholar]
- 6.Catalona W.J., Smith D.S., Wolfert R.L., Wang T.J., Rittenhouse H.G., Ratliff T.L. Evaluation of percentage of free serum prostate-specific antigen to improve specificity of prostate cancer screening. JAMA. 1995:1214–1220. [PubMed] [Google Scholar]
- 7.Catalona W.J., Partin A.W., Slawin K.M., Brawer M.K., Flanigan R.C., Patel A. Use of the percentage of free prostate-specific antigen to enhance differentiation of prostate cancer from benign prostatic disease: a prospective multicenter clinical trial. JAMA. 1998;279:1542–1547. doi: 10.1001/jama.279.19.1542. [DOI] [PubMed] [Google Scholar]
- 8.Lee R., Localio A.R., Armstrong K., Malkowicz S.B., Schwartz J.S., Free PSA Study Group A meta-analysis of the performance characteristics of the free prostate-specific antigen test. Urology. 2006;67:762–768. doi: 10.1016/j.urology.2005.10.052. [DOI] [PubMed] [Google Scholar]
- 9.GLOBOCAN 2012 v1.0, Cancer incidence and mortality worldwide IARC CancerBase No. 11 [Internet]. Available online at:http://globocan.iarc.fr [accessed on 28.08.2014].
- 10.Liu Z.Y., Sun Y.H., Xu C.L., Gao X., Zhang L.M., Ren S.C. Age-specific PSA reference ranges in Chinese men without prostate cancer. Asian J Androl. 2009;11:100–103. doi: 10.1038/aja.2008.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oesterling J.E., Kumamoto Y., Tsukamoto T., Girman C.J., Guess H.A., Masumori N. Serum prostate-specific antigen in a community-based population of healthy Japanese men: lower values than for similarly aged white men. Br J Urol. 1995;75:347–353. doi: 10.1111/j.1464-410x.1995.tb07347.x. [DOI] [PubMed] [Google Scholar]
- 12.Huang M., Lin Y., Xu A., Uhlman M., Deng X., Lin X. Percent free prostate-specific antigen does not improve the effectiveness of prostate cancer detection in Chinese men with a prostate-specific antigen of 2.5–20.0 ng/ml: a multicenter study. Med Oncol. 2014;31:925. doi: 10.1007/s12032-014-0925-4. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y., Sun G., Pan J.G., Guo Z.J., Li T. Performance of tPSA and f/tPSA for prostate cancer in Chinese. A systematic review and meta-analysis. Prostate Cancer Prostat Dis. 2006;9:374–378. doi: 10.1038/sj.pcan.4500906. [DOI] [PubMed] [Google Scholar]
- 14.Gelmann E.P., Chia D., Pinsky P.F., Andriole G.L., Crawford E.D., Reding D. Relationship of demographic and clinical factors to free and total prostate-specific antigen. Urology. 2001;58:561–566. doi: 10.1016/s0090-4295(01)01305-x. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki H., Komiya A., Kamiya N., Imamoto T., Kawamura K., Miura J. Development of a nomogram to predict probability of positive initial prostate biopsy among Japanese patients. Urology. 2006;67:131–136. doi: 10.1016/j.urology.2005.07.040. [DOI] [PubMed] [Google Scholar]
- 16.Jeong I.G., Lee K.H., Korean Urological Oncologic Society Prostate Cancer Study Group Percent free prostate specific antigen does not enhance the specificity of total prostate specific antigen for the detection of prostate cancer in Korean men 50 to 65 years old: a prospective multicenter study. J Urol. 2008;179:111–116. doi: 10.1016/j.juro.2007.08.153. [DOI] [PubMed] [Google Scholar]
- 17.Omar J., Jaafar Z., Abdullah M.R. A pilot study on percent free prostate specific antigen as an additional tool in prostate cancer screening. Malays J Med Sci. 2009;16:44–47. [PMC free article] [PubMed] [Google Scholar]
- 18.Oesterling J.E., Jacobsen S.J., Klee G.G., Pettersson K., Piironen T., Abrahamsson P.A. Free, complexed and total serum prostate specific antigen: the establishment of appropriate reference ranges for their concentrations and ratios. J Urol. 1995;154:1090–1095. doi: 10.1016/s0022-5347(01)66984-2. [DOI] [PubMed] [Google Scholar]
- 19.Kalish L.A., McKinlay J.B. Serum prostate-specific antigen levels (PSA) in men without clinical evidence of prostate cancer: age-specific reference ranges for total PSA, free PSA, and percent free PSA. Urology. 1999;54:1022–1027. doi: 10.1016/s0090-4295(99)00349-0. [DOI] [PubMed] [Google Scholar]
- 20.Catalona W.J., Partin A.W., Slawin K.M., Naughton C.K., Brawer M.K., Flanigan R.C. Percentage of free PSA in black versus white men for detection and staging of prostate cancer: a prospective multicenter clinical trial. Urology. 2000;55:372–376. doi: 10.1016/s0090-4295(99)00547-6. [DOI] [PubMed] [Google Scholar]
- 21.Hara N., Kitamura Y., Saito T., Komatsubara S. Total and free prostate-specific antigen indexes in prostate cancer screening: value and limitation for Japanese populations. Asian J Androl. 2006;8:429–434. doi: 10.1111/j.1745-7262.2006.00155.x. [DOI] [PubMed] [Google Scholar]
- 22.Yang W.J., Lee D.H., Chung B.H., Cho J.S., Choi Y.D., Kim S.J. Detection rate of prostate cancer on biopsy according to serum prostate-specific antigen in Korean men: a multicenter study. Urology. 2006;67:333–336. doi: 10.1016/j.urology.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 23.Thompson I.M., Ankerst D.P., Chi C., Goodman P.J., Tangen C.M., Lucia M.S. Assessing prostate cancer risk: results from the prostate cancer prevention trial. J Natl Cancer Inst. 2006;98:529–534. doi: 10.1093/jnci/djj131. [DOI] [PubMed] [Google Scholar]
- 24.Catalona W.J., Partin A.W., Finlay J.A., Chan D.W., Rittenhouse H.G., Wolfert R.L. Use of percentage of free prostate-specific antigen to identify men at high risk of prostate cancer when PSA levels are 2.51 to 4 ng/mL and digital rectal examination is not suspicious for prostate cancer: an alternative model. Urology. 1999;54:220–224. doi: 10.1016/s0090-4295(99)00185-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.