Abstract
Recent studies demonstrated that bladder cancers can be grouped into basal and luminal molecular subtypes that possess distinct biological and clinical characteristics. Basal bladder cancers express biomarkers characteristic of cancer stem cells and epithelial-to-mesenchymal transition (EMT). Patients with basal cancers tend have more advanced stage and metastatic disease at presentation. In preclinical models basal human orthotopic xenografts are also more metastatic than luminal xenografts are, and they metastasize via an EMT-dependent mechanism. However, preclinical and clinical data suggest that basal cancers are also more sensitive to neoadjuvant chemotherapy (NAC), such that most patients with basal cancers who are aggressively managed with NAC have excellent outcomes. Importantly, luminal bladder cancers can also progress to become invasive and metastatic, but they appear to do so via mechanisms that are much less dependent on EMT and may involve help from stromal cells, particularly cancer-associated fibroblasts (CAFs). Although patients with luminal cancers do not appear to derive much clinical benefit from NAC, the luminal tumors that are infiltrated with stromal cells appear to be sensitive to anti-PDL1 antibodies and possibly other immune checkpoint inhibitors. Therefore, neoadjuvant and/or adjuvant immunotherapy may be the most effective approach in treating patients with advanced or metastatic infiltrated luminal bladder cancers.
Keywords: Bladder cancer, Metastasis, Basal subtypes, Luminal subtypes, Epithelial-to-mesenchymal transition
1. Introduction
Urothelial bladder cancer is a highly heterogeneous disease with variable patterns of progression and responses to conventional and targeted agents [1]. Histopathologically, bladder cancers are grouped into two major subsets (papillary and non-papillary) that pose distinct challenges for clinical management [2]. Papillary bladder cancers rarely progress to become muscle-invasive and metastatic, but they are highly prone to recurrence, necessitating expensive life-long clinical surveillance with repeated surgical intervention [1], [2]. Thus, a top research priority is to identify agents that produce long-term durable remissions in patients with this form of the disease. There is also a need to identify biomarkers that distinguish papillary cancers that pose no risk of progression to muscle invasion from the smaller fraction that do, so that more aggressive interventions can be applied early when the chance of cure is greatest.
On the other hand, non-papillary bladder cancers have a high risk of progression to muscle invasive and metastatic disease [1], and they therefore pose a major threat to the patient. It is believed that many of these cancers evolve from carcinoma in situ (CIS) [2], although many patients have advanced or metastatic disease at presentation. High risk papillary and non-papillary non-muscle invasive cancers are currently treated uniformly with intravesical bacillus Calmette-Guerin (BCG) [1], which is a tuberculosis-like mycobacterium that produces a local immune response that mediates tumor regression [3]. However, even though most (>70%) patients are initially rendered free of clinically detectable disease, most will also develop recurrences that can become BCG-unresponsive [4]. These tumors have a high risk of progression, and at this point clinicians are faced with the decision of whether to remove the bladder (cystectomy) or to make another attempt at bladder preservation with a different intravesical or systemic regimen. There are currently few viable candidates for the latter, but new immunotherapy approaches hold promise. For example, adenoviral interferon-alpha (Ad-IFNα) gene therapy produced durable clinical responses in over a third of patients with BCG-unresponsive disease in completed clinical trials [5], and a phase III registration trial is now open that could lead to FDA approval. There are also open clinical trials examining the potential efficacy of immune checkpoint blockade in these patients [6].
Muscle-invasive bladder cancers are also clinically heterogeneous [1]. Approximately half of patients are cured by cystectomy with or without perioperative cisplatin-based combination chemotherapy, but the others experience very rapid disease progression to cisplatin-refractory and metastatic disease [1]. Bladder cancers tend to metastasize to the liver, lung, brain, and bone, but the molecular mechanisms underlying organ-specific metastasis have not been identified. Until very recently there were no effective treatment options for patients with advanced and/or metastatic disease, but exciting recent studies established that immunotherapy with blocking anti-PD1 or -PDL1 antibodies produces significant and durable benefit in about a quarter of these patients [7], [8], which prompted the FDA to approve the anti-PDL1 antibody atezolizumab for advanced bladder cancer in May 2016.
Papillary and non-papillary bladder cancers appear to be caused by distinct genomic alterations [2]. Papillary cancers are characterized by very high frequencies (>70%) of activating mutations in the type 3 receptor for fibroblast growth factor receptor (FGFR3) [9]. The mutations cause constitutive ligand-independent dimerization of FGFR3, leading to downstream MAP kinase activation that drives proliferation. So far no mouse models of FGFR3-driven papillary bladder tumorigenesis have been developed, but expression of constitutively active mutant Ha-ras under the control of a urothelium-specific (uroplakin) promoter causes papillary tumorigenesis [10], suggesting that FGFR3-induced Ras pathway activation probably plays a central role in transformation.
Non-papillary bladder cancers are more closely associated with inactivation of classical tumor suppressors, most notably TP53 and RB1 [2], [9]. It appears that TP53 mutations are present in precursor lesions (CIS) [2], [9], but most of the published studies that support this conclusion used indirect methods to identify p53-mutant tumors (i.e., immunohistochemistry to detection high levels of p53 protein), so more direct methods, such as next generation DNA sequencing, will be required to confirm these results. Preclinical studies confirmed that TP53 inactivation promotes the emergence of CIS and muscle-invasive tumors in mice exposed to the cigarette smoke nitrosamine carcinogen, BBN [11] and combined inactivation of TP53 and RB1 via expression of the SV40 large T antigen in the urothelium also drives CIS and non-papillary tumorigenesis in mice [12]. Inactivation of TP53 and PTEN in the mouse urothelium produced similar effects [13].
