Abstract
Recent cancer research has demonstrated the existence of circulating tumor cells (CTCs) in cancer patient's blood. Once identified, CTC biomarkers will be invaluable tools for clinical diagnosis, prognosis and treatment. In this review, we propose ex vivo culture as a rational strategy for large scale amplification of the limited numbers of CTCs from a patient sample, to derive enough CTCs for accurate and reproducible characterization of the biophysical, biochemical, gene expressional and behavioral properties of the harvested cells. Because of tumor cell heterogeneity, it is important to amplify all the CTCs in a blood sample for a comprehensive understanding of their role in cancer metastasis. By analyzing critical steps and technical issues in ex vivo CTC culture, we developed a cost-effective and reproducible protocol directly culturing whole peripheral blood mononuclear cells, relying on an assumed survival advantage in CTCs and CTC-like cells over the normal cells to amplify this specified cluster of cancer cells.
Keywords: Cancer metastasis, Peripheral blood, Circulating tumor cell, ex vivo culture
1. Introduction
Given its life-threatening consequences, cancer metastasis could be considered the most crucial topic in cancer research. The mechanism of metastasis should be intensely investigated to define the critical players for therapeutic targeting to improve patient survival. Based on historical precedents, in which once-incurable diseases became curable or successfully managed by medical oncologists after advances in biomedical and clinical science [1], [2], cancer metastasis will be curable in the future. The overall cancer death rate in the United State, for example, has been falling over the last few decades [3], thanks mainly to the identification of tobacco use as an oncogenic risk factor and a protracted period involving in multistep nature of carcinogenesis. In addition, the recognition of extensive tumor cell heterogeneity has led to personalized oncology, which is markedly improving cancer treatment and patient survival [4], [5], [6]. A full understanding of metastatic tumor pathophysiology, in combination with a careful examination of the tumor microenvironment for a clear definition of participant cell types and the signaling network in cancer-stromal and cancer-host immune interaction, may provide clinicians with critical mechanistic insights into the treatment of cancer metastasis [7], [8].
This review discusses one of our ongoing research projects focusing on isolation and characterization of circulating tumor cells (CTCs) following ex vivo culture. Expanding CTCs in large quantities will facilitate detailed genotypical and phenotypical examination, allowing causal abnormalities driving cancer metastasis to be accurately identified and exploited as biomarkers for cancer diagnosis, prognosis and treatment. Thus, establishment of a highly reproducible and efficient CTC propagation protocol holds the promise for advancing precision medicine and personalized oncology. This review summarizes the current status of high quality CTC research results with special emphasis on ex vivo culture of this specific cancer cell type. Based on published literature and our own work, we comment on critical issues facing the development of CTC culture methodologies and propose a rational strategy for culturing CTCs from cancer patient peripheral blood samples. The goal of this discussion is to attract research interest to the development of a cost-effective and reproducible protocol for ex vivo culture of a limited number of CTCs from a given clinical blood sample into a large population amendable to molecular characterization.
2. Limitations in the research of cancer metastasis
Many confounding factors make the study of cancer metastasis more difficult than the study of oncogenic initiation, promotion and progression. Firstly, the study of cancer initiation is based on a unified hypothesis, which postulates that cancer initiation is due to genomic mutation or genetic abnormality, while environmental risk factors promote these perturbations and culminate in cancer development and local invasion. In contrast, metastasis is shown to be the result of interplay between cancer cells and resident cells of the tumor microenvironment, involving mesenchymal, endothelial and immune cells at secondary organ sites [9], [10], [11], where many of these bystander cell types potentially affect cancer cells through multiple reciprocity [12], [13]. The presence of plural variants both in heterogeneous cancer cells and host microenvironment makes identification of critical effectors difficult. Secondly, the study of cancer development has a longer history facilitated by having immortalized cell lines as study subjects and genetically reconstituted animal models to investigate how specific genetic mutations affect cancer development. The study of cancer metastasis is less granular, due to the difficulty of manipulating the drivers of cancer metastasis, while animal models often fail to recapitulate human metastatic profiles. Thirdly, cancer initiation and development can be studied with defined experimental systems, in which the effect of a suspected causal factor can be quantitatively evaluated by multiple modalities such as cell proliferation, apoptosis, migration, invasion and xenograft tumor formation. The study of cancer metastasis, by contrast, is often hindered by a lack of optimal model systems to decipher the interaction between cancer cells and the tumor microenvironment at the metastatic site. Finally, from the therapeutic perspective, many primary cancers can be cured either by surgery and/or adjuvant therapy, while there is a general lack of confidence in the curability of cancer metastasis [14], [15], [16], which often leads to the patient's demise. It is challenging for investigators to establish reliable in vitro models to delineate the mechanism of cancer metastasis at an organismic level. It would be ideal if metastatic cancer cell lines could be established routinely from each individual cancer patient.
Human cancers are broadly categorized into two groups according to their lineage origin, tumors from mesenchymal lineage and cancers of epithelial cell origin. Though the true cause of either cancer type remains to be defined, histopathologic analysis has shown that these two groups of malignancies have distinctive natures of development, progression and metastasis [17], [18]. In the following discussion, the term “cancer” refers mainly to malignancies of epithelial origin.
Many cancer theories assume that compared to normal cells, tumor cells acquire survival and proliferation advantages through tumor cell evolution in situ. Counterintuitively, tumor cells of epithelial origin are difficult to amplify by ex vivo culture [19], [20], [21]. Only a few successes have been achieved establishing immortalized cancer cell lines. Pancreatic cancer, for example, is a fast progressing, highly metastatic and devastating disease, but only a few invasive human pancreatic cancer cell lines have been established to date [22], [23]. The same is true of prostate cancer, another highly lethal disease with high incidence in Western countries [24]. Cell line scarcity makes it especially difficult to study cancer cell heterogeneity, known to contribute ultimately to cancer progression and to anti-tumor therapeutic resistance. More importantly, the inability to culture cancer cells from patient tumor tissues makes it difficult to practice precision oncology at the personalized level. It would be ideal to grow tumor cells ex vivo from every cancer patient, and to then subject these cells to screening for the most effective regimen specifically tailored to the individual patient. An improvement in primary human cell culture would contribute to the advancement of cancer research and precision-based cancer treatment.
Our laboratory's research focus on the mechanism of cancer metastasis has revealed the importance of cellular interactions, which collectively contributing to metastatic dissemination of prostate cancer cells to the mouse skeleton [7], [8], [25], [26], [27], [28], [29]. We have found that cancer cells can recruit and reprogram naïve non-tumorigenic bystander cells to participate in tumorigenic and metastatic cascade via activation of critical transcription factors that confer mesenchymal, stemness and neuroendocrine phenotypes to the bystander cells [25], [30], [31]. These results exposed a previously unrecognized facet of cancer metastasis: though the mechanism of cancer initiation and development in primary tumors may be a tumor cell-centered event, cancer metastasis is a bona fide systemic disease, in which many innocent cell types in the tumor microenvironment can be “transdifferentiated” to express malignant phenotypes and become active participants in the process of tumor cell spreading. Detection of these newly converted cells may provide sensitive biomarkers for metastasis detection, and blockade of the recruitment and reprogramming mechanism could be a new approach to delay metastasis and prolong patient survival.
3. CTCs as a marker of cancer metastasis
There is a general lack of sensitive biomarkers for diagnosis, treatment, and prognosis of many metastatic cancers [32]. Conventional cancer research relies on comparative analysis of cancer versus normal control cells to identify differential biomarkers. Extensive biomarker search has yielded few bona fide cancer biomarkers, partly because the survival advantage of cancer cells is achieved by coopting normal regulatory mechanisms, rather than by activating cancer-specific “oncogenes”. Shared structure and gene expression between cancer and normal cells would make it almost impossible to use “cancer-specific” biomarkers to distinguish the presence of cancer from other nonmalignant cells. In addition, published data in the literature suggest that abnormalities of cancer cells are dynamic, changing from one pathologic state to another along with abnormal cell division [6], [33], [34]. Clinically, only the appearance of certain morphologically distinct cells, indicative of cancer, in the secondary organ sites is taken as a concrete sign of cancer metastasis.
Many forms of patient samples can be used for disease diagnosis, but the most common and least invasive sampling is liquid biopsy of peripheral blood. In cancer research, it has long been postulated that the circulation system is a thoroughfare for cancer spreading [35], [36], [37]. This postulation has now been supported by accumulating evidence, as cancer cells have been detected and isolated from blood samples both experimentally [38], [39], [40] and from clinical cancer patients [41], [42], [43]. CTCs in cancer patient blood form an attractive liquid biopsy compartment that can be subjected to molecular interrogation to unveil genomic abnormality in tumor cells and their fate in the metastatic cascade. With in-depth characterization, CTCs per se or unique features of the CTCs may be used clinically for the diagnosis, prognosis and treatment of cancer metastasis in the future [44].
4. Argument for the necessity of ex vivo CTC culture
There are two strategies for studying CTCs. The popular strategy presently is to study CTCs directly from patient blood. In a few cases, CTCs are amplified ex vivo into a large population before investigation. We believe that for reproducible investigation, especially for behavioral characterization, CTCs have to be amplified, preferably into stable cell lines.
