Abstract
Prostate cancers (PCa) have been reported to actively suppress antitumor immune responses by creating an immune-suppressive microenvironment. There is mounting evidence that PCas may undergo an ‘‘Epithelial Immune Cell-like Transition’’ (EIT) by expressing molecules conventionally associated with immune cells (e.g., a variety of cytokines/receptors, immune transcription factors, Ig motifs, and immune checkpoint molecules), which subsequently results in the suppression of anti-cancer immune activity within the tumor microenvironment. Recent progress within the field of immune therapy has underscored the importance of immune checkpoint molecules in cancer development, thus leading to the development of novel immunotherapeutic approaches. Here, we review the expression of select immune checkpoint molecules in PCa epithelial and associated immune cells, with particular emphasis on clinical data supporting the concept of an EIT-mediated phenotype in PCa. Furthermore, we summarize current advances in anti-immune checkpoint therapies, and provide perspectives on their potential applicability.
Keywords: Prostate cancer, Immune checkpoint, Epithelial immune cell-like transition, Immune suppression, Immune therapy
1. Introduction
Prostate cancer (PCa) is the most commonly-diagnosed non-cutaneous cancer and the second leading cause of cancer-related death in North American males. Although PCas typically present as androgen-dependent cancers, and initial androgen ablation can lead to substantial remissions, they frequently return in an androgen-independent, castration-resistant form. Within the last few years, a number of new agents have been developed and approved for treatment of metastatic castration-resistant prostate cancer (mCRPC). These include second generation anti-androgen agents (abiraterone acetate and enzalutamide), cytotoxic drugs (cabazitaxel), and radiopharmaceuticals (radium-223 dichloride) [1], [2], [3], [4]. However, while these advancements have significantly improved patient survival and quality of life, and slowed disease progression, relapse invariably occurs. Therefore, there still remains an urgent need for novel therapeutic targets and agents for mCRPC.
During the past decade, immune therapy has become one of the most active and prolific areas of cancer research. Sipuleucel-T, an autologous cellular immune therapy, is the first therapeutic cancer vaccine and currently the only FDA-approved immune therapy for PCa. Recent advances have also underscored the crucial role of immune checkpoint molecules in facilitating cancer development, thus leading to numerous novel immunotherapeutic approaches. In particular, studies have demonstrated that PCa cells employ various mechanisms, including the aberrant expression of immune checkpoint molecules, in order to elude the immune system. Therefore, these insights have served to provide corroborating evidence for the “Epithelial Immune Cell-like Transition” (EIT) hypothesis [5]. In this review, we will revisit the EIT concept with regards to the expression of immune checkpoint molecules on PCa cells, with an emphasis on clinical data. Additionally, we will summarize current advances in immune checkpoint-based therapies and provide perspectives on their potential applicability.
2. EIT and PCa
Although the immune system plays an invaluable role in the elimination of cancer cells, there is mounting evidence that epithelial cancers avoid immune destruction by expressing certain immune genes and products (e.g., cytokines and immune-inhibitory molecules) not physiologically-expressed by the originating normal tissues. On the basis of these observations, we propose that the immune-suppressive activity of epithelial cancers may arise from acquired immune-suppressive characteristics via a transdifferentiation process we term EIT. These properties could enable cross-talk between cancer and immune cells, thereby facilitating evasion of immune surveillance and co-optation of immune mechanisms to promote tumor growth.
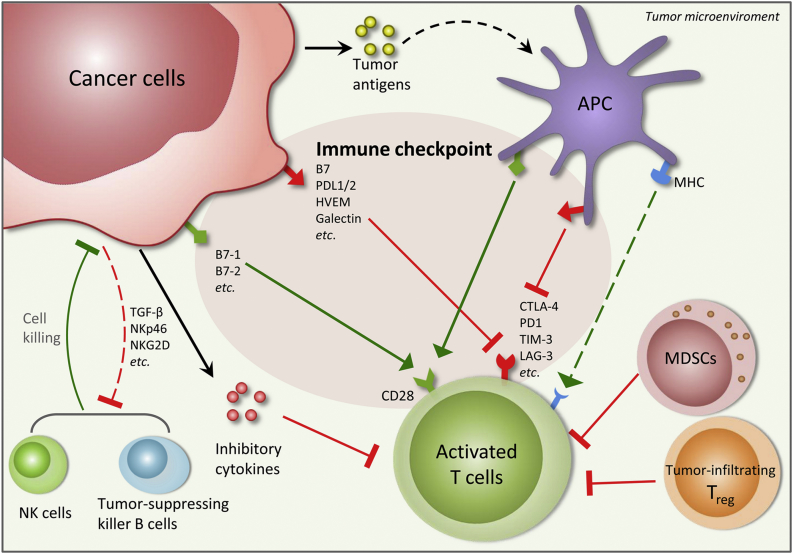
Recent reports have confirmed that PCa cells are able to use various mechanisms in order to evade the immune system (Fig. 1). Unsurprisingly, the presence of multiple immunosuppressive cell types within the PCa microenvironment has been found to be associated with a poor prognosis. In particular, an increased number of regulatory T cells (the archetypal immunosuppressive cell type), have been found within the peripheral blood of PCa patients [6], [7], [8]. Furthermore, M2 macrophages and myeloid-derived suppressor cells (MDSCs) also appear to be significant contributors in maintaining the immunosuppressive microenvironment, with multiple reports associating increased numbers with poor prognosis in PCa patients [9], [10], [11], [12]. Conversely, the abundance of cytotoxic natural killer (NK) cells within the PCa tumor could potentially confer a protective effect [13].
