Abstract
Key points
We investigated whether intramuscular temperature affects the acute recovery of exercise performance following fatigue‐induced by endurance exercise.
Mean power output was better preserved during an all‐out arm‐cycling exercise following a 2 h recovery period in which the upper arms were warmed to an intramuscular temperature of ~ 38°C than when they were cooled to as low as 15°C, which suggested that recovery of exercise performance in humans is dependent on muscle temperature.
Mechanisms underlying the temperature‐dependent effect on recovery were studied in intact single mouse muscle fibres where we found that recovery of submaximal force and restoration of fatigue resistance was worsened by cooling (16–26°C) and improved by heating (36°C).
Isolated whole mouse muscle experiments confirmed that cooling impaired muscle glycogen resynthesis.
We conclude that skeletal muscle recovery from fatigue‐induced by endurance exercise is impaired by cooling and improved by heating, due to changes in glycogen resynthesis rate.
Abstract
Manipulation of muscle temperature is believed to improve post‐exercise recovery, with cooling being especially popular among athletes. However, it is unclear whether such temperature manipulations actually have positive effects. Accordingly, we studied the effect of muscle temperature on the acute recovery of force and fatigue resistance after endurance exercise. One hour of moderate‐intensity arm cycling exercise in humans was followed by 2 h recovery in which the upper arms were either heated to 38°C, not treated (33°C), or cooled to ∼15°C. Fatigue resistance after the recovery period was assessed by performing 3 × 5 min sessions of all‐out arm cycling at physiological temperature for all conditions (i.e. not heated or cooled). Power output during the all‐out exercise was better maintained when muscles were heated during recovery, whereas cooling had the opposite effect. Mechanisms underlying the temperature‐dependent effect on recovery were tested in mouse intact single muscle fibres, which were exposed to ∼12 min of glycogen‐depleting fatiguing stimulation (350 ms tetani given at 10 s interval until force decreased to 30% of the starting force). Fibres were subsequently exposed to the same fatiguing stimulation protocol after 1–2 h of recovery at 16–36°C. Recovery of submaximal force (30 Hz), the tetanic myoplasmic free [Ca2+] (measured with the fluorescent indicator indo‐1), and fatigue resistance were all impaired by cooling (16–26°C) and improved by heating (36°C). In addition, glycogen resynthesis was faster at 36°C than 26°C in whole flexor digitorum brevis muscles. We conclude that recovery from exhaustive endurance exercise is accelerated by raising and slowed by lowering muscle temperature.
Keywords: cold‐water immersion, fatigue, glycogen, recovery, skeletal muscle, temperature
Key points
We investigated whether intramuscular temperature affects the acute recovery of exercise performance following fatigue‐induced by endurance exercise.
Mean power output was better preserved during an all‐out arm‐cycling exercise following a 2 h recovery period in which the upper arms were warmed to an intramuscular temperature of ~ 38°C than when they were cooled to as low as 15°C, which suggested that recovery of exercise performance in humans is dependent on muscle temperature.
Mechanisms underlying the temperature‐dependent effect on recovery were studied in intact single mouse muscle fibres where we found that recovery of submaximal force and restoration of fatigue resistance was worsened by cooling (16–26°C) and improved by heating (36°C).
Isolated whole mouse muscle experiments confirmed that cooling impaired muscle glycogen resynthesis.
We conclude that skeletal muscle recovery from fatigue‐induced by endurance exercise is impaired by cooling and improved by heating, due to changes in glycogen resynthesis rate.
Abbreviations
- [Ca2+]i
myoplasmic free [Ca2+]
- FDB
flexor digitorum brevis
- HR
heart rate
- PLFFD
prolonged low‐frequency force depression
- RER
respiratory exchange ratio
- SR
sarcoplasmic reticulum
Introduction
Recovery of skeletal muscle force generation following fatigue‐inducing exercise can take from minutes to hours, or even days before returning to pre‐fatigued values (Edwards et al. 1977). This prolonged depression of contractile force is often more pronounced at low than at high stimulation frequencies (Edwards et al. 1977) and has been referred to as prolonged low‐frequency force depression (PLFFD) (Allen et al. 2008). Thus, higher stimulation frequencies are necessary in order to attain a given submaximal force output. Given that the majority of locomotion involves submaximal contractions, PLFFD can markedly contribute to the sensation of muscle weakness that persists long after a fatiguing exercise has been completed, even though maximum force capacity can still be attained upon a maximal voluntary effort. The development of PLFFD is consistent with an impaired sarcoplasmic reticulum (SR) Ca2+ release and/or a decreased myofibrillar Ca2+ sensitivity, with the relative importance depending on the type of exercise and recovery time (Skurvydas et al. 2016). For instance, increased reactive oxygen–nitrogen species generated during high‐intensity type fatigue tasks have been implicated in depressing both SR Ca2+ release and myofibrillar Ca2+ sensitivity (Bruton et al. 2008; Cheng et al. 2015; Place et al. 2015; Watanabe & Wada, 2016; Dutka et al. 2017), whereas myofibrillar damage may cause PLFFD after repeated eccentric contractions (Skurvydas et al. 2016). Furthermore, the muscle glycogen level may also affect SR Ca2+ regulation (Ørtenblad & Nielsen, 2015).
Glycogen is a primary energy source for muscle contraction, and depletion of glycogen from mainly intra‐myofibrillar pools close to the SR has been associated with impaired SR Ca2+ release during prolonged, endurance‐type exercise in elite cross‐country skiers (Nielsen et al. 2009; Ørtenblad et al. 2011b), following fatiguing contractions in mechanically skinned rat fibres (Nielsen et al. 2009), and in intact mouse fibres (Nielsen et al. 2014). Given that decreased SR Ca2+ release can be long lasting after fatigue induction (Westerblad et al. 2000), it is feasible that glycogen depletion contributes to the depressed SR Ca2+ release and consequently PLFFD. In humans, glycogen depletion provides a strong drive for its own resynthesis and the resynthesis rate is highest in the early post‐exercise period (0–4 h) and slows down as muscle glycogen increases (Piehl, 1974; Jentjens & Jeukendrup, 2003; Marchand et al. 2007; Nielsen et al. 2011). Depending on the extent of glycogen depletion and exercise mode, full glycogen recovery may last up to 24 h or several days (Piehl, 1974; Kiens & Richter, 1998; Jentjens & Jeukendrup, 2003; Pilegaard et al. 2005; Krustrup et al. 2011). Thus, glycogen repletion and PLFFD follow a similar time course (Edwards et al. 1977), although a causative relationship has not been established.
Among the numerous approaches to maximizing post‐exercise recovery, cryotherapy (i.e. cooling the exercised limbs) has become widely popular. Although whole‐body cooling appears to be clearly beneficial for counteracting hyperthermia when exercising in a warm environment (Todd et al. 2005; Thomas et al. 2006; Tyler et al. 2015; Zhang et al. 2015), the benefits of cryotherapy of the exercised limbs alone are more uncertain (Leeder et al. 2012; Ihsan et al. 2016). Rates of enzymatic processes generally increase as the temperature rises, and thus we hypothesized that muscle cooling might actually delay recovery following prolonged glycogen‐depleting exercise, particularly during the first hours following fatigue when regeneration of force and glycogen resynthesis are most rapid (Piehl, 1974; Marchand et al. 2007; Nielsen et al. 2011). Conversely, heating muscles may promote both of these processes. To test these hypotheses we performed experiments with exercising human subjects and electrically stimulated single mouse muscle fibres. Altogether our current results reveal that recovery of contractile function and muscle endurance as well as glycogen repletion were faster at elevated than at lowered muscle temperatures.
Methods
Healthy recreationally active young subjects (4 females, 1 male) participated in this study. The age, height, weight and percentage body fat (as measured by bioelectrical impedance, InBody720, Seoul, Korea; mean ± SEM) were 26 ± 2 years, 171 ± 4 cm, 64 ± 2 kg, 19 ± 3%. All human experiments were performed at Mid Sweden University and were approved by the Umeå Regional Ethics Committee (no. 2014‐53‐31), and complied with the Declaration of Helsinki. Subjects were fully informed of any potential risk associated with the experiments before written and oral consent was obtained from all subjects prior to participation in this study.
All experiments in mice were performed at Karolinska Institutet and complied with the Swedish Animal Welfare Act, the Swedish Welfare Ordinance, and applicable regulations and recommendations from Swedish authorities. The parts of the study involving mice were approved by the Stockholm North Ethical Committee on Animal Experiments (no. N120/13). Female C57BL/6J mice (n = 50) (Scanbur Research, Sweden) were used for this study. Animals were housed in a temperature‐controlled environment with a 12 h light–dark cycle, and were provided with standard rodent chow and water ad libitum.
