Abstract
Objective
Inflammatory serum markers have proven to be a powerful predictive tool of patient prognosis in cancer treatment for a wide variety of solid organ malignancies, predominantly in the context of localized disease. In this study we evaluated the preoperative neutrophil-to-lymphocyte ratio (NLR) as a predictive tool in patients with metastatic clear cell renal cell carcinoma (RCC).
Methods
Sixty-four patients with metastatic clear cell RCC undergoing nephrectomy were selected. Only patients with preoperative NLR were included for survival analysis. Patients were categorized into high and low NLR score determined by plotting the NLR ROC curve. Multivariable analysis was performed.
Results
Median age was 60.8 years (38.2–81.2). Median follow-up time was 8.1 months (0.1–106.3). Fuhrman grade distribution was: 2 (3.1%) grade 1, 6 (9.4%) grade 2, 24 (37.5%) grade 3 and 32 (50.0%) grade 4. Median NLR score was 3.5 (1.4–31.0). NLR ≥ 4 was associated with decreased overall survival compared to NLR < 4 (p = 0.017). Multivariable survival analysis showed NLR ≥ 4 as an independent predictor of survival (Hazard ratio (HR) 2.41, 95%CI 1.05–5.50, p = 0.03).
Conclusion
Elevated preoperative NLR is associated with poor prognosis in patients with metastatic kidney cancer. Preoperative NLR is a useful tool, which can predict prognosis, stratify patients for postoperative surveillance, and help guide decisions for therapy.
Keywords: Renal cancer, Neutrophil-to-lymphocyte ratio, Cytoreductive nephrectomy, Prognosis
1. Introduction
Renal cancer is among the 10 most common cancers in the United States, with 63,920 new cases and 13,860 deaths estimated in 2014 [1]. Approximately 30% of patients with apparent localized disease will ultimately develop metastasis with a 5-year survival rate of less than 10% [2], [3]. As such, there has been a tremendous, long-standing interest in accurately identifying those patients most likely to suffer from disease progression. Research in recent years has focused on the development of prognostic models to aid in surveillance strategies and patient counseling. Currently, the most commonly used tool to predict outcome in renal cell carcinoma (RCC) is the TNM staging system and nuclear grade [4]. However, there is considerable overlap in survival between stages [2], which has promoted the search for new prognostic markers to better stratify patients with expected poor outcomes.
In past years, efforts at identifying markers of disease progression in RCC have focused on the available and cost-effective preoperative laboratory blood tests. It is becoming increasingly clear that cancer progression depends on a coordinated interface between tumor biology and the host inflammatory response [5].
The systemic inflammatory response, which is usually measured by blood-based parameters, such as C-reactive protein, neutrophil or platelet count, among others has been shown to independently predict the clinical outcome of various human cancer types [6]. With the context of genitourinary malignancies elevated neutrophil-to-lymphocyte ratio (NLR) has been associated with high T stage and worse survival in a variety of tumor types including bladder and kidney cancer [7]. Of these inflammatory parameters, an increased NLR has been proposed as an easily accessible and reliable marker to predict cancer survival [6]. Increasing evidence in metastatic RCC suggests that a high NLR might represent an independent adverse prognostic factor in interferon treated [8], interleukin-2-treated [9], as well as in sunitinib-treated [10] patients. Therefore, the aim of our study is to provide further evidence of the prognostic significance of the preoperative NLR in metastatic clear cell RCC and to evaluate whether this parameter provides additional prognostic information [11].
2. Patients and methods
2.1. Patients
A total of 1871 patients underwent nephrectomy at Emory University Hospital for renal tumors between 2004 and 2014. The database contains information on the demographics, pathological findings, preoperative laboratory parameters and survival of consecutive patients. Inclusion criteria consisted of clear cell histology and radiological or histopathological evidence of distant metastases at the time of intervention, available preoperative NLR measurements, and no concomitant immunosuppression therapy. We chart reviewed the medical records of 81 patients following cytoreductive nephrectomy for confirmed metastatic RCC and all clinical records, including follow-up. Seventeen patients with non-clear cell RCC were excluded from the study. Consequently, the remaining 64 patients were included in the present study (Fig. 1). The Institutional Review Board approved the study.
