Abstract
When compared with maintenance dialysis, renal transplantation affords patients with end-stage renal disease better long-term survival and a better quality of life. Approximately 9% of patients will develop a major urologic complication following kidney transplantation. Ureteral complications are most common and include obstruction (intrinsic and extrinsic), urine leak and vesicoureteral reflux. Ureterovesical anastomotic strictures result from technical error or ureteral ischemia. Balloon dilation or endoureterotomy may be considered for short, low-grade strictures, but open reconstruction is associated with higher success rates. Urine leak usually occurs in the early postoperative period. Nearly 60% of patients can be successfully managed with a pelvic drain and urinary decompression (nephrostomy tube, ureteral stent, and indwelling bladder catheter). Proximal, large-volume, or leaks that persist despite urinary diversion, require open repair. Vesicoureteral reflux is common following transplantation. Patients with recurrent pyelonephritis despite antimicrobial prophylaxis require surgical treatment. Deflux injection may be considered in recipients with low-grade disease. Grade IV and V reflux are best managed with open reconstruction.
Keywords: Renal transplantation, Ureteral stricture, Ureteral obstruction, Urine leak, Vesicoureteral reflux
1. Introduction
Annually, more than 40 billion dollars are spent in the United States treating end-stage renal disease (ESRD) [1]. Over 870,000 individuals suffer from the disease, with approximately 400,000 patients managed by dialysis and 172,553 people living with functioning renal allografts [1]. Compared to dialysis, renal transplantation affords patients dramatically improved 5-year survival rates (85.5% vs. 35.8%), while costing the health care system nearly three times less [1].
Although kidney transplantation is associated with significant survival and cost benefits, urologic complications after surgery do occur. In 2002, Streeter and colleagues [2] reported an overall major urologic complication rate of 9.2% following 1535 consecutive renal transplants. Ureteral complications were most common with urine leak and obstruction occurring in 2.9% and 3.0% of recipients, respectively. A more recent series of 1670 consecutive transplants published in 2015 found a urologic complication rate of 8% [3]. Urine leak occurred in 1.8% of men and 4% of women, while ureteral stricture formation was observed in 2.4% of male and 1.2% of female recipients. Vesicoureteral reflux (VUR) following transplantation is common with an incidence ranging from 50% to 86% [4], [5].
Here we review the etiology, clinical presentation, diagnostic work-up and management of ureteral complications following renal transplantation. Pertinent studies are discussed and recommendations are provided to help guide treatment decisions.
2. Evidence acquisition
A literature search was performed using the PubMed database and the terms “transplant”,“ureteral stricture”, “ureteral obstruction”, “urine leak”, and “vesicoureteral reflux”. Case reports and non-English manuscripts were excluded. Full text case series and their references were reviewed. When feasible, data were combined from multiple series. However, a formal meta-analysis was not possible due to the limited and heterogeneous data available.
3. Evidence synthesis
3.1. Ureteral obstruction
Approximately 1%–4.5% of renal transplant recipients will develop ureteral obstruction at some time after surgery [2], [3], [6]. Distal obstruction is most common. Ureteral devascularization leading to intrinsic stricture formation is the principle cause in nearly 90% of cases [7]. Technical errors during the ureteroneocystostomy, extrinsic compression (e.g., hematoma, lymphocele, abscess), kinking of a redundant ureter, collecting system hematoma, a stone transplanted with the kidney, and anastomotic edema can be causes of obstruction during the early postoperative (<3 months) period. Late obstruction (>3 months) usually results from ureteral ischemia, but vasculitis secondary to acute rejection, lymphocele, fibrosis from immunosuppressant medications, and ureterolithiasis may also occur.
A variety of recipient, donor and operative details have been evaluated as predictors of ureteral obstruction following transplantation [8]. Allografts with more than two renal arteries and from donors older than 65 years of age are at increased risk [9]. The authors theorized that multiple renal arteries might correlate with “insufficient inferior pole perfusion, producing relative ischemia to the ureter”. Prolonged ischemia time and ureteroneocystostomy without ureteral stent placement have also been correlated with stricture formation [8]. Operative parameters not associated with obstruction include retrieval modality (open versus laparoscopic), preservation of the gonadal vessels during donation, and reimplantation technique (intra- vs. extravesical) [8].