Figure 1.
Cross-talks between cancer and immune cells in the tumor microenvironment. Several anti-tumor immunities exist in the tumor microenvironment, including activated tumor-specific T cells induced through tumor antigen presentation and T cell receptor (TCR) recognition, natural killer (NK) cells and tumor-suppressing killer B cells. Cancer cells are able to use various mechanisms to suppress the anti-tumor immunities, e.g., by increased expression of immune checkpoint proteins, release of inhibitory cytokines, facilitation of immunosuppressive effects of tumor-infiltrating regulatory T cells and myeloid-derived suppressor cells (MDSCs) and inhibition of the NK cell activations. The immunosuppressive activities of cancer cells, which may arise from acquired immunosuppressive characteristics via a transdifferentiation process termed as “Epithelial Immune Cell-like Transition” (EIT), create a complex immunosuppressive network in the tumor microenvironment that facilitates evasion of immune surveillance and co-optates immune mechanisms to promote tumor growth. APC, antigen-presenting cell.
Mechanistically, several well-known immunosuppressive molecules have been implicated in the inhibition of cytotoxic cell functions within the PCa microenvironment. For example, the cytokine milieu of the prostate tumor was found to contain high levels of TGF-β, and in conjunction with decreased expression of activating receptors (NKp46 and NKG2D) and increased expression of the inhibitory receptor ILT2 on NK cell surfaces, suppress the cytotoxic immune response against PCa cells [14]. Furthermore, high levels of the NKG2D ligand have been found on the surfaces of PCa-derived exosomes, resulting in the downregulation of NKG2D on NK and CD8+ T cells, thus inhibiting their activation [15]. Additionally, the upregulation of CSF1 and IL1β in Pten-null prostatic epithelium have been shown to facilitate the immunosuppressive effects of MDSCs, inhibiting T cell proliferation via high levels of arginase-1 and iNOS expression [16].
Standard therapies used for the treatment of PCa have also been found to induce immune suppression. In particular, upregulation of CSF1 following radiotherapy results in an increased MDSC population in PCa patients. When a selective inhibitor of CSF1R was administered in conjunction with radiation, the treatment suppresses tumor growth more effectively than with irradiation alone. This indicates that an alleviation of the immunosuppressive tumor microenvironment can enhance anticancer therapeutic effects [17]. Additionally, androgen deprivation therapy, the current first-line treatment for metastatic PCa, has been shown to mediate immune suppression via impairment of initial T cell activation priming and IFN-γ production [18]. Furthermore, although there have been reports suggesting that concurrent immune therapy and castration could initially enhance the number and function of cytotoxic CD8+ T cells, the synergy is short lived and ultimately offset by a parallel expansion of regulatory T cells [19].
Finally, the increased expression of immune checkpoint molecules has also been observed in PCa, thus indicating an important immune-suppressive mechanism underlying PCa development. Below, we will focus on the details of expression, function, and clinical applications of these immune checkpoint molecules in PCa.
3. Immune checkpoint molecules and PCa – expression and function
3.1. PD-1/PD-L1
First described by Ishida et al. in 1992 [20], programmed death 1 (PD-1) is a transmembrane glycoprotein and T cell co-inhibitory receptor expressed on T cells, B cells, NK cells, dendritic cells (DCs), and activated monocytes [21]. As important mediators of immune suppression, PD-1 and its ligand PD-L1 are part of co-inhibitory pathways that serve to maintain peripheral tolerance by restricting the lytic activity of immune cells [22]. The immediate consequences of PD-1 stimulation are the inhibition of cytokine secretion and immune cell proliferation [23].
PD-1/PD-L1 expression within cancer cells and the surrounding microenvironment is observed to be a dynamic process. Various immune checkpoint molecules, such as PD-1, are up-regulated as T cells enter the tumor. This induces the production of IFN-γ, which results in the up-regulation of PD-L1 [24]. High levels of PD-1 expression have been demonstrated in both CD8+ prostate-infiltrating T-lymphocytes and tumor-infiltrating regulatory T cells [25], [26].
Normally, PD-L1 expression is restricted to monocytes in peripheral blood and macrophages in the lungs, liver, and tonsils [27]. However, PD-L1 has been found to be highly expressed in certain epithelial cancer cells and is correlated with negative clinical outcomes, supporting the EIT hypothesis [28]. Regarding PD-L1 expression on PCa cells, the evidence remains mixed. Several studies have showed negative staining of PD-L1 in mCRPC samples using immunohistochemistry [29], [30]. Martin et al. [31] also reported that PD-L1 is rarely expressed in primary PCas and independent of PTEN loss. On the contrary, within a cohort of 16 mCRPC patients, 50% of patients showed PD-L1 expression on PCa cells (with 19% considered high-expressing) while 56% of patients had tumor-infiltrating lymphocytes expressing PD-1 (with 19% considered high-expressing) [32]. Finally, Gevensleben et al. [33] recently evaluated PD-L1 expression using a new anti-PD-L1 antibody in two independent cohorts including 873 primary radical prostatectomy specimens (derived from treatment-naive patients) and demonstrated an increased level of PD-L1 expression (52.2% and 61.7% in the respective cohorts). This study also demonstrated that PD-L1 is an independent prognosticator of patients' disease free survival.