Figure 1.
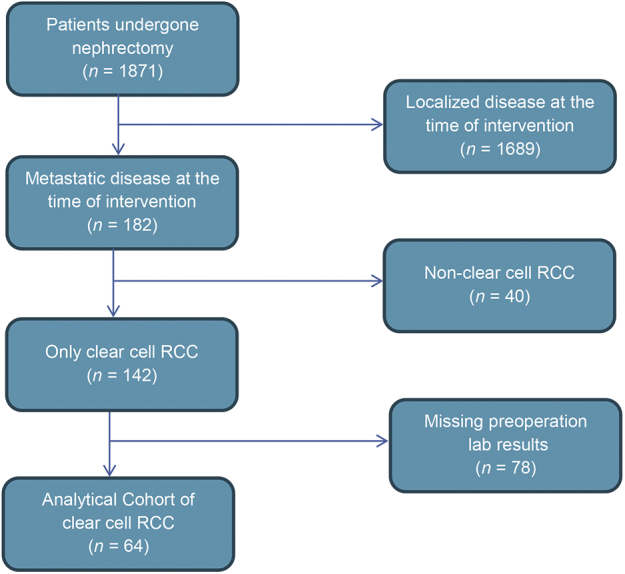
Flow chart of patients who met study inclusion/exclusion criteria. RCC, renal cell carcinoma.
2.2. Clinical and laboratory assessment
The clinical variables recorded included age, gender, race, ethnicity, date of intervention, surgical approach (open vs. laparoscopic), Eastern Cooperative Oncology Group (ECOG) performance status, body mass index (BMI), obesity (BMI ≥ 30 kg/m2), neutrophil count, lymphocyte count, preoperative NLR. NLR within 1 month prior to the intervention was used for analysis. All the clinicopathologic data were retrieved from medical records of the Department of Urology, as well as from the pathology reports from the Department of Pathology at Emory University Hospital.
The pathologic features studied included histologic subtype classified according to the Union for International Cancer Control, American Joint Committee on Cancer, and Heidelberg guidelines, tumor size, the 2009 primary tumor and regional lymph node classifications, nuclear grade, tumor necrosis, and sarcomatoid differentiation. Staging was initially based on six stages (T1a, T1b, T2, T3a, T3b, and T3c). However, one-way analysis of variance demonstrated no significant difference in outcomes between T1a and T1b and between T3a, T3b and T3c. Therefore, patients were divided into two groups based on low and high T-stages: T1–2 and T3–4, respectively.
Lymph node dissection was performed at the discretion of the treating surgeon at the time of nephrectomy. Dates of death were obtained from United States National death index (http://www.cdc.gov/nchs/ndi.htm), as well as from hospital medical records and the hospital tumor registry. Overall survival (OS) time was defined as time (in months) from the date of surgery to the individual's death from any cause.
2.3. Outcome measures
OS was the primary endpoint. Metastases were diagnosed radiologically using computerized tomography and/or magnetic resonance imaging. Postoperative clinical evaluations were generally performed every 3–6 months for the first 5 years at the discretion of the treating physician and then annually.