A kidney transplant recipient rarely develops symptoms of ureteral obstruction unless urinary tract reconstruction was done by pyeloureterostomy or ureteroureterostomy to the native ureter because the renal allograft is denervated. As a result, recipients typically present with an asymptomatic decline in renal function and a decrease in urine output. Less commonly, patients will complain of a dull ache or feeling of fullness over the allograft due to irritation of adjacent peritoneum.
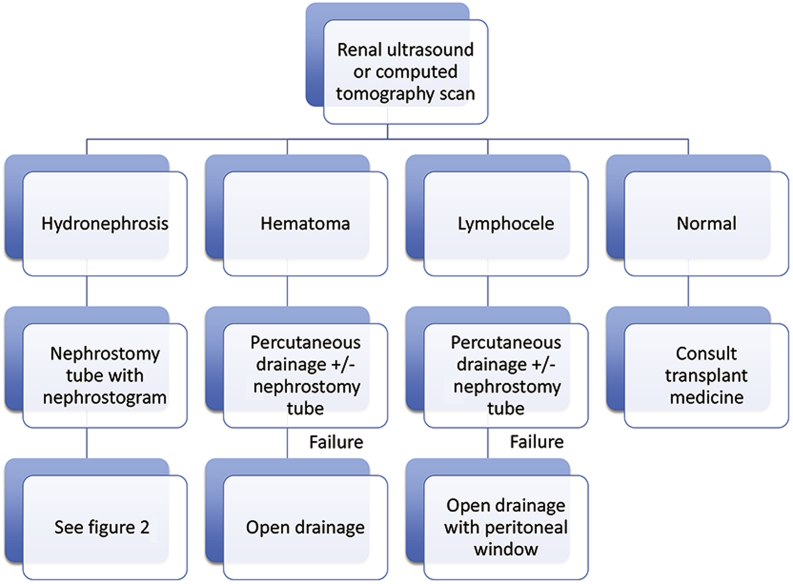
Transplant recipients presenting with an acute decline in renal function should undergo anatomic imaging (Fig. 1). Renal ultrasonography (US) is an excellent study to screen for hydroureteronephrosis. If present, computed tomography (CT) should be considered, especially in the early postoperative period, because of its ability to identify both intrinsic (e.g., ureteral calculus) and extrinsic (e.g., lymphocele, hematoma) sources of obstruction. Collecting system dilation without a clear etiology should prompt a nuclear medicine scan with furosemide washout to confirm the presence of obstruction (Tip: be certain the bladder is empty during the study). Hydroureteronephrosis without blockage is concerning for VUR and a voiding cystourethrogram (VCUG) should be done.
Figure 1.
Work-up and management of acute renal insufficiency after transplantation.
Decompression of the collecting system to minimize allograft injury is the initial priority in recipients with ureteral obstruction. Both ureteral stent and nephrostomy tube placement are options. However, ureteral stent placement is often challenging due to the anterolateral location of the ureteroneocystostomy, anastomotic edema, ureteral tortuosity, and distal obstruction. Consequently, it is the authors' opinion that percutaneous nephrostomy tube placement should be considered first-line treatment in most recipients.
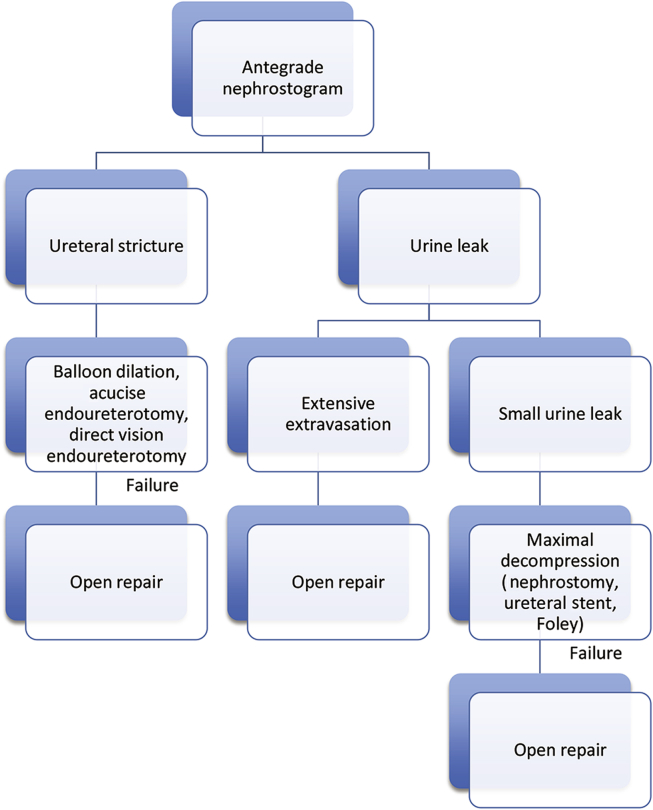
Following decompression, the cause of obstruction must be fully characterized unless it is obvious (e.g., ureteral stone). Antegrade pyelography during nephrostomy tube placement is usually diagnostic (Fig. 2). The location and severity of urine leaks and ureteral strictures can often be defined. On occasion combined antegrade and retrograde contrast studies will be required to accurately determine stricture length. Pyelography should be delayed in patients presenting with a febrile urinary tract infection (UTI) until the infection has resolved.