4.1. The practical value of ex vivo CTC culture
A manifest disadvantage of directly detecting CTCs for cancer diagnosis is the sparse and sporadic nature of this abnormal cell type, which is mixed among the vast populations of peripheral blood mononuclear cells (PBMCs) [42], [45], [46]. This feature makes it difficult to perform any comprehensive and reproducible analyses directly, either for CTC detection and enumeration at the cellular level, or for genomic and gene expression studies at the molecular level. Indeed, partly due to CTC heterogeneity and partly due to present-day technological limitations, even a confident counting of CTCs in any given patient blood sample is hard, as is correlation of CTC counts to disease progression or metastasis. Contributing factors to the conundrum and the anemic understanding of CTCs as a pathologic and tumor progression entity will be discussed in detail in the following sections. It seems that for CTC biomarker identification, an assessment of the heterogeneity of genomic and gene expression abnormalities can be made only by acquiring a large amount of CTCs for concise molecular analyses and reproducible validation [47].
4.2. Unknown fundamental biophysical and biochemical information of the CTCs
Our marginal knowledge of CTCs is in stark contrast to our profound understanding of the normal constituents of the peripheral blood. Whereas each blood cell type has been meticulously studied for its biophysical, biochemical, gene expressional and behavioral characteristics, none of these aspects is clearly defined for CTCs in the same sample. Individual PBMC constituents, for instance, are well characterized for their total counts, differential ratios, lineage relationships, distinct cell sizes at resting state, cellular densities, cytoplasmic granularities, nucleus morphologies, surface markers, growth and differentiation capacities, half-lives in circulation, biological functions and possible pathophysiologic abnormalities. All these features could be explored for the enumeration and isolation of a specific PBMC fraction. In contrast, none of these features has been fully explored in CTCs, making it difficult to conduct unbiased detection and isolation of this abnormal cell type from the vast blood cell population. Ultimately the unique features of CTCs can only be identified by detailed characterization of live CTCs.
The objective of ex vivo CTC culture is to expand the limited numbers of CTCs in a given blood sample into a large population, preferably into an immortal cell line, so that molecular, cellular and behavioral investigations can be conducted on these cells with high accuracy and reproducibility.
5. Low success rate as the default of ex vivo CTC culture
Many attempts have made to propagate CTCs through ex vivo culture, with only limited success. This can be demonstrated by the fact that though a huge literature on CTC research is available, only about 50 publications in the last decade address culturing CTCs from clinical patient blood samples. Most of these cultures are short-term, lasting from 3 days to 14 days [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61]. Only a few long-term cultures have reported successfully established CTC lines with demonstrated immortality that could be cultured stably for many passages [62], [63], [64], [65]. These successes are summarized in Table 1.
Table 1.
Summary of successes in long-term CTC culture with clinical cancer patient blood samples.
| Disease | Method of CTC isolation | CTC culture conditions | Group size | CTC lines established | Morphology | Reference |
|---|---|---|---|---|---|---|
| Breast cancer | CD45/CD66 iChip negative selection | RPMI 1640 medium, serum-free, with EGF, FGF, and B27, in 4% O2, spheroid culture | 36 | 6 | Spheroids | Yu, et al., 2014 [62] |
| Colon cancer | CD45 RosetteSep negative selection | DMEM: F12 medium, 2% FBS, with insulin, EGF, and bFGF, supplemented with N2, in 2% O2 for 2 weeks; then in RPMI 1640 medium, 2% FBS, with insulin, EGF, bFGF, supplemented with transferrin and selenite, in normoxia | 71 | 1 | Spheroids | Cayrefourcq, et al., 2015 [63] |
| Prostate cancer | Ficoll-Paque density gradient and CD45 RosetteSep negative selection | DMEM: F12 medium, with EGF, bFGF, FGF10, supplemented with R-spondin 1, DHT, B27, nicotinamide, A83-01, SB202190 and Y27632, in Matrigel | 17 | 1 | Spheroids | Gao, et al., 2014 [64] |
| Small cell lung cancer | Ficoll-Hypaque density gradient | RPMI 1640 medium, serum-free, with insulin, IGF-1 and selenite | 30 | 3 | Spheroids or attached | Hamilton, et al., 2015 [65] |
bFGF, basic fibroblast growth factor; CTC, circulating tumor cells; DHT, dihydrotestosterone; EGF, epidermal growth factor; FBS, fetal bovine serum; FGF, fibroblast growth factor; IGF-1, insulin-like growth factor 1.
Though ex vivo CTC culture currently has low success rates, the successes indicate that some CTCs in patient blood are immortal and could be cultured ex vivo into stable cell lines. In this discussion, long-term CTC culture will be the main issue of examination, mainly because long-term CTC culture is hindered by technical difficulties [19].
5.1. Rarity of CTCs by detection and enumeration
The main difficulty is the extreme rarity of CTCs. For instance, a peripheral blood sample contains huge numbers of red blood cells (around 5 × 109/mL), platelets (around 4 × 108/mL), and PBMCs (around 6 × 106/mL). In contrast, CTC counts in published studies have been detected as two-digit numbers in a 7.5 mL blood [66]. CTCs thus exist as a minute fraction among the vast number of blood cells, and without ex vivo expansion it would be difficult to conduct any comprehensive characterization on this elusive cell type.
5.2. The caveat of using peripheral blood for translational CTC study
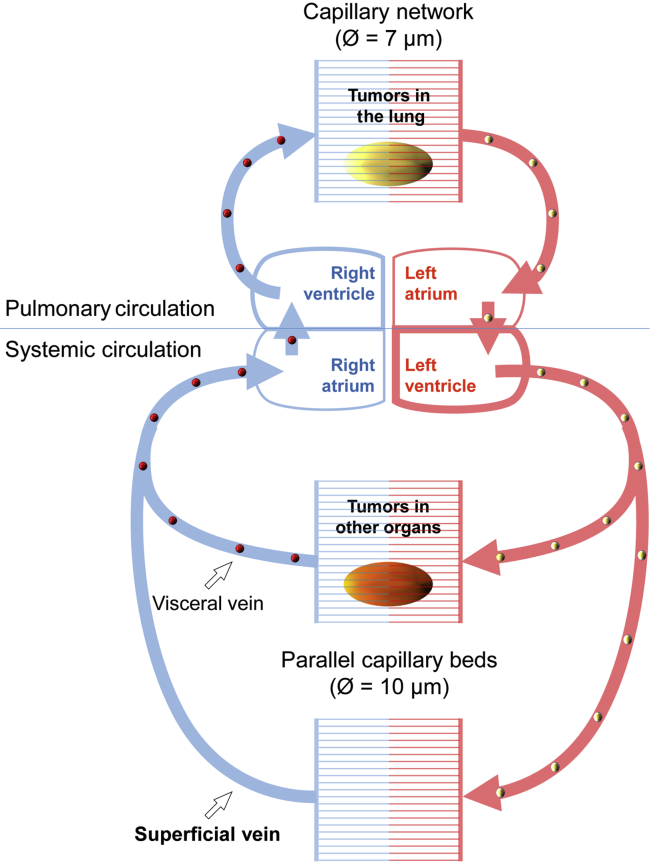
CTCs are presumed to be in a dynamic equilibrium with homeostasis of the tumor in situ, and are considered as a surrogate marker for tumor status. CTCs, however, may not be uniformly distributed in the blood. Analogous to the non-uniform distribution of cells, proteins, hormones, and gases in the circulation system, a non-uniform CTC distribution should be expected, based on anatomy and physiology of the circulation system. As depicted in Fig. 1, distribution of CTCs may be determined by two factors: origin of the CTCs, and size of the CTCs. Individual CTCs will be filtered through two serial capillary beds; and in periphery, CTCs are transported in parallel circuits. Structure of the systemic circulation implies that peripheral blood may only contain the least and smallest CTCs. Nonetheless, it is this fraction of rare and small CTCs that are mediating tumor spreading. The clinical value of peripheral blood sampling for CTC detection and enumeration should be critically evaluated.
Figure 1.
Size-limiting structure of the blood circulation system. A diagram of the human blood circulation system shows the likeliest site to find CTCs. Pulmonary circulation (upper portion) may be considered as a serial perfusion system, transporting CTCs (golden balls) from lung tumors to the oxygenated blood (red), where the CTCs will be filtered by peripheral capillary beds. Systemic circulation (lower portion) is comprised of parallel vascular systems. Two parallel capillary beds are used to show scenarios of CTC circulation. In one scenario, CTCs (red balls) from tumors of visceral organs may be shed to the internal vein, and will be filtered by the pulmonary capillary network. In another scenario, blood samples from upper limb superficial veins are commonly used for CTC studies. Though this is convenient and less invasive for routine clinical phlebotomy, cancer patient blood samples from these veins may contain only the smallest CTCs in size and the fewest CTCs in number. According to this diagram, CTCs from lung tumors may be easier to detect in arterial blood, whereas CTCs from tumors in other organs may be easier to be detected from the blood approximate to the right side of the heart.
5.3. CTC immortality
Though immortality is ideal for ex vivo CTC culture, in the clinical setting immortality is not essential for CTCs to metastasize from the primary tumor to a distant site. A CTC does not have to be immortalized to cause metastatic growth, as long as a viable CTC can be transported to distant sites by the circulation system, which is known to be swift and highly efficient for corporal distribution. This presumption is in agreement with the finding that CTCs from patient blood are vulnerable [67], [68], just as they are susceptible to ex vivo culture conditions [19]. Based on extremely low published success rates, and our own CTC culture experience, it is intriguing to postulate that until late cancer stages, when heterogeneity becomes so thorough that certain tumor cells have extensively evolved, immortal CTCs may be rare in the patient's blood. This observation predicts that, by default, ex vivo culture should be a low-success-rate practice, especially when a large number of cancer patients are sampled without pre-selection for late-stage cases. Development of an optimal protocol for ex vivo culture is thus a critical subject for reproducible CTC research.