2.4. Statistical analysis
The primary study endpoint was OS, calculated as the months from the date of the surgery to the date of death from any cause. Cox proportional analyses were used to find the ideal cut-off value for the continuous NLR by testing all possible cut-offs that would discriminate between survival and death from any cause, which was then rounded to clinically relevant values. The relationship between NLR and other clinicopathologic parameters was tested with nonparametric tests. Kaplan–Meier method and log–rank test were used to test the clinical endpoints. Variables in univariate analysis with p ≤ 0.1 were included in multivariate analysis. We decided a priori to include age, T stage, and grade in the multivariate analysis, irrespective of its significance in univariate analysis, as these important factors are used very often in prognostication schemes. Backward stepwise multivariate Cox proportional analysis was performed to determine the influence of pathologic T stage, grade, age, gender, and tumor necrosis on OS after the models were assessed for co-linearity and interactions. Hazard ratio (HR) estimated from Cox analysis was reported as relative risks with corresponding 95% confidence intervals (CI). All statistical analysis was performed using SAS v9.3 (SAS Institute, Cary, NC, USA). Statistical significance in this study was set at p < 0.05.
3. Results
Sixty-four patients were included in the analysis. The study population was 78.13% Caucasian and 72.58% males. Median age at the time of surgery is 60.8 year (38.2–81.2). About 23.44% of the patients had BMI greater than 30 kg/m2. Pathologic T stage was pT1–T2 in 9 (14.06%) and pT3–T4 in 55 (85.94%) of the patients while Fuhrman tumor grading was G1–G3 in 32 (50.00%) and G4 in 32 (50.00%) of patients. Overall, histologic necrosis was noted in 31 patients. The median neutrophil count was 5.608 × 109/L, median lymphocyte count was 1.659 × 109/L and median NLR was 3.5 (min–max 1.4–31.0). Patients' demographic and pathological characteristics are summarized in Table 1. Cox proportional analysis of NLR as a dichotomous variable confirmed a cut point of 4 as the strongest prognostic value in our dataset. Therefore, this level was chosen for further analysis. At baseline, 26 (40.6%) patients had NLR greater than or equal to 4, whereas 38 (59.4%) patients had NLR lower than 4. Most of the baseline characteristics did not significantly differ according to these groups, apart from the significant association of histologic sarcomatoid change in the tumor with a higher NLR. Of the 64 patients included in the analysis, sunitinib, temsirolimus, pazopanib, sorafenib and bevacizumab were given to 21 (32.8%), 8 (12.5%), 6 (9.3%), 5 (7.8%) and 3 (4.7%) patients, respectively. Two (3.1%) patients received interleukin-2, 1 (1.6%) patient received axitinib and 1 (1.6%) patient was given carboplatin/paclitaxel. The other 17 (26.5%) patients had no post-cytoreductive nephrectomy therapy due to patient preference or reasons not recorded in the clinical chart.
Table 1.
Clinical and pathological parameters in patients with metastatic ccRCC and low (<4) or high (≥4) NLR.
| Covariate | Level | High NLR (n = 26) | Low NLR (n = 38) | Total (n = 64) | p-Value# |
|---|---|---|---|---|---|
| Gendera | Female | 6 (24) | 11 (29.73) | 17 (27.42) | 0.620 |
| Male | 19 (76) | 26 (70.27) | 45 (72.58) | ||
| Race | Other | 5 (19.23) | 9 (23.68) | 14 (21.88) | 0.672 |
| White | 21 (80.77) | 29 (76.32) | 50 (78.13) | ||
| Age (year) | 60.6 (38.2–78.5) | 62.2 (41.1–81.2) | 60.8 (38.2–81.2) | 0.547 | |
| BMI ≥ 30 kg/m2 | No | 21 (80.77) | 28 (73.68) | 49 (76.56) | 0.511 |
| Yes | 5 (19.23) | 10 (26.32) | 15 (23.44) | ||
| T stage | pT1–T2 | 1 (3.85) | 8 (21.05) | 9 (14.06) | 0.052 |
| pT3–T4 | 25 (96.15) | 30 (78.95) | 55 (85.94) | ||
| Grade | G1–G3 | 10 (38.46) | 22 (57.89) | 32 (50.00) | 0.127 |
| G4 | 16 (61.54) | 16 (42.11) | 32 (50.00) | ||
| Necrosisa | No | 1 (5.26) | 9 (40.91) | 10 (24.39) | 0.008 |
| Yes | 18 (94.74) | 13 (59.09) | 31 (75.61) | ||
| SSIGN score | 9 (5–10) | 9 (2–12) | 9 (2–12) | 0.571 | |
| Max tumor size | 11.3 (2–16) | 9 (0.9–16.1) | 9.5 (0.9–16.1) | 0.030 | |
| Follow-up (month) | 6.9 (1.0–48.4) | 11.1 (0.1–106.3) | 8.1 (0.1–106.3) | 0.138 | |
| Neutrophil count ( × 109/L) | 8.608 (4.380–22.878) | 4.957 (2.583–14.212) | 5.608 (2.583–22.878) | <0.001 | |
| Lymphocyte count ( × 109/L) | 1238.5 (582–3230) | 1880 (738–4180) | 1659 (582–4180) | 0.004 | |
| NLR | 6.0 (4.1–31.0) | 2.7 (1.4–4) | 3.5 (1.4–31.0) | <0.001 |
Values expressed as median (min–max) and n (%) # Chi-square and Wilcoxon sum rank test.