Human experiments
The experiment consisted of four visits. The first visit was used for familiarization and initial testing of during arm cycling to determine exercise intensities in subsequent visits. In visits 2–4, subjects performed arm cycling on a modified ergometer (SRM GmbH, Jülich, Germany) (Fig. 1). First, subjects performed two 4 min warm‐up exercises at submaximal intensity (40 W), and then a fatigue test which consisted of 3 × 5 min all‐out exercise at a constant pedaling frequency of 100 rpm, with 5 min recovery between sets. Mean power output, blood lactate, cardiorespiratory data and heart rate were continuously measured; all methods were well established and regularly used in the laboratory (Zinner et al. 2016). Five minutes after the last set of the fatigue test, subjects performed exhaustive exercise consisting of 4 × 15 min of arm cycling at an intensity of 50% . Then followed 2 h of recovery during which both arms were either: (1) continuously cooled with ice‐chilled water‐perfused arm cuffs, (2) kept at physiological temperature (∼33°C), or (3) heated to 5˚C above physiological temperatures (i.e. 38°C) using modified arm cuffs (Breg PolarPad Multi‐Use, Carlsbad, CA, USA) continuously perfused by a temperature‐controlled water bath (Julabo ED‐5, Seelbach, Germany). The cooling experiment mimicked other cold‐water immersion studies in which exercised limbs are immersed in ice‐cooled water (Ihsan et al. 2016), which is well tolerated over prolonged periods. Preliminary experiments revealed that a 5°C increase in arm muscle temperature with heating was the upper limit of what subjects could comfortably tolerate for 2 h. The arm cuffs were modified by removing the flow restrictor valve in the tubing to allow for more rapid water perfusion through the cuff. To prevent skin irritation with the temperature manipulations, a thin dressing was wrapped over top of the skin before applying the arm cuffs. The order of the cooling, control, and heating condition was randomized between subjects at visits 2–4. Intramuscular temperature was recorded with probes (IT‐23, Physitemp Instruments, USA) inserted 1.5 cm deep into the lateral head of the triceps brachii muscle of both arms using a 23‐gauge catheter. Core temperature was measured with an infrared radiation device measuring temperature from the tympanic membrane (Braun ThermoScan 5, Germany).
Figure 1. Schematic of the exercise protocol performed in humans.
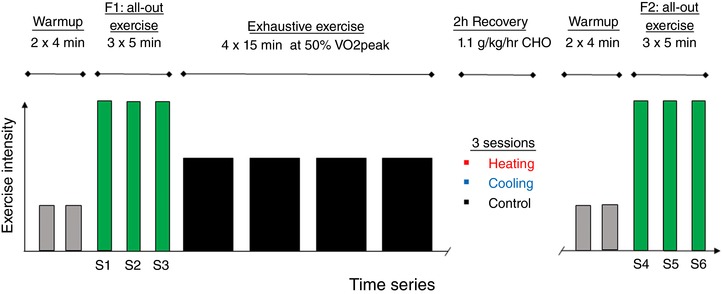
Warmup at low intensity (grey bars) was followed by a 3 × 5 min all‐out exercise fatigue bout (F1) (green bars), and then 4 × 15 min of exhaustive exercise (black bars). During the subsequent 2 h recovery, subjects were given 1.1 g kg−1 h−1 carbohydrate at one of the three different muscle temperature conditions at each visit. After the recovery period, a warmup was followed by a second fatigue bout (F2). [Color figure can be viewed at wileyonlinelibrary.com]
During the recovery period, subjects consumed 1.1 g−1 (kg body weight)−1 h−1 carbohydrates in the form of sports drinks and bars (High5 Sports Nutrition, Leicester, UK) to maximize glycogen repletion (Jentjens & Jeukendrup, 2003). The subjects were instructed to consume the drinks and bars continuously during the recovery period and to be finished 20 min before the end of the recovery period. After the 2 h recovery, subjects performed two 4‐min submaximal warm‐up stages at 100 rpm pedalling frequency and at an intensity that elicited a respiratory exchange ratio of <1.0, which was followed by the 3 × 5 min fatigue test to determine whether exercise performance was affected by the recovery intervention.
The oxygen uptake , carbon dioxide , and pulmonary ventilation were continuously monitored using an open‐circuit metabolic cart (AMIS 2001 model C, Innovision A/S, Odense, Denmark) calibrated with a 3 L syringe prior to each test (Hans Rudolph Inc., Kansas City, KS, USA) with a certified mixture of 16.0% O2 and 4.5% CO2 in N2. Values were analysed as an average of the highest 30 s for the and an average of the entire 5 min time trial for . Heart rate (HR) was monitored (RS400, Polar Electro Oy, Kempele, Finland) during all testing and the average of each trial is reported. Before and immediately after the fatigue tests, capillary blood samples from the earlobe were analysed for lactate concentration (Biosen 5140, EKF Diagnostic GmbH, Magdeburg, Germany).
Single fibre experiments
Mice were killed by rapid cervical dislocation and whole flexor digitorum brevis (FDB) muscles were removed from the hindlimbs. Intact single muscle fibres with attached tendons were mechanically dissected from whole FDB muscles (Cheng & Westerblad, 2017). Aluminum T‐clips were attached to both tendons of the single fibre and the fibre was placed longitudinally in a perfusion chamber between two platinum plates that delivered the electrical currents. One tendon of the single fibre was mounted to a platinum hook attached to a force transducer (Akers 801, Kronex Technologies, Oakland, CA, USA) and the other tendon was mounted to a platinum hook attached to an adjustable holder. The fibre was electrically stimulated with 70 Hz tetani of 350 ms duration (0.5 ms pulse width) and the fibre length was adjusted until the fibre achieved its optimum length of force production. A camera and display monitor were used to determine the fibre cross‐sectional area at optimal length, which was measured by taking the average diameter measured at two different portions of the fibre. Before fatiguing stimulation, all fibres were continuously perfused with Tyrode solution containing (in mm): 121 NaCl, 5.0 KCl, 1.8 CaCl2, 0.5 MgCl2, 0.4 NaH2PO4, 24.0 NaHCO3, 0.1 EDTA, and 5.5 glucose. The solution was bubbled with 95% O2–5% CO2, giving a bath pH of 7.4. Experiments were performed at physiological temperature for mouse FDB fibres (31°C) (Bruton et al. 1998), and fibres were perfused with Tyrode containing glucose unless otherwise mentioned. Single fibres were pressure injected with the fluorescent Ca2+ indicator indo‐1 (no. 0146, TEFLabs Inc., Austin, TX, USA) to measure myoplasmic free [Ca2+] ([Ca2+]i]. Indo‐1 was excited at 360 ± 5 nm wavelength and emission was recorded at 405 ± 5 nm and 495 ± 5 nm wavelengths using a system consisting of a xenon lamp, a monochromator, and two photomultiplier tubes (Photon Technology International, Wedel, Germany). Ratiometric indo‐1 emission values were converted to [Ca2+]i using an in vivo calibration as reported previously (Cheng & Westerblad, 2017). Indo‐1 fluorescence decreased over time, and thus measurements of tetanic [Ca2+]i at >1 h recovery were not used because the 495 nm signal, which declines when [Ca2+]i increases, became too low to allow reliable ratio measurements.
Initially, each fibre was stimulated with 350 ms duration tetani once every 1 min at 30, 70 and 120 Hz, and the peak force and spatial and time‐averaged [Ca2+]i were measured during tetani. After 5 min of rest, fibres were perfused with glucose‐free Tyrode solution and then fatigue was induced by repetitively stimulating fibres with 350 ms duration 70 Hz tetani given once every 10 s until peak force was decreased to 30% of the starting value. This low‐intensity stimulation protocol has been shown to cause a marked 60–70% glycogen decrease in single mouse FDB fibres (Nielsen et al. 2014). To determine temperature effects on the recovery of force and [Ca2+]i after fatigue, fibres were perfused with glucose‐containing Tyrode solution for up to 2 h at one of four temperatures: 16°C (i.e. 10°C below physiological temperature), 26°C (i.e. 5°C below physiological temperature), 31°C (physiological temperature of the FDB muscle) (Bruton et al. 1998), or 36°C (i.e. 5°C above physiological temperature). To further assess whether force recovery was dependent on glycogen restoration, additional experiments were performed where fibres were perfused with glucose‐free Tyrode solution at the different temperatures. Every 30 min during the recovery period, the temperature was briefly changed to 31°C (for ∼3 min) and fibres stimulated at 30, 70 and 120 Hz. Temperatures were briefly restored to 31°C when fibres were tested during the recovery period since temperature in itself alters force production, that is, lowering muscle temperature increases submaximal force while raising the temperature decreases submaximal force (Lännergren & Westerblad, 1987); thus, measuring force and [Ca2+]i at the same temperature allowed a comparison of recovery characteristics between conditions. To assess the effect of temperature and glucose availability on the recovery of muscle fatigue resistance, a second fatigue bout matching the initial fatiguing stimulation was performed after the recovery period. There is a positive correlation between the force produced and the energy consumed by cross‐bridges and SERCA (Crow & Kushmerick, 1983; Rall, 2005). In some instances, fibres entered a marked PLFFD and the 70 Hz force in these fibres was significantly lower before the second vs. first fatigue bout. For these fibres, the stimulation frequency during tetani was increased in the second fatigue bout in order to match the initial forces, and hence energy consumption, in the two fatigue bouts.
