Abstract
The treatment of metastatic renal cell carcinoma (RCC) and urothelial carcinoma (UC) remains a major challenge. Past research has implicated the immune system in tumor surveillance of both malignancies, leading to the application of immunotherapy agents for both cancers. Among them, the most promising agents are the checkpoint blockade drugs, such as antibodies targeting the cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), programmed death receptor 1 (PD-1), and PD-1 ligand (PD-L1). In normal physiology, these immune checkpoints act as inhibitory signals to fine-tune the duration and strength of immune reactions, which is pivotal for maintaining self-tolerance. However, tumor cells also utilize immune checkpoint pathways to evade anti-tumor immune response, leading to disease progression and metastasis. Thus, there has been intense preclinical and clinical effort focused on the application of checkpoint inhibitors in metastatic RCC and UC. To date, nivolumab (anti-PD-1) and atezolizumab (anti-PD-L1) have been approved for the treatment of metastatic RCC and UC, respectively. Despite these successes, challenges remain in how to further improve response rates to immunotherapy and how to select patients that will benefit from this approach. In this report, we review existing data and research on immunotherapy in metastatic RCC and UC.
Keywords: Immune checkpoint inhibitors, Nivolumab, Atezolizumab, Pembrolizumab, Renal cell carcinoma, Urothelial carcinoma
1. Introduction
An estimated 338,000 new cases of renal cell carcinoma (RCC) and 429,000 new cases of urothelial carcinoma (UC) were diagnosed worldwide in 2012 [1]. Of all genitourinary cancers, RCC has the highest mortality rate, at 30% per year [1], [2]. Meanwhile, UC, the vast majority of which is urothelial cancer of the bladder (UBC), is the 9th most common cancer with 165,000 related deaths worldwide in 2012 [1]. For patients with advanced RCC or UC, the median survival duration from time of diagnosis is less than 2 years. The poor prognosis of RCC and UC may be attributed to the lack of major advances for the treatment of patients with metastatic disease [3], [4].
The immune system has long been theorized to play an intrinsic role in tumor surveillance of RCC. Spontaneous tumor regressions, prolonged stable disease intervals and delayed relapse rates have been detected in patients with RCC after cytoreductive nephrectomies [5]. Many groups have described the presence of tumor infiltration with various immune cells, including T-cells, natural killer (NK) cells, dendritic cells (DCs) and macrophages. These observations have promoted the development of immunotherapy of RCC over the past few decades [6]. In 1992, high dose interleukin-2 (IL-2) was approved by United States Food and Drug Administration (U.S. FDA) to treat patients with metastatic RCC (mRCC). However, the clinical benefits were limited since durable complete responses occurred in only a rare subset of patients. Furthermore, high dose IL-2 resulted in substantial treatment-related toxicities, which required administration with intensive supportive care in an inpatient setting [7]. In order to reduce toxicity and enhance efficacy, a series of phase II and phase III trials were conducted to compare high-dose IL-2 alone with low-dose outpatient administrations or combination of low-dose IL-2 and interferon-alpha (IFN-α) in patients with mRCC. Unfortunately, these attempts of dose reduction schedules or combination cytokine programs failed to improve the survival benefits and led to more severe toxicities [7], [8], [9], [10], [11]. Overall, the previous mainstays of cytokine immunotherapies such as high dose IL-2 and IFN-α only delivered limited objective responses in 10%–15% of patients with mRCC, with an overall median survival time of about 12 months [12]. Until November 2015, there was no optimal immunotherapy for patients with mRCC.
The treatment strategy of metastatic UC (mUC) has depended heavily on systematic chemotherapies, with cisplatin-containing regimens having the most success. Far less attention has been drawn to this lethal disease [13]. The local susceptibility of bladder cancer to the immune system has been confirmed for more than 2 decades [14], [15]. The presence of tumor-infiltrating lymphocytes (TILs) and secreted cytokines play a critical role in the host antitumor response, and also is the basis of using the Bacillus Calmette–Guerin (BCG) vaccine to treat patients with superficial bladder cancer [16]. However, the applications of immunotherapy have presented a great challenge in muscle-invasive bladder cancer (MIBC) and metastatic UBC.