Figure 2.
Work-up and management of transplant ureteral complications.
Obstruction from blood clots within the collecting system and edema of the ureteroneocystostomy will resolve with conservative management. Clot obstruction is usually apparent on noncontrast CT as high-density fluid (60–90 Housfield Units) within the collecting system and filling defects on antegrade pyelography. Nephrostomy tube drainage is continued until urokinase has lysed the hematoma and the patient's hematuria has resolved. The nephrostomy tube is then capped and removed 48 to 72 h later if the urine output remains appropriate and there is no decline in renal function. A similar strategy may be employed for obstruction due to anastomotic edema.
Fluid collections that cause extrinsic ureteral obstruction should be drained percutaneously. The aspirate is sent for cell count, gram stain, culture, and creatinine determination. A fluid creatinine well above the serum level is consistent with aurinoma. Management of urinary extravasation is detailed below. Small lymphoceles may resolve following percutaneous drainage but conservative management fails in over 50% of recipients [10]. Improved success rates following sclerosis of the lymphocele cavity with doxycycline, ethanol, povidone-iodine, bleomycin, talc and fibrin glue have been reported, but periureteral fibrosis is a potential risk. Persistent or recurrent lymphoceles are treated with either laparoscopic or open unroofing into the peritoneal cavity. Success rates with both techniques exceed 88% [11]. However, laparoscopic drainage is usually not feasible for lymphoceles lateral to the renal allograft [12].
Depending upon the length of the involved segment, ureteral strictures have traditionally been managed by open ureteroneocystostomy or more complex reconstructive procedures. In select cases, endourologic techniques have been utilized in an effort to spare patients the potential morbidity of open reconstruction. Treatment options include ureteral balloon dilation and endoureterotomy. Endoureterotomy may be performed with a cold knife, Acucise (Applied Medical Systems, Laguna Hill, CA, USA) balloon-cutting catheter or Holmium laser. Ureteral balloon dilation and Acucise endoureterotomy are performed under fluoroscopic guidance making them ideal for both antegrade and retrograde treatment. Cold knife and laser endoureterotomy require endoscopic visualization of the strictured ureteral segment. As a result, treatment of distal strictures frequently requires an antegrade approach.
Multiple contemporary series have been published evaluating the efficacy of primary balloon dilation [13], [14], [15], [16]. These studies included a total of 94 patients with a mean follow-up of 37.3 months (range 17–78 months). Their overall success rate was 51% (range 44%–62%) following initial treatment but declined to 25% with repeat dilation. One series found improved success if treatment was performed within 3 months of transplantation (74% vs. 44%) [16].
First reported in 1993, the Acucise balloon-cutting device has been primarily used to treat ureteropelvic junction obstructions. Several small series have detailed its use for post-transplant ureteral strictures [17], [18], [19], [20]. A total of 21 patients were treated with a mean follow-up of 19 months (range 13–27 months) and an overall success rate of 78% (range 60%–100%). Although success rates are better when compared to primary balloon dilation, the Acucise device is rarely used because of the bleeding risk associated with blind ureteral incision [21].