Glycogen measurements
Muscle glycogen content was determined in whole FDB muscles. Silk suture thread was tied to the distal and proximal tendons of isolated FDB muscles and then the muscles were mounted in a stimulation chamber (World Precision Instruments ‐ SI‐MB4, Hertfordshire, UK) containing Tyrode solution bubbled with 95% O2–5% CO2. Whole FDBs were adjusted to optimal length by stimulating with 70 Hz tetani. Muscles were then fatigued at 31°C in glucose‐free Tyrode solution with repeated 70 Hz tetani. Two stimulation protocols were performed: a moderately glycogen‐depleting protocol involving 700 ms duration contractions given every 10 s for 150 tetani, and a more severely glycogen‐depleting protocol with 1 s contractions given every 5 s for 300 tetani. Tendons were cut, muscles weighed, and frozen in liquid nitrogen at 5 min after the repeated stimulations to assess the extent of glycogen depletion during fatiguing stimulations. In another set of experiments, control muscles were frozen in liquid nitrogen without prior stimulation to represent glycogen content before fatigue. Glycogen resynthesis was assessed for the different stimulation protocols by bathing muscles at temperatures of 26°C or 36°C with Tyrode solution containing glucose, and snap freezing muscles at 30 min of recovery. Frozen FDB muscles were dissolved in 25 μl 1 n HCl for 30 min with agitation. Homogenates were neutralized with the addition of 25 μl 1 n NaOH, and 5 μl homogenate were added in duplicates to a 96 well plate. Glycogen content was established using a fluorometric kit (MAK016, Sigma‐Aldrich, St Louis, USA). Glycogen content was normalized to muscle wet weight.
Statistics
Two‐way repeated measures ANOVA, one‐way ANOVA, and Student's t tests were performed as appropriate using Graphpad Prism Software (Graphpad Prism 7.0, San Diego, CA, USA). Post hoc analysis was performed using a Tukey's or Sidak's test. Data are presented as means ± SEM. Statistically significant differences were determined at P < 0.05.
Results
Decreased fatigue resistance with cooling in humans
Three sets of 5 min all‐out arm cycling were performed before and after a 2 h recovery period at different muscle temperatures to assess the effect of recovery temperature on subsequent fatigue resistance. Intramuscular triceps brachii temperature was recorded under the different recovery conditions, with muscle warming elevating the temperature to ∼38°C (5°C above the control temperature of ∼33°C) by 20 min, whereas muscle cooling gradually reduced muscle temperatures down to 15°C (Fig. 2 A). Core temperature was not affected by warming or cooling (two‐way repeated measures ANOVA, P = 0.70, n = 5) (Fig. 2 B).
Figure 2. Decreased mean power output in humans following recovery at low muscle temperatures.
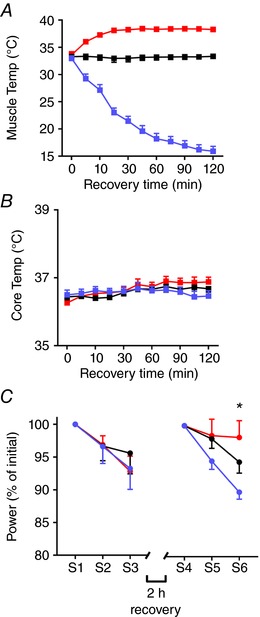
Mean data (± SEM; n = 5) showing changes in lateral triceps brachii intramuscular temperature (A) and body core temperature (B) during the 2 h recovery period, and relative power (C) during the 3 × 5 min fatigue test performed before (S1–S3) and after 2 h recovery (S4–S6). *Main temperature effect (one‐way ANOVA, P = 0.027). Heating (red), control (black), and cooling (blue). [Color figure can be viewed at wileyonlinelibrary.com]
Mean power output in the first set (S1) of the fatigue test (i.e. prior to heating, control, or cooling) was not different across the three sessions, averaging 97 ± 7 W, 94 ± 7 W, and 94 ± 5 W (one‐way ANOVA, P = 0.45, n = 5). Furthermore, the mean relative power output was also similar during the first 3 × 5 min fatigue test (S1–S3) before the different recovery conditions (two‐way repeated measures ANOVA, P = 0.94) (Fig. 2 C). Thus, the performance did not differ at the start of the three testing sessions.
After 2 h recovery, with three different temperatures, mean power output in the first set (S4) of the fatigue test did not differ across sessions, averaging 93 ± 5 W after heating, 89 ± 5 W in control, and 93 ± 6 W with cooling (one‐way ANOVA, P = 0.50, n = 5). However, there was a significant main effect (one‐way ANOVA, P = 0.027, n = 5) of altering muscle temperature in the final set of the fatigue test (S6), with power being markedly better maintained after heating than after cooling (Fig. 2 C).
There was no observed difference between cardiorespiratory parameters during the all‐out arm cycling performed before and after the different recovery conditions (see Table 1). However, blood lactate was higher in the final set of the fatigue test (S6) following the recovery condition with muscle heating than in control or with cooling (one‐way ANOVA, P = 0.0064, n = 5).
Table 1.
Cardiorespiratory and blood lactate measurements from all‐out arm cycling exercise performed before and after different recovery temperatures (n = 5)
| Fatigue test before recovery | Fatigue test after recovery | ||||
|---|---|---|---|---|---|
| 1st set (S1) | 3rd set (S3) | Recovery condition | 4th set (S4) | 6th set (S6) | |
| (L min−1) | 2.3 ± 0.2 | 2.3 ± 0.2 | Heating | 2.4 ± 0.2 | 2.4 ± 0.2 |
| 2.3 ± 0.2 | 2.3 ± 0.2 | Control | 2.3 ± 0.2 | 2.3 ± 0.2 | |
| 2.2 ± 0.2 | 2.2 ± 0.2 | Cooling | 2.4 ± 0.2 | 2.4 ± 0.2 | |
| HRmean (beats min−1) | 159 ± 5 | 164 ± 5 | Heating | 160 ± 6 | 167 ± 6 |
| 161 ± 6 | 165 ± 5 | Control | 158 ± 6 | 162 ± 7 | |
| 167 ± 8 | 169 ± 6 | Cooling | 163 ± 4 | 166 ± 5 | |
| (L min−1) | 1.9 ± 0.1 | 1.9 ± 0.1 | Heating | 1.9 ± 0.1 | 2.0 ± 0.1 |
| 1.8 ± 0.1 | 1.9 ± 0.1 | Control | 1.8 ± 0.1 | 1.9 ± 0.1 | |
| 1.8 ± 0.1 | 1.8 ± 0.1 | Cooling | 1.9 ± 0.1 | 1.9 ± 0.1 | |
| (L min−1) | 2.0 ± 0.2 | 1.8 ± 0.1 | Heating | 2.0 ± 0.1 | 1.9 ± 0.1 |
| 2.0 ± 0.2 | 1.8 ± 0.2 | Control | 1.9 ± 0.1 | 1.7 ± 0.1 | |
| 2.0 ± 0.1 | 1.8 ± 0.2 | Cooling | 2.0 ± 0.1 | 1.8 ± 0.1 | |
| RERmean | 1.07 ± 0.04 | 0.98 ± 0.02 | Heating | 1.06 ± 0.03 | 0.96 ± 0.02 |
| 1.07 ± 0.03 | 0.97 ± 0.02 | Control | 1.05 ± 0.02 | 0.97 ± 0.02 | |
| 1.11 ± 0.03 | 0.98 ± 0.02 | Cooling | 1.02 ± 0.03 | 0.94 ± 0.02 | |
| Lactate [mm] | 7.3 ± 0.6 | 8.1 ± 1.0 | Heating | 6.6 ± 0.7 | 6.6 ± 1.1* |
| 7.4 ± 0.6 | 8.3 ± 1.0 | Control | 5.8 ± 0.7 | 4.6 ± 0.9 | |
| 8.2 ± 1.0 | 8.1 ± 1.3 | Cooling | 6.4 ± 0.7 | 5.2 ± 0.8 | |
*Denotes heating trial greater than control or cooling recovery condition (one‐way ANOVA main effect, P = 0.0064; Tukey post hoc test, P < 0.05). Data are shown as means ± SEM.