6. Technical issues in the various CTC isolation methods
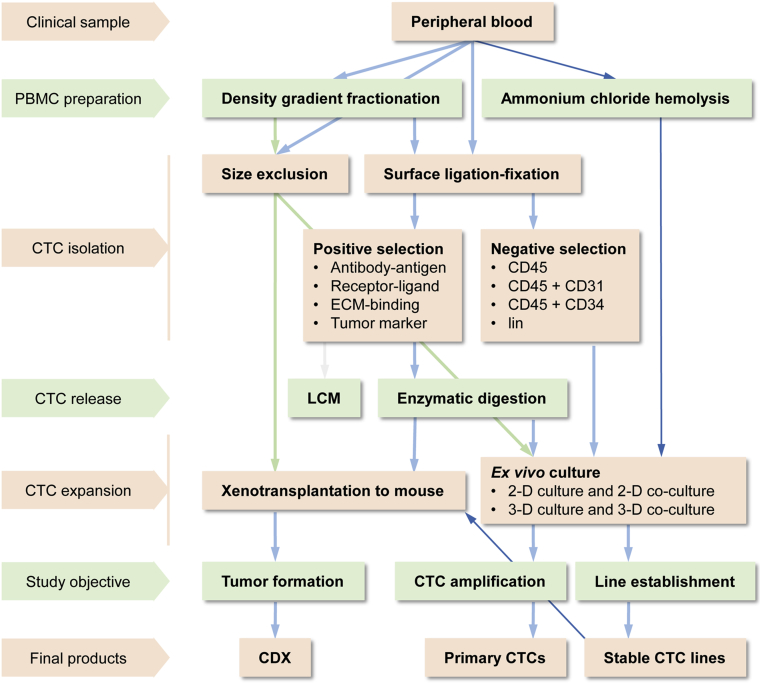
It is habitual to assume that, in order to culture CTCs, these cells should first be isolated from patient blood samples. Most reported CTC culture protocols employ a two-step strategy: isolating the CTCs from whole blood and then subjecting the isolated cells to ex vivo culture under defined conditions. A summary of CTC isolation strategies is shown in Fig. 2. Though there are many conventional technologies for isolating a specific cell type from a mixed sample, none is suitable for the isolation of CTCs from cancer patient blood, because specific traits that can be explored for a clean isolation of CTCs have not yet been defined.
Figure 2.
Current methodologies for ex vivo CTC expansion. Current strategies, methods, and technologies employed in CTC culture are summarized in this flowchart. Arrowed lines denote blood sample manipulative steps. Thin blue lines in the flowchart indicate the unique experimental steps developed in our laboratory. CDX, CTC-derived xenograft; CTC, circulating tumor cells; LCM, laser capture micro-dissection; 2-D, two-dimension; 3-D, three-dimension.
6.1. CTC isolation by density gradient fractionation
In many reports, CTCs are initially isolated from erythrocytes together with the PBMC population. Cellular density-based isolation with Ficoll polysaccharides is one of the best-established methods for isolating PBMCs from a blood sample. This method, nonetheless, only allows the isolation of a lighter fraction of the PBMCs. Ficoll-Paque medium with a density of 1.077 g/mL (Ficoll-Paque 1077), for example, selectively isolates monocytes and lymphocytes that in comparison to red blood cells, have lighter densities [69], [70]. The use of Ficoll-Paque with a density of 1.084 g/mL may additionally isolate basophils, but leaves behind heavier neutrophils and eosinophils. In our study of CTCs from prostate and pancreatic cancer patient blood samples [42], some candidate CTCs appeared much heavier, and could not be isolated by Ficoll-Paque 1.077 or 1.084 banding. Because the density of CTCs is not defined and probably is not uniform, using density gradient to isolate CTCs in monocyte/lymphocyte banding is not supported by biophysical data.
Alternative strategies for isolating CTCs directly from blood sample encounter difficulties as well. Most such methods are based on surface marker expression: detecting and isolating CTCs that bear a predetermined surface marker absent from normal blood cells. Both positive and negative selection strategies are commonly used. Neither of these strategies guarantees a comprehensive isolation of all the CTCs in a given sample, but may introduce complications to later analyses.
6.2. CTC isolation by positive selection
To isolate by positive selection, CTCs are presumed to have retained surface marker expression of the primary tumor cells. Since primary cancers are of epithelial origin, and since most cancer cells carry epithelial surface markers, these markers are often applied for fishing CTCs from the blood. Epithelial marker expression, however, is known to be altered and may even be lost in cancers. The epithelial cell adhesion molecule (EpCAM), for example, is universally expressed on normal epithelial cells. It is involved in maintenance of intercellular adhesion in an epithelial layer, clenching epithelial cells into a static and docile state [71], [72]. During cancer development and progression, however, its expression would be reduced or lost [73], [74], [75]due to multiple oncogenic signaling, or through epithelial to mesenchymal transition. Significantly, loss of these types of epithelial markers accounts for the acquired mobility, and invasive and migratory potential, of cancer cells. The formation and shedding of CTCs into blood circulation, therefore, could imply that these special cancer cell types have lost most of their EpCAM surface expression. Due to tumor cell heterogeneity in epithelial marker expression, the use of epithelial proteins as surrogate cancer cell markers may detect certain CTCs, but may not be an ideal strategy for comprehensive isolation of all the CTCs in a given blood sample. Importantly, it should be kept in mind that the CTCs whose epithelial behavior is completely lost might be the ones that are more representative of the malignant stage of disease [76], [77], [78].
Another drawback to positive selection is that this method is inherently harmful to CTCs, as isolated CTCs are no longer viable for ex vivo culture. Firstly, antibody-binding could be harmful to cell survival. Some antibodies against EpCAM, for example, may directly cause cancer cell death [79] or indirectly induce antibody-dependent cell-mediated cytotoxicity (ADCC). Indeed, anti-EpCAM mediated cancer cell killing is a mechanism sought in studies involving adecatumumab, edrecolomab and catumaxomab. Secondly, positive selection relies on mechanisms such as antibody-antigen ligation, ligand-receptor binding, or integrin-extracellular matrix (ECM) adhesion for CTC recognition. To physically isolate CTCs, the recognition part of the ligands in the forms of antibodies, peptides, or organic molecules, has to be affixed onto a solid or semi-solid surface, either magnetic beads, microfluidic chips, or polysaccharides, so the CTCs can be tethered onto the surface and then isolated by physical means. In many cases, isolated CTCs remain affixed onto the solid surface and are easily killed by dehydration. In some cases, live CTCs may actively attach onto the surface due to close adjacency. To free the isolated CTCs from the solid surface, either enzymatic digestion to break the affixation or laser capture micro-dissection (LCM) would most likely kill the CTC as well.
6.3. CTC isolation by negative selection
Technical difficulties are similarly seen in CTC isolation by negative selection, which is designed to deplete all normal PBMCs from a blood sample using well-known hematopoietic surface markers, leaving only CTCs behind. The major drawback with this strategy is that not all normal cells in a blood sample can be removed by negative selection, because a fraction of hematopoietic cells does not express known hematopoietic surface markers [80]. In addition, not all blood cells are hematopoietic, so a peripheral blood sample may contain many non-hematopoietic cell types besides CTCs [81].
CD45 isoforms, for example, are said to be expressed on all cells of the hematopoietic lineage and are commonly used in negative selection to deplete hematopoietic cells from blood. Not all blood cells, not even all hematopoietic cells, are CD45-positive [80]. Though the frequency of CD45-negative cells in human blood has not been systematically quantitated, this fraction of cells is attracting more research. In different patient groups and with different detection settings, CD45-negative cells are found to range from 0.02% [82] to almost 2% [80] of PBMCs. These may translate into a CD45-negative count ranging from 1200/mL to 120,000/mL blood sample, still much higher than the assumed two-digit CTC counts in the same volume. The result of a CD45-negative selection, therefore, is CTCs mixed with many poorly characterized but otherwise normal cells of the blood. Similarly, an alternative negative depletion with human lineage marker (Lin, a cocktail of antibodies against known cell types of the entire hematopoietic lineage) would result in CTCs mixed with various Lin-negative normal cells [80], [83]. Though negative selection may have less effect on CTC viability, this method fails to yield a pure CTC population and will potentially be misleading by totaling enriched normal blood cells as CTCs.
6.4. CTC isolation by size-based technology
In addition to negative selection, there are other isolation methods that may not guarantee a successful CTC isolation and may instead introduce distortions in CTC quality and quantity. Size-based isolation, for example, may not be able to isolate all the CTCs from a given blood sample, simply because the size distribution of CTCs in a given blood sample is unknown. It is possible that, similar to established cancer cell lines, CTCs in a given patient blood sample may have varied cell sizes, overlapping the size range of resident hematopoietic cells. This complexity is compounded by the fact that PBMCs in the same blood sample highly vary in size as well, with diameters ranging from 7 μm for resting lymphocytes up to 30 μm for monocytes.
On the other hand, lumen dimensions of the limiting capillary micro-vessels range from 7.5 μm to 10 μm in diameter, and pulmonary capillaries could be even narrower [84]. To be free to travel throughout the circulating system, a CTC may have to adopt a small size, or not be markedly larger than PBMCs; otherwise CTCs may not circulate through capillary vessels. The relationship of size-limiting capillary beds and CTC distribution is shown in Fig. 1.
As shown in Fig. 1, a size-limiting structure of the blood circulation system additionally indicates that, unless blood has to be sampled from large internal veins of the patient, it would be difficult to expect that a size-based method could isolate out any pure CTC population. In this regard, a more invasive sampling from large visceral organ-draining veins could indeed catch large cancer cells and even large tumor cell clusters [85], [86], [87], while our group has identified a type of CTCs with a small nucleus from peripheral blood samples, which correlated with the state of castration-resistant progression and metastasis of prostate cancer [45].