In this review, we discuss the rationale of novel immunotherapeutic strategies for mRCC and mUC, recent advances of immune checkpoint inhibitors in these two lethal diseases, and other potential targets in the future.
2. Brief rationale of novel immunotherapy
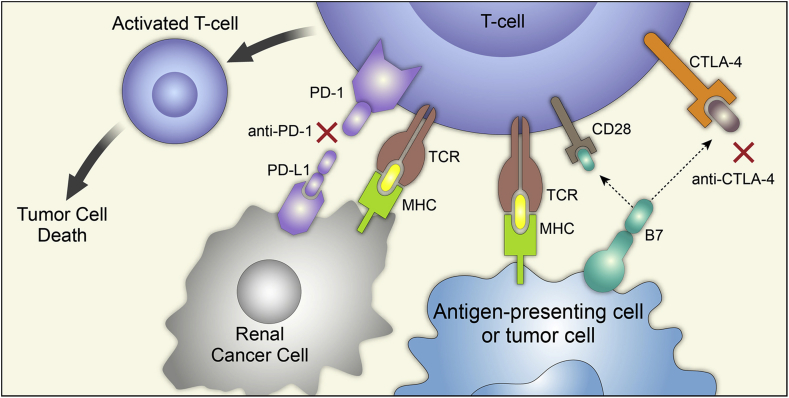
T-cell mediated immunity is a complex process with multiple sequential steps, and each step is regulated by a delicate balance of stimulatory and inhibitory signals (Fig. 1). Antigen-specific naïve T-cells are activated through signal one, between the interaction of the T-cell receptor (TCR) and the major histocompatibility complex (MHC) on tumor cells, and signal two, between the CD28 receptor on T-cells and the B7 receptor on tumor cells. The B7 receptor can potentially also bind the cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) receptor on T-cells, which then suppresses T-cells and leads to immune tolerance or anergy. Another major inhibitor pathway signals from the interaction of programmed cell death 1 (PD-1) on T-cells and its ligand, PD-L1 on tumor cells. These immune checkpoint pathways counterbalance the stimulatory pathways to stop an immune response. In normal tissues, these signals act as the “brake” to fine-tune the duration and strength of physiologic immune reactions and to avoid collateral damage caused by an overactive host immune response. Comparatively, tumor cells may overexpress immune checkpoint molecules to escape from tumor-specific T-cell immunity in the cancer microenvironment; this tumor escape can lead to subsequent progression and metastasis. Thus, insufficient T-cell activation caused by the redundant negative regulation may partially explain some resistance to antitumor treatments and the poor response to conventional immunotherapy, such as cytokine treatments. Recently, increasing attention has been drawn to “loosen the brake” by blocking these CTLA-4 and PD-1 inhibitory pathways to restore host immune response against tumors [17], [18], [19].
Figure 1.
Mechanism of antitumor immunity and immune checkpoint inhibitors. Tumor surveillance is mediated through a series of activating and inhibitory signals. T-cell activation occurs through signal 1 (between the TCR and the MHC complex) and signal 2 (between CD28 and the B7 receptor on APCs). The CTLA-4 receptor on T-cells competes with CD28 for B7 ligands to terminate T-cell activation in the priming phase. PD-1 is expressed on effector T-cells and binds to PD-L1 on tumor cells to exhaust T-cells and suppress its antitumor effect. Blockade of CTLA-4 (anti-CTLA-4), PD-1 (anti-PD-1) or PD-L1 (anti-PD-L1, not included in this figure) restores host immune function and induces antitumor activity. Reproduced with permission from Raman R and Vaena D. Biomed Res Int 2015; 2015:367354 [5], copyright ©2015. TCR, T-cell receptor; MHC, major histocompatibility complex; APCs, antigen-presenting cells; CTLA-4, cytotoxic T-lymphocyte antigen-4; PD-1, programmed death-1; PD-L1, programmed death-ligand 1. Figure from Immunotherapy in metastatic renal cell carcinoma: a comprehensive review[5].