3.2. B7-H3
The B7 family is comprised of proteins that interact with receptors to modulate T-lymphocyte activity. Although several members of the B7 family (including PD-L1, otherwise known as B7-H1) have been well characterized, the mechanisms of other B7 ligands (with regards to their immune-stimulatory or immune-suppressive role) are still not well understood [34].
First described in 2001 by Chapoval et al. [35], B7-H3 is a type I transmembrane protein that has inducible expression on several types of immune cells, such as T cells, dendritic cells, and monocytes. In human tissue, B7-H3 exhibits varying levels of expression in the heart, liver, and prostate. Although the precise receptor(s) have yet to be identified, B7-H3 is thought to have different immune-modulatory capabilities (stimulatory or inhibitory) depending on the receptor. B7-H3 is able to exert immune stimulatory functions by promoting T cell proliferation and expression of IFN-γ, while it is able to suppress immune responses by suppressing cytokine production, type I T-helper (Th1) cell responses, and NK cell-mediated cytolysis [34], [36], [37].
Numerous studies have found strong IHC staining of B7-H3 in PCa samples (including adenocarcinomas, high-grade prostatic intraepithelial neoplasias, and PCa cell lines) [38], [39], [40], [41]. From 823 prostatectomy samples, 93% were found to have high levels of B7-H3 expression. These elevated levels of B7-H3 were correlated with metastases in addition to a higher risk of recurrence and death. Similarly, other studies have shown that B7-H3 expression on PCa cell surfaces correlate with aggressive histopathological features (such as higher Gleason scores, increased tumor volumes, extra-prostatic extensions, and infiltration of seminal vesicles) [42], proliferation markers [42], increased risk of tumor recurrence and progression [40], [42], [43], and poor outcomes [40], [41], [42], [43]. Finally, B7-H3 expression has also been found in samples of bone metastases and hormone-resistant PCa [44].
3.3. B7-H4
B7-H4 is a type I transmembrane protein and is predominantly expressed on activated T cells, B cells, dendritic cells, and monocytes. B7-H4 exerts several co-inhibitory functions, such as inhibition of T cell proliferation and cytokine production in CD4+ and CD8+ T cells [45], [46], [47], [48]. Although its receptor(s) are yet to be determined, over-expression of B7-H4 has been implicated in cancer cell growth. Tumor expression of B7-H4 has been associated with aggressive cancer features (increased neo-angiogenesis, high tumor burden, and advanced tumor stage) and poor clinical outcomes [48], [49], [50]. Notably, in both PCa and renal cell carcinoma, robust expression of B7-H4 has been related to metastatic disease, disease recurrence, and a higher risk of death due to cancer [38], [51]. A B7-H4 immunohistochemical analysis conducted by Qian et al. found diffusely positive cytoplasm and/or membrane staining in PCa samples to be correlated with higher tumor grades [52].
3.4. TIM-3
Monney et al. [53] first identified T cell immunoglobulin mucin-3 (TIM-3) in 2002 as a negative immunomodulatory molecule. TIM-3 acts in conjunction with its ligand, galectin-9 (commonly up-regulated in various types of cancers) [54], to negatively-regulate Th1 cell immunity, induce peripheral tolerance, and cause the phagocytosis of apoptotic cells [55]. Physiologically, Th1 cells and innate immune cells (e.g., dendritic cells) express TIM-3 [56]. However, recent murine studies have found co-expression of TIM-3 with PD-1 on tumor-specific CD8+ T cells, which were able to significantly inhibit effector cytokine secretion [54].
TIM-3 also supports the progression of cancer by contributing towards the immunosuppressive microenvironment via induction of T cell exhaustion [57], [58], [59]. Piao et al. [60] recently reported increased expression of TIM-3 on both CD4+ and CD8+ T cells within peripheral blood and PCa tissue specimens, with a strong correlation to characteristics associated with advanced disease stage (metastatic spread, prostate specific antigen (PSA) levels, and Gleason score). A comparison of benign and cancer tissues from 137 patients with treatment-naive PCa found an up-regulation of TIM-3 in samples with prostatic intraepithelial neoplasia and invasive carcinomas [61].
3.5. LAG3
LAG3 (lymphocyte-activation gene 3) is an inhibitory receptor with a dual function [62]. LAG3 binds to HLA class II molecules to inhibit T cell functions, particularly those of CD8+ T cells. Additionally, LAG3 can strengthen the immuno-inhibitory functions of T-regulatory cells. Elevated expression of LAG3 has been found in tumor-infiltrating antigen-presenting cells and macrophages. Further, LAG3 has been found to be upregulated in tumor cells [62]. However, this molecule remains under-evaluated with regards to PCa.
3.6. CTLA-4
Considered a prototypical immune checkpoint, cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) is a co-inhibitory receptor that restricts early-stage T cell activation [63], [64]. Structurally homologous to CD28, CTLA-4 has markedly higher affinity and avidity for the same ligands (B7-1 and B7-2) as CD28 (the main receptor needed for T cell activation). Thus, binding of CTLA-4 effectively hijacks the mechanisms that promote IL-2 mRNA production and cell cycle progression [63], [64], [65].
Although CD28 is expressed by both naïve and activated T cells, CTLA-4 is expressed by activated CD8+ effector T-cells and regulatory T cells (where interactions serve to enhance regulatory T cell immunosuppressive activity) [66]. However, unlike the previously described immune checkpoint molecules, CTLA-4 is expressed exclusively by T cells.