The distribution of CTCs in blood circulation is not uniform, but is limited to certain segments of the vasculature. As pointed out in Fig. 1, any CTCs will be filtered against two in-serial capillary beds, which must have enormous anti-tumor capacities for purging all the trapped CTCs. Also, the anatomical site of blood draw has to be considered in CTC research. Arterial blood may be CTC-rich for cancers of the pulmonary circulation, and vena cava blood should be rich for CTCs from other organs perfused by systemic circulation. In any case, because of capillary filtration and the parallel pattern of systemic circulation, peripheral blood always represents a CTC-poor sample (Fig. 1), although peripheral phlebotomy is the most convenient and least invasive sampling route.
As discussed above, the term “CTC” is presently an operational concept, simply denoting any cancer cells being shed from a tumor into blood circulation. Cancer cells are highly plastic and are in dynamic evolution with processes mimetic to stem cell differentiation [88], transdifferentiation [89], or somatic cell fusion [26]. Cancer cell evolution ultimately results in a highly heterogeneous cell population, with individual cells in a given patient displaying a wide spectrum of biophysical, biochemical, gene expressional and behavioral alterations, none of which will be representative of the entire tumor cell population. Similar scenarios of cellular heterogeneity may be present in the CTCs in a patient's blood sample, and any CTC studies based on a predetermined parameter would only detect and isolate a fraction of the whole CTC population.
7. Current status of ex vivo CTC culture
Presently, isolated CTCs are difficult to maintain in a viable state, and are rarely cultured ex vivo into a large population, least of all into stable CTC cell lines. Furthermore, CTCs have been said to be an inherently vulnerable cell type, possibly because the circulating blood may not be an amiable environment for CTC survival [67], [68]. All these confounding factors make the isolation of viable CTCs difficult.
7.1. Studying CTCs without amplification
Because isolated CTCs are not viable and cannot be expanded in numbers by ex vivo culture, most ensuing molecular and genetic investigations on CTCs have to be performed on very limited numbers of CTCs, which yield too few and too little starting materials for extensive and comprehensive examinations. Presently, only a few analytical methods may be adopted for CTC examination, most as nucleotide-based assays, while the starting material has to be pre-amplified with polymerase chain reaction (PCR). Popular CTC assay methods include microarray expression profiling, single-cell sequencing, and quantitative PCR (qPCR) detection.
While single-cell analysis is a cutting-edge advancement, accumulating results suggest that many technological shortcomings of single-cell analyses remain to be overcome. A single cell, for instance, can now be used as starting material for whole exon sequencing with the next-generation sequencing platform, but the result is often suboptimal. As a single run on the platform routinely put out more than one million sequencing reads, the reads from a single-cell analysis are found with frequent base ambiguities, while computational analysis can assign only a small fraction of these reads to a small fraction of encoding genes in the human genome.
The scenario becomes even more problematic for comparative studies, because many assigned reads in one single CTC are not detectable in another single CTC, due to randomness and the sporadic nature of whole exon sequencing with limited starting material [90], [91]. For “profiling” expression in a single CTC, the potential power of next-generation sequencing is severely limited in single-cell sequencing. It would be ideal if limited numbers of CTCs could be amplified into a larger population by ex vivo culture, so comprehensive genomic and genetic analyses could be performed with high reproducibility and accuracy, and then compared confidently among different CTC cultures from individual patients against the stages of disease development and progression.
7.2. CTC culture by short-term cultivation
Several laboratories have subjected isolated CTCs from patient blood samples to short term ex vivo culture. Enriched CTCs from PBMCs in free form or on solid surfaces were cultured ex vivo for 3–14 days. In these studies, choice of culture medium was mostly based on each laboratory's experience, as with long-term culture studies (Table 1).
Reports with short-term culture demonstrated successful ex vivo expansion of isolated cells, as determined by amplification of cell numbers and colony formation [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61]. In many cases, the expanded cells were shown to be suitable subjects for immunohistological analysis, karyotypic examination, and gene expressional profiling.
As discussed above, short-term CTC culture may be compromised by impurity of the CTC isolation or by a distortion of the mixed blood cells. As the limited numbers of colonies grown in short-term culture may alleviate the limitations seen in single-CTC analysis, results of CTC characterization from these studies have to be further validated by additional means, because of the limits on CTC reproducibility. Nonetheless, these results provide supporting evidence that CTCs in patient blood samples can be cultured. With careful optimization, these culture conditions may be applied to successful long-term culture.
7.3. Long-term culture to establish stable CTC lines
It is difficult in general to culture human cells in vitro, and even harder to culture human cells into cell lines. It is similarly difficult to culture CTCs from cancer patient blood samples, even though cancer cells are believed to have evolved survival and proliferation advantages. As summarized in Table 1, there are nonetheless a few reports of long-term culture success, in which CTCs in cancer patient peripheral blood samples have been expanded ex vivo into large population, and a few of these CTCs even showed the capability of continuous passage for numbers of generations [62], [63], [64], [65], suggesting that similar to established cancer cell lines, these CTCs may harbor immortality.
The capability for independent survival and growth is said to be a watershed feature of cancer progression. Tumor cells at advanced stages are thought to be able to survive adversities in their surroundings and proliferate indifferent to regulatory cues in the microenvironment. Paradoxically, many ex vivo CTC cultures resort to the help of cytokines, growth factors, tissue organ extracts, hormones, and small molecules to facilitate CTC survival and growth in vitro [62], [63], [64], [65]. The optimal conditions for ex vivo CTC culture remain to be defined.
Current CTC culture recipes are mostly varied adoptions of stem cell culture conditions. Besides cocktails of conventional cell culture mediums, proprietary mediums are often used in CTC culture, but may not guarantee CTC amplification. In addition to alternative choices of animal serum, different recipes are found using epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF, or FGF2), which are often seen in recipes for stem cell culture. Additional growth factors used include fibroblast growth factor-10 (FGF10), granulocyte macrophage colony-stimulating factor (GM-CSF), insulin and insulin-like growth factor 1 (IGF-1). In some cases, R-spondin 1 protein is added in an attempt to sustain survival and growth of epithelial cells through WNT/β-catenin signaling [92]. In cases when serum-free CTC culture media are used, the recipes mostly include proprietary B27 and N2 supplements, together with insulin, transferrin and selenium (ITS) supplementation.
Other factors that may promote stem cell growth are also used, including dihydrotestosterone (DHT) and nicotinamide. In addition, small molecule inhibitors for specific signaling pathways have been tested for promoting CTC growth. Inhibitors of A83-01 and ALX-270-445, for example, may specifically inhibit the function of TGFβ type 1 receptor kinase (ALK5) and so prevent epithelial to mesenchymal transition and promote CTC survival and proliferation. SB202190, a specific P38 inhibitor, may prevent apoptosis due to P38 activation. Y27632, a cell permeable inhibitor of Rho-associated coiled-coil containing protein kinase (ROCK), is used in CTC culture to promote organoid growth and formation.
The most interesting phenomena in all the published CTC culture studies is how few of the cultured CTCs display any expected morphologies or behaviors of an epithelial lineage. Instead, most of the successful cultures yielded CTCs growing in suspension in 3-dimensions (3-D), aggregating to a spheroid or organoid organization. Intriguingly, these morphologic and behavioral features are commonly seen with stem cell culture, and are taken as a sign of stemness [93], [94]. Though it remains to be determined whether CTCs amplified by ex vivo culture are cancer stem cells, these results confirmed that CTCs represent cancer cells that have completely lost epithelial marker expression.
In addition to conventional suspension culture setups, more ex vivo CTC cultures are adopting various 3-D formats. Soft agar colony formation, non-adherent culture, and spheroid or organoid culture on growth factor-reduced Matrigel matrix are popular CTC culture systems. To induce survival responses in CTCs, hypoxic culture conditions or consecutive culture in hypoxic and normoxic environment are used.
It should be noted that, because optimal CTC culture condition has yet to be defined, current research relies on stem cell culture methods to ensure a maximal CTC expansion. Each protocol is designed based on the researcher's own expertise and empirical results, not by a rationale based on CTC biological features, which are presently not defined. Published protocols yielded very low success rates, generally less than 5% of the patient blood samples cultured. Rationale-based modifications should be tested to improve the success rate.
One of the future modifications probably should be a reduction or complete avoidance of exogenous growth factors and small molecules in CTC culture, because CTCs grown with the support of these factors may have limited applications for the mechanistic investigation of cancer progression and metastasis. Cancer cells already have acquired growth and survival autonomy. On the other hand, cancer metastasis, homing, and colonization are characterized by cancer cell interaction with resident cells of the mesenchymal lineage in the tumor microenvironment, and the interaction has been shown to be mediated by growth factors and can be modulated by small molecule signaling pathway inhibitors. CTCs cultured in the presence of growth factors and small molecule inhibitors thus may not be ideal subjects for modeling cancer and stromal interaction.
8. Our experience with ex vivo CTC culture
Our laboratories study the mechanism of cancer progression and metastasis. As CTCs are plausible mediators in distant tumor cell spreading, we used mouse xenograft models to track CTCs during metastasis [39], [95]. In recent years, we have established close collaborations with clinical oncologists and surgeons in translational studies with clinical blood samples from cancer patients. These intercalated research interests led to a platform for translational research on critical issues relevant to the welfare of cancer patients.
8.1. Modeling CTC culture with experimental animals
We initially carried out studies in tumor-bearing mice to confirm that there indeed were CTCs in animal blood. To facilitate tracking live CTCs, we tagged the ARCaPE human prostate cancer cells with a stable expression of red fluorescence protein (RFP). Selected ARCaPE-RFP clones were inoculated orthotopically to the prostates of athymic mice for xenograft tumor formation, which caused a wide spread of cancer cells to internal organs in addition to skeleton [39], [95]. In one of the experiments, intra-cardiac blood samples were observed to contain red fluorescent cells, indicating that metastasis of the xenograft tumor was mediated by these cells.