Recent lineage tracing studies suggest that papillary and non-papillary bladder cancers also arise from different cells of origin [14], [15]. The papillary non-muscle invasive tumors that arise spontaneously in BBN-treated mice originate via transformation of a cell within the intermediate and/or superficial (luminal) layer [14], whereas CIS and muscle-invasive tumors arise from SONIC Hedgehog-expressing basal cell(s) [15]. It will be interesting to determine whether the genomic abnormalities that are observed in papillary and non-papillary cancers are only permissive for transformation in these more luminal or basal cells, respectively.
2. Intrinsic subtypes of bladder cancer
The introduction of whole genome technologies to catalog all of the mRNA expression patterns and DNA alterations in a given tumor has transformed our understanding of human cancers. The Cancer Genome Atlas (TCGA) and the International Cancer Genomics Consortium (ICGC) are examples of two high profile collaborative public projects that have exploited these capabilities to obtain comprehensive genomic portraits of dozens of human cancers, and parallel private efforts have further enhanced these efforts. One approach that has been used very successfully has been to use transcriptome profiling data to identify “molecular subtypes” of cancers that share gene expression signatures and by inference, biological properties. The highest profile early example of the successful utilization of this approach can be found in the work of Perou and colleagues [16] in human breast cancer. Their results revealed that breast cancers can be grouped into at least 5 distinct subtypes (luminal A, luminal B, HER2-enriched, basal-like, and claudin-low) that behave clinically as distinct disease entities [17]. Luminal tumors are associated with good long-term outcomes, and patients with them obtain major benefit from adjuvant therapy with selective estrogen receptor modulators (SERMs) but not from perioperative chemotherapy [18], [19], [20]. On the other hand, basal-like, HER2-enriched, or claudin-low tumors tend to be highly aggressive, progressing rapidly to produce local and distant metastases and shorter disease-specific survival. Patients with these cancers do not benefit from adjuvant therapy with SERMs, but many do obtain major benefit from perioperative chemotherapy, and patients with HER2-enriched tumors also benefit from ERBB2 antagonists (i.e., Herceptin) [18], [19], [20].
The gene expression signatures associated with the breast cancer subtypes provide important information about their likely biological properties. Luminal cancers express genes characteristic of estrogen receptor activation and terminal differentiation within the normal mammary epithelium [16]. Luminal B cancers are largely distinguished from luminal A by higher expression of proliferation biomarkers (i.e., Ki-67) [19], and they also contain more TP53 mutations [21], [22]. In contrast, basal-like cancers express higher levels of biomarkers that characterize the basal layer of the normal mammary epithelium and of other epithelial tissues, including TP63, CD44, and high molecular weight cytokeratins (KRT5,6, and 14) [16], [21], [23]. These biomarkers are also enriched in normal epithelial stem cells and cancer-associated stem cells derived from epithelial lineage tumors. Basal-like tumors are also enriched with biomarkers associated with epithelial-to-mesenchymal transition (EMT) [19], [21], a developmental program that mediates wound healing and can be reactivated to promote metastasis in solid tumors. The claudin-low tumors are a subset of the basal-like cancers that express even higher levels of EMT and stem cell biomarkers with lower expression of canonical epithelial biomarkers, including E-cadherin, the claudins, and the cytokeratins [19], [24]. They express somewhat lower levels of Ki-67 than do the basal-like tumors, and consequently they are somewhat less sensitive to neoadjuvant chemotherapy (NAC) [19]. Preclinical studies concluded that basal-like cancers can readily and spontaneously “switch” between more epithelial and more mesenchymal states [25], which may be important for their aggressive behaviors (Fig. 1).
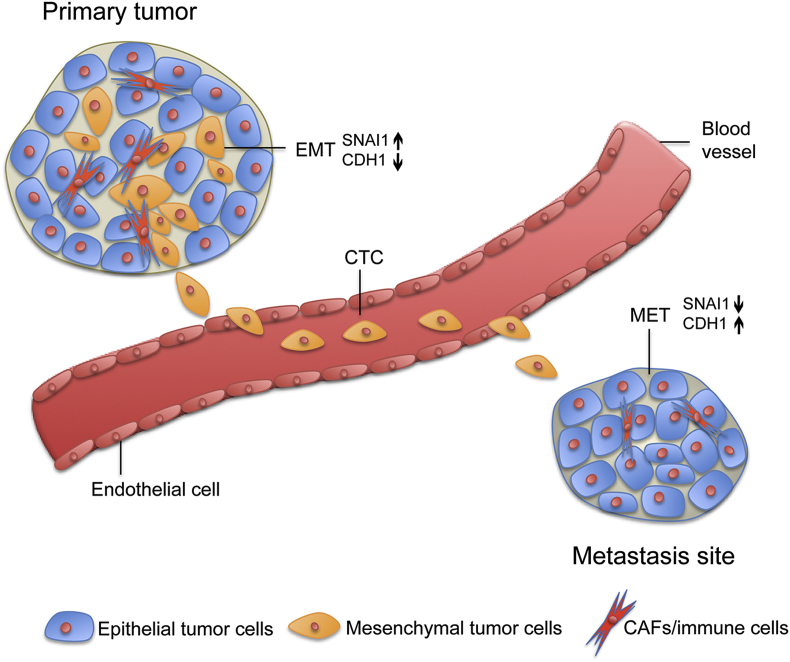
Figure 1.