3. Novel immunotherapy approaches
3.1. CTLA-4
During the priming phase of T-cell mediated immunity, one of the best characterized co-stimulatory molecules is the CD28 superfamily, which regulates the interaction between antigen presenting cells (APCs) and T-cells. CTLA-4 is exclusively expressed on T-cells and was initially thought to be a co-stimulatory molecule similar to CD28, but further studies proved its inhibitory effect, dampening the early activity of naïve and memory T-cells [20], [21]. APCs present two subtypes of the B7 protein, B7.1 (CD80) and B7.2 (CD86), which can bind either CD28 or CTLA-4. Once a cognate antigen is recognized by the TCR, CD28 signaling strongly augments TCR signaling to activate T-cells. On the other hand, CTLA-4 may outcompete CD28 by binding B7.1 and B7.2 with a much higher affinity in order to terminate T-cell activation at an early stage [22]. However, the entirety of other downstream effects of the CTLA-4 pathway is still under investigation.
3.1.1. Anti-CTLA-4 in mRCC
The inhibitory role of CTLA-4 has been elucidated using genetically engineered mice which are deficient in CTLA-4 [23]. These mice develop lymphoproliferative disorders with multi-organ lymphocytic infiltration and tissue destruction. Preclinical models of tumor-bearing mice also showed tumor shrinkage related to antitumor immunity via CTLA-4 blockade [24]. These promising preclinical results encouraged the development of anti-CTLA-4 therapy for cancer patients. The first and most clinically studied anti-CTLA-4 strategy to date is ipilimumab (also known as MDX-010, Yervoy®), a monoclonal antibody targeting CTLA-4 that is FDA approved for treatment of unresectable or metastatic melanoma [25], [26]. For patients with mRCC, a single institution phase II trial showed that only one of 21 patients had a partial response (PR) with low-dose ipilimumab (3 mg/kg followed by 1 mg/kg every 3 weeks), and five of 40 patients had PRs in the high-dose cohort (3 mg/kg every 3 weeks), including two responses from patients had failed high dose IL-2 therapy [27]. Intriguingly, the response rate was 30% in patients who developed immune-related adverse events (irAEs) but 0% in patients free of autoimmune toxicities (p = 0.0007), indicating that tumor regression was strikingly associated with autoimmune events. This effect may be explained by the autoimmunity induced in a variety of other target tissues in addition to tumor tissues.
Another CTLA-4 antibody that has been studied in patients with mRCC is tremelimumab (CP-675206). A phase I trial (NCT00372853) enrolled 28 adult patients with mRCC who had previously received systemic treatments. In a 3 + 3 dose escalation scheme, they were treated with intravenous tremelimumab (6 mg/kg, 10 mg/kg or 15 mg/kg) every 12 weeks combined with oral sunitinib (50 mg daily for 4 weeks with 2 weeks off or 37.5 mg daily as a continuous dose). Dose limited toxicities (DLT) were detected in two of five patients receiving tremelimumab 6 mg/kg and sunitinib 50 mg, and in three of six patients treated with tremelimumab 15 mg/kg and continuous sunitinib at 37.5 mg. In addition, one of seven patients treated with tremelimumab 10 mg/kg and continuous sunitinib 37.5 mg suffered a sudden death, and three DLT events occurred in an expansion cohort of seven additional patients at the same dose level [28]. Although PRs were detected in 43% of patients, the toxicity was significantly with the combination compared with historical data of sunitinib alone, especially the high incidence of rapid onset renal failure [29]. Thus, further combination investigations may move back to the preclinical setting to evaluate the increased renal toxicity.