Direct vision endoureterotomy affords patients improved success rates when compared to balloon dilation, and decreases the risk of perioperative hemorrhage associated with blind ureteral incision. Several series have been published with a mean success rate and follow-up of 79% (range 63%–100%) and 29 months, respectively [6], [22], [23], [24], [25]. It should be highlighted that these series are small and heterogeneous. They include patients treated by the cold knife, electrocautery and Holmium laser techniques.
3.2. Urinary leak/urinary fistula
Urine leak is the most common early urologic complication following renal transplantation, with an incidence ranging from 1.2% to 8.9% [2], [3], [26]. Devascularization of the distal ureter during organ harvest is the primary risk factor for this complication. Poor construction of the ureteroneocystostomy may also result in a non-watertight anastomosis.
Data regarding the effectiveness of routine ureteral stent placement during transplantation to prevent urine leak are equivocal. One randomized study involving 194 transplant recipients found a benefit [27]. The incidence of urine leak in the stented group was only 1% compared to 6% in the unstented group. A larger randomized study involving 280 recipients found no significant difference between the two groups (3.5% stented vs. 6.6% unstented; p = 0.23) [28].
Patients presenting with a urine leak following transplantation are initially managed with placement of a Foley catheter and percutaneous nephrostomy tube to divert urine away from the region of extravasation. Large urinomas should be drained percutaneously to minimize the risk of infection and prevent ureteral obstruction.
An antegrade nephrostogram at the time of nephrostomy tube placement will determine the extent and location of the extravasation. If the urine leak is distal and low-volume, then conservative management can be considered. This requires maximal decompression with a nephrostomy tube, ureteral stent and bladder catheter. Periodic contrast studies are performed. The patient's nephrostomy tube and bladder catheter are removed once the leak has resolved. The ureteral stent is removed 4–6 weeks later. Close monitoring (plasma creatinine and renal US) is required following stent removal because of the risk of secondary stricture.
The effectiveness of conservative therapy has been reported in several small series [29], [30], [31], [32], [33]. An overall success rate of 62% (range 36%–87%) was reported at a mean follow-up of 35 months (range 24–67 months). However, two patients died from sepsis and three grafts were lost in one series [29].
Patients with proximal or extensive urine leaks should not be managed conservatively. Extravasation that does not resolve following maximal urinary diversion also requires exploration. Urinomas are drained, devitalized ureter is resected, and a repeat ureteral reimplantation is performed. If the residual, viable ureter is short, a bladder flap may be required. Patients with ureterocutaneous or vesicocutaneous fistula should undergo resection of the tract and omental interposition as well.
3.3. Vesicoureteral reflux
Vesicoureteral reflux following renal transplantation is common, and some authors report an incidence as high as 50%–86% [4], [5]. Reflux is largely due to surgical technique and many surgeons favor a patulous ureteroneocystostomy over a tunneled reimplant in an effort to minimize the risk of ureteral stricture.
The long-term impact of VUR on renal transplant function and pyelonephritis is unclear. A study published in 2009 compared 15 recipients with reflux to 22 without [34]. Each patient had at least one UTI in the previous year and was 2 or more years out from surgery. No difference was found in the number of infections per year, plasma creatinine levels, graft and overall patient survival between the two groups. However, the study did not include any patients with grade IV or V reflux. Jung and colleagues [35] published a similar study involving 75 transplant recipients, but 61.3% of the VUR group had grade IV or V disease. Similarly, no difference was noted in renal function or the UTI rate between the two cohorts, but follow-up was less than 1 year. A study of recipients followed up to 5 years after surgery found a higher incidence of hypertension and a trend towards an increased risk of urosepsisin VUR patients [5].