4. Targeting immune checkpoint molecules in PCa
4.1. PD-1/PD-L1 blockade
Although PD-1/PD-L1 blockade therapies have shown promising results in various types of cancers, the effect of these therapies in PCa remains ambiguous. In a large phase I trial for testing the safety and efficacy of nivolumab (an anti-PD1 antibody) among 296 patients with advanced solid tumors, no objective responses to single-agent PD-1 blockade were reported for any of the 17 PCa patients. This lackluster response of PCa to PD-1 blockade may be attributed to the phenotype of prostate-infiltrating lymphocytes, which are generally refractory to stimulation [67]. Another possible explanation may involve the low expression levels of PD-L1 in PCa cells, which were negative in both samples examined by the study. However, the sample size (for PCa) used in this study was too small to be sufficiently indicative, particularly since only two of the 17 tissue specimens were available for immunohistochemical staining. Therefore, further investigations into the role of anti-PD-1/PD-L1 molecules for PCa treatment, especially in larger cohorts, is necessary for determination of therapeutic efficacy.
Currently, there are a number of ongoing clinical trials testing the safety and efficacy of PD-1/PD-L1 blockade therapy as a single agent in PCa. In terms of PD-L1 blockade, a phase II trial for atezolizumab (a humanized antibody against PD-L1) is currently underway for patients with advanced solid tumors, including PCa (NCT02458638). Furthermore, a number of phase I trials are also initiated for anti-PD-L1 drugs (avelumab, nivolumab/MDX-1106) alone or in combination with other therapies (MEDI4736 and olaparib/cediranib) in multiple tumor types, including PCa (NCT01772004, NCT00730639, NCT02484404).
With regards to PD-1 blockade, pembrolizumab (an anti-PD-1 antibody) is currently under evaluation as a single agent for mCRPC patients previously-treated with enzalutamide (NCT02312557). Concurrently, several other clinical trials are assessing pembrolizumab in combination with other therapies. These include pembrolizumab in conjunction with cryosurgery (for treatment of patients with newly diagnosed, oligo-metastatic PCa [NCT02489357]), in combination with pTVG-HP plasmid DNA vaccine (in mCRPC patients [NCT02499835]), and in combination with ADXS31-142 (a Listeria monocyto-genes/PSA [Lm-LLO-PSA] vaccine [ADXS-PSA]) in pre-treated mCRPC patients (NCT02325557).
Finally, the effect of anti-PD-1 therapy in combination with other immunotherapies, specifically within PCa, is also under evaluation. A phase II trial of nivolumab and ipilimumab in AR-V7 positive mCRPC patients is assessing the efficacy of concurrent PD-1 and CTLA-4 blockade (NCT02601014). Additionally, another phase II trial is assessing the effect of CT-011 (an anti-PD-1 antibody) in combination with sipuleucel-T and cyclophosphamide in advanced mCRPC patients (NCT01420965).
4.2. B7-H3 blockade
In a phase I dose-escalation study of enoblituzumab (MGA271, an antibody targeting B7-H3) monotherapy, anti-tumor activity was observed for several tumor types, including patients with PCa, bladder cancer, and melanoma [68]. Two phase I trials are ongoing in order to evaluate the safety of enoblituzumab in combination with Keytruda (pembrolizumab) and Yervoy (ipilimumab), respectively, in patients with B7-H3-expressing cancers (NCT02475213, NCT02381314).
4.3. CTLA-4 blockade
Even though CTLA-4 is expressed exclusively by T cells, it remains one of the most well-characterized immune checkpoint molecules. As such, CTLA-4 blockade therapies are of particular significance. Notably, ipilimumab was the first anti-immune checkpoint agent approved by the FDA to treat cancer. It produced significant improvements in the overall survival of metastatic melanoma patients [69], [70]. Furthermore, a number of phase I and phase II trials have evaluated the effect of ipilimumab (as a single agent or in combination therapy) in mCRPC patients, and have demonstrated some benefits associated with its clinical use [71], [72], [73], [74]. More recently, a phase III trial assessed the efficacy of ipilimumab treatment following radiotherapy in patients with mCRPC arising from docetaxel chemotherapy. Ipilimumab was reported to significantly improve progression-free survival and PSA response. Furthermore, two other studies (phase III and phase II) have recently been completed, the results of which are expected in 2016. The first is a large, randomized phase III trial which examined the efficacy of ipilimumab in mCRPC patients who have yet to undergo chemotherapy (NCT01057810). The latter is a neoadjuvant phase II trial, which tested ipilimumab in combination with Lupron, in patients who have yet to undergo radical prostatectomy (NCT01194271). Finally, other combinatorial therapies of ipilimumab with other FDA-approved agents are also under investigation. These include two ongoing phase I/II trials evaluating the impact of ipilimumab in combination with sipuleucel-T (NCT01804465), and in combination with abiraterone acetate plus prednisone (NCT01688492), in patients with PCa.
4.4. Other checkpoint molecules (i.e., TIM-3, LAG3 blockade)
The development of TIM-3 and LAG3 blockade therapy is in its early stages. A phase I, first-in-human study of MBG453 (a TIM-3 inhibitor) has been designed to characterize the safety, tolerability, and anti-tumor activity of MBG453, as a single agent or in combination with PDR001, in patients with advanced solid tumors, including PCa. Additionally, another phase I trial is ongoing to assess the safety and tolerability of BMS-986016 (an anti-LAG3 monoclonal antibody), alone and in combination with nivolumab, in subjects with select advanced (metastatic and/or unresectable) solid tumors.