Recovery in mouse single fibres is temperature and glucose dependent
Single muscle fibre experiments were used to determine potential cellular mechanisms underlying the greater fatigue resistance following a recovery period with muscle heating vs. cooling. These single fibre experiments allow temperature to be strictly controlled and hence temperature effects on the recovery of muscle contractile function can be directly assessed. Figure 3 A shows a significant main effect of temperature on the recovery of 30 Hz force (two‐way repeated measures ANOVA, P = 0.0001, n = 4—5 per group), with the slowest recovery evident at 16°C and the fastest recovery at 36°C. There was also a significant main effect observed for temperature on the recovery of 70 Hz force (two‐way repeated measures ANOVA, P = 0.0001, n = 5 per group), although the differences in force were smaller than observed with 30 Hz stimulation. In contrast, 120 Hz force recovered rapidly to >90% of the control and showed no significant temperature dependency (two‐way repeated measures ANOVA, P = 0.56, n = 5 per group).
Figure 3. Recovery of submaximal force is temperature and glucose dependent.
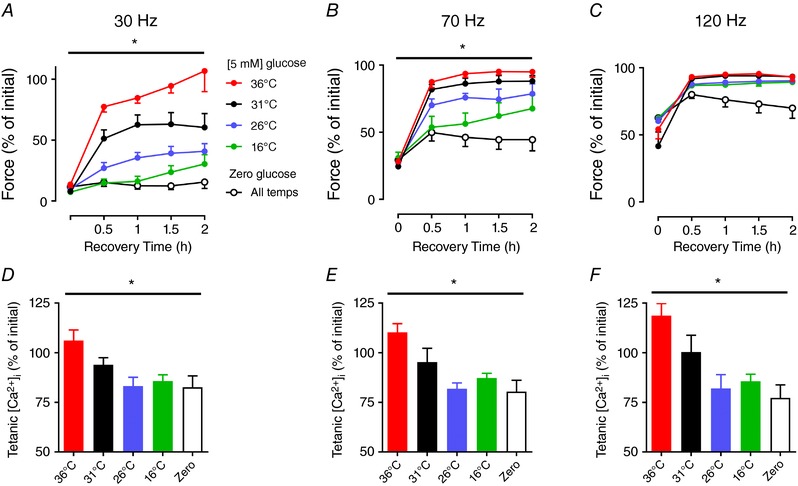
Mean data (± SEM) showing relative force at 30 Hz (A), 70 Hz (B), and 120 Hz (C) during 2 h recovery with glucose (5 mm) at 36°C (red), 31°C (black), 26°C (blue), 16°C (green), and without glucose (open symbols). Tetanic [Ca2+]i at 30 Hz (D), 70 Hz (E), and 120 Hz (F) at 30 min of recovery. Force and [Ca2+]i before the first fatigue test were set to 100% at each frequency. *Two‐way repeated measures ANOVA showed main temperature effect for 30 Hz force (P = 0.0001), 70 Hz force (P = 0.0001), 30 Hz tetanic [Ca2+]i (P = 0.036), 70 Hz tetanic [Ca2+]i (P = 0.006), and 120 Hz tetanic [Ca2+]i (P = 0.002) (n = 4—11 per group). [Color figure can be viewed at wileyonlinelibrary.com]
The recovery of force in the absence of glucose at different temperatures (26°C, 31°C, 36°C) resulted in consistently lower mean force than in the presence of glucose. Moreover, the force depression showed no temperature dependency in the absence of glucose (two‐way repeated measures ANOVA, P = 0.97, n = 3–5 per group), and hence grouped data from all eleven fibres recovering without glucose are plotted in Fig. 3 A–C. Thus, the effect of temperature on recovery of force is abolished if glucose is not provided to the fibres.
The largest temperature‐dependent differences in force were observed after 30 min of recovery. Therefore, we measured [Ca2+]i at this time point and observed a relationship between higher tetanic [Ca2+]i and increased temperature (one‐way ANOVA main effect, P < 0.05, n = 4–11 per group) (Fig. 3 D–F). Importantly, this temperature effect was absent when fibres recovered in the absence of glucose. Together, these data are consistent with the idea that PLFFD following fatiguing contractions is caused by a decrease in tetanic [Ca2+]i which is dependent on muscle glycogen levels. It can be noted that although [Ca2+]i during 30 Hz tetani was fully recovered at 36°C, force was still decreased by ∼20%, which implies that there was a contributing component of reduced myofibrillar Ca2+ sensitivity (compare Fig. 3 A and D). Furthermore, the temperature‐dependent differences in recovery of tetanic [Ca2+]i at 120 Hz stimulation was not reflected in the force, which was restored close to the pre‐fatigue force levels under all conditions. Thus, although tetanic [Ca2+]i was decreased by ∼25% at 16°C and 26°C, it was still high enough to reach saturating levels.
Decreased fatigue resistance after recovery without glucose and at low temperatures
After the 2 h recovery, FDB fibres were fatigued again with repeated tetani (350 ms duration every 10 s) to determine whether the temperature, and the presence or absence of glucose affected fatigue development. Fibres that recovered without glucose required higher stimulation frequencies of 100–150 Hz to reach the same initial forces as in the first fatigue bout (Fig. 4). These glycogen‐deprived fibres showed a rapid decline in force during fatigue induction. Typical force records illustrating this decrease in fatigue resistance are shown in Fig. 4 A and B. The more rapid fatigue development in Fig. 4 B is not due to the higher stimulation frequency per se, as a typical force recording from one of three control experiments shows well‐maintained force during stimulation with repeated 150 Hz tetani in a previously unfatigued fibre. All fibres that underwent a recovery period without glucose showed severely impaired fatigue resistance after the recovery period (paired t test, P < 0.05, n = 5) (Fig. 4 D). In the presence of glucose over the 2 h recovery period, some, but not all, fibres recovered at 16°C, 26°C, 31°C, which may reflect slight variations in muscle glycogen levels in the fibres and a relatively sharp glycogen–SR Ca2+ release association. However, the recovering fibres required higher stimulation frequencies of 100–120 Hz to reach the same initial forces as in the first fatigue bout. Fibres that recovered at 26°C, 31°C and 36°C sustained a comparable number of contractions in the second fatigue bout. Conversely, fatigue resistance was severely impaired following 2 h recovery at the lowest temperature of 16°C (Fig. 4 C).
Figure 4. Repeated bout fatigue resistance is glucose and temperature dependent.
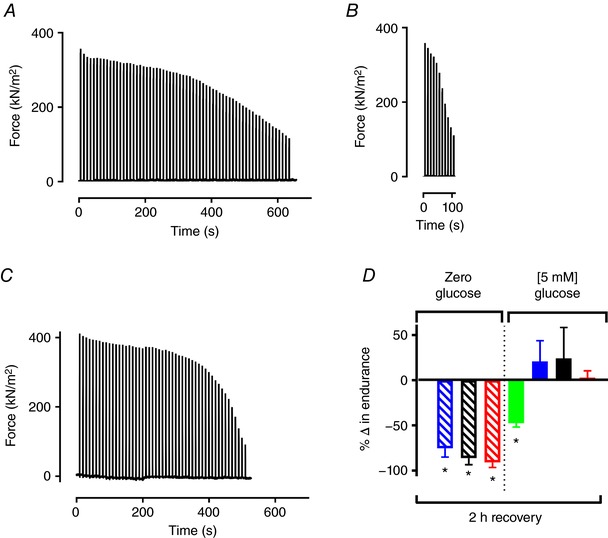
Typical records from a single fibre fatigued with 70 Hz repeated tetani before 2 h recovery (A) and fatigued after 2 h recovery with 120 Hz tetani (B) at 31°C in the absence of glucose. Note the similar starting forces in panels A and B, and the markedly faster fatigue development in panel B. C, typical record from a control experiment with 150 Hz repeated tetani showing that the faster fatigue development in B is not caused by a higher stimulation frequency. D, mean data (± SEM) of relative endurance, expressed as the relative number of contractions performed in the first vs. second fatigue bout for fibres that recovered at 16°C (green), 26°C (blue), 31°C (black), and 36°C (red). *Significantly different from the number of contractions performed in the first fatigue run, (Student's paired t test, P < 0.05, n = 3–5 per group); thick horizontal line at 0% indicates no difference between the first and second fatigue bouts. [Color figure can be viewed at wileyonlinelibrary.com]
Next, with the rationale that a temperature‐dependent effect on glycogen restoration might be discerned when the recovery time was shortened, we evaluated fatigue resistance after only 1 h recovery at 26°C and 36°C, i.e. temperatures spanning the interval where fatigue resistance was fully restored after 2 h recovery (see Fig. 4 C). All fibres recovering at 26°C required higher stimulation frequencies of 100–120 Hz to reach the same initial forces as in the first fatigue bout. The number of contractions sustained until force was decreased to 30% of the starting force in the second fatigue bout was markedly fewer after 1 h recovery at 26°C than at 36°C (Fig. 5 A–D) and mean data show a significantly impaired endurance after recovery at 26°C than at 36°C (unpaired t test, P = 0.0035, n = 6–7) (Fig. 5 E). Hence, endurance is worsened following recovery at 26 than at 36°C.