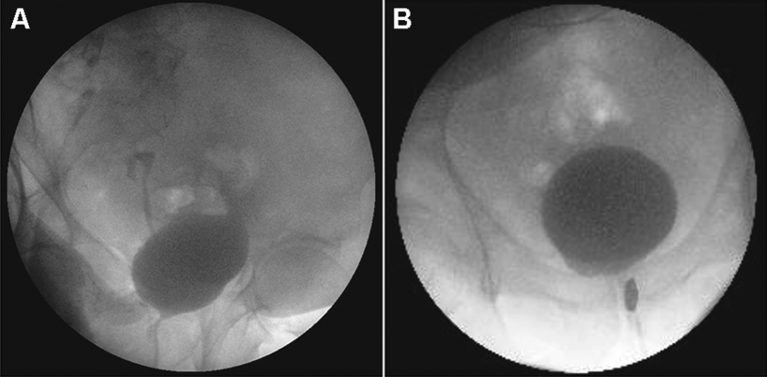
Transplant patients with recurrent UTIs, in particular pyelonephritis, should be evaluated for vesicoureteral reflux with a VCUG. Recipients with reflux should also be worked-up for voiding dysfunction. High storage pressures from reduced bladder compliance and capacity may require augmentation cystoplasty and clean intermittent catheterization. Bladder outlet obstruction is a frequent cause of secondary reflux in men. Transurethral bladder outlet procedures have been shown to be safe and effective in transplant recipients, even in the early postoperative period (Fig. 3) [36].
Figure 3.
Sixty-two years old man with end-stage renal disease from polycystic kidney disease who underwent a living unrelated renal transplant. Work-up for worsening allograft function included urodynamics and a voiding cystourethrogram (A), which demonstrating grade IV reflux and primary bladder neck obstruction. Following a transurethral incision of the bladder neck a repeat voiding cystourethrogram (B) revealed resolution of his reflux. His allograft function has remained stable.
A trial of prophylactic antibiotics may be considered in patients with low-grade reflux (grade I-III). Breakthrough infections or the diagnosis of high-grade VUR should prompt surgical therapy. A redo ureteroneocystostomy or end-to-side ureteroureterostomy to the ipsilateral native ureter may be considered. Although success rates are high with these reconstructive techniques, they are invasive and may result in an anastomotic stricture, ureteral necrosis, urine leak, and persistent reflux.
Stenberg and Lackgren [37] first reported the use of dextranomer/hyaluronic acid copolymer (Deflux) to endoscopically treat VUR in 1995. In contrast to other agents, Deflux has minimal particle migration and rarely incites an immunogenic foreign body response. Consequently, it has been used extensively to treat primary VUR in children. Success rates of up to 85% have been reported in well-selected patients [38].
In 2007 the first case series of transplant-associated VUR treated with Deflux was published [39]. The study included four women with VUR and worsening graft function. Reflux resolved following a single injection in one patient and after two treatments in two recipients. The last woman required an open ureteral reimplant.
In 2011 Pichler and colleagues [40] published their experience in a total of 19 recipients who had suffered three or more UTIs per year. Reflux resolved in 57.9% of patients after an initial injection, and this increased to 78.9% after two treatments. The authors found a reduction in the mean number of infections per year from 4.89 to 1.31. Temporary ureteral obstruction that required nephrostomy tube placement was encountered in two patients.
The largest study to date included 26 recipients with a reported overall success rate of 53.8% [41]. Success was correlated with VUR grade. Ninety percent of patients with Grade I and II disease were cured following injection compared to 31% with Grade III and IV reflux.
In summary, vesicoureteral reflux is common after renal transplantation. Its long-term impact on graft function, hypertension and recurrent UTIs has not been clearly delineated. Nonetheless, surgical management should be strongly considered in patients with worsening renal function and recurrent pyelonephritis. Deflux is usually successful in patients with Grade I and II disease, but patients with high-grade VUR are best managed with open reconstruction.
4. Conclusion
Ureteral obstruction, urine leak and vesicoureteral reflux are the most common urologic complications following kidney transplantation. Traditionally these complications have been managed by open reconstruction. Over time, advances in equipment and endourologic techniques have allowed many patients to be successfully treated without the morbidity of open repair. However, endoscopic management should be approached with caution given the limited data in the medical literature.
Conflicts of interest
The authors declare no conflict of interest.
Footnotes
Peer review under responsibility of Shanghai Medical Association and SMMU.