5. Conclusions and further directions
There is an increasing body of evidence that supports the hypothesis that an EIT-mediated immune-suppressive microenvironment is a critical mechanism underlying the development and progression/metastasis of PCa. Selective targeting of this acquired immune-like phenotype, in particular through certain aberrantly-expressed immune checkpoint molecules, could likely lead to effective therapeutic approaches. Although the blockade of CTLA-4 and PD-1/PD-L1 have already demonstrated significant efficacy in various types of cancers, ongoing clinical trials will clarify its applicability towards PCa. Novel therapies, targeting other immune checkpoint molecules, are currently under evaluation in patients with various stages of PCa.
The success of immune checkpoint blockade as a monotherapy likely requires a pre-existing antitumor immune response in the patient. Some studies have shown various cancer therapies to be capable of inciting or amplifying antitumor immunity. In view of this, the coupling of therapies with immune therapy may enhance its overall efficacy, on the basis of both inducing de novo antitumor immune responses and alleviating the immune suppressive environment through immune checkpoint blockade therapy. In the future, further clinical trials evaluating combinatorial immune therapy or simultaneous blockade of multiple immune checkpoint molecules, or immunotherapies in combination with other therapies (such as androgen ablation therapy, chemotherapy and radiotherapy), will demonstrate potential synergistic effects. Although this review has outlined a selection of well-documented immune checkpoint molecules, immune checkpoints are still a component of the overarching immune-suppressive mechanism. Therefore, further investigations are necessary in order to clarify the full scope of an EIT-mediated immune-suppressive phenotype, with regards to the development of malignancies derived from prostate and other epithelial origins. As such, an improved understanding of the mechanisms underlying immune-suppression and cancer development could provide valuable knowledge with which to inform the discovery of additional therapeutic targets and agents.
Conflicts of interest
The authors declare no conflict of interest.
Footnotes
Peer review under responsibility of Second Military Medical University.
References
- 1.de Bono J.S., Logothetis C.J., Molina A., Fizazi K., North S., Chu L. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richard C.C., Nancy Lee H., Eric S.R., Jo-Anne O.S., Alice M.C., Sally H.E. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 3.de Bono J.S., Oudard S., Ozguroglu M., Hansen S., Machiels J.P., Kocak I. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 4.Parker C., Nilsson S., Heinrich D., Helle S.I., O'Sullivan J.M., Fossa S.D. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–223. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 5.Choi S.Y., Gout P.W., Collins C.C., Wang Y. Epithelial immune cell-like transition (EIT): a proposed transdifferentiation process underlying immune-suppressive activity of epithelial cancers. Differentiation. 2012;83:293–298. doi: 10.1016/j.diff.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Huen N.Y., Pang A.L., Tucker J.A., Lee T.L., Vergati M., Jochems C. Up-regulation of proliferative and migratory genes in regulatory T cells from patients with metastatic castration-resistant prostate cancer. Int J Cancer. 2013;133:373–382. doi: 10.1002/ijc.28026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller A.M., Lundberg K., Ozenci V., Banham A.H., Hellstrom M., Egevad L. CD4+CD25high T cells are enriched in the tumor and peripheral blood of prostate cancer patients. J Immunol. 2006;177:7398–7405. doi: 10.4049/jimmunol.177.10.7398. [DOI] [PubMed] [Google Scholar]
- 8.Flammiger A., Weisbach L., Huland H., Tennstedt P., Simon R., Minner S. High tissue density of FOXP3+ T cells is associated with clinical outcome in prostate cancer. Eur J Cancer. 2013;49:1273–1279. doi: 10.1016/j.ejca.2012.11.035. [DOI] [PubMed] [Google Scholar]
- 9.Idorn M., Kollgaard T., Kongsted P., Sengelov L., Thor Straten P. Correlation between frequencies of blood monocytic myeloid-derived suppressor cells, regulatory T cells and negative prognostic markers in patients with castration-resistant metastatic prostate cancer. Cancer Immunol Immunother. 2014;63:1177–1187. doi: 10.1007/s00262-014-1591-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vuk-Pavlovic S., Bulur P.A., Lin Y., Qin R., Szumlanski C.L., Zhao X. Immunosuppressive CD14+HLA-DRlow/− monocytes in prostate cancer. Prostate. 2010;70:443–455. doi: 10.1002/pros.21078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lanciotti M., Masieri L., Raspollini M.R., Minervini A., Mari A., Comito G. The role of M1 and M2 macrophages in prostate cancer in relation to extracapsular tumor extension and biochemical recurrence after radical prostatectomy. Biomed Res Int. 2014;2014:486798. doi: 10.1155/2014/486798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nonomura N., Takayama H., Nakayama M., Nakai Y., Kawashima A., Mukai M. Infiltration of tumour-associated macrophages in prostate biopsy specimens is predictive of disease progression after hormonal therapy for prostate cancer. BJU Int. 2011;107:1918–1922. doi: 10.1111/j.1464-410X.2010.09804.x. [DOI] [PubMed] [Google Scholar]