Reversible EMT mediates metastasis of basal bladder cancers. Cells from orthotopic UM-UC3 tumors expressed higher levels of “epithelial” biomarkers than did circulating tumor cells (CTCs) harvested from tumor-bearing animals. The CTCs expressed particularly high levels of the EMT transcription factor, SNAIL (SNAI1). Interestingly, expression of EMT biomarkers returned to baseline in established metastases. Very similar observations were made previously by Tsai et al. [49] in a mouse model of squamous cell carcinoma. Based on Roth et al., Oncotarget 2016 [59]. CAFs, cancer-associated fibroblasts; EMT, epithelial-to-mesenchymal transition.
The application of similar methods to whole genome mRNA expression profiling data from multiple independent cohorts of bladder cancers revealed that they can also be grouped into basal and luminal subtypes (Table 1) [26], [27], [28], [29]. Most of this work was focused on muscle-invasive bladder cancers, but the first deep characterization of non-muscle invasive cancers was just published and reveals similarities [30]. At the highest level, unsupervised hierarchical analyses revealed that non-muscle and muscle-invasive cancers are highly distinct [31], [32], [33], driven largely by differential expression of genes related to cell cycle progression, invasion, migration, metastasis, and the DNA damage response [34]. Muscle-invasive cancers also contain more TP53 mutations and copy number variations characteristic of chromosomal instability [34]. The work that has been performed to date suggests that non-muscle invasive cancers can be further subdivided into 2–3 molecular subtypes [30], [35], and at least one of them appears to be enriched with basal biomarkers [30], but additional work is required to more precisely define the relationships between the molecular subtypes of non-muscle invasive and muscle-invasive cancers.
Table 1.
Molecular subtypes of bladder cancer.
| TCGA | Lund | MDACC | UNC | Properties |
|---|---|---|---|---|
| Cluster I | UroA, GU | Luminal | Luminal | FGFR3 mutations, papillary features |
| Cluster II | Infiltrated | P53-like | Luminal | CAFs, immune cells |
| Cluster III | SCC-like, UroB | Basal | Basal-like | Stem cell biomarkers, squamous features |
| Cluster IV | Infiltrated | P53-like | Claudin-low | EMT, CAFs, immune cells |
Transcriptome profiling and unsupervised analyses were used by several groups to identify candidate molecular subtypes of bladder cancer. Cross comparisons of the results obtained by 4 of the groups are presented above as examples. These comparisons are meant to be illustrative rather than definitive, but they show how the overall concordance among the approaches was very high. Also see Aine et al., Sci Rep 2015 [37] for a more detailed analysis.
While the molecular portraits of non-muscle invasive cancers are still emerging, much more is known about the molecular subtypes of muscle-invasive bladder cancers. Pioneering studies performed by Höglund and colleagues at the University of Lund identified 5 distinct subtypes of muscle-invasive cancers that exhibited biologically informative gene expression signatures (Table 1) [35]. One of the subtypes (SCC-like) was enriched with squamous histopathological features and biomarkers that characterize squamous tumors in other organ sites, and patients with these tumors exhibited relatively poor clinical outcomes. In two of the subtypes (urobasal A and urobasal B) the hierarchical patterns of differentiation-associated urothelial biomarker expression were preserved. These tumors expressed FGFR3-associated gene expression signatures [35], suggesting that these tumors might be dependent on FGFR3 signaling for their growth. Another subtype, termed genomically unstable, was characterized by homogeneous expression of E-cadherin and dysregulated expression of biomarkers associated with cell cycle and the DNA damage response.
Subsequently, TCGA [26] and several other groups [27], [28], [36] independently identified molecular subtypes of bladder cancer. Although each group defended the existence of different numbers of subtypes (n = 2–4), the overall concordance among them (and with the 5 subtypes identified previously by the group at Lund) was extremely high [37]. At the highest level, muscle-invasive bladder cancers can be subdivided into basal and luminal subtypes (Table 1). Basal bladder cancers can be further subdivided into basal and claudin-low, and luminal bladder cancers can be subdivided based on differential expression of biomarkers associated with stromal cell infiltration and proliferation (Table 1). The Lund group's uroA and genomically unstable (GU) tumors correspond to luminal tumors identified by the other groups that possess different patterns of driver mutations (W. Choi, manuscript in preparation). UroA tumors are enriched with mutations in FGFR3 and other mutations that are also enriched in low-grade papillary tumors, whereas the GU tumors contain more inactivating TP53 and RB1 alterations and copy number variations (W. Choi, manuscript in preparation). Interestingly, the Lund group's uroB subtype is also enriched with activating FGFR3 mutations but is assigned to the basal subtypes using the other classifiers, is enriched with squamous features, and is associated with poor clinical outcomes [35]. The uroB tumors may therefore correspond to a “progressed” subset of the uroA cancers that have acquired more aggressive properties.