NLR, neutrophil-to-lymphocyte ratio; ccRCC, clear cell renal cell carcinoma; SSIGN, tumor stage, size, grade and necrosis.
Numbers may not add up due to missing data.
Survival analysis was conducted to investigate if NLR is associated with clinical outcome of metastatic clear cell RCC. Median follow-up time from the surgery was 8.1 months. Thirty-five patients died during the follow-up period. Univariate analysis identified an NLR of 4 as a significant prognosticator of poor outcome for patient OS (p = 0.017). Since there were only a small number of patients with low grade, low stage (pathologic T1–T2, low histologic grade, no necrosis) disease, these variables cannot be significantly associated with OS. One-year survival rate for the cohort was 49.0% ± 11.0%, while 3-year survival rate was 17.5% ± 10.5%.
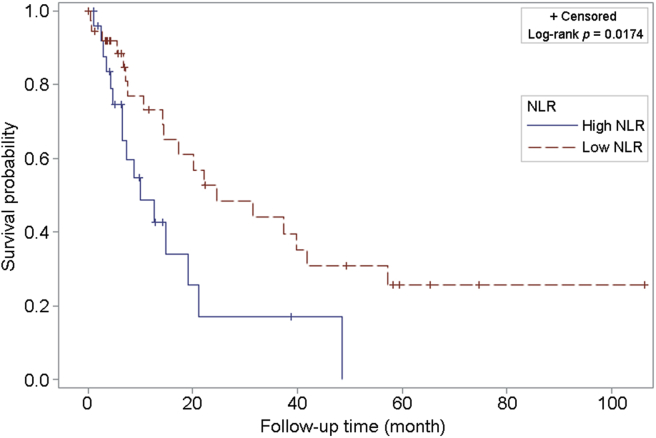
To determine the independent prognostic significance of the NLR for OS, we a priori decided to include pathologic T stage, histologic grade of the tumor, and age at surgery, as covariates for multivariable survival analysis using Cox proportional hazards model. In our multivariate analysis, only NLR of ≥4 was found to be significantly and independently associated with OS (HR = 2.41; 95%CI 1.05–5.50; p = 0.036) (Table 2). Kaplan–Meier curves stratified by low and high NLR group are shown in Fig. 2.
Table 2.
Univariate and multivariate regression models.
| Univariate |
Multivariate |
|||
|---|---|---|---|---|
| HR (95%CI) | p-Value | HR (95%CI) | p-Value | |
| NLR ≥ 4 | 2.29 (1.14–4.63) | 0.017 | 2.41 (1.05–5.50) | 0.036 |
| Age ≥ 65 years | 0.89 (0.46–1.74) | 0.744 | 0.74 (0.34–1.59) | 0.448 |
| Gender | 2.59 (1.10–6.09) | 0.024 | 2.33 (0.90–6.02) | 0.079 |
| Tumor stage | 1.68 (0.65–4.35) | 0.281 | 0.98 (0.34–2.79) | 0.978 |
| Grade | 1.00 (0.51–1.95) | 0.996 | 1.30 (0.63–2.65) | 0.469 |
NLR, neutrophil-to-lymphocyte ratio.