- 13.Gannon P.O., Poisson A.O., Delvoye N., Lapointe R., Mes-Masson A.M., Saad F. Characterization of the intra-prostatic immune cell infiltration in androgen-deprived prostate cancer patients. J Immunol Methods. 2009;348:9–17. doi: 10.1016/j.jim.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Pasero C., Gravis G., Guerin M., Granjeaud S., Thomassin-Piana J., Rocchi P. Inherent and tumor-driven immune tolerance in the prostate microenvironment impairs natural killer cell antitumor activity. Cancer Res. 2016;76:2153–2165. doi: 10.1158/0008-5472.CAN-15-1965. [DOI] [PubMed] [Google Scholar]
- 15.Lundholm M., Schroder M., Nagaeva O., Baranov V., Widmark A., Mincheva-Nilsson L. Prostate tumor-derived exosomes down-regulate NKG2D expression on natural killer cells and CD8+ T cells: mechanism of immune evasion. PLoS One. 2014;9:e108925. doi: 10.1371/journal.pone.0108925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia A.J., Ruscetti M., Arenzana T.L., Tran L.M., Bianci-Frias D., Sybert E. Pten null prostate epithelium promotes localized myeloid-derived suppressor cell expansion and immune suppression during tumor initiation and progression. Mol Cell Biol. 2014;34:2017–2028. doi: 10.1128/MCB.00090-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu J., Escamilla J., Mok S., David J., Priceman S., West B. CSF1R signaling blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Res. 2013;73:2782–2794. doi: 10.1158/0008-5472.CAN-12-3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pu Y., Xu M., Liang Y., Yang K., Guo Y., Yang X. Androgen receptor antagonists compromise T cell response against prostate cancer leading to early tumor relapse. Sci Transl Med. 2016;8 doi: 10.1126/scitranslmed.aad5659. 333ra47. [DOI] [PubMed] [Google Scholar]
- 19.Tang S., Moore M.L., Grayson J.M., Dubey P. Increased CD8+ T-cell function following castration and immunization is countered by parallel expansion of regulatory T cells. Cancer Res. 2012;72:1975–1985. doi: 10.1158/0008-5472.CAN-11-2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishida Y., Agata Y., Shibahara K., Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keir M.E., Butte M.J., Freeman G.J., Sharpe A.H. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agata Y., Kawasaki A., Nishimura H., Ishida Y., Tsubata T., Yagita H. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8:765–772. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- 23.Okazaki T., Chikuma S., Iwai Y., Fagarasan S., Honjo T. A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat Immunol. 2013;14:1212–1218. doi: 10.1038/ni.2762. [DOI] [PubMed] [Google Scholar]
- 24.Sharma P., Allison J.P. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 25.Sfanos K.S., Bruno T.C., Meeker A.K., De Marzo A.M., Isaacs W.B., Drake C.G. Human prostate-infiltrating CD8+ T lymphocytes are oligoclonal and PD-1+ Prostate. 2009;69:1694–1703. doi: 10.1002/pros.21020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zou W., Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 27.Dong H., Strome S.E., Salomao D.R., Tamura H., Hirano F., Flies D.B. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 28.Zang X., Allison J.P. The B7 family and cancer therapy: costimulation and coinhibition. Clin Cancer Res. 2007;13:5271–5279. doi: 10.1158/1078-0432.CCR-07-1030. [DOI] [PubMed] [Google Scholar]
- 29.Topalian S.L., Hodi F.S., Brahmer J.R., Gettinger S.N., Smith D.C., McDermott D.F. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taube J.M., Klein A., Brahmer J.R., Xu H., Pan X., Kim J.H. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20:5064–5074. doi: 10.1158/1078-0432.CCR-13-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin A.M., Nirschl T.R., Nirschl C.J., Francica B.J., Kochel C.M., van Bokhoven A. Paucity of PD-L1 expression in prostate cancer: innate and adaptive immune resistance. Prostate Cancer Prostatic Dis. 2015;18:325–332. doi: 10.1038/pcan.2015.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Massari F., Ciccarese C., Calio A., Munari E., Cima L., Porcaro A.B. Magnitude of PD-1, PD-L1 and T lymphocyte expression on tissue from castration-resistant prostate adenocarcinoma: an exploratory analysis. Target Oncol. 2015;11:345–351. doi: 10.1007/s11523-015-0396-3. [DOI] [PubMed] [Google Scholar]
- 33.Gevensleben H., Dietrich D., Golletz C., Steiner S., Jung M., Thiesler T. The immune checkpoint regulator PD-L1 is highly expressed in aggressive primary prostate cancer. Clin Cancer Res. 2016;22:1969–1977. doi: 10.1158/1078-0432.CCR-15-2042. [DOI] [PubMed] [Google Scholar]