We then drew blood from the tumor-bearing mice and tested a basic protocol for expanding CTCs through ex vivo culture [39]. This series of experiments led to the development of a simple and cost-effective protocol, with which CTCs in a drop of blood (about 75 μL) from either a tail cut or from the left ventricle were enough to form multiple colonies of red fluorescent cells. Interestingly, these colonies were markedly different from parental ARCaPE-RFP cells in both morphological, behavioral and gene expressional senses; and the changes were irreversible, with CTC-derived clones losing most epithelial features and acquiring conspicuous mesenchymal cell traits. We subsequently repeated the ex vivo CTC culture with a different xenograft model, in which RANKL overexpression converted androgen-dependent non-tumorigenic LNCaP prostate cancer cells to androgen-independent, highly tumorigenic, metastatic cells [25]. From these mice, blood samples from retro-orbital sinus, submandibular vein, tail cut and left ventricle could all be cultured to form cancer cell colonies (unpublished data). These preliminary results prompted us to test CTC ex vivo culture from clinical cancer patient blood samples.
8.2. Modification of PBMC isolation
We obtained cancer patient samples in the form of packed blood cells, the cellular fraction after plasma was taken for clinical use. In some cases, both blood and surgical tumor from the same patient were sampled and subjected to identical ex vivo culture, in order to validate the culture method.
We first tested if CTCs were present in cancer patient peripheral blood as well. PBMCs were isolated from prostate cancer patient peripheral blood samples with two methods. With the first method, density gradient banding in Ficoll-Paque 1077 or 1084 was used to isolate the monocyte and lymphocyte fraction. Candidate CTCs in this fraction were detected with fluorescein-conjugated anti-EpCAM antibody, together with NIR-783, a prototype heptamethine carbocyanine near infrared organic dye, which we have determined to have tumor cell specificity [96]. This examination demonstrated CTC-like signals in the patient blood, and the count of these cells correlated well with disease progression in general trends [42]. These results confirmed that there were detectable numbers of CTCs in blood samples of prostate cancer patients, especially in those with advanced disease.
On the other hand, this series of experiments revealed that density gradient centrifugation was not an ideal method for isolating all CTCs from a given patient blood sample, because after removal of the monocyte and lymphocyte band, additional EpCAM+NIR+ signals could be detected from the remaining heavier Ficoll-Paque fractions. The signal was even detected from the heaviest erythrocyte pellet. This finding indicated that in a given cancer patient blood sample, heterogeneous CTCs might vary in density, making gradient fractionation a less than optimal method for comprehensive isolation of all CTCs from the sample.
With the Ficoll-Paque isolated PBMCs, we additionally tested whether CTCs could be sorted from PBMCs by flow cytometry. Though anti-EpCAM antibody and NIR-783 stained cells could be isolated by flow cytometric sorting in all the patient blood samples tested, the number of sorted cells was mostly too low and too diluted in sorting fluid for successful ex vivo culture.
The second method we tested was whole PBMC isolation by ammonium chloride hemolysis. Because it recovers all the nucleated cells from a blood sample in a cost-effective and highly reproducible manner, ammonium chloride hemolysis is now becoming the mainstay of PBMC isolation. To assess whether it could be a favorable method for ex vivo CTC culture, we found that some hemolysis formulas had marked time-dependent toxicity to PBMCs and even to established human prostate cancer cell lines. Accordingly, we evaluated a series of formulas to identify a modified recipe, which was potent for hemolysis but carried little cytotoxicity to nucleated cells. In subsequent CTC cultures, this modified PBMC isolation protocol has been found to be markedly cost-effective, less labor intensive, and highly reproducible.
8.3. Culturing entire PBMC populations for ex vivo CTC expansion
As discussed above, the process of isolation either affected CTC viability or yielded a mixed cell population. In addition, CTC isolation is a costly practice not suitable to large scale studies or routine CTC culture. We reasoned that the isolation step may not be essential to ex vivo culture of CTCs, if these specified cancer cells indeed had survival and proliferation advantages over other constituent cells in PBMCs. We explored the possibility of culturing CTCs by skipping the isolation step.
It is well known that most blood cells of hematopoietic lineage are terminally differentiated and have lost proliferation capability. Though the T and B lymphocytes harbor proliferation potential, proliferation has to be triggered by specific antigen receptor ligation [97], by a Ca2+ influx together with a PKC activation [98], or by super-antigen cross-linking [99]. Moreover, the activated lymphocyte proliferation is transient, ending with a programmed activation-induced cell death [100], [101], [102]. In general, without additional stimulation, PBMCs in resting state would perish in ex vivo culture within days, each cell type dying in a programmed manner. Macrophages are the only cell type in PBMCs capable of survival for weeks in culture. Macrophages, however, are also a terminally differentiated cell type incapable of ex vivo proliferation. The constant presence of some macrophages may not affect the survival and proliferation of non-hematopoietic cells, including CTCs. These observations suggested that CTCs could be amplified naturally by culturing whole PBMCs, should CTCs indeed have survival and proliferation advantages.
8.4. Choosing a culture medium
Different from culturing immortalized cell lines, which would survive and proliferate in vitro under varied culture conditions, culturing primary human cells is thought to require specific culture conditions. For ex vivo CTC culture, however, an optimal culture medium has yet to be defined, because we do not know the preferred conditions for CTC survival and proliferation, and because we are not even sure whether the determinants for CTC survival and growth reside within the cell or outside the cell.
To choose a practical medium formula for ex vivo CTC culture, we tested various culture mediums and conditions, with reference to published methods for various primary human cells. We concluded that, though nutrition-rich or stem cell culture mediums may prolong the survival of primary tumor cells, some PBMCs, and probably CTCs, none promoted cellular proliferation. Rather, the subject cells adopted a “blast” morphology but undertook little cell division. In contrast, some regular recipes for routine maintenance of commonly used cancer cell lines were amiable for the proliferation of CTC-like cells, which could survive and grow into large population, some even showing immortality behaviors, capable of proliferation beyond 60 continuous passages [103], [104].
Our choice of conventional medium for CTC culture was also supported by our results culturing surgical tumor specimens from cancer patients. We have established new prostate cancer and pancreatic cancer cell lines by culturing clinical surgical tumor specimens (unpublished data), and these cell lines are currently being characterized. As both nutrition-rich stem cell culture mediums and conventional mediums facilitated outgrowth of tumor cells, we found that immortalized cancer cell lines could only be established from cultures in conventional medium, albeit at low success rates. These observations strongly suggested that, for primary human cell culture, survival and proliferation is determined by mechanisms inside the cell, rather than by the conditions of the ex vivo culture.
8.5. Establishing stable cultures of CTC-like cell preparations
Using our ex vivo culture protocol, we have accumulated a panel of immortalized cells from clinical cancer patient blood samples. While these cells have yet to be fully characterized, results from xenograft tumor formation have confirmed their malignancy to be similar to the patients' primary disease (unpublished data). With a cost-effective and straightforward method established, further fine tuning of culture conditions and standardizing phlebotomy and sample preparation protocols may markedly improve the success rates of CTC culture. Additionally, we are identifying biomarkers from the established CTC-like cell lines. Through CTC biomarker detection from prospective patient blood samples, these CTC-like cell lines will be validated as true CTCs. It is rational to predict that in the future these markers will serve well as surrogates for clinical CTC detection during cancer diagnosis, treatment and prognosis, so time-consuming CTC culture from every cancer patient would not always be needed for precision oncology and personalized cancer therapy.
Though ex vivo CTC survival and proliferation are determined by endogenous mechanisms, our preliminary studies suggested that these abilities could be modulated by exogenous growth factors and specific small molecule pathway inhibitors, similar to published observations by other laboratories. The application of exogenous factors, however, could not increase the success rate of CTC culture and might introduce undesired complications to further investigation. For instance, upon intra-cardiac inoculation, tissue-specificity of homing and colonization of the inoculated CTCs in host mice would be determined by exogenous cues in the tumor microenvironment [8]. We reasoned that addition of growth factors in the ex vivo CTC expansion step may pre-condition these cells into a peculiar state, and thus complicate data interpretation in studies particularly relevant to CTC colonization at secondary organ sites.
8.6. Establishment of CTC-derived xenograft tumor models
Our ex vivo culture has resulted in the establishment of a panel of CTC cultures from peripheral blood samples of both pre-surgical and non-resectable pancreatic cancer cases, and also from both primary pre-surgical and castration-resistant and metastatic prostate cancer patients. Besides determining the malignant characteristics of these CTCs in vitro, we assessed the tumorigenicity of each of these preparations with a CTC-derived xenograft (CDX) model. CTCs in mixture with Matrigel were inoculated subcutaneously, intracardially or orthotopically to the pancreas or the prostate of athymic mice. Prevalent and rapid xenograft tumor formation and lethality were observed in host mice, confirming the malignant nature of these cells [103], [104]. Importantly, the tumor-bearing mice displayed frequent and multiple metastases in distant organs. As it is well known that human cancer cell xenograft tumors rarely cause metastasis in experimental mice, these CDX models may have inherited the highly invasive and metastatic nature of the patients' in situ tumor. Characterizations of the CDX models, in the context of correlation with the original clinical specimens, and evaluation of their application in prostate and pancreatic cancer translational research are underway.