Figure 2.
Kaplan–Meier curve for overall survival among patients with metastatic clear cell RCC with high (≥ 4) vs. low (< 4) NLR. NLR, neutrophil-to-lymphocyte ratio; RCC, renal cell carcinoma.
4. Discussion
The outcome of patients with RCC is remarkably heterogeneous, and its diagnosis at an early stage remains a significant challenge. Up to 30% of patients with localized kidney cancer will experience recurrence. These patients are typically treated with non-curative therapies such as targeted therapies including tyrosine kinase inhibitor (TKI), mammalian target of rapamycin inhibitor (mTORi) and recently targeted immunotherapy. Despite recent progress in the identification of genetic and molecular alterations, the routine diagnostic and prognostic assessment of RCC currently relies on pathological tissue examination and traditional clinicopathological prognostic variables. Therefore, numerous studies have focused on identifying various objective measures, for both diagnostic as well as prognostic use, to define risk groups for preoperative patient counseling and postoperative surveillance strategies in patients with metastatic RCC [12], [13], [14], [15], [16].
In 1999, Motzer et al. [17] utilizing a series of 670 patients treated with a wide range of therapy regimens developed a prognostic model based on the absence of prior nephrectomy and a series of factors including Karnofsky performance status, serum lactate dehydrogenase, hemoglobin, and corrected serum calcium. However that was during the era before targeted therapy. Today, patients are treated with targeted therapy worldwide. Therefore, in 2009 and then in 2014, Heng et al. [18], [19] published a new prognostic model in the new environment for clinical trials development and patient care in patients with metastatic RCC. The model was partially based on the MSKCC (Memorial Sloan-Kettering Cancer Center) criteria [20] which included hemoglobin, corrected serum calcium, Karnofsky performance status and less than 1 year between initial diagnosis and initiation of therapy. Additionally, absolute neutrophil and platelets count were added to the model. They showed that patients who underwent a cytoreductive nephrectomy had a higher OS respect to patients not receiving a cytoreductive nephrectomy (20.6 vs. 9.6 months) and it was associated to a better progression-free survival (HR = 0.75; 95%CI = 0.66–0.85; p = 0.001). The Heng criteria have been used and validated by many other groups [21], [22], [23], [24]. While these criteria are of significant value, it is possible that even simpler models can be implemented in the prognostication of metastatic RCC patients, especially in busy clinical environments.
The systemic inflammatory response, such as C-reactive protein, neutrophil, and platelet count, has been shown to be independent predictors of clinical outcome of various cancer types including gastrointestinal, soft tissue sarcoma, nasopharyngeal and lung cancer. Of those inflammatory-based parameters, an increased NLR has been proposed as an easily calculated, accessible to almost all clinicians, reliable, and low-cost marker to predict cancer survival [6]. Previous groups have examined pretreatment NLR in RCC patients. In a large European cohort of 678 patients with non-metastatic clear cell RCC, Pichler et al. [25] demonstrated that an increased NLR was an independent negative predictor for patient OS (HR = 1.59; 95%CI = 1.10–2.31; p = 0.014), but not a predictor for direct cancer-related end points such as cancer specific survival (CSS) (HR = 1.59; 95%CI = 0.84–2.99; p = 0.148) and metastatic-free survival. Our study differs from Pichler et al. [25] because it focuses purely on metastatic patients. In 2010 and 2012, Ohno et al. [26], [27] demonstrated the prognostic role of pre- and post-treatment NLR in localized and metastatic RCC patients who underwent radical nephrectomy and the association between postoperative NLR and recurrence-free survival. Recently, Santoni et al. [28] demonstrated in a retrospective analysis of 97 patients with metastatic RCC that pre-treatment NLR was an independent prognostic factor (HR = 2.27; 95%CI = 1.57–5.57; p < 0.001) as well as increased pre-treatment NLR was significantly associated with worse progression-free survival and OS in the overall population and in the cohorts of patients treated with second- or third-line everolimus after vascular endothelial growth factor receptor-tyrosine kinase inhibitor (VEGFR-TKI) therapy. Our study showed the same conclusions regardless of the post-cytoreductive nephrectomy treatment. In addition, Kobayashi et al. [29] showed in a prospective study that changes in NLR during the early phase of targeted therapy may be a strong discriminator of who will benefit from subsequent treatment with targeted therapy. Patients who sustained low NLR at their baseline during the initial course of treatments could expect a more favorable outcome in the sequential targeted therapy.