3. Implications of the basal and luminal subtypes for metastasis
The clinical properties associated with the basal and luminal subtypes of muscle-invasive bladder cancer provide additional clues about their origins. Luminal bladder cancers are enriched with papillary histopathological features [26], consistent with the idea that they correspond to low-grade papillary tumors that acquired additional genomic abnormalities and progressed to become muscle-invasive. Conversely, basal tumors are often enriched with squamous histopathological features [27], [33], [35], consistent with clinical experience indicating that tumors with squamous features tend to be more aggressive. It seems likely that other bladder cancer histopathological variants will also display luminal or basal biases. For example, we recently showed that micropapillary bladder cancers correspond to a clinically aggressive luminal variant characterized by downregulation of miR-296 [38].
Like their breast cancer counterparts, basal and luminal UCs are associated with different levels of benefit from NAC. Initial studies demonstrated that rates of pathological down staging were lower in “infiltrated” (p53-like) basal and luminal tumors [27], although more recent results raise questions about the relative impact of down staging on survival in patients with basal or luminal cancers. We recently performed subtype assignments on tumors collected from patients enrolled in a phase II neoadjuvant clinical trial of dose-dense MVAC plus bevacizumab (Avastin). Rates of down staging in patients with basal or luminal tumors were equivalent, but the patients with basal tumors had significantly better long-term outcomes [39]. Indeed, patients with basal tumors benefited much more from NAC than would have been expected based on down staging. There are many possible explanations for this uncoupling of pathological down staging of the primary tumor and survival, including differential effects of surgery, different levels of subclinical metastases, and differential sensitivity of those micro-metastases to NAC. All of these possibilities are currently under investigation.
As compared with luminal tumors, basal UCs are associated with advanced stage and metastatic disease at clinical presentation [27]. Like their breast cancer counterparts, basal UCs are characterized by high levels of stem cell and EMT biomarkers [26], [27], [28], which are overexpressed even further in the claudin-low subset of basal UCs [28]. Thus, the lethality associated with basal UCs in patients who do not receive NAC appears to be linked more tightly to their intrinsic invasive and metastatic potential as opposed to intrinsic drug resistance.
3.1. EMT and UC metastasis
A very large body of preclinical and clinical evidence has linked EMT to invasion, migration, stemness, and metastasis [40], [41], [42], [43]. During EMT polarized epithelial cells downregulate the homotypic adhesion molecule E-cadherin via upregulation of families of transcription factors, including ZEB1/2, SNAIL, SLUG, and TWIST, that block E-cadherin expression [40]. Under normal conditions the epithelial phenotype is maintained by expression of members of the miR-200 family of microRNAs [44], [45], [46], which interact directly with the transcripts encoding ZEB1 and ZEB2, blocking their translation and promoting their degradation. Conversely, ZEB1 and ZEB2 can bind to the promoters that control expression of the miR-200 family and inhibit their expression. Interestingly, TP63, which has been implicated in epithelial “stemness” [47], can also block EMT by inducing expression of miR-205 [48], which functions like the members of the miR-200 family to inhibit ZEB1 and ZEB2. In normal epithelial cells reversible EMT is initiated by cytokines (particularly TGFβ) that induce expression of the EMT-associated transcription factors [40]. However, in many cancers EMT appears to be driven by endogenous mechanisms.
Reversible EMT plays a central role in wound healing by enabling normal epithelial cells to temporarily adopt a more invasive and migratory phenotype that promotes wound closure [40]. These effects are coopted by cancer cells to enable them to become invasive and metastatic [40]. Using whole genome mRNA expression profiling, Weinberg's group [43] demonstrated that metastatic murine breast cancer cells overexpressed TWIST, and TWIST knockdown blocked metastasis. Indeed, enforced overexpression of TWIST was sufficient to promote “stemness” in human breast cancer cells [42].
As is true in wound healing, it appears that EMT reversibility also promotes metastasis. Using a conditional TWIST mouse model, Yang's group demonstrated that sustained, enforced TWIST expression promoted tumor cell invasion and migration in carcinogen-induced squamous cell carcinomas and resulted in the emergence of large numbers of circulating tumor cells (CTCs) in the peripheral blood, but it did not lead to efficient formation of large metastases [49]. Rather, transient upregulation of TWIST resulted in a transient EMT and downstream CTC production that was associated with the formation of large macro-metastases [49]. These effects were attributable to the differential effects of EMT on invasion/migration on the one hand and on proliferation on the other – the increase in the former was associated with a significant decrease in the latter [49]. It is interesting and potentially significant that there are similar differences in baseline proliferation rates (as measured by Ki-67 immunohistochemistry) in claudin-low and basal-like breast cancers [19].
Weinberg's group [25] also demonstrated that basal breast cancer cell lines have a much higher tendency to undergo spontaneous EMT than luminal breast cancer cell lines do. They linked these effects to a “poised” chromatin conformation within the ZEB1 promoter, enabling it to respond rapidly to environmental cues [25]. The molecular mechanisms that mediate this chromatin phenotype have not been defined, although as discussed above, a role for TP63 is very attractive given its established roles in maintaining both “stemness” and an “epithelial” phenotype. Modulation of TP63 expression could represent an important mechanism for effecting rapid and reversible changes in EMT transcription factor expression. Another possible mechanism could involve fluctuations in the expression of members of the miR-200 family of microRNAs [45].