3.1.2. Anti-CTLA-4 in mUC
Active investigation of CTLA-4 as an immunotherapy target is also ongoing in the treatment of mUC. Given the standard use of systemic neoadjuvant chemotherapy prior to cystectomy, a phase II study detected the safety and immune effects of neoadjuvant ipilimumab for localized UBC. Twelve patients were enrolled to receive ipilimumab at an escalating dose between 3 and 10 mg/kg for two cycles. The results showed a tolerable safety profile in the pre-operative setting. Furthermore, an increased number of CD4+ T lymphocytes expressing ICOS (inducible costimulatory; CD4+ICOShi T-cells) was also detected in both tumor tissue and the peripheral blood after anti-CTLA-4 treatment. CD4+ICOShi T-cells were found to produce IFN-γ and recognize the tumor antigen NY-ESO-1, resulting in the elevated ratio of effector T-cells to regulatory T-cells and enhanced tumor-related immune responses. Therefore, it might be a potential biomarker to monitor the clinical outcome of ipilimumab [30], [31]. Based on the considerable activity of ipilimumab, an ongoing phase II trial (NCT01524991) was conducted to evaluate ipilimumab combined with gemcitabine and cisplatin as first-line treatment of patients with mUC. Furthermore, another phase 1b/2 trial (NCT02205333) is underway to deliver an OX-40 agonist (MEDI6469) alone or combined with either tremelimumab, durvalumab (anti-PD-L1 monoclonal antibody; MEDI4736), or rituximab (anti-CD20 monoclonal antibody) to treat a variety of advanced solid tumors or aggressive B-cell lymphomas [32].
3.2. PD-1
PD-1 is a pivotal immune checkpoint receptor on the surface of activated T-cells, which can be engaged by PD-1 ligands, including PD-L1 (i.e., B7-H1 or CD274) and PD-L2 (i.e., B7-DC or CD273) [33], [34]. In contrast to the CTLA-4 receptor, the main role of PD-1 is to limit T-cell activity at the time of an inflammatory response to infection rather than regulate T-cell activation. More specifically, the interaction between PD-1 and its ligands may inhibit T-cell proliferation, cytokine production, and cytotoxic function. After exposure to INF-γ produced by activated T-cells, PD-L1 can be highly expressed by diverse subsets of cells, such as T-cells, epithelial cells and cancer cells [35]. Thus, PD-1 signaling may translate into an immune resistance mechanism in the tumor microenvironment, which may be explained as a consequence of either constitutive activation of an oncogenic pathway, or infiltrating immune cell production of interferons [36]. Preclinical studies have demonstrated that blockade of the interaction between PD-1 and PD-L1 can restore host immune function and induce antitumor activity.
3.2.1. PD-1 inhibitors in mRCC
Nivolumab (i.e., Opdivo®, ONO-4538, BMS-936558 or MDX1106), the most researched PD-1 blocking antibody, is the only therapeutic checkpoint inhibitor with U.S. FDA approval to treat mRCC after disease progression with anti-angiogenic agents. Nivolumab has also been approved to treat patients with metastatic melanoma and non-small cell lung cancer.
A phase I trial (NCT00441337) of single-agent nivolumab primarily demonstrated its safety and efficacy in 39 patients with various types of advanced solid tumors, including one patient with mRCC that had a PR in the study [37]. This was the first time that a PD-1 inhibitor was studied in a clinical trial form RCC. Subsequently, another phase I trial with a dose expansion phase in mRCC (NCT0730639) enrolled more heavily pretreated patients to receive nivolumab. In 34 patients with mRCC, 10 (29%) obtained an objective response (OR) with a median duration of 12.9 months. Meanwhile, stable disease (SD) was achieved in nine patients (27%) and lasted for at least 24 weeks. Subjects developed predominantly grade 1 or 2 AEs (e.g., rash, hypothyroidism, hepatitis, nausea, adrenal insufficiency, diarrhea and vitiligo). Grade 3 or 4 treatment-related toxicities occurred in six of 34 patients (18%) and were all manageable [38], [39]. In addition, neither of these two phase I studies reached the maximum tolerated dose. All of these results indicate that nivolumab is well tolerated and can deliver a durable antitumor activity in a subset of patients with mRCC. Also, it is feasible to administrate nivolumab in an outpatient setting.