Figure 5. Fatigue resistance is decreased after 1 h recovery at low temperature.
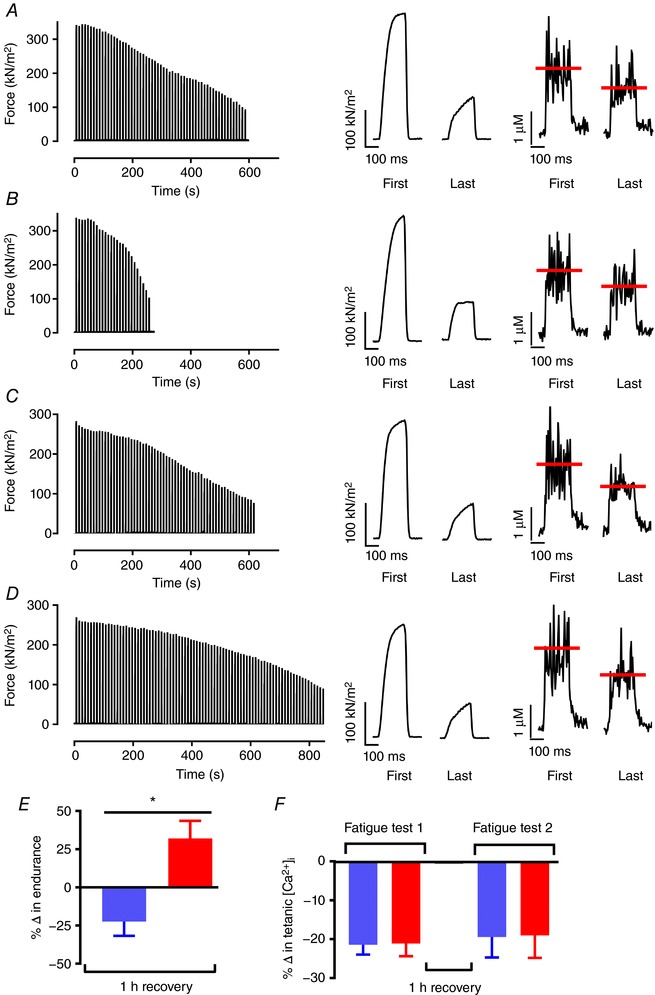
Typical force records from a single fibre during repeated tetanic stimulation performed before (A) and after 1 h of recovery at 26°C (B), and before (C) and after 1 h of recovery at 36°C (D). Right panels show force and [Ca2+]i for the first and last contractions of the fatigue runs. The line (red) indicates the mean tetanic [Ca2+]i during the tetanus. E, mean data (± SEM) comparing relative fibre endurance, which is expressed as the number of contractions performed in the first vs. second fatigue test for fibres that recovered at 26°C (blue), and 36°C (red). *Endurance was significantly different following recovery at 26°C vs. 36°C (Student's unpaired t test, P = 0.0035, n = 6–7). F, mean data (± SEM) of the relative fatigue‐induced decline in tetanic [Ca2+]i during each fatigue test. Thick horizontal line at 0% signifies no change in endurance between the first and second fatigue bouts (E) and no fatigue‐induced change in tetanic [Ca2+]i (F). [Color figure can be viewed at wileyonlinelibrary.com]
To assess whether the difference in fatigue resistance after recovery at the two temperatures reflected a major difference in fatigue mechanisms, tetanic [Ca2+]i was assessed at the start and end of the fatigue bouts. Tetanic [Ca2+]i at the start of fatiguing stimulation before and after 1 h recovery at 26°C was similar (0.76 ± 0.09 μm vs. 0.88 ± 0.14 μm; P = 0.70); comparable values were obtained before and after recovery at 36°C (0.81 ± 0.07 μm vs. 0.92 ± 0.08 μm; P = 0.69). The typical tetanic [Ca2+]i records in Fig. 5 A–D show a similar reduction in the fatigued state in all four conditions and mean data show a ∼20% decrease in tetanic [Ca2+]i at the end of the first fatigue bouts as well as after recovery at 26°C and 36°C (Fig. 5 F). Altogether these experiments show the same fatigue‐induced reduction in tetanic [Ca2+]i at the end of fatigue, but that the reduction occurs faster following recovery at 26°C compared to 36°C.
Slower glycogen resynthesis during recovery at low temperature
To determine whether muscle glycogen resynthesis was temperature dependent, muscle glycogen content was measured in whole FDB muscles before and after fatigue induction, and then 30 min after fatigue at two recovery temperatures, 26°C and 36°C. Following the milder fatiguing stimulation protocol, there was a modest depletion of glycogen (one‐way ANOVA main effect, P = 0.0038; Sidak's post hoc test, P = 0.0003, n = 5 per group), followed by complete recovery at both 26°C (Sidak's post hoc test, P = 0.15, n = 5 per group) and 36°C (Sidak's post hoc test, P = 0.86, n = 5 per group) relative to the unfatigued (PRE) value, with no difference in glycogen content between temperatures (Fig. 6 A) (unpaired t test, P = 0.17, n = 5 per group). Following the more severe fatiguing stimulation protocol, glycogen was more depleted and a clear temperature dependence in glycogen resynthesis was revealed (one‐way ANOVA main effect, P < 0.0001, n = 6 per group), with muscle glycogen content being fully restored after 30 min recovery at 36°C relative to the PRE value (Sidak's post hoc test, P = 0.766, n = 6 per group), whereas it had only recovered to 61 ± 11% of the PRE value at 26°C (Fig. 6 B) (Sidak's post hoc test, P = 0.0047, n = 6 per group).
Figure 6. Glycogen resynthesis is slower at low temperature.
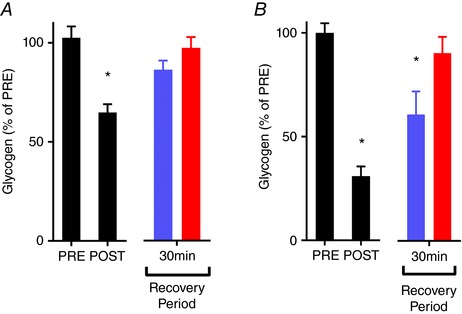
Mean data (± SEM) of muscle glycogen content before (PRE), after (POST), and after 30 min recovery at 26°C (blue bars) and 36°C (red bars) with repeated stimulation that caused moderate (A) and severe glycogen depletion (B). One‐way ANOVA showed significant differences over time for the moderate (P = 0.0038, n = 5) and severe glycogen depletion protocol (P < 0.0001, n = 6); *Significantly different from PRE (P < 0.005) with Sidak's post hoc test. Muscle glycogen returned to PRE values following recovery from moderate glycogen depletion at 26°C and 36°C, whereas muscle glycogen content was restored to PRE values at 36°C but not at 26°C following the severe glycogen depletion. [Color figure can be viewed at wileyonlinelibrary.com]
Discussion
By using an integrative approach from human whole muscle to mice single fibre experiments, our results reveal that intramuscular temperature affects the acute recovery of exercise performance following fatigue. Specifically, cooling slowed whereas heating accelerated recovery from fatigue. At the cellular level, the slower recovery of low‐frequency (submaximal) force and fatigue resistance at low temperature was linked to decreased tetanic [Ca2+]i and reduced muscle glycogen resynthesis.