We have shown that preoperative measurement of NLR is an independent prognostic factor of outcome of patients who have metastatic renal cancer undergoing cytoreductive nephrectomy. That can be incorporated in counseling about expected outcome as well as consideration of new clinical trial design base upon systemic inflammatory markers.
This calculation can be used in real-time clinical practice to help patients and health care providers to make a decision proceeding with surgical therapy.
To our knowledge, there is no study that analyzes predictive associations of NLR with overall mortality in patients with metastatic clear cell RCC, regardless of the patient's postoperative treatment. Multivariate survival analysis showed that preoperative NLR ≥ 4 was independently and significantly associated with an increased risk of overall mortality in patients with metastatic clear cell RCC undergoing cytoreductive nephrectomy. In univariate and multivariate analysis, we were not able to show independent prognostic effect of pathologic T stage and grade because of the minimal number of patients in lower stage and grade. Additional studies should focus on the reliable and cost-effective prognostic markers.
Nonetheless, there are limitations to this study. First, this is a retrospective study, which is susceptible to bias in data selection and analysis. It is a small cohort as it included patients with metastatic clear cell RCC only. This study warrants validation in large multicenter trials. No data were available about the cause of death for calculations of CSS, but given the overall diagnosis of metastatic RCC, it is likely almost all patients would have died of RCC. In addition, the effect of specific targeted therapy was not investigated in this study. The patients were treated with a large variety of therapy regimens. All of those are modern targeted therapy treatments. While heterogeneous array of therapies is a study limitation, this study represents a real-time, busy clinical practice with a diverse patient population regardless of postoperative therapy. The strength of this study is that we have only focused on patients with metastatic RCC, while other studies have had a focus on localized disease.
5. Conclusion
In conclusion, preoperative NLR appears to be an independent survival predictor in patients with metastatic RCC undergoing cytoreductive nephrectomy. A high serum level of NLR is associated with poor prognosis. Ultimately, the results suggest that preoperative NLR may be useful in patients risk stratification, postoperative surveillance, patient counseling and planning therapy strategies [15].
Conflicts of interest
The authors declare no conflict of interest.
Footnotes
Peer review under responsibility of Shanghai Medical Association and SMMU.