A subsequent phase II study (NCT01354431) further added to a growing body of evidence supporting the efficacy and safety of nivolumab. One hundred and sixty-eight mRCC patients previously treated with at least one vascular endothelial growth factor receptor (VEGFR) inhibiting agent were randomized to receive intravenous nivolumab at three dose levels (0.3 mg/kg, 2 mg/kg or 10 mg/kg every 3 weeks). A dose–response relationship was not observed for progression free survival (PFS), and there was no distinction of response rates among the three dose groups. Although PFS was not impressive, prolonged overall survival (OS) was noted in 2 and 10 mg/kg groups compared with that in 0.3 mg/kg group (25.5 and 24.7 months, respectively, vs. 18.2 months) [40].
Based on these promising results, a pivotal phase III trial (NCT01668784) was conducted to compare nivolumab with everolimus, an approved mTOR inhibitor for patients with mRCC that have failed previous anti-angiogenic treatment. In total, 821 previously treated patients with advanced RCC were assigned in a 1:1 ratio to have either nivolumab 3 mg/kg intravenous every 2 weeks or everolimus 10 mg by mouth daily. Although the median PFS was almost the same in the two groups (4.6 months with nivolumab vs. 4.4 months with everolimus), the median OS with nivolumab was significantly longer than that with everolimus (25.0 months vs. 19.6 months), and the hazard ratio for any death was 0.73 (nivolumab vs. everolimus, 98.5%CI, 0.57–0.93; p = 0.002). Meanwhile, the objective response rate (ORR) in nivolumab group was 25% compared with only 5% in the everolimus group (odds ratio, 5.98; 95%CI, 3.7–5.4; p < 0.001). In addition to substantial response rates and prolonged long-term survival, nivolumab was also better tolerated than everolimus with fewer grade 3–4 treatment-related AEs [41]. Interestingly, neither the phase II nor phase III studies had impressive PFS. Patients with nivolumab often experienced transient progression initially after treatment delivery [40], [41]. This unconventional immune-related response (termed pseudo-progression) has also been demonstrated with nivolumab and ipilimumab in treating malignant melanoma [42]. Possible explanations include 1) transient progression imitated by immune-cell infiltration of the tumor and 2) lack of tumor cell death immediately after the immune activation triggered by nivolumab early on at the time of initial restaging scans [40], [42]. Therefore, the clinical activity of immunotherapies should be carefully monitored.
Pembrolizumab (Keytruda®, MK-3475), a humanized monoclonal immunoglobulin G4 (IgG4) PD-1 antibody, was initially approved by the U.S. FDA in 2014 for patients with unresectable or metastatic melanoma and the label was expanded to include first-line treatment in December 2015. For patients with mRCC, pembrolizumab is still under early phase clinical development. A phase I study of pembrolizumab (NCT01295827) has been conducted in order to expand its application in more cancer types. The results showed durable anticancer activity in multiple solid tumors; however no data on RCC were reported [43]. On the other hand, a neoadjuvant study of pembrolizumab (NCT02212730) is underway for mRCC only. The primary endpoints are safety and increase of intratumoral lymphocytic infiltration.