References
- 1.National Kidney and Urologic Diseases Information Clearinghouse . National Institutes of Health; 2012. Kidney disease statistics for the United States. Publication No. 12–3895. [Google Scholar]
- 2.Streeter E.H., Little D.M., Cranston D.W., Morris P.J. The urological complications of renal transplantation: a series of 1535 patients. BJU Int. 2002;90:627–634. doi: 10.1046/j.1464-410x.2002.03004.x. [DOI] [PubMed] [Google Scholar]
- 3.Lempinen J., Stenman J., Kyllonen L., Salmela K. Surgical complications following 1670 consecutive adult renal transplantations: a single center study. Scand J Surg. 2015 doi: 10.1177/1457496914565419. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Ostrowski M., Wlodarczyk Z., Wesolowski T., Gracz H., Sluzar T., Sienko J. Influence of ureterovesical anastomosis technique on the incidence of vesicoureteral reflux in renal transplant recipients. Ann Transplant. 1999;4:54–58. [PubMed] [Google Scholar]
- 5.Mastrosimone S., Pignata G., Maresca M.C., Calconi G., Rabassini A., Butini R. Clinical significance of vesicoureteral reflux after kidney transplantation. Clin Nephrol. 1993;40:38–45. [PubMed] [Google Scholar]
- 6.Mano R., Golan S., Holland R., Livne P.M., Lifshitz D.A. Retrograde endoureterotomy for persistent ureterovesical anastomotic strictures in renal transplant kidneys after failed antegrade balloon dilation. Urology. 2012;80:255–259. doi: 10.1016/j.urology.2012.02.030. [DOI] [PubMed] [Google Scholar]
- 7.Kumar S., Ameli-Renani S., Hakim A., Jeon J.H., Shrivastava S., Patel U. Ureteral obstruction following renal transplantation: causes, diagnosis and management. Br J Radiol. 2014;87 doi: 10.1259/bjr.20140169. 20140169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giessing M. Transplant ureter stricture following renal transplantation: surgical options. Transplant Proc. 2011;43:383–386. doi: 10.1016/j.transproceed.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 9.Fuller T.F., Deger S., Büchler A., Roigas J., Schonberger B., Schnorr D. Ureteral complications in the renal transplant recipient after laparoscopic living donor nephrectomy. Eur Urol. 2006;50:535–540. doi: 10.1016/j.eururo.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 10.Bailey S.H., Mone M.C., Holman J.M., Nelson W. Laparoscopic treatment of postrenal transplant lymphoceles. Surg Endosc. 2003;17:1896–1899. doi: 10.1007/s00464-003-8814-5. [DOI] [PubMed] [Google Scholar]
- 11.Kolonko R.K.A., Chudek J., Ziaja J., Pawlicki J., Maly A., Kunsdorf-Wnuk A. Did volume of lymphocele after kidney transplantation determine the choice of treatment modality? Transplant Proc. 2007;39:2740–2743. doi: 10.1016/j.transproceed.2007.08.039. [DOI] [PubMed] [Google Scholar]
- 12.Gruessner R.W., Fasola C., Benedetti E., Foshager M.C., Gruessner A.C., Matas A.J. Laparoscopic drainage of lymphoceles after kidney transplantation: indications and limitations. Surgery. 1995;117:288–295. doi: 10.1016/s0039-6060(05)80204-1. [DOI] [PubMed] [Google Scholar]
- 13.Bachar G.N., Mor E., Bartal G., Atar E., Goldberg N., Belenky A. Percutaneous balloon dilation for the treatment of early and late ureteral strictures after renal transplantation: long-term follow-up. Cardiovasc Intervent Radiol. 2004;27:335–338. doi: 10.1007/s00270-004-0163-9. [DOI] [PubMed] [Google Scholar]
- 14.Bromwich E., Coles S., Atchley J., Fairley I., Brown J.L., Keoghane S.R. A 4-year review of balloon dilation of ureteral strictures in renal allografts. J Endourol. 2006;20:1060–1061. doi: 10.1089/end.2006.20.1060. [DOI] [PubMed] [Google Scholar]