Although luminal breast cancers are associated with lower rates of early metastasis, a significant fraction of women with luminal cancers do develop late (mostly bone) metastases. Therefore, luminal cancer cells are clearly capable of metasizing, but they may require “help” from cells in the tumor microenvironment [50], [51], [52], including cancer-associated fibroblasts (CAFs) [51]. Work from Massague's group [51] demonstrated that fibroblasts promoted bone metastasis in human breast cancer xenografts via Src pathway activation and production of specific chemokines and cytokines, most notably CXCL12 and IGF-1. CAFs appeared to “prime” breast cancer cells within the primary tumor for subsequent growth in the bone microenvironment by upregulating these Src-related pathways [51].
A significant fraction of primary luminal human breast cancers is infiltrated with CAFs [25], and it is possible that they enable them to metastasize without undergoing full EMT. The existence of EMT-dependent and -independent pathways of metastasis has recently been documented in two high profile studies performed in genetically engineered mouse models of breast and pancreatic cancer [53], [54].
3.2. EMT, CAFs and UC metastasis
Our group has a longstanding interest in defining the molecular mechanisms that mediate UC metastasis. Early studies demonstrated that invasion and metastasis in patients were associated with upregulation of matrix metalloproteinase-9 (MMP9) and downregulation of E-cadherin [55], [56], changes that are consistent with EMT [57]. We also developed some of the first preclinical mouse models to study UC metastasis using an approach termed “orthotopic recycling” in nude mice [58]. The technique involves implanting human cancer cells into the anatomically correct organ microenvironment (in this case, the bladder wall), allowing the tumors to grow until the animals become moribund, harvesting cells from any metastases that develop, and reimplanting them back into the bladder. This process is repeated until metastases are consistently observed in almost all of the mice [58].
We initially used this approach to generate metastatic variants of the 253J human bladder cancer cell line [58]. More recently we launched studies to systematically measure the tumorigenic and metastatic potentials of the 30 human cell lines in our panel, and we recently published results obtained with the first 5 (UM-UC3, UM-UC6, UM-UC9, UM-UC13, and UM-UC14) [59]. Of them, only the UM-UC3 and UM-UC13 lines reliably produced metastases after orthotopic recycling in nude mice [59]. The UM-UC3 and UM-UC13 lines were also the only cells in the panel that expressed biomarkers characteristic of EMT [48].
We used the complete TCGA dataset to develop a 515-gene prediction analysis of microarrays (PAM) classifier that assigned primary human tumors to the basal or luminal subtype with a high degree of accuracy (A. Ochoa, manuscript under revision). Using this classifier, we assigned all 30 lines in our panel of human bladder cancer cells to the basal or luminal subtype. The UM-UC3 and UM-UC13 cells were stably assigned to the basal subtype, whereas the UM-UC6, UM-UC9, and UM-UC14 lines were all stably assigned to the luminal subtype. This result was not surprising given that EMT biomarkers are generally enriched in basal cancers, but it supported the overall hypothesis that basal cancers are intrinsically more metastatic than luminal cancers are.
As described above, a recent study concluded that reversible EMT plasticity was required for optimal formation of macro-metastases in a mouse model of squamous cell carcinoma. To investigate whether the same effects were required for metastasis in our preclinical models, we performed whole genome mRNA expression profiling on matched primary tumors, CTCs, and established macro-metastases from the UM-UC3 model [59]. Consistent with the previous work, many EMT markers were upregulated in the CTCs relative to the primary tumors or established metastases, but the most striking change observed was upregulation of SNAIL (SNAI1) in the CTCs [59]. Therefore, in order to directly determine whether this upregulation of SNAIL was required for metastasis, we transduced the “recycled” UM-UC3 cells with a conditional, doxycycline-regulated lentiviral shRNA construct specific for SNAIL and examined the effects of transient knockdown on CTC production and metastasis. Administration of doxycycline to the drinking water blocked both in mice bearing established UM-UC3 tumors, and removal of doxycycline restored CTC production and subsequent metastasis [59]. Therefore, SNAIL knockdown caused a reversible inhibition of both events, consistent with the hypothesis.
Although basal bladder cancers (like their breast cancer counterparts) appear to be intrinsically more metastatic, luminal bladder cancers can progress to become muscle-invasive and metastatic as well. It seems likely that CAFs play an important role in promoting metastasis of luminal cancers. Indirect support for this hypothesis came from our recently completed neoadjuvant phase II trial of dose-dense MVAC plus the blocking anti-VEGF antibody, bevacizumab (Avastin) [39]. Post-treatment tumors from resistant patients were enriched with CAFs and membership in the MDACC “p53-like”/infiltrated subtype [39], consistent with our original findings in an independent cohort [27]. Importantly, many patients with these tumors developed bone metastases, and p53-like tumors were associated with the shortest disease-specific survival [39]. We have optimized methods for isolating CAFs from bladder cancers and have confirmed that they express high levels of the biomarkers that are associated with the p53-like subtype. We plan to combine them with recycled basal and luminal human bladder cancer cells to determine whether the CAFs promote chemoresistance and metastasis. If so, the models will serve as powerful model systems to identify the molecular mechanisms involved.