References
- 1.Siegel R., Naishadham D., Jemal A. Cancer statistics. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Ramsey S., Lamb G.W., Aitchison M., McMillan D.C. Prospective study of the relationship between the systemic inflammatory response, prognostic scoring systems and relapse-free and cancer-specific survival in patients undergoing potentially curative resection for renal cancer. BJU Int. 2008;101:959–963. doi: 10.1111/j.1464-410X.2007.07363.x. [DOI] [PubMed] [Google Scholar]
- 3.Klatte T., Lam J.S., Shuch B., Belldegrun A.S., Pantuck A.J. Surveillance for renal cell carcinoma: why and how? when and how often? Urol Oncol. 2008;26:550–554. doi: 10.1016/j.urolonc.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 4.Fuhrman S.A., Lasky L.C., Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol. 1982;6:655–663. doi: 10.1097/00000478-198210000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Magera J.S., Leibovich B.C., Lohse C.M., Sengupta S., Cheville J.C., Kwon E.D. Association of abnormal preoperative laboratory values with survival after radical nephrectomy for clinically confined clear cell renal cell carcinoma. Urology. 2008;71:278–282. doi: 10.1016/j.urology.2007.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roxburgh C.S., McMillan D.C. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010;6:149–163. doi: 10.2217/fon.09.136. [DOI] [PubMed] [Google Scholar]
- 7.Downs T.M., Schultzel M., Shi H., Sanders C., Tahir Z., Sadler G.R. Renal cell carcinoma: risk assessment and prognostic factors for newly diagnosed patients. Crit Rev Oncol Hematol. 2009;70:59–70. doi: 10.1016/j.critrevonc.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atzpodien J., Royston P., Wandert T., Reitz M. DGCIN – German Cooperative Renal Carcinoma Chemo-Immunotherapy Trials Group. Metastatic renal carcinoma comprehensive prognostic system. Br J Cancer. 2003;88:348–353. doi: 10.1038/sj.bjc.6600768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donskov F., von der Maase H. Impact of immune parameters on long-term survival in metastatic renal cell carcinoma. J Clin Oncol. 2006;24:1997–2005. doi: 10.1200/JCO.2005.03.9594. [DOI] [PubMed] [Google Scholar]
- 10.Keizman D., Ish-Shalom M., Huang P., Eisenberger M.A., Pili R., Hammers H. The association of pre-treatment neutrophil to lymphocyte ratio with response rate, progression free survival and overall survival of patients treated with sunitinib for metastatic renal cell carcinoma. Eur J Cancer. 2012;48:202–208. doi: 10.1016/j.ejca.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leibovich B.C., Blute M.L., Cheville J.C., Lohse C.M., Frank I., Kwon E.D. Prediction of progression after radical nephrectomy for patients with clear cell renal cell carcinoma. Cancer. 2003;97:1663–1671. doi: 10.1002/cncr.11234. [DOI] [PubMed] [Google Scholar]
- 12.Johnson T., Abbasi A., Owen-Smith A., Young A., Ogan K., Pattaras J. Absolute preoperative C-reactive protein predicts metastasis and mortality in the first year following potentially curative nephrectomy for clear cell renal cell carcinoma. J Urol. 2010;184:1570–1571. doi: 10.1016/j.juro.2009.10.014. [author reply 1571] [DOI] [PubMed] [Google Scholar]
- 13.Hannisdal E., Bostad L., Grøttum K., Langmark F. Erythrocyte sedimentation rate as a prognostic factor in renal cell carcinoma. Eur J Surg Oncol. 1989;15:333–336. [PubMed] [Google Scholar]
- 14.Sengupta S., Lohse C.M., Cheville J.C., Leibovich B.C., Thompson R.H., Webster W.S. The preoperative erythrocyte sedimentation rate is an independent prognostic factor in renal cell carcinoma. Cancer. 2006;106:304–312. doi: 10.1002/cncr.21617. [DOI] [PubMed] [Google Scholar]
- 15.Kawai Y., Matsuyama H., Korenaga Y., Misumi T., Eguchi S., Hara T. Preoperative erythrocyte sedimentation rate is an independent prognostic factor in Japanese patients with localized clear cell renal cell carcinoma. Urol Int. 2009;83:306–310. doi: 10.1159/000241673. [DOI] [PubMed] [Google Scholar]