- 34.Ceeraz S., Nowak E.C., Noelle R.J. B7 family checkpoint regulators in immune regulation and disease. Trends Immunol. 2013;34:556–563. doi: 10.1016/j.it.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chapoval A.I., Ni J., Lau J.S., Wilcox R.A., Flies D.B., Liu D. B7-H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol. 2001;2:269–274. doi: 10.1038/85339. [DOI] [PubMed] [Google Scholar]
- 36.Katayama A., Takahara M., Kishibe K., Nagato T., Kunibe I., Katada A. Expression of B7-H3 in hypopharyngeal squamous cell carcinoma as a predictive indicator for tumor metastasis and prognosis. Int J Oncol. 2011;38:1219–1226. doi: 10.3892/ijo.2011.949. [DOI] [PubMed] [Google Scholar]
- 37.Gregosiewicz A., Wosko I., Kandzierski G., Drabik Z. Double-elevating osteotomy of tibiae in the treatment of severe cases of Blount's disease. J Pediatr Orthop. 1989;9:178–181. [PubMed] [Google Scholar]
- 38.Zang X., Thompson R.H., Al-Ahmadie H.A., Serio A.M., Reuter V.E., Eastham J.A. B7-H3 and B7x are highly expressed in human prostate cancer and associated with disease spread and poor outcome. Proc Natl Acad Sci U S A. 2007;104:19458–19463. doi: 10.1073/pnas.0709802104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tekle C., Nygren M.K., Chen Y.W., Dybsjord I., Nesland J.M., Maelandsmo G.M. B7-H3 contributes to the metastatic capacity of melanoma cells by modulation of known metastasis-associated genes. Int J Cancer. 2012;130:2282–2290. doi: 10.1002/ijc.26238. [DOI] [PubMed] [Google Scholar]
- 40.Roth T.J., Sheinin Y., Lohse C.M., Kuntz S.M., Frigola X., Inman B.A. B7-H3 ligand expression by prostate cancer: a novel marker of prognosis and potential target for therapy. Cancer Res. 2007;67:7893–7900. doi: 10.1158/0008-5472.CAN-07-1068. [DOI] [PubMed] [Google Scholar]
- 41.Yuan H., Wei X., Zhang G., Li C., Zhang X., Hou J. B7-H3 over expression in prostate cancer promotes tumor cell progression. J Urol. 2011;186:1093–1099. doi: 10.1016/j.juro.2011.04.103. [DOI] [PubMed] [Google Scholar]
- 42.Liu Y., Vlatkovic L., Saeter T., Servoll E., Waaler G., Nesland J.M. Is the clinical malignant phenotype of prostate cancer a result of a highly proliferative immune-evasive B7-H3-expressing cell population? Int J Urol. 2012;19:749–756. doi: 10.1111/j.1442-2042.2012.03017.x. [DOI] [PubMed] [Google Scholar]
- 43.Parker A.S., Heckman M.G., Sheinin Y., Wu K.J., Hilton T.W., Diehl N.N. Evaluation of B7-H3 expression as a biomarker of biochemical recurrence after salvage radiation therapy for recurrent prostate cancer. Int J Radiat Oncol Biol Phys. 2011;79:1343–1349. doi: 10.1016/j.ijrobp.2010.01.061. [DOI] [PubMed] [Google Scholar]
- 44.Chavin G., Sheinin Y., Crispen P.L., Boorjian S.A., Roth T.J., Rangel L. Expression of immunosuppresive B7-H3 ligand by hormone-treated prostate cancer tumors and metastases. Clin Cancer Res. 2009;15:2174–2180. doi: 10.1158/1078-0432.CCR-08-2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zang X., Loke P., Kim J., Murphy K., Waitz R., Allison J.P. B7x: a widely expressed B7 family member that inhibits T cell activation. Proc Natl Acad Sci U S A. 2003;100:10388–10392. doi: 10.1073/pnas.1434299100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prasad D.V., Richards S., Mai X.M., Dong C. B7S1, a novel B7 family member that negatively regulates T cell activation. Immunity. 2003;18:863–873. doi: 10.1016/s1074-7613(03)00147-x. [DOI] [PubMed] [Google Scholar]
- 47.Sica G.L., Choi I.H., Zhu G., Tamada K., Wang S.D., Tamura H. B7-H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity. 2003;18:849–861. doi: 10.1016/s1074-7613(03)00152-3. [DOI] [PubMed] [Google Scholar]
- 48.Krambeck A.E., Thompson R.H., Dong H., Lohse C.M., Park E.S., Kuntz S.M. B7-H4 expression in renal cell carcinoma and tumor vasculature: associations with cancer progression and survival. Proc Natl Acad Sci U S A. 2006;103:10391–10396. doi: 10.1073/pnas.0600937103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu W., Shibata K., Koya Y., Kajiyama H., Senga T., Yamashita M. B7-H4 overexpression correlates with a poor prognosis for cervical cancer patients. Mol Clin Oncol. 2014;2:219–225. doi: 10.3892/mco.2013.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen L.J., Sun J., Wu H.Y., Zhou S.M., Tan Y., Tan M. B7-H4 expression associates with cancer progression and predicts patient's survival in human esophageal squamous cell carcinoma. Cancer Immunol Immunother. 2011;60:1047–1055. doi: 10.1007/s00262-011-1017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thompson R.H., Zang X., Lohse C.M., Leibovich B.C., Slovin S.F., Reuter V.E. Serum-soluble B7x is elevated in renal cell carcinoma patients and is associated with advanced stage. Cancer Res. 2008;68:6054–6058. doi: 10.1158/0008-5472.CAN-08-0869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qian Y., Yao H.P., Shen L., Cheng L.F., Zhang L.H. Expression of B7-H4 in prostate cancer and its clinical significance. Zhejiang Univ Med Sci. 2010;39:345–349. doi: 10.3785/j.issn.1008-9292.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 53.Monney L., Sabatos C.A., Gaglia J.L., Ryu A., Waldner H., Chernova T. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415:536–541. doi: 10.1038/415536a. [DOI] [PubMed] [Google Scholar]