9. Conclusion
In this review, we critically examined the major steps of ex vivo culture protocols, to identify the technical challenges to successful CTC amplification from cancer patient blood samples. We think that the most critical issue facing CTC research is the lack of information on the fundamental characteristics of CTCs in terms of their biophysical, biochemical, gene expressional, and behavioral features. Due to this lack of information, no reliable methods can yet be designed for isolating CTCs from vast numbers of blood cells. A full illustration of the fundamental characteristics of CTCs is an urgent topic in cancer research.
In keeping with overall cancer cell heterogeneity, CTCs may be highly heterogeneous as well. Because it is challenging to isolate all the heterogeneous CTCs from a given patient peripheral blood, in our laboratory we skipped the CTC isolation step by culturing whole PBMCs in order to amplify CTCs, relying on their survival and proliferation advantages. Using this strategy, we have established a panel of CTC-like cell lines from pancreatic, prostate, kidney, breast and gastric cancer patient blood samples. Full characterizations of these cells are underway. These molecular interrogations could lead to the identification of CTC biomarkers and molecular signaling network underlying therapeutic responses and resistance observed in patients.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgments
This work is supported by US NIH/NCI research grant (2PO1CA098912), Cedars-Sinai Medical Center Board of Governors Cancer Research Chair (LWKC), and US NIH/NCI (UO1CA198900) (HRT/EMP).
Footnotes
Peer review under responsibility of Second Military Medical University.
References
- 1.Dubey A.K., Gupta U., Jain S. Epidemiology of lung cancer and approaches for its prediction: a systematic review and analysis. Chin J Cancer. 2016;35:71. doi: 10.1186/s40880-016-0135-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanna N., Einhorn L.H. Testicular cancer: a reflection on 50 years of discovery. J Clin Oncol. 2014;32:3085–3092. doi: 10.1200/JCO.2014.56.0896. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 4.Cresswell G.D., Apps J.R., Chagtai T., Mifsud B., Bentley C.C., Maschietto M. Intra-tumor genetic heterogeneity in wilms tumor: clonal evolution and clinical implications. EBioMedicine. 2016;9:120–129. doi: 10.1016/j.ebiom.2016.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parker J.S., Perou C.M. Tumor heterogeneity: focus on the leaves, the trees, or the forest? Cancer Cell. 2015;28:149–150. doi: 10.1016/j.ccell.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 6.Sun X.X., Yu Q. Intra-tumor heterogeneity of cancer cells and its implications for cancer treatment. Acta Pharmacol Sin. 2015;36:1219–1227. doi: 10.1038/aps.2015.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung L.W., Huang W.C., Sung S.Y., Wu D., Odero-Marah V., Nomura T. Stromal-epithelial interaction in prostate cancer progression. Clin Genitourin Cancer. 2006;5:162–170. doi: 10.3816/CGC.2006.n.034. [DOI] [PubMed] [Google Scholar]
- 8.Sung S.Y., Hsieh C.L., Law A., Zhau H.E., Pathak S., Multani A.S. Coevolution of prostate cancer and bone stroma in three-dimensional coculture: implications for cancer growth and metastasis. Cancer Res. 2008;68:9996–10003. doi: 10.1158/0008-5472.CAN-08-2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chappard D., Bouvard B., Basle M.F., Legrand E., Audran M. Bone metastasis: histological changes and pathophysiological mechanisms in osteolytic or osteosclerotic localizations. A review. Morphologie. 2011;95:65–75. doi: 10.1016/j.morpho.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Miles F.L., Sikes R.A. Insidious changes in stromal matrix fuel cancer progression. Mol Cancer Res. 2014;12:297–312. doi: 10.1158/1541-7786.MCR-13-0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan Y. Spatial heterogeneity in the tumor microenvironment. Cold Spring Harb Perspect Med. 2016;6 doi: 10.1101/cshperspect.a026583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liotta L.A., Kohn E.C. The microenvironment of the tumour-host interface. Nature. 2001;411:375–379. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- 13.Place A.E., Jin Huh S., Polyak K. The microenvironment in breast cancer progression: biology and implications for treatment. Breast Cancer Res. 2011;13:227. doi: 10.1186/bcr2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schofield P.E., Stockler M.R., Zannino D., Tebbutt N.C., Price T.J., Simes R.J. Hope, optimism and survival in a randomised trial of chemotherapy for metastatic colorectal cancer. Support Care Cancer. 2016;24:401–408. doi: 10.1007/s00520-015-2792-8. [DOI] [PubMed] [Google Scholar]
- 15.Seah D.S., Scott S., Guo H., Najita J., Lederman R., Frank E. Variation in the attitudes of medical oncologists toward research biopsies in patients with metastatic breast cancer. Oncologist. 2015;20:992–1000. doi: 10.1634/theoncologist.2015-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanriverdi O., Yavuzsen T., Akman T., Senler F.C., Taskoylu B.Y., Turhal S. The perspective of non-oncologist physicians on patients with metastatic cancer and palliative care (ALONE study): a study of the palliative care working committee of the Turkish oncology group (TOG) J Cancer Educ. 2015;30:253–259. doi: 10.1007/s13187-015-0794-3. [DOI] [PubMed] [Google Scholar]
- 17.Mohseny A.B., Hogendoorn P.C. Concise review: mesenchymal tumors: when stem cells go mad. Stem Cells. 2011;29:397–403. doi: 10.1002/stem.596. [DOI] [PubMed] [Google Scholar]
- 18.Pinchuk I.V., Mifflin R.C., Saada J.I., Powell D.W. Intestinal mesenchymal cells. Curr Gastroenterol Rep. 2010;12:310–318. doi: 10.1007/s11894-010-0135-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maheswaran S., Haber D.A. Ex vivo culture of CTCs: an emerging resource to guide cancer therapy. Cancer Res. 2015;75:2411–2415. doi: 10.1158/0008-5472.CAN-15-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centenera M.M., Raj G.V., Knudsen K.E., Tilley W.D., Butler L.M. Ex vivo culture of human prostate tissue and drug development. Nat Rev Urol. 2013;10:483–487. doi: 10.1038/nrurol.2013.126. [DOI] [PubMed] [Google Scholar]
- 21.Mitra A., Mishra L., Li S. Technologies for deriving primary tumor cells for use in personalized cancer therapy. Trends Biotechnol. 2013;31:347–354. doi: 10.1016/j.tibtech.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hwang C.I., Boj S.F., Clevers H., Tuveson D.A. Preclinical models of pancreatic ductal adenocarcinoma. J Pathol. 2016;238:197–204. doi: 10.1002/path.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deer E.L., Gonzalez-Hernandez J., Coursen J.D., Shea J.E., Ngatia J., Scaife C.L. Phenotype and genotype of pancreatic cancer cell lines. Pancreas. 2010;39:425–435. doi: 10.1097/MPA.0b013e3181c15963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gunderson K., Wang C.Y., Wang R. Global prostate cancer incidence and the migration, settlement, and admixture history of the Northern Europeans. Cancer Epidemiol. 2011;35:320–327. doi: 10.1016/j.canep.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chu G.C., Zhau H.E., Wang R., Rogatko A., Feng X., Zayzafoon M. RANK- and c-Met-mediated signal network promotes prostate cancer metastatic colonization. Endocr Relat Cancer. 2014;21:311–326. doi: 10.1530/ERC-13-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang R., Sun X., Wang C.Y., Hu P., Chu C.Y., Liu S. Spontaneous cancer-stromal cell fusion as a mechanism of prostate cancer androgen-independent progression. PLoS One. 2012;7:e42653. doi: 10.1371/journal.pone.0042653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang R., Xu J., Juliette L., Castilleja A., Love J., Sung S.Y. Three-dimensional co-culture models to study prostate cancer growth, progression, and metastasis to bone. Semin Cancer Biol. 2005;15:353–364. doi: 10.1016/j.semcancer.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 28.Zhau H.E., Goodwin T.J., Chang S.M., Baker T.L., Chung L.W. Establishment of a three-dimensional human prostate organoid coculture under microgravity-simulated conditions: evaluation of androgen-induced growth and PSA expression. In Vitro Cell Dev Biol Anim. 1997;33:375–380. doi: 10.1007/s11626-997-0008-3. [DOI] [PubMed] [Google Scholar]
- 29.Zhau H.E., He H., Wang C.Y., Zayzafoon M., Morrissey C., Vessella R.L. Human prostate cancer harbors the stem cell properties of bone marrow mesenchymal stem cells. Clin Cancer Res. 2011;17:2159–2169. doi: 10.1158/1078-0432.CCR-10-2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pathak S., Nemeth M.A., Multani A.S., Thalmann G.N., von Eschenbach A.C., Chung L.W. Can cancer cells transform normal host cells into malignant cells? Br J Cancer. 1997;76:1134–1138. doi: 10.1038/bjc.1997.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shiao S.L., Chu G.C., Chung L.W. Regulation of prostate cancer progression by the tumor microenvironment. Cancer Lett. 2016;380:340–348. doi: 10.1016/j.canlet.2015.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beketic-Oreskovic L., Maric P., Ozretic P., Oreskovic D., Ajdukovic M., Levanat S. Assessing the clinical significance of tumor markers in common neoplasms. Front Biosci Elite Ed. 2012;4:2558–2578. doi: 10.2741/e566. [DOI] [PubMed] [Google Scholar]