4. Summary and conclusion
Like their breast cancer counterparts, basal bladder cancers are enriched with EMT and cancer stem cell biomarkers and are intrinsically aggressive. In preclinical models basal human bladder cancer cell lines are more invasive and metastatic, and they appear to metastasize via EMT-dependent mechanisms. However, basal bladder cancers also appear to be sensitive to NAC, and many patients with basal cancers treated with NAC have excellent long-term outcomes. Therefore, patients with basal cancers should be identified as quickly as possible after diagnosis so that they can be managed with frontline therapy aggressively to prevent metastasis.
Although luminal cancers appear to be less prone to metastasize, a significant fraction of them still do. NAC appears to produce less benefit in patients with luminal tumors in general and particularly in patients whose tumors are infiltrated with CAFs. In patients treated with NAC, those with p53-like infiltrated tumors appear to be at the highest risk for developing metastases, particularly to the bone, and they exhibit the shortest disease-specific survival. Therefore, novel approaches are urgently needed for patients with these CAF-infiltrated tumors. The preliminary observation that the anti-PDL1 antibody atezolizumab produced the most clinical impact in patients with infiltrated (TCGA cluster II) tumors suggests that perioperative therapy with immune checkpoint blockade might be the best option for these patients. This hypothesis will need to be tested prospectively in future clinical trials.
Conflicts of interest
The authors declare no conflict of interest.
Footnotes
Peer review under responsibility of Second Military Medical University.
References
- 1.Kamat A.M., Hahn N.M., Efstathiou J.A., Lerner S.P., Malmstrom P.U., Choi W. Bladder cancer. Lancet. 2016 doi: 10.1016/S0140-6736(16)30512-8. pii: S0140–6736(16)30512-8. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Czerniak B., Dinney C., McConkey D. Origins of bladder cancer. Annu Rev Pathol. 2016;11:149–174. doi: 10.1146/annurev-pathol-012513-104703. [DOI] [PubMed] [Google Scholar]
- 3.Biot C., Rentsch C.A., Gsponer J.R., Birkhauser F.D., Jusforgues-Saklani H., Lemaitre F. Preexisting BCG-specific T cells improve intravesical immunotherapy for bladder cancer. Sci Transl Med. 2012;4 doi: 10.1126/scitranslmed.3003586. 137ra72. [DOI] [PubMed] [Google Scholar]
- 4.Lerner S.P., Dinney C., Kamat A., Bivalacqua T.J., Nielsen M., O'Donnell M. Clarification of bladder cancer disease states following treatment of patients with intravesical BCG. Bladder Cancer. 2015;1:29–30. doi: 10.3233/BLC-159002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dinney C.P., Fisher M.B., Navai N., O'Donnell M.A., Cutler D., Abraham A. Phase I trial of intravesical recombinant adenovirus mediated interferon-alpha2b formulated in Syn3 for Bacillus Calmette-Guerin failures in nonmuscle invasive bladder cancer. J Urol. 2013;190:850–856. doi: 10.1016/j.juro.2013.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghasemzadeh A., Bivalacqua T.J., Hahn N.M., Drake C.G. New strategies in bladder cancer: a second coming for immunotherapy. Clin Cancer Res. 2016;22:793–801. doi: 10.1158/1078-0432.CCR-15-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenberg J.E., Hoffman-Censits J., Powles T., van der Heijden M.S., Balar A.V., Necchi A. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387:1909–1920. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Powles T., Eder J.P., Fine G.D., Braiteh F.S., Loriot Y., Cruz C. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558–562. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 9.Knowles M.A., Hurst C.D. Molecular biology of bladder cancer: new insights into pathogenesis and clinical diversity. Nat Rev Cancer. 2015;15:25–41. doi: 10.1038/nrc3817. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Z.T., Pak J., Huang H.Y., Shapiro E., Sun T.T., Pellicer A. Role of Ha-ras activation in superficial papillary pathway of urothelial tumor formation. Oncogene. 2001;20:1973–1980. doi: 10.1038/sj.onc.1204315. [DOI] [PubMed] [Google Scholar]
- 11.Ozaki K., Sukata T., Yamamoto S., Uwagawa S., Seki T., Kawasaki H. High susceptibility of p53(+/−) knockout mice in N-butyl-N-(4-hydroxybutyl)nitrosamine urinary bladder carcinogenesis and lack of frequent mutation in residual allele. Cancer Res. 1998;58:3806–3811. [PubMed] [Google Scholar]
- 12.Cheng J., Huang H., Zhang Z.T., Shapiro E., Pellicer A., Sun T.T. Overexpression of epidermal growth factor receptor in urothelium elicits urothelial hyperplasia and promotes bladder tumor growth. Cancer Res. 2002;62:4157–4163. [PubMed] [Google Scholar]
- 13.Puzio-Kuter A.M., Castillo-Martin M., Kinkade C.W., Wang X., Shen T.H., Matos T. Inactivation of p53 and Pten promotes invasive bladder cancer. Genes Dev. 2009;23:675–680. doi: 10.1101/gad.1772909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Batavia J., Yamany T., Molotkov A., Dan H., Mansukhani M., Batourina E. Bladder cancers arise from distinct urothelial sub-populations. Nat Cell Biol. 2014;16:982–991. doi: 10.1038/ncb3038. 1-5. [DOI] [PubMed] [Google Scholar]