Our current findings in humans show that skeletal muscle cooling during the recovery period after fatiguing contractions results in reduced endurance, shown as a greater relative decline in mean power output during a bout of 3 × 5 min all‐out exercise. We ascribe the impaired recovery at low temperature to slowed glycogen resynthesis. This assumption is based on the present results obtained in isolated mouse FDB muscle where there was a clear association between the recovery of submaximal force and muscle temperature, which was glucose dependent. Furthermore, glycogen resynthesis was slower at 26°C than at 36°C (see Fig. 6 B). This is consistent with the fact that the rate of biological or chemical system metabolic reactions generally becomes slower when the temperature is lowered. These results are consistent with previous data showing lower glycogen content in vastus lateralis muscles 4 h after glycogen‐depleting exercise when ice was applied at 30 min intervals that decreased intramuscular temperature by ∼10°C (Tucker et al. 2012). On the other hand, others have shown no effect of lower limb cold water immersion on muscle glycogen resynthesis following glycogen‐depleting cycling exercise (Gregson et al. 2013). However, in that study cold water immersion was only applied for a brief 10 min and muscle temperature only decreased to 30°C in superficial portions, and even less in deeper parts of the muscle, which was only minimally cooler than in the control condition (∼35°C). In comparison, in the present study the triceps brachii was cooled to an average temperature of ∼20°C over 2 h (see Fig. 1 A). Thus, it appears safe to conclude that glycogen resynthesis is temperature dependent, becoming slower when the temperature is reduced for a prolonged period. Cooling of the muscle and surrounding tissue may also decrease glycogen resynthesis rate due to a decreased blood flow thereby limiting glucose delivery. In line with an increased glycogen resynthesis in the heated muscle, we observed a higher blood lactate concentration (see Table 1), which is consistent with previous studies showing that higher muscle glycogen content in exercising muscle is associated with increased muscle glycogenolysis and greater lactate production (Hargreaves et al. 1995; Blomstrand & Saltin, 1999). The present results provide a mechanism for this association as a higher glycogen content allows muscles to sustain greater power output, and hence produce more lactate, but eventually glycogen depletion inhibits SR Ca2+ release during fatigue.
The present experiments on isolated mouse FDB fibres show glucose‐ and temperature‐dependent recovery of force and [Ca2+]i during contractions (see Fig. 3). During recovery from fatiguing contractions there was a significant main effect of temperature on tetanic [Ca2+]i at all three stimulation frequencies (30, 70 and 120 Hz) with glucose. Importantly, there was no temperature effect when recovering with zero glucose. Further, the depressive effect on force from lack of glucose and low temperature was pronounced at 30 Hz, intermediate at 70 Hz, and absent at 120 Hz. The small or absent glucose‐ or temperature‐effect on high‐frequency force is likely due to the fact that the tetanic [Ca2+]i at 120 Hz is high enough to reach close to saturating levels (see Fig. 3 C and F). For submaximal contractions that occur on the steep portion of the force–[Ca2+]i relation, the fatigue protocol revealed a more sensitive glucose‐ and temperature‐dependent influence on PLFFD, which could be explained by decreased SR Ca2+ release during contractions. Notably, this is consistent with the idea that PLFFD following fatiguing contractions is caused by a decrease in tetanic [Ca2+]i, which in turn is dependent on muscle glycogen levels. A recent study showed that the intramyofibrillar glycogen content, which is the largest store of glycogen within muscle fibres, regulates SR Ca2+ release in isolated mouse FDB fibres (Nielsen et al. 2014). Glycogen per se is not required for SR Ca2+ release as this process primarily depends on action potential‐mediated activation of the t‐tubular voltage sensors, the dihydropyridine receptors (DHPRs), which mechanically activate the SR Ca2+ release channels, the ryanodine receptors 1 (RyR1) (Rebbeck et al. 2014). On the other hand, [ATP], [Mg2+], and [Pi] are regulators of RyR1 opening (Duke & Steele, 2001; Laver, 2006), and their myoplasmic concentrations and spatial distribution in muscle can be altered during fatigue (Cady et al. 1989; Westerblad & Allen, 1992). This may especially be evident in the restricted space of the triad junction, with a diffusional restricted space for more than 90% of t‐systems length, and with a high metabolic activity (Dulhunty et al. 1984). Studies on mechanically skinned muscle fibres have shown impaired SR Ca2+ release in glycogen‐depleted fibres even when bulk myoplasmic [ATP] is kept at baseline values (Stephenson et al. 1999; Barnes et al. 2001; Nielsen et al. 2009), suggesting that low global intracellular [ATP] as such cannot explain the impaired RyR1 opening. In line with this, studies on SR vesicles from both trained and untrained human muscle have demonstrated an association between reduced muscle glycogen and decreased SR vesicle Ca2+ release rate (Duhamel et al. 2006; Ørtenblad et al. 2011a; Gejl et al. 2014). The fact that SR Ca2+ release is impaired at low [glycogen] might be considered a protective mechanism since inhibiting SR Ca2+ release in glycogen‐depleted fibres would reduce downstream ATP consumption by the cross‐bridges and SR Ca2+ pumps, thereby preventing deleterious consequences of critically low [ATP] (Ørtenblad & Nielsen, 2015; Cheng et al. 2017).
Also, the present experiments on isolated mouse FDB fibres show (1) a glucose‐dependent and (2) a temperature‐dependent recovery of endurance. As expected, fatigue occurred very rapidly after 2 h of recovery without glucose. This fits with previous studies which showed severely impaired fatigue resistance when limited or no glucose is provided during the recovery period to isolated mouse muscle (Chin & Allen, 1997; Helander et al. 2002) and in humans (Alghannam et al. 2016). Furthermore, muscle glycogen resynthesis is indeed severely blunted with minimal or no carbohydrate provision following exercise (Maehlum & Hermansen, 1978; Alghannam et al. 2016). In line with this, muscle glucose utilization is higher when plasma glucose levels are increased during exercise by ingestion of carbohydrate (McConell et al. 1994). Furthermore, a temperature effect on fatigue resistance was observed with more rapid fatigue after 2 h recovery at 16°C (see Fig. 4). Also, fibres recovering for 1 h were more fatigue resistant after recovery at 36°C than at 26°C (see Fig. 5). A reasonable explanation for these observations is that fibres recovering at lower temperatures (16°C or 26°C) contained less glycogen at the start of the second fatigue bout than those recovering at higher temperatures (36°C), such that a glycogen‐dependent decrease in SR Ca2+ release occurred faster during repeated contractions as can be observed for instance in Fig. 5 A vs. B at the same time point (e.g. at ∼250 s). This is in line with the idea of a critical set quantity of glycogen below which SR Ca2+ release is impaired (Ørtenblad & Nielsen, 2015). Supporting this argument is the finding that fibres that recovered at 26°C and 36°C reached the same reduction in tetanic [Ca2+]i at the end of fatigue (Fig. 5 F), suggestive of fibres reaching a critical glycogen concentration which eventually impairs SR Ca2+ release.
We show that PLFFD develops after fatigue‐induced by endurance exercise. This PLFFD is related to reduced tetanic [Ca2+]i, although there is also a component of decreased myofibrillar Ca2+ sensitivity; for instance, after 30 min recovery at 36°C 30 Hz force was still decreased by ∼20% despite full recovery of [Ca2+]i (see Fig. 3 A and D). Previously, PLFFD following metabolically demanding, high‐intensity exercise has been linked to defective SR Ca2+ release and/or decreased myofibrillar Ca2+ sensitivity via reactive oxygen–nitrogen species‐dependent mechanisms (Cheng et al. 2016), whereas PLFFD following repeated high‐force eccentric contractions is related to myofibrillar damage that impairs force generating capacity (Skurvydas et al. 2016). Our current findings add a glycogen‐dependent mechanism to PLFFD.