- 54.Ngiow S.F., Teng M.W., Smyth M.J. Prospects for TIM3-targeted antitumor immunotherapy. Cancer Res. 2011;71:6567–6571. doi: 10.1158/0008-5472.CAN-11-1487. [DOI] [PubMed] [Google Scholar]
- 55.Sakuishi K., Jayaraman P., Behar S.M., Anderson A.C., Kuchroo V.K. Emerging Tim-3 functions in antimicrobial and tumor immunity. Trends Immunol. 2011;32:345–349. doi: 10.1016/j.it.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anderson A.C., Anderson D.E., Bregoli L., Hastings W.D., Kassam N., Lei C. Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science. 2007;318:1141–1143. doi: 10.1126/science.1148536. [DOI] [PubMed] [Google Scholar]
- 57.Gao X., Zhu Y., Li G., Huang H., Zhang G., Wang F. TIM-3 expression characterizes regulatory T cells in tumor tissues and is associated with lung cancer progression. PLoS One. 2012;7:e30676. doi: 10.1371/journal.pone.0030676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fourcade J., Sun Z., Benallaoua M., Guillaume P., Luescher I.F., Sander C. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207:2175–2186. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu J., Liu C., Qian S., Hou H. The expression of Tim-3 in peripheral blood of ovarian cancer. DNA Cell Biol. 2013;32:648–653. doi: 10.1089/dna.2013.2116. [DOI] [PubMed] [Google Scholar]
- 60.Piao Y.R., Jin Z.H., Yuan K.C., Jin X.S. Analysis of Tim-3 as a therapeutic target in prostate cancer. Tumour Biol. 2014;35:11409–11414. doi: 10.1007/s13277-014-2464-1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 61.Piao Y.R., Piao L.Z., Zhu L.H., Jin Z.H., Dong X.Z. Prognostic value of T cell immunoglobulin mucin-3 in prostate cancer. Asian Pac J cancer Prev. 2013;14:3897–3901. doi: 10.7314/apjcp.2013.14.6.3897. [DOI] [PubMed] [Google Scholar]
- 62.Norde W.J., Hobo W., van der Voort R., Dolstra H. Coinhibitory molecules in hematologic malignancies: targets for therapeutic intervention. Blood. 2012;120:728–736. doi: 10.1182/blood-2012-02-412510. [DOI] [PubMed] [Google Scholar]
- 63.Collins A.V., Brodie D.W., Gilbert R.J., Iaboni A., Manso-Sancho R., Walse B. The interaction properties of costimulatory molecules revisited. Immunity. 2002;17:201–210. doi: 10.1016/s1074-7613(02)00362-x. [DOI] [PubMed] [Google Scholar]
- 64.Rudd C.E., Taylor A., Schneider H. CD28 and CTLA-4 coreceptor expression and signal transduction. Immunol Rev. 2009;229:12–26. doi: 10.1111/j.1600-065X.2009.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Linsley P.S., Ledbetter J.A. The role of the CD28 receptor during T cell responses to antigen. Annu Rev Immunol. 1993;11:191–212. doi: 10.1146/annurev.iy.11.040193.001203. [DOI] [PubMed] [Google Scholar]
- 66.Walunas T.L., Lenschow D.J., Bakker C.Y., Linsley P.S., Freeman G.J., Green J.M. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 67.Bronte V., Kasic T., Gri G., Gallana K., Borsellino G., Marigo I. Boosting antitumor responses of T lymphocytes infiltrating human prostate cancers. J Exp Med. 2005;201:1257–1268. doi: 10.1084/jem.20042028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Powderly J., Cote G., Flaherty K., Szmulewitz R.Z., Ribas A., Weber J. Interim results of an ongoing Phase I, dose escalation study of MGA271 (Fc-optimized humanized anti-B7-H3 monoclonal antibody) in patients with refractory B7-H3-expressing neoplasms or neoplasms whose vasculature expresses B7-H3. J Immunother Cancer. 2015;3:O8. [Google Scholar]
- 69.Hodi F.S., O'Day S.J., McDermott D.F., Weber R.W., Sosman J.A., Haanen J.B. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Robert C., Thomas L., Bondarenko I., O'Day S., Weber J., Garbe C. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 71.Small E.J., Tchekmedyian N.S., Rini B.I., Fong L., Lowy I., Allison J.P. A pilot trial of CTLA-4 blockade with human anti-CTLA-4 in patients with hormone-refractory prostate cancer. Clin Cancer Res. 2007;13:1810–1815. doi: 10.1158/1078-0432.CCR-06-2318. [DOI] [PubMed] [Google Scholar]
- 72.O'Mahony D., Morris J.C., Quinn C., Gao W., Wilson W.H., Gause B. A pilot study of CTLA-4 blockade after cancer vaccine failure in patients with advanced malignancy. Clin Cancer Res. 2007;13:958–964. doi: 10.1158/1078-0432.CCR-06-1974. [DOI] [PubMed] [Google Scholar]
- 73.Fong L., Kwek S.S., O'Brien S., Kavanagh B., McNeel D.G., Weinberg V. Potentiating endogenous antitumor immunity to prostate cancer through combination immunotherapy with CTLA4 blockade and GM-CSF. Cancer Res. 2009;69:609–615. doi: 10.1158/0008-5472.CAN-08-3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Slovin S.F., Higano C.S., Hamid O., Tejwani S., Harzstark A., Alumkal J.J. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Ann Oncol. 2013;24:1813–1821. doi: 10.1093/annonc/mdt107. [DOI] [PMC free article] [PubMed] [Google Scholar]