- 15.Shin K., Lim A., Odegaard J.I., Honeycutt J.D., Kawano S., Hsieh M.H. Cellular origin of bladder neoplasia and tissue dynamics of its progression to invasive carcinoma. Nat Cell Biol. 2014;16:469–478. doi: 10.1038/ncb2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perou C.M., Sorlie T., Eisen M.B., van de Rijn M., Jeffrey S.S., Rees C.A. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 17.Prat A., Ellis M.J., Perou C.M. Practical implications of gene-expression-based assays for breast oncologists. Nat Rev Clin Oncol. 2012;9:48–57. doi: 10.1038/nrclinonc.2011.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cortazar P., Zhang L., Untch M., Mehta K., Costantino J.P., Wolmark N. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 19.Prat A., Parker J.S., Karginova O., Fan C., Livasy C., Herschkowitz J.I. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res BCR. 2010;12:R68. doi: 10.1186/bcr2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Minckwitz G., Untch M., Blohmer J.U., Costa S.D., Eidtmann H., Fasching P.A. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30:1796–1804. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 21.Cancer Genome Atlas Network Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ellis M.J., Perou C.M. The genomic landscape of breast cancer as a therapeutic roadmap. Cancer Discov. 2013;3:27–34. doi: 10.1158/2159-8290.CD-12-0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoadley K.A., Yau C., Wolf D.M., Cherniack A.D., Tamborero D., Ng S. Multiplatform analysis of 12 cancer types reveals molecular classification within and across tissues of origin. Cell. 2014;158:929–944. doi: 10.1016/j.cell.2014.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taube J.H., Herschkowitz J.I., Komurov K., Zhou A.Y., Gupta S., Yang J. Core epithelial-to-mesenchymal transition interactome gene-expression signature is associated with claudin-low and metaplastic breast cancer subtypes. Proc Natl Acad Sci U S A. 2010;107:15449–15454. doi: 10.1073/pnas.1004900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaffer C.L., Marjanovic N.D., Lee T., Bell G., Kleer C.G., Reinhardt F. Poised chromatin at the ZEB1 promoter enables breast cancer cell plasticity and enhances tumorigenicity. Cell. 2013;154:61–74. doi: 10.1016/j.cell.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cancer Genome Atlas Research N Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507:315–322. doi: 10.1038/nature12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi W., Porten S., Kim S., Willis D., Plimack E.R., Hoffman-Censits J. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell. 2014;25:152–165. doi: 10.1016/j.ccr.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Damrauer J.S., Hoadley K.A., Chism D.D., Fan C., Tiganelli C.J., Wobker S.E. Intrinsic subtypes of high-grade bladder cancer reflect the hallmarks of breast cancer biology. Proc Natl Acad Sci U S A. 2014;111:3110–3115. doi: 10.1073/pnas.1318376111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim J., Akbani R., Creighton C.J., Lerner S.P., Weinstein J.N., Getz G. Invasive bladder cancer: genomic insights and therapeutic promise. Clin Cancer Res. 2015;21:4514–4524. doi: 10.1158/1078-0432.CCR-14-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hedegaard J., Lamy P., Nordentoft I., Algaba F., Hoyer S., Ulhoi B.P. Comprehensive transcriptional analysis of early-stage urothelial carcinoma. Cancer Cell. 2016;30:27–42. doi: 10.1016/j.ccell.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 31.Dyrskjot L., Thykjaer T., Kruhoffer M., Jensen J.L., Marcussen N., Hamilton-Dutoit S. Identifying distinct classes of bladder carcinoma using microarrays. Nat Genet. 2003;33:90–96. doi: 10.1038/ng1061. [DOI] [PubMed] [Google Scholar]
- 32.Lindgren D., Frigyesi A., Gudjonsson S., Sjodahl G., Hallden C., Chebil G. Combined gene expression and genomic profiling define two intrinsic molecular subtypes of urothelial carcinoma and gene signatures for molecular grading and outcome. Cancer Res. 2010;70:3463–3472. doi: 10.1158/0008-5472.CAN-09-4213. [DOI] [PubMed] [Google Scholar]
- 33.Blaveri E., Simko J.P., Korkola J.E., Brewer J.L., Baehner F., Mehta K. Bladder cancer outcome and subtype classification by gene expression. Clin Cancer Res. 2005;11:4044–4055. doi: 10.1158/1078-0432.CCR-04-2409. [DOI] [PubMed] [Google Scholar]
- 34.Lindgren D., Sjodahl G., Lauss M., Staaf J., Chebil G., Lovgren K. Integrated genomic and gene expression profiling identifies two major genomic circuits in urothelial carcinoma. PLoS One. 2012;7:e38863. doi: 10.1371/journal.pone.0038863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sjodahl G., Lauss M., Lovgren K., Chebil G., Gudjonsson S., Veerla S. A molecular taxonomy for urothelial carcinoma. Clin Cancer Res. 2012;18:3377–3386. doi: 10.1158/1078-0432.CCR-12-0077-T. [DOI] [PubMed] [Google Scholar]
- 36.Rebouissou S., Bernard-Pierrot I., de Reynies A., Lepage M.L., Krucker C., Chapeaublanc E. EGFR as a potential therapeutic target for a subset of muscle-invasive bladder cancers presenting a basal-like phenotype. Sci Transl Med. 2014;6 doi: 10.1126/scitranslmed.3008970. 244ra91. [DOI] [PubMed] [Google Scholar]