- 16.Cross B.W., Johnson T.V., DeRosa A.B., Ogan K., Pattaras J.G., Nieh P.T. Preoperative erythrocyte sedimentation rate independently predicts overall survival in localized renal cell carcinoma following radical nephrectomy. Int J Surg Oncol. 2012;2012:524981. doi: 10.1155/2012/524981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Motzer R.J., Mazumdar M., Bacik J., Berg W., Amsterdam A., Ferrara J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. 1999;17:2530–2540. doi: 10.1200/JCO.1999.17.8.2530. [DOI] [PubMed] [Google Scholar]
- 18.Heng D.Y., Xie W., Regan M.M., Warren M.A., Golshayan A.R., Sahi C. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor–targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27:5794–5799. doi: 10.1200/JCO.2008.21.4809. [DOI] [PubMed] [Google Scholar]
- 19.Heng D.Y., Wells J.C., Rini B.I., Beuselinck B., Lee J.-L., Knox J.J. Cytoreductive nephrectomy in patients with synchronous metastases from renal cell carcinoma: results from the international metastatic renal cell carcinoma database consortium. Eur Urol. 2014;66:704–710. doi: 10.1016/j.eururo.2014.05.034. [DOI] [PubMed] [Google Scholar]
- 20.Motzer R.J., Bacik J., Murphy B.A., Russo P., Mazumdar M. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol. 2002;20:289–296. doi: 10.1200/JCO.2002.20.1.289. [DOI] [PubMed] [Google Scholar]
- 21.Escudier B., Eisen T., Porta C., Patard J.J., Khoo V., Algaba F. Renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl. 7) doi: 10.1093/annonc/mds227. vii65-71. [DOI] [PubMed] [Google Scholar]
- 22.Choueiri T.K. Clinical treatment decisions for advanced renal cell cancer. J Natl Compr Canc Netw. 2013;11:694–697. doi: 10.6004/jnccn.2013.0204. [DOI] [PubMed] [Google Scholar]
- 23.Manola J., Royston P., Elson P., McCormack J.B., Mazumdar M., Négrier S. Prognostic model for survival in patients with metastatic renal cell carcinoma: results from the international kidney cancer working group. Clin Cancer Res. 2011;17:5443–5450. doi: 10.1158/1078-0432.CCR-11-0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finley D.S., Pantuck A.J., Belldegrun A.S. Tumor biology and prognostic factors in renal cell carcinoma. Oncologist. 2011;16(Suppl. 2):4–13. doi: 10.1634/theoncologist.2011-S2-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pichler M., Hutterer G., Stoeckigt C., Chromecki T., Stojakovic T., Golbeck S. Validation of the pre-treatment neutrophil–lymphocyte ratio as a prognostic factor in a large European cohort of renal cell carcinoma patients. Br J Cancer. 2013;108:901–907. doi: 10.1038/bjc.2013.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohno Y., Nakashima J., Ohori M., Hatano T., Tachibana M. Pretreatment neutrophil-to-lymphocyte ratio as an independent predictor of recurrence in patients with nonmetastatic renal cell carcinoma. J Urol. 2010;184:873–878. doi: 10.1016/j.juro.2010.05.028. [DOI] [PubMed] [Google Scholar]
- 27.Ohno Y., Nakashima J., Ohori M., Gondo T., Hatano T., Tachibana M. Followup of neutrophil-to-lymphocyte ratio and recurrence of clear cell renal cell carcinoma. J Urol. 2012;187:411–417. doi: 10.1016/j.juro.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 28.Santoni M., De Giorgi U., Iacovelli R., Conti A., Burattini L., Rossi L. Pre-treatment neutrophil-to-lymphocyte ratio may be associated with the outcome in patients treated with everolimus for metastatic renal cell carcinoma. Br J Cancer. 2013;109:1755–1759. doi: 10.1038/bjc.2013.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kobayashi M., Kubo T., Komatsu K., Fujisaki A., Terauchi F., Natsui S. Changes in peripheral blood immune cells: their prognostic significance in metastatic renal cell carcinoma patients treated with molecular targeted therapy. Med Oncol. 2013;30:556. doi: 10.1007/s12032-013-0556-1. [DOI] [PubMed] [Google Scholar]