- 15.Aytekin C., Boyvat F., Harman A., Ozyer U., Colak T., Haberal M. Percutaneous therapy of ureteral obstructions and leak after renal transplantation: long-term results. Cardiovasc Intervent Radiol. 2007;30:1178–1184. doi: 10.1007/s00270-007-9031-8. [DOI] [PubMed] [Google Scholar]
- 16.Juaneda B., Alcaraz A., Bujons A., Guirado L., Diaz J.M., Marti J. Endourological management is better in early-onset ureteral stenosis in kidney transplantation. Transplant Proc. 2005;37:3825–3827. doi: 10.1016/j.transproceed.2005.09.199. [DOI] [PubMed] [Google Scholar]
- 17.Erturk E., Burzon D.T., Waldman D. Treatment of transplant ureteral stenosis with endoureterotomy. J Urol. 1999;161:412–414. [PubMed] [Google Scholar]
- 18.Schwartz B.F., Chathman J.R., Bretan P., Goharderakhshan R., Stoller M.L. Treatment of refractory kidney transplant ureteral strictures using balloon cautery endoureterotomy. Urology. 2001;58:536–539. doi: 10.1016/s0090-4295(01)01275-4. [DOI] [PubMed] [Google Scholar]
- 19.Seseke F., Heuser M., Zoller G., Plothe K.D., Ringert R.H. Treatment of iatrogenic postoperative ureteral strictures with Acucise endoureterotomy. Eur Urol. 2002;42:370–375. doi: 10.1016/s0302-2838(02)00322-6. [DOI] [PubMed] [Google Scholar]
- 20.Bhayani S.B., Landman J., Slotoroff C., Figenshau R.S. Transplant ureter stricture: Acucise endoureterotomy and balloon dilation are effective. J Endourol. 2003;17:19–22. doi: 10.1089/089277903321196733. [DOI] [PubMed] [Google Scholar]
- 21.el-Nahas A.R. Retrograde endopyelotomy: a comparison between laser and Acucise balloon cutting catheter. Curr Urol Rep. 2007;8:122–127. doi: 10.1007/s11934-007-0061-1. [DOI] [PubMed] [Google Scholar]
- 22.Katz R., Pode D., Gofrit O.N., Shenfeld O.Z., Landau E.H., Golijanin D. Transurethral incision of ureteroneocystostomy strictures in kidney transplant recipients. BJU Int. 2003;92:769–771. doi: 10.1046/j.1464-410x.2003.04455.x. [DOI] [PubMed] [Google Scholar]
- 23.Kristo B., Phelan M.W., Gritsch H.A., Schulam P.G. Treatment of renal transplant ureterovesical anastomotic strictures using antegrade balloon dilation with or without holmium:YAG laser endoureterotomy. Urology. 2003;62:831–834. doi: 10.1016/s0090-4295(03)00655-1. [DOI] [PubMed] [Google Scholar]
- 24.He Z., Li X., Chen L., Zeng G., Yuan J., Chen W. Endoscopic incision for obstruction of vesico-ureteric anastomosis in transplanted kidneys. BJU Int. 2008;102:102–106. doi: 10.1111/j.1464-410X.2008.07604.x. [DOI] [PubMed] [Google Scholar]
- 25.Gdor Y., Gabr A.H., Faerber G.J., Wolf J.S., Jr. Holmium:yttrium-aluminum-garnet laser endoureterotomy for the treatment of transplant kidney ureteral strictures. Transplantation. 2008;85:1318–1321. doi: 10.1097/TP.0b013e31816c7f19. [DOI] [PubMed] [Google Scholar]
- 26.Nie Z.L., Zhang K.Q., Li Q.S., Jin F.S., Zhu F.Q., Huo W.Q. Treatment of urinary fistula after kidney transplantation. Transplant Proc. 2009;41:1624–1626. doi: 10.1016/j.transproceed.2008.10.103. [DOI] [PubMed] [Google Scholar]
- 27.Benoit G., Blanchet P., Eschwege P., Alexandre L., Bensadoun H., Charpentier B. Insertion of a double pigtail ureteral stent for the prevention of urological complications in renal transplantation: a prospective randomized study. J Urol. 1996;156:881–884. [PubMed] [Google Scholar]
- 28.Dominguez J., Clase C.M., Mahalati K., MacDonald A.S., McAlisster V.C., Belitsky P. Is routine ureteric stenting needed in kidney transplantation? A randomized trial. Transplantation. 2000;70:597–601. doi: 10.1097/00007890-200008270-00011. [DOI] [PubMed] [Google Scholar]