- 37.Aine M., Eriksson P., Liedberg F., Sjodahl G., Hoglund M. Biological determinants of bladder cancer gene expression subtypes. Sci Rep. 2015;5:10957. doi: 10.1038/srep10957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo C.C., Dadhania V., Zhang L., Majewski T., Bondaruk J., Sykulski M. Gene expression profile of the clinically aggressive micropapillary variant of bladder cancer. Eur Urol. 2016 doi: 10.1016/j.eururo.2016.02.056. pii: S0302–2838(16)00246-3. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McConkey D.J., Choi W., Shen Y., Lee I.L., Porten S., Matin S.F. A prognostic gene expression signature in the molecular classification of chemotherapy-naive urothelial cancer is predictive of clinical outcomes from neoadjuvant chemotherapy: a phase 2 trial of dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin with bevacizumab in urothelial cancer. Eur Urol. 2016;69:855–862. doi: 10.1016/j.eururo.2015.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Polyak K., Weinberg R.A. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 41.Guo W., Keckesova Z., Donaher J.L., Shibue T., Tischler V., Reinhardt F. Slug and Sox9 cooperatively determine the mammary stem cell state. Cell. 2012;148:1015–1028. doi: 10.1016/j.cell.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mani S.A., Guo W., Liao M.J., Eaton E.N., Ayyanan A., Zhou A.Y. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang J., Mani S.A., Donaher J.L., Ramaswamy S., Itzykson R.A., Come C. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 44.Wellner U., Schubert J., Burk U.C., Schmalhofer O., Zhu F., Sonntag A. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat Cell Biol. 2009;11:1487–1495. doi: 10.1038/ncb1998. [DOI] [PubMed] [Google Scholar]
- 45.Brabletz S., Brabletz T. The ZEB/miR-200 feedback loop–a motor of cellular plasticity in development and cancer? EMBO Rep. 2010;11:670–677. doi: 10.1038/embor.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burk U., Schubert J., Wellner U., Schmalhofer O., Vincan E., Spaderna S. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep. 2008;9:582–589. doi: 10.1038/embor.2008.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blanpain C., Fuchs E. p63: revving up epithelial stem-cell potential. Nat Cell Biol. 2007;9:731–733. doi: 10.1038/ncb0707-731. [DOI] [PubMed] [Google Scholar]
- 48.Tran M.N., Choi W., Wszolek M.F., Navai N., Lee I.L., Nitti G. The p63 protein isoform DeltaNp63alpha inhibits epithelial-mesenchymal transition in human bladder cancer cells: role of MIR-205. J Biol Chem. 2013;288:3275–3288. doi: 10.1074/jbc.M112.408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsai J.H., Donaher J.L., Murphy D.A., Chau S., Yang J. Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell. 2012;22:725–736. doi: 10.1016/j.ccr.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Acharyya S., Oskarsson T., Vanharanta S., Malladi S., Kim J., Morris P.G. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell. 2012;150:165–178. doi: 10.1016/j.cell.2012.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang X.H., Jin X., Malladi S., Zou Y., Wen Y.H., Brogi E. Selection of bone metastasis seeds by mesenchymal signals in the primary tumor stroma. Cell. 2013;154:1060–1073. doi: 10.1016/j.cell.2013.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang X.H., Wang Q., Gerald W., Hudis C.A., Norton L., Smid M. Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer Cell. 2009;16:67–78. doi: 10.1016/j.ccr.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zheng X., Carstens J.L., Kim J., Scheible M., Kaye J., Sugimoto H. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527:525–530. doi: 10.1038/nature16064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fischer K.R., Durrans A., Lee S., Sheng J., Li F., Wong S.T. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature. 2015;527:472–476. doi: 10.1038/nature15748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Inoue K., Kamada M., Slaton J.W., Fukata S., Yoshikawa C., Tamboli P. The prognostic value of angiogenesis and metastasis-related genes for progression of transitional cell carcinoma of the renal pelvis and ureter. Clin Cancer Res. 2002;8:1863–1870. [PubMed] [Google Scholar]
- 56.Slaton J.W., Millikan R., Inoue K., Karashima T., Czerniak B., Shen Y. Correlation of metastasis related gene expression and relapse-free survival in patients with locally advanced bladder cancer treated with cystectomy and chemotherapy. J Urol. 2004;171:570–574. doi: 10.1097/01.ju.0000108845.91485.20. [DOI] [PubMed] [Google Scholar]
- 57.McConkey D.J., Choi W., Marquis L., Martin F., Williams M.B., Shah J. Role of epithelial-to-mesenchymal transition (EMT) in drug sensitivity and metastasis in bladder cancer. Cancer Metastasis Rev. 2009;28:335–344. doi: 10.1007/s10555-009-9194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dinney C.P., Fishbeck R., Singh R.K., Eve B., Pathak S., Brown N. Isolation and characterization of metastatic variants from human transitional cell carcinoma passaged by orthotopic implantation in athymic nude mice. J Urol. 1995;154:1532–1538. [PubMed] [Google Scholar]
- 59.Roth B., Jayaratna I., Sundi D., Cheng T., Melquist J., Choi W. Employing an orthotopic model to study the role of epithelial-mesenchymal transition in bladder cancer metastasis. Oncotarget. 2016 doi: 10.18632/oncotarget.11009. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]