4. PD-1 inhibitors in mUC
Previous studies showed that PD-L1 expression increased with advanced tumor staging, and intense PD-L1 expression was detected in roughly 40% of specimens with carcinoma in situ (CIS) from patients who failed BCG treatment [44]. Therefore, aberrant expression of PD-L1 in UC cells may be predictive of aggressive disease and failure of BCG immunotherapy, and therapeutic blockade of PD-1/PD-L1 pathway may be effective in UC treatment [44], [45]. Pembrolizumab targets PD-1 and has been studied in mUC. The phase 1b KEYNOTE-012 study (NCT01848834) enrolled patients with advanced solid tumor including 33 patients with recurrent, metastatic, or persistent UC. The preliminary results presented at ASCO 2015 showed durable antitumor activity in patients who responded to pembrolizumab. The ORR to pembrolizumab was 28% (95%CI 13%–47%) including three complete responses (CR) and five partial responses (PR), and the response rate was higher in patients with positive PD-L1 expression. Grade 3–4 AEs occurred in five patients (15%), suggesting that pembrolizumab was well tolerated in this patient cohort. Further analysis from this study is awaited to evaluate PD-L1 as a predictive biomarker [46]. There are several ongoing studies of pembrolizumab in urothelial cancer: in mUC (NCT02335424), as neoadjuvant treatment of muscle-invasive localized UC as monotherapy and in combination with chemotherapy (NCT02736266 and NCT02690558), and as maintenance therapy for mUC patients who have achieved stable disease or better after first-line chemotherapy (NCT02500121).
4.1. PD-L1
4.1.1. Anti-PD-L1 in mRCC
Atezolizumab (Tecentriq™, MPDL3280A) is an engineered humanized lgG1 monoclonal antibody (mAb), which targets PD-L1 and prevents binding of PD-L1 and its receptors, PD-1 and B7.1 [47]. A phase Ia study (NCT01375842) enrolled 70 patients with previously treated mRCC to receive atezolizumab at doses between 3 mg/kg and 20 mg/kg for up to 1 year. The results showed good tolerance with 17% treatment-related grade 3 AEs and 4% immune-mediated grade 3 AEs. Evaluable patients treated with atezolizumab had a median PFS of 5.6 months (95%CI, 3.9–8.2 months) and median OS of 28.9 months (95%CI, 20.0 months to not reached). Furthermore, the correlative study also defined some potential predictive and pharmacodynamic biomarkers may guide the studies in the future [48]. An ongoing multicenter, phase II trial (NCT01984242) is evaluating atezolizumab alone or combined with Avastin (bevacizumab) compared with sunitinib monotherapy in patients with previously untreated advanced RCC. Another ongoing phase III trial comparing the combination of atezolizumab with bevacizumab against sunitinib in first-line treatment of mRCC (NCT02420821) will be a pivotal study in evaluating PD-L1 therapy in combination with VEGF-targeting treatments.
BMS-936559 (MDX-1105) is a fully human IgG4 mAb that also binds to PD-L1. A multicenter, phase I trial evaluated its efficacy and safety in 270 patients with metastatic solid tumors. Among 17 patients with mRCC, BMS-936559 was administered at the dose of 10 mg/kg every 2 weeks. Two patients in this cohort (12%) obtained an objective response (complete or partial response) for a duration of 4 months and 17 months, respectively. Meanwhile, an additional seven patients (41%) experienced SD for at least 24 weeks, and the 24-week PFS was 53% [49].