Conclusion
Recovery interventions involving muscle temperature manipulation have been commonly adopted by athletes to improve recovery following different forms of exercise. A major assumption underlying the usage of muscle cooling is that it reduces inflammation induced by high‐load, high‐power exercise, e.g. during resistance exercise that might induce some muscle damage (Leeder et al. 2012; Ihsan et al. 2016). However, recent results show that local cooling does not decrease the muscle inflammatory response, or reduce indicators of muscle damage following acute resistance exercise (Peake et al. 2017), and it may blunt muscle protein synthesis during long‐term resistance training (Roberts et al. 2015). The extent to which muscle cooling influences acute recovery of muscle performance following endurance exercise has not been previously addressed. Our results show a clear positive relationship between increased muscle temperature and the recovery of force and endurance of exercised muscle, given that carbohydrate is provided during the recovery period. Cryotherapy is a popular post‐exercise recovery modality, but we provide mechanistic evidence that lowering the temperature of limb muscles following endurance‐type exercise exacerbates PLFFD, slows glycogen resynthesis, and impairs fatigue resistance during subsequent exercise. Indeed, temperature directly affects metabolism in all living organisms, with increasing temperature generally accelerating biochemical reactions (Gillooly et al. 2001). Critical to intramuscular glycogen resynthesis is muscle glucose uptake via GLUT4 in the sarcolemma, and glycogen polymer resynthesis through glycogen synthase activation (Jensen & Richter, 2012). While it seems that the number of GLUT4 glucose transporters translocating to the sarcolemma is not markedly affected by temperature (Somwar et al. 2001), glucose uptake into the fibre (Somwar et al. 2001), and the activity of glycogen synthase would slow down when the temperature is reduced. Whether or not used by endurance athletes, the current results argue against prolonged cryotherapy as an effective recovery method for athletes performing prolonged exhaustive exercise where the rate of recovery of muscle force‐generating capacity, endurance and glycogen resynthesis can play a critical role; for instance, in competitive sports that require multiple races to be performed in the same day, or in prolonged events performed over consecutive days.
Additional information
Competing interests
The authors have no competing interests.
Author contributions
Conception of the study design was done by A.J.C., S.J.W., C.Z., T.C., H.C.H. and H.W. Human experiments were performed at Mid Sweden University by A.J.C., S.W. and C.Z. Animal experiments were performed at Karolinska Institutet by A.J.C., T.C. and N.I. Initial drafting of the work was done by A.J.C. and H.W., and critical interpretation and revisions of the work was performed by all authors. All authors approved the final version of the manuscript, and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was supported by the Swedish Research Council to H.W. (K2014‐52X‐10842‐21‐5), the Swedish Research Council for Sport Science to A.J.C. (P2014‐0037) and H.W. (FO2016‐0033), and by a fellowship from Wenner‐Gren Foundation to T.C.
Linked articles This article is highlighted by a Perspective by Allen. To read this Perspective, visit https://doi.org/10.1113/JP275370.
This is an Editor's Choice article from the 15 December 2017 issue.
Edited by: Michael C. Hogan & Bruno Grassi
References
- Alghannam AF, Jedrzejewski D, Tweddle MG, Gribble H, Bilzon J, Thompson D, Tsintzas K & Betts JA (2016). Impact of muscle glycogen availability on the capacity for repeated exercise in man. Med Sci Sports Exerc 48, 123–131. [DOI] [PubMed] [Google Scholar]
- Allen DG, Lamb GD & Westerblad H (2008). Skeletal muscle fatigue: cellular mechanisms. Physiol Rev 88, 287–332. [DOI] [PubMed] [Google Scholar]
- Barnes M, Gibson LM & Stephenson DG (2001). Increased muscle glycogen content is associated with increased capacity to respond to T‐system depolarisation in mechanically skinned skeletal muscle fibres from the rat. Pflügers Archiv 442, 101–106. [DOI] [PubMed] [Google Scholar]
- Blomstrand E & Saltin B (1999). Effect of muscle glycogen on glucose, lactate and amino acid metabolism during exercise and recovery in human subjects. J Physiol 514, 293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruton J, Lännergren J & Westerblad H (1998). Effects of CO2‐induced acidification on the fatigue resistance of single mouse muscle fibers at 28 degrees C. J Appl Physiol 85, 478–483. [DOI] [PubMed] [Google Scholar]
- Bruton JD, Place N, Yamada T, Silva JP, Andrade FH, Dahlstedt AJ, Zhang SJ, Katz A, Larsson NG & Westerblad H (2008). Reactive oxygen species and fatigue‐induced prolonged low‐frequency force depression in skeletal muscle fibres of rats, mice and SOD2 overexpressing mice. J Physiol 586, 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cady EB, Jones DA, Lynn J & Newham DJ (1989). Changes in force and intracellular metabolites during fatigue of human skeletal muscle. J Physiol 418, 311–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng AJ, Bruton JD, Lanner JT & Westerblad H (2015). Antioxidant treatments do not improve force recovery after fatiguing stimulation of mouse skeletal muscle fibres. J Physiol 593, 457–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng AJ, Place N & Westerblad H (2017). Molecular basis for exercise‐induced fatigue: the importance of strictly controlled cellular Ca2+ handling. Cold Spring Harb Perspect Med, https://doi.org/10.1101/cshperspect.a029710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng AJ & Westerblad H (2017). Mechanical isolation, and measurement of force and myoplasmic free [Ca2+] in fully‐intact single skeletal muscle fibers. Nat Protoc 12, 1763–1776. [DOI] [PubMed] [Google Scholar]
- Cheng AJ, Yamada T, Rassier DE, Andersson DC, Westerblad H & Lanner JT (2016). Reactive oxygen/nitrogen species and contractile function in skeletal muscle during fatigue and recovery. J Physiol 594, 5149–5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin E & Allen D (1997). Effects of reduced muscle glycogen concentration on force, Ca2+ release and contractile protein function in intact mouse skeletal muscle. J Physiol 498, 17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow MT & Kushmerick MJ (1983). Correlated reduction in velocity of shortening and the rate of energy utilization in mouse fast‐twitch muscle during a continuous tetanus. J Gen Physiol 82, 703–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhamel TA, Perco JG & Green HJ (2006). Manipulation of dietary carbohydrates after prolonged effort modifies muscle sarcoplasmic reticulum responses in exercising males. Am J Physiol Regul Integr Comp Physiol 291, R1100–R1110. [DOI] [PubMed] [Google Scholar]
- Duke AM & Steele DS (2001). Mechanisms of reduced SR Ca2+ release induced by inorganic phosphate in rat skeletal muscle fibers. Am J Physiol Cell Physiol 281, C418–429. [DOI] [PubMed] [Google Scholar]
- Dulhunty A, Carter G & Hinrichsen C (1984). The membrane capacity of mammalian skeletal muscle fibres. J Muscle Res Cell Motil 5, 315–332. [DOI] [PubMed] [Google Scholar]
- Dutka TL, Mollica JP, Lamboley CR, Weerakkody VC, Greening DW, Posterino GS, Murphy RM & Lamb GD (2017). S‐Nitrosylation and S‐glutathionylation of Cys134 on troponin I have opposing competitive actions on Ca2+ sensitivity in rat fast‐twitch muscle fibers. Am J Physiol Cell Physiol 312, C316–C327. [DOI] [PubMed] [Google Scholar]
- Edwards R, Hill D, Jones D & Merton P (1977). Fatigue of long duration in human skeletal muscle after exercise. J Physiol 272, 769–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gejl KD, Hvid LG, Frandsen U, Jensen K, Sahlin K & Ørtenblad N (2014). Muscle glycogen content modifies SR Ca2+ release rate in elite endurance athletes. Med Sci Sports Exerc 46, 496–505. [DOI] [PubMed] [Google Scholar]
- Gillooly JF, Brown JH, West GB, Savage VM & Charnov EL (2001). Effects of size and temperature on metabolic rate. Science 293, 2248–2251. [DOI] [PubMed] [Google Scholar]
- Gregson W, Allan R, Holden S, Phibbs P, Doran D, Campbell I, Waldron S, Joo CH & Morton JP (2013). Postexercise cold‐water immersion does not attenuate muscle glycogen resynthesis. Med Sci Sports Exerc 45, 1174–1181. [DOI] [PubMed] [Google Scholar]
- Hargreaves M, McConell G & Proietto J (1995). Influence of muscle glycogen on glycogenolysis and glucose uptake during exercise in humans. J Appl Physiol 78, 288–292. [DOI] [PubMed] [Google Scholar]