- 29.Matalon T.A., Thompson M.J., Patel S.K., Ramos M.V., Jensik S.C., Merkel F.K. Percutaneous treatment of urine leaks in renal transplantation patients. Radiology. 1990;174(3 Pt 2):1049–1051. doi: 10.1148/radiology.174.3.174-3-1049. [DOI] [PubMed] [Google Scholar]
- 30.Campbell S.C., Streem S.B., Zelch M., Hodge E., Novick A.C. Percutaneous management of transplant ureteral fistulas: patient selection and long-term results. J Urol. 1993;150:1115–1117. doi: 10.1016/s0022-5347(17)35701-4. [DOI] [PubMed] [Google Scholar]
- 31.Swierzewski S.J., Konnak J.W., Ellis J.H. Treatment of renal transplant ureteral complications by percutaneous technique. J Urol. 1993;149:986–987. doi: 10.1016/s0022-5347(17)36274-2. [DOI] [PubMed] [Google Scholar]
- 32.Bhagat V.J., Gordon R.L., Osorio R.W., LaBerge J.M., Kerlan R.K., Jr., Melzer J.S. Ureteral obstructions and leaks after renal transplantation: outcome of percutaneous antegrade ureteral stent placement in 44 patients. Radiology. 1998;209:159–167. doi: 10.1148/radiology.209.1.9769827. [DOI] [PubMed] [Google Scholar]
- 33.Alcaraz A., Bujons A., Pascual X., Juaneda B., Marti J., de la Torre P. Percutaneous management of transplant ureteral fistulae is feasible in selected cases. Transplant Proc. 2005;37:2111–2114. doi: 10.1016/j.transproceed.2005.03.118. [DOI] [PubMed] [Google Scholar]
- 34.Favi E., Spagnoletti G., Valentini A.L., Tondolo V., Nanni G., Citterio F. Long-term clinical impact of vesicoureteral reflux in kidney transplantation. Transplant Proc. 2009;41:1218–1220. doi: 10.1016/j.transproceed.2009.03.052. [DOI] [PubMed] [Google Scholar]
- 35.Jung G.O., Chun J.M., Park J.B., Choi G.S., Kwon C.H., Joh J.W. Clinical significance of posttransplantation vesicoureteral reflux during short-term period after kidney transplant. Transplant Proc. 2008;40:2339–2341. doi: 10.1016/j.transproceed.2008.06.027. [DOI] [PubMed] [Google Scholar]
- 36.Koziolek M.J., Wolfram M., Muller G.A., Scheel A.K., Strutz F., Scheuermann E.H. Benign prostatic hyperplasia (BPH) requiring transurethral resection in freshly transplanted renal allograft recipients. Clin Nephrol. 2004;62:8–13. doi: 10.5414/cnp62008. [DOI] [PubMed] [Google Scholar]
- 37.Stenberg A., Lackgren G. A new bioimplant for the endoscopic treatment of vesicoureteral reflux: experimental and short-term clinical results. J Urol. 1995;154(2 Pt 2):800–803. doi: 10.1097/00005392-199508000-00127. [DOI] [PubMed] [Google Scholar]
- 38.Elder J.S., Diaz M., Caldamone A.A., Cendron M., Greenfield S., Hurwitz R. Endoscopic therapy for vesicoureteral reflux: a meta-analysis. I. Reflux resolution and urinary tract infection. J Urol. 2006;175:716–722. doi: 10.1016/S0022-5347(05)00210-7. [DOI] [PubMed] [Google Scholar]
- 39.Seifert H.H., Mazzola B., Ruszat R., Muller A., Steiger J., Bachmann A. Transurethral injection therapy with dextranomer/hyaluronic acid copolymer (Deflux) for treatment of secondary vesicoureteral reflux after renal transplantation. J Endourol. 2007;21:1357–1360. doi: 10.1089/end.2007.0020. [DOI] [PubMed] [Google Scholar]
- 40.Pichler R., Buttazzoni A., Rehder P., Bartsch G., Steiner H., Oswald J. Endoscopic application ofdextranomer/hyaluronic acid copolymer in the treatment of vesico-ureteric reflux after renal transplantation. BJU Int. 2011;107:1967–1972. doi: 10.1111/j.1464-410X.2010.09792.x. [DOI] [PubMed] [Google Scholar]
- 41.Yucel S., Akin Y., Celik O., Erdogru T., Baykara M. Endoscopic vesicoureteral reflux correction in transplanted kidneys: does injection technique matter? J Endourol. 2010;24:1661–1664. doi: 10.1089/end.2010.0219. [DOI] [PubMed] [Google Scholar]