4.1.2. Anti-PD-L1 in mUC
Results from the phase Ia study (NCT01375842) mentioned above showed noteworthy efficacy and safety of atezolizumab in 67 patients with mUC. The subgroup analysis showed that the ORRs were higher in patients with higher levels of PD-L1 expression on tumor-infiltrating immune cells compared with their counterparts with low expression levels (43% vs. 11%). Atezolizumab was well-tolerated in this population; grade 3 treatment-related AEs only occurred in 4% of patients, with no grade 4 or 5 AEs [3]. To confirm the preliminary anti-tumor activity, a phase II, multicenter, single-arm trial of atezolizumab was conducted in patients with mUC progressing after platinum-based chemotherapy (NCT02108652). Three hundred and ten patients received the treatment with ORR of 15% (95%CI, 11%–20%, p = 0.005). Stratified further, patients with higher PD-L1 expression levels were more likely to derive clinical benefit from atezolizumab, with ORR of 26%. With a median follow-up of 11.7 months, 38 of 45 responders (84%) had durable responses. The median PFS based on RECIST criteria and median OS were 2.1 months and 7.9 months, respectively, in the entire cohort of patients, Grade 3–4 treatment-related AEs occurred in 16% of all patients, and any grade immune-mediated AEs occurred in 7% of patients [50]. These results indicate that atezolizumab has disease activity in mUC, with some patients having durable responses; moreover, atezolizumab has a favorable toxicity profile. Based on these results, the US FDA granted accelerated approval of atezolizumab for use in cisplatin-refractory bladder cancer on May 18, 2016. An ongoing phase III study will compare outcomes from atezolizumab monotherapy and chemotherapy (either paclitaxel or docetaxel per provider's choice) in patients with cisplatin-refractory mUC (NCT02302807; IMvigor211).
There are two other IgG1 mAb targeting PD-L1 (MED14736 (durvalumab) and MSB0010718C) currently undergoing early phase studies in various solid tumors. No data for RCC or UC cohorts are yet available [51], [52].
5. PD-L1 expression
A great challenge of tumor immunotherapy is to identify a specific biomarker for patient selection and disease management. Increased PD-L1 expression on tumor cells is likely driven by constitutive oncogenic pathways, and recent research also indicates that PD-L1 may have dual roles both to suppress the immune response and also to induce endogenous inflammatory immune responses. The final outcome is determined by the balance of the host immune response and negative feedback modulation [53]. Preclinical and translational studies have demonstrated that high PD-L1 expression levels in tumors are associated with aggressive disease and poor prognosis in various types of cancer including RCC and UC [44], [45], [54]. This is also the basis of therapeutic blockade of PD-1/PD-L1 pathway. However, whether the PD-L1 expression can predict clinical efficacy of PD-1/PD-L1 inhibitors is still controversial and depends on tumor types. An association of tumor PD-L1 expression level and clinical efficacy of nivolumab has been detected in metastatic melanoma and non-squamous cell non-small cell lung cancer [55], [56]. When exploring in other cancer types, two phase I trials of nivolumab in various solid tumors showed that 75% and 36% of PD-L1 positive patients, respectively, responded to the study drug while none of the PD-L1 negative patients did [37], [38] in the phase II study of mRCC, nivolumab showed a higher response rate (31%) in patients with PD-L1 positive disease compared to 18% in patients with PD-L1 negative disease [40]. However, in the phase III RCC study, there was no correlation between PD-L1 status and response to nivolumab. Similarly, a phase Ia study of atezolizumab demonstrated that anti-PD-L1 therapy was effective in both PD-L1 positive and negative patients with solid tumors, including RCC and UC, although the response rate was lower in PD-L1 negative group (ORR, 39% vs. 13%) [57].
Based on the results above, PD-L1 expression in tumor cells likely cannot be used as the sole biomarker to predict for treatment responses to PD-1/PD-L1 inhibitors; this inability to accurately serve as a predictive biomarker may be due to several reasons. First, PD-L1 expression is a dynamic parameter that may change over time, and many clinical trials use archival tissue to evaluate for PD-L1 positivity. PD-L1 expression has been found to be discordant between primary tumors and metastatic lesions, as well as between metastatic lesions from the same patient [18], [58]. Secondly, there is no technical standard for PD-L1 detection with different thresholds of defining PD-L1 positivity (tumor cells showed membranous staining on immunohistochemistry of ≥5% or ≥1%), specimen collection (archival or fresh tumor samples) and antibodies for IHC testing (murine or rabbit) [37], [38], [40], [41], [49], [57]. Recent studies have demonstrated that it might be