- Helander I, Westerblad H & Katz A (2002). Effects of glucose on contractile function, [Ca2+]i, and glycogen in isolated mouse skeletal muscle. Am J Physiol Cell Physiol 282, C1306–C1312. [DOI] [PubMed] [Google Scholar]
- Ihsan M, Watson G & Abbiss CR (2016). What are the physiological mechanisms for post‐exercise cold water immersion in the recovery from prolonged endurance and intermittent exercise? Sports Med 46, 1095–1109. [DOI] [PubMed] [Google Scholar]
- Jensen TE & Richter EA (2012). Regulation of glucose and glycogen metabolism during and after exercise. J Physiol 590, 1069–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentjens R & Jeukendrup A (2003). Determinants of post‐exercise glycogen synthesis during short‐term recovery. Sports Med 33, 117–144. [DOI] [PubMed] [Google Scholar]
- Kiens B & Richter EA (1998). Utilization of skeletal muscle triacylglycerol during postexercise recovery in humans. Am J Physiol Endocrinol Metab 275, E332–E337. [DOI] [PubMed] [Google Scholar]
- Krustrup P, Ørtenblad N, Nielsen J, Nybo L, Gunnarsson TP, Iaia FM, Madsen K, Stephens F, Greenhaff P & Bangsbo J (2011). Maximal voluntary contraction force, SR function and glycogen resynthesis during the first 72 h after a high‐level competitive soccer game. Eur J Appl Physiol 111, 2987–2995. [DOI] [PubMed] [Google Scholar]
- Lännergren J & Westerblad H (1987). The temperature dependence of isometric contractions of single, intact fibres dissected from a mouse foot muscle. J Physiol 390, 285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver DR (2006). Regulation of ryanodine receptors from skeletal and cardiac muscle during rest and excitation. Clin Exp Pharmacol Physiol 33, 1107–1113. [DOI] [PubMed] [Google Scholar]
- Leeder J, Gissane C, van Someren K, Gregson W & Howatson G (2012). Cold water immersion and recovery from strenuous exercise: a meta‐analysis. Br J Sports Med 46, 233–240. [DOI] [PubMed] [Google Scholar]
- McConell G, Fabris S, Proietto J & Hargreaves M (1994). Effect of carbohydrate ingestion on glucose kinetics during exercise. J Appl Physiol 77, 1537–1541. [DOI] [PubMed] [Google Scholar]
- Maehlum S & Hermansen L (1978). Muscle glycogen concentration during recovery after prolonged severe exercise in fasting subjects. Scand J Clin Lab Invest 38, 557–560. [DOI] [PubMed] [Google Scholar]
- Marchand I, Tarnopolsky M, Adamo K, Bourgeois J, Chorneyko K & Graham T (2007). Quantitative assessment of human muscle glycogen granules size and number in subcellular locations during recovery from prolonged exercise. J Physiol 580, 617–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J, Cheng AJ, Ørtenblad N & Westerblad H (2014). Subcellular distribution of glycogen and decreased tetanic Ca2+ in fatigued single intact mouse muscle fibres. J Physiol 592, 2003–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J, Holmberg HC, Schrøder HD, Saltin B & Ørtenblad N (2011). Human skeletal muscle glycogen utilization in exhaustive exercise: role of subcellular localization and fibre type. J Physiol 589, 2871–2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J, Schrøder H, Rix C & Ørtenblad N (2009). Distinct effects of subcellular glycogen localization on tetanic relaxation time and endurance in mechanically skinned rat skeletal muscle fibres. J Physiol 587, 3679–3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ørtenblad N & Nielsen J (2015). Muscle glycogen and cell function – location, location, location. Scand J Med Sci Sports 25, 34–40. [DOI] [PubMed] [Google Scholar]
- Ørtenblad N, Nielsen J, Saltin B & Holmberg HC (2011a). Role of glycogen availability in sarcoplasmic reticulum Ca2+ kinetics in human skeletal muscle. J Physiol 589, 711–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ørtenblad N, Nielsen J, Saltin B & Holmberg HC (2011b). Role of glycogen availability in sarcoplasmic reticulum Ca2+ kinetics in human skeletal muscle. J Physiol 589, 711–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peake JM, Roberts LA, Figueiredo VC, Egner I, Krog S, Aas SN, Suzuki K, Markworth JF, Coombes JS, Cameron‐Smith D & Raastad T (2017). The effects of cold water immersion and active recovery on inflammation and cell stress responses in human skeletal muscle after resistance exercise. J Physiol 595, 695–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piehl K (1974). Time course for refilling of glycogen stores in human muscle fibres following exercise‐induced glycogen depletion. Acta Physiol Scand 90, 297–302. [DOI] [PubMed] [Google Scholar]
- Pilegaard H, Osada T, Andersen LT, Helge JW, Saltin B & Neufer PD (2005). Substrate availability and transcriptional regulation of metabolic genes in human skeletal muscle during recovery from exercise. Metabolism 54, 1048–1055. [DOI] [PubMed] [Google Scholar]
- Place N, Ivarsson N, Venckunas T, Neyroud D, Brazaitis M, Cheng AJ, Ochala J, Kamandulis S, Girard S, Volungevicius G, Pauzas H, Mekideche A, Kayser B, Martinez‐Redondo V, Ruas JL, Bruton J, Truffert A, Lanner JT, Skurvydas A & Westerblad H (2015). Ryanodine receptor fragmentation and sarcoplasmic reticulum Ca2+ leak after one session of high‐intensity interval exercise. Proc Natl Acad Sci USA 112, 15492–15497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall JA (2005). Energetics, mechanics and molecular engineering of calcium cycling in skeletal muscle. Adv Exp Med Biol 565, 183–192. [DOI] [PubMed] [Google Scholar]
- Rebbeck RT, Karunasekara Y, Board PG, Beard NA, Casarotto MG & Dulhunty AF (2014). Skeletal muscle excitation–contraction coupling: who are the dancing partners? Int J Biochem Cell Biol 48, 28–38. [DOI] [PubMed] [Google Scholar]
- Roberts LA, Raastad T, Markworth JF, Figueiredo VC, Egner IM, Shield A, Cameron‐Smith D, Coombes JS & Peake JM (2015). Post‐exercise cold water immersion attenuates acute anabolic signalling and long‐term adaptations in muscle to strength training. J Physiol 593, 4285–4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurvydas A, Mamkus G, Kamandulis S, Dudoniene V, Valanciene D & Westerblad H (2016). Mechanisms of force depression caused by different types of physical exercise studied by direct electrical stimulation of human quadriceps muscle. Eur J Appl Physiol 116, 2215–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somwar R, Kim DY, Sweeney G, Huang C, Niu W, Lador C, Ramlal T & Klip A (2001). GLUT4 translocation precedes the stimulation of glucose uptake by insulin in muscle cells: potential actiation of GLUT4 via p38 mitogen‐activated protein kinase. Biochem J 359, 639–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson DG, Nguyen LT & Stephenson GM (1999). Glycogen content and excitation‐contraction coupling in mechanically skinned muscle fibres of the cane toad. J Physiol 519, 177–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MM, Cheung SS, Elder GC & Sleivert GG (2006). Voluntary muscle activation is impaired by core temperature rather than local muscle temperature. J Appl Physiol 100, 1361–1369. [DOI] [PubMed] [Google Scholar]
- Todd G, Butler JE, Taylor JL & Gandevia SC (2005). Hyperthermia: a failure of the motor cortex and the muscle. J Physiol 563, 621–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker T, Slivka D, Cuddy J, Hailes W & Ruby B (2012). Effect of local cold application on glycogen recovery. J Sports Med Phys Fitness 52, 158–164. [PubMed] [Google Scholar]
- Tyler CJ, Sunderland C & Cheung SS (2015). The effect of cooling prior to and during exercise on exercise performance and capacity in the heat: a meta‐analysis. Br J Sports Med 49, 7–13. [DOI] [PubMed] [Google Scholar]
- Watanabe D & Wada M (2016). Predominant cause of prolonged low‐frequency force depression changes during recovery after in situ fatiguing stimulation of rat fast‐twitch muscle. Am J Physiol Regul Integr Comp Physiol 311, R919–R929. [DOI] [PubMed] [Google Scholar]
- Westerblad H & Allen DG (1992). Myoplasmic free Mg2+ concentration during repetitive stimulation of single fibres from mouse skeletal muscle. J Physiol 453, 413–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Bruton JD, Allen DG & Lännergren J (2000). Functional significance of Ca2+ in long‐lasting fatigue of skeletal muscle. Eur J Appl Physiol 83, 166–174. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Davis JK, Casa DJ & Bishop PA (2015). Optimizing cold water immersion for exercise‐induced hyperthermia: a meta‐analysis. Med Sci Sports Exerc 47, 2464–2472. [DOI] [PubMed] [Google Scholar]
- Zinner C, Morales‐Alamo D, Ørtenblad N, Larsen FJ, Schiffer TA, Willis SJ, Gelabert‐Rebato M, Perez‐Valera M, Boushel R, Calbet JA & Holmberg HC (2016). The physiological mechanisms of performance enhancement with sprint interval training differ between the upper and lower extremities in humans. Front Physiol 7, 426. [DOI] [PMC free article] [PubMed] [Google Scholar]