- 33.Laughney A.M., Elizalde S., Genovese G., Bakhoum S.F. Dynamics of tumor heterogeneity derived from clonal karyotypic evolution. Cell Rep. 2015;12:809–820. doi: 10.1016/j.celrep.2015.06.065. [DOI] [PubMed] [Google Scholar]
- 34.Masramon L., Vendrell E., Tarafa G., Capella G., Miro R., Ribas M. Genetic instability and divergence of clonal populations in colon cancer cells in vitro. J Cell Sci. 2006;119:1477–1482. doi: 10.1242/jcs.02871. [DOI] [PubMed] [Google Scholar]
- 35.Fidler I.J., Gersten D.M., Hart I.R. The biology of cancer invasion and metastasis. Adv Cancer Res. 1978;28:149–250. doi: 10.1016/s0065-230x(08)60648-x. [DOI] [PubMed] [Google Scholar]
- 36.Warner T.F., Krueger R.G. Circulating lymphocytes and the spread of myeloma. Review of the evidence. Lancet. 1978;1:1174–1176. doi: 10.1016/s0140-6736(78)90966-2. [DOI] [PubMed] [Google Scholar]
- 37.Weiss L., Ward P.M. Cell detachment and metastasis. Cancer Metastasis Rev. 1983;2:111–127. doi: 10.1007/BF00048965. [DOI] [PubMed] [Google Scholar]
- 38.Feo-Zuppardi F.J., Taylor C.W., Iwato K., Lopez M.H., Grogan T.M., Odeleye A. Long-term engraftment of fresh human myeloma cells in SCID mice. Blood. 1992;80:2843–2850. [PubMed] [Google Scholar]
- 39.He H., Yang X., Davidson A.J., Wu D., Marshall F.F., Chung L.W. Progressive epithelial to mesenchymal transitions in ARCaPE prostate cancer cells during xenograft tumor formation and metastasis. Prostate. 2010;70:518–528. doi: 10.1002/pros.21086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang X., Shao C., Wang R., Chu C.Y., Hu P., Master V. Optical imaging of kidney cancer with novel near infrared heptamethine carbocyanine fluorescent dyes. J Urol. 2013;189:702–710. doi: 10.1016/j.juro.2012.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang X., Qian X., Beitler J.J., Chen Z.G., Khuri F.R., Lewis M.M. Detection of circulating tumor cells in human peripheral blood using surface-enhanced Raman scattering nanoparticles. Cancer Res. 2011;71:1526–1532. doi: 10.1158/0008-5472.CAN-10-3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shao C., Liao C.P., Hu P., Chu C.Y., Zhang L., Bui M.H. Detection of live circulating tumor cells by a class of near-infrared heptamethine carbocyanine dyes in patients with localized and metastatic prostate cancer. PLoS One. 2014;9:e88967. doi: 10.1371/journal.pone.0088967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu S., Tian Z., Zhang L., Hou S., Hu S., Wu J. Combined cell surface carbonic anhydrase 9 and CD147 antigens enable high-efficiency capture of circulating tumor cells in clear cell renal cell carcinoma patients. Oncotarget. August 1 2016 doi: 10.18632/oncotarget.10979. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ankeny J.S., Court C.M., Hou S., Li Q., Song M., Wu D. Circulating tumour cells as a biomarker for diagnosis and staging in pancreatic cancer. Br J Cancer. 2016;114:1367–1375. doi: 10.1038/bjc.2016.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen J.F., Ho H., Lichterman J., Lu Y.T., Zhang Y., Garcia M.A. Subclassification of prostate cancer circulating tumor cells by nuclear size reveals very small nuclear circulating tumor cells in patients with visceral metastases. Cancer. 2015;121:3240–3251. doi: 10.1002/cncr.29455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ignatiadis M., Riethdorf S., Bidard F.C., Vaucher I., Khazour M., Rothe F. International study on inter-reader variability for circulating tumor cells in breast cancer. Breast Cancer Res. 2014;16:R43. doi: 10.1186/bcr3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Court C.M., Ankeny J.S., Sho S., Hou S., Li Q., Hsieh C. Reality of single circulating tumor cell sequencing for molecular diagnostics in pancreatic cancer. J Mol Diagn. 2016;18:688–696. doi: 10.1016/j.jmoldx.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bobek V., Gurlich R., Eliasova P., Kolostova K. Circulating tumor cells in pancreatic cancer patients: enrichment and cultivation. World J Gastroenterol. 2014;20:17163–17170. doi: 10.3748/wjg.v20.i45.17163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bobek V., Kacprzak G., Rzechonek A., Kolostova K. Detection and cultivation of circulating tumor cells in malignant pleural mesothelioma. Anticancer Res. 2014;34:2565–2569. [PubMed] [Google Scholar]
- 50.Bobek V., Matkowski R., Gurlich R., Grabowski K., Szelachowska J., Lischke R. Cultivation of circulating tumor cells in esophageal cancer. Folia Histochem Cytobiol. 2014;52:171–177. doi: 10.5603/FHC.2014.0020. [DOI] [PubMed] [Google Scholar]
- 51.Cegan M., Kolostova K., Matkowski R., Broul M., Schraml J., Fiutowski M. In vitro culturing of viable circulating tumor cells of urinary bladder cancer. Int J Clin Exp Pathol. 2014;7:7164–7171. [PMC free article] [PubMed] [Google Scholar]
- 52.Kolostova K., Broul M., Schraml J., Cegan M., Matkowski R., Fiutowski M. Circulating tumor cells in localized prostate cancer: isolation, cultivation in vitro and relationship to T-stage and Gleason score. Anticancer Res. 2014;34:3641–3646. [PubMed] [Google Scholar]
- 53.Kolostova K., Cegan M., Bobek V. Circulating tumour cells in patients with urothelial tumours: enrichment and in vitro culture. Can Urol Assoc J. 2014;8:E715–E720. doi: 10.5489/cuaj.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kolostova K., Matkowski R., Gurlich R., Grabowski K., Soter K., Lischke R. Detection and cultivation of circulating tumor cells in gastric cancer. Cytotechnology. 2016;68:1095–1102. doi: 10.1007/s10616-015-9866-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kolostova K., Spicka J., Matkowski R., Bobek V. Isolation, primary culture, morphological and molecular characterization of circulating tumor cells in gynecological cancers. Am J Transl Res. 2015;7:1203–1213. [PMC free article] [PubMed] [Google Scholar]
- 56.Kolostova K., Zhang Y., Hoffman R.M., Bobek V. In vitro culture and characterization of human lung cancer circulating tumor cells isolated by size exclusion from an orthotopic nude-mouse model expressing fluorescent protein. J Fluoresc. 2014;24:1531–1536. doi: 10.1007/s10895-014-1439-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Malara N., Trunzo V., Foresta U., Amodio N., De Vitis S., Roveda L. Ex-vivo characterization of circulating colon cancer cells distinguished in stem and differentiated subset provides useful biomarker for personalized metastatic risk assessment. J Transl Med. 2016;14:133. doi: 10.1186/s12967-016-0876-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sheng W., Ogunwobi O.O., Chen T., Zhang J., George T.J., Liu C. Capture, release and culture of circulating tumor cells from pancreatic cancer patients using an enhanced mixing chip. Lab Chip. 2014;14:89–98. doi: 10.1039/c3lc51017d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen J.Y., Tsai W.S., Shao H.J., Wu J.C., Lai J.M., Lu S.H. Sensitive and specific biomimetic lipid coated microfluidics to isolate viable circulating tumor cells and microemboli for cancer detection. PLoS One. 2016;11:e0149633. doi: 10.1371/journal.pone.0149633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Z., Shiratsuchi H., Lin J., Chen G., Reddy R.M., Azizi E. Expansion of CTCs from early stage lung cancer patients using a microfluidic co-culture model. Oncotarget. 2014;5:12383–12397. doi: 10.18632/oncotarget.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paris P.L., Kobayashi Y., Zhao Q., Zeng W., Sridharan S., Fan T. Functional phenotyping and genotyping of circulating tumor cells from patients with castration resistant prostate cancer. Cancer Lett. 2009;277:164–173. doi: 10.1016/j.canlet.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 62.Yu M., Bardia A., Aceto N., Bersani F., Madden M.W., Donaldson M.C. Cancer therapy. Ex vivo culture of circulating breast tumor cells for individualized testing of drug susceptibility. Science. 2014;345:216–220. doi: 10.1126/science.1253533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cayrefourcq L., Mazard T., Joosse S., Solassol J., Ramos J., Assenat E. Establishment and characterization of a cell line from human circulating colon cancer cells. Cancer Res. 2015;75:892–901. doi: 10.1158/0008-5472.CAN-14-2613. [DOI] [PubMed] [Google Scholar]
- 64.Gao D., Vela I., Sboner A., Iaquinta P.J., Karthaus W.R., Gopalan A. Organoid cultures derived from patients with advanced prostate cancer. Cell. 2014;159:176–187. doi: 10.1016/j.cell.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hamilton G., Burghuber O., Zeillinger R. Circulating tumor cells in small cell lung cancer: ex vivo expansion. Lung. 2015;193:451–452. doi: 10.1007/s00408-015-9725-7. [DOI] [PubMed] [Google Scholar]
- 66.Court C.M., Ankeny J.S., Sho S., Tomlinson J.S. Circulating tumor cells in gastrointestinal cancer: current practices and future directions. In: Bentrem D., Benson A.B., editors. Springer International Publishing; 2016. pp. 345–376. (Cancer Treatment and Research. Gastrointentional malignancies). [DOI] [PubMed] [Google Scholar]
- 67.den Toonder J. Circulating tumor cells: the grand challenge. Lab Chip. 2011;11:375–377. doi: 10.1039/c0lc90100h. [DOI] [PubMed] [Google Scholar]
- 68.van de Stolpe A., Pantel K., Sleijfer S., Terstappen L.W., den Toonder J.M. Circulating tumor cell isolation and diagnostics: toward routine clinical use. Cancer Res. 2011;71:5955–5960. doi: 10.1158/0008-5472.CAN-11-1254. [DOI] [PubMed] [Google Scholar]
- 69.Kelbaek H. Sterile isolation of polymorphonuclear leukocytes from large blood volumes. J Clin Chem Clin Biochem. 1985;23:17–20. [PubMed] [Google Scholar]
- 70.Zipursky A., Bow E., Seshadri R.S., Brown E.J. Leukocyte density and volume in normal subjects and in patients with acute lymphoblastic leukemia. Blood. 1976;48:361–371. [PubMed] [Google Scholar]
- 71.Martowicz A., Seeber A., Untergasser G. The role of EpCAM in physiology and pathology of the epithelium. Histol Histopathol. 2016;31:349–355. doi: 10.14670/HH-11-678. [DOI] [PubMed] [Google Scholar]
- 72.Schnell U., Cirulli V., Giepmans B.N. EpCAM: structure and function in health and disease. Biochim Biophys Acta. 2013;1828:1989–2001. doi: 10.1016/j.bbamem.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 73.Fong D., Moser P., Kasal A., Seeber A., Gastl G., Martowicz A. Loss of membranous expression of the intracellular domain of EpCAM is a frequent event and predicts poor survival in patients with pancreatic cancer. Histopathology. 2014;64:683–692. doi: 10.1111/his.12307. [DOI] [PubMed] [Google Scholar]
- 74.Gosens M.J., van Kempen L.C., van de Velde C.J., van Krieken J.H., Nagtegaal I.D. Loss of membranous Ep-CAM in budding colorectal carcinoma cells. Mod Pathol. 2007;20:221–232. doi: 10.1038/modpathol.3800733. [DOI] [PubMed] [Google Scholar]
- 75.Songun I., Litvinov S.V., van de Velde C.J., Pals S.T., Hermans J., van Krieken J.H. Loss of Ep-CAM (CO17-1A) expression predicts survival in patients with gastric cancer. Br J Cancer. 2005;92:1767–1772. doi: 10.1038/sj.bjc.6602519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Beuran M., Negoi I., Paun S., Ion A.D., Bleotu C., Negoi R.I. The epithelial to mesenchymal transition in pancreatic cancer: a systematic review. Pancreatology. 2015;15:217–225. doi: 10.1016/j.pan.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 77.May C.D., Sphyris N., Evans K.W., Werden S.J., Guo W., Mani S.A. Epithelial-mesenchymal transition and cancer stem cells: a dangerously dynamic duo in breast cancer progression. Breast Cancer Res. 2011;13:202. doi: 10.1186/bcr2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.May M., Brookman-May S., Burger M., Koch S., Otto W., Brundl J. A switch from epithelial to mesenchymal properties correlates with lymphovascular invasion in squamous cell carcinoma of the penis. Pathol Res Pract. 2015;211:641–645. doi: 10.1016/j.prp.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 79.Liao M.Y., Lai J.K., Kuo M.Y., Lu R.M., Lin C.W., Cheng P.C. An anti-EpCAM antibody EpAb2-6 for the treatment of colon cancer. Oncotarget. 2015;6:24947–24968. doi: 10.18632/oncotarget.4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ciraci E., Della Bella S., Salvucci O., Rofani C., Segarra M., Bason C. Adult human circulating CD34–Lin–CD45–CD133– cells can differentiate into hematopoietic and endothelial cells. Blood. 2011;118:2105–2115. doi: 10.1182/blood-2010-10-316596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Anversa P., Kajstura J., Leri A. Circulating progenitor cells: search for an identity. Circulation. 2004;110:3158–3160. doi: 10.1161/01.CIR.0000148679.30170.78. [DOI] [PubMed] [Google Scholar]
- 82.Williams E.S., Rodriguez-Bravo V., Chippada-Venkata U., De Ia Iglesia-Vicente J., Gong Y., Galsky M. Generation of prostate cancer patient derived xenograft models from circulating tumor cells. J Vis Exp. 2015;105:53182. doi: 10.3791/53182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Preffer F.I., Dombkowski D., Sykes M., Scadden D., Yang Y.G. Lineage-negative side-population (SP) cells with restricted hematopoietic capacity circulate in normal human adult blood: immunophenotypic and functional characterization. Stem Cells. 2002;20:417–427. doi: 10.1634/stemcells.20-5-417. [DOI] [PubMed] [Google Scholar]
- 84.Townsley M.I. Structure and composition of pulmonary arteries, capillaries, and veins. Compr Physiol. 2012;2:675–709. doi: 10.1002/cphy.c100081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hart I.R. New evidence for tumour embolism as a mode of metastasis. J Pathol. 2009;219:275–276. doi: 10.1002/path.2616. [DOI] [PubMed] [Google Scholar]
- 86.Tien Y.W., Kuo H.C., Ho B.I., Chang M.C., Chang Y.T., Cheng M.F. A high circulating tumor cell count in portal vein predicts liver metastasis from periampullary or pancreatic cancer: a high portal venous CTC count predicts liver metastases. Med Baltim. 2016;95:e3407. doi: 10.1097/MD.0000000000003407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Catenacci D.V., Chapman C.G., Xu P., Koons A., Konda V.J., Siddiqui U.D. Acquisition of portal venous circulating tumor cells from patients with pancreaticobiliary cancers by endoscopic ultrasound. Gastroenterology. 2015;149 doi: 10.1053/j.gastro.2015.08.050. 1794–803.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Marjanovic N.D., Weinberg R.A., Chaffer C.L. Cell plasticity and heterogeneity in cancer. Clin Chem. 2013;59:168–179. doi: 10.1373/clinchem.2012.184655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ansieau S. EMT in breast cancer stem cell generation. Cancer Lett. 2013;338:63–68. doi: 10.1016/j.canlet.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 90.Hou Y., Wu K., Shi X., Li F., Song L., Wu H. Comparison of variations detection between whole-genome amplification methods used in single-cell resequencing. Gigascience. 2015;4:37. doi: 10.1186/s13742-015-0068-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liang J., Cai W., Sun Z. Single-cell sequencing technologies: current and future. J Genet Genomics. 2014;41:513–528. doi: 10.1016/j.jgg.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 92.de Lau W.B., Snel B., Clevers H.C. The R-spondin protein family. Genome Biol. 2012;13:242. doi: 10.1186/gb-2012-13-3-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mueller-Klieser W. Multicellular spheroids. A review on cellular aggregates in cancer research. J Cancer Res Clin Oncol. 1987;113:101–122. doi: 10.1007/BF00391431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sutherland R.M., Durand R.E. Growth and cellular characteristics of multicell spheroids. Recent Results Cancer Res. 1984;95:24–49. doi: 10.1007/978-3-642-82340-4_2. [DOI] [PubMed] [Google Scholar]
- 95.Xu J., Wang R., Xie Z.H., Odero-Marah V., Pathak S., Multani A. Prostate cancer metastasis: role of the host microenvironment in promoting epithelial to mesenchymal transition and increased bone and adrenal gland metastasis. Prostate. 2006;66:1664–1673. doi: 10.1002/pros.20488. [DOI] [PubMed] [Google Scholar]
- 96.Yang X., Shi C., Tong R., Qian W., Zhau H.E., Wang R. Near IR heptamethine cyanine dye-mediated cancer imaging. Clin Cancer Res. 2010;16:2833–2844. doi: 10.1158/1078-0432.CCR-10-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Berridge M.J. Lymphocyte activation in health and disease. Crit Rev Immunol. 1997;17:155–178. doi: 10.1615/critrevimmunol.v17.i2.30. [DOI] [PubMed] [Google Scholar]
- 98.Gupta S. Mechanisms of transmembrane signalling in human T cell activation. Mol Cell Biochem. 1989;91:45–50. doi: 10.1007/BF00228078. [DOI] [PubMed] [Google Scholar]
- 99.Kilpatrick D.C. Mechanisms and assessment of lectin-mediated mitogenesis. Mol Biotechnol. 1999;11:55–65. doi: 10.1007/BF02789176. [DOI] [PubMed] [Google Scholar]
- 100.Facchinetti A., Panozzo M., Pertile P., Tessarollo L., Biasi G. In vivo and in vitro death of mature T cells induced by separate signals to CD4 and alpha beta TCR. Immunobiology. 1992;185:380–389. doi: 10.1016/s0171-2985(11)80654-6. [DOI] [PubMed] [Google Scholar]
- 101.Sharma K., Wang R.X., Zhang L.Y., Yin D.L., Luo X.Y., Solomon J.C. Death the Fas way: regulation and pathophysiology of CD95 and its ligand. Pharmacol Ther. 2000;88:333–347. doi: 10.1016/s0163-7258(00)00096-6. [DOI] [PubMed] [Google Scholar]
- 102.Green D.R., Scott D.W. Activation-induced apoptosis in lymphocytes. Curr Opin Immunol. 1994;6:476–487. doi: 10.1016/0952-7915(94)90130-9. [DOI] [PubMed] [Google Scholar]
- 103.Wang R.X., Chu C.Y., Nissen N.N., Lewis M.S., Palanisamy N., Edderkaoui M. American Pancreatic Association 2015 Annual Meeting, Loews Coronado Bay, San Diego, November 4–7. 2015. Novel patient-derived CTC-xenograft models for the study of pancreati cancer biology, metastasis and therapy. [Google Scholar]
- 104.Wang R.X., Posadas E.M., Hu P., Chu C.Y., Sievert M., Bhowmick I. 2012 Society for Basic Urological Research Fall Symposium, Trump International Beach Resort, Miami Beach, FL, November 15–18. 2012. Expansion of circulating tumor cells from prostate ancer patients by culturing circulating peripheral blood mononuclear cells. [Google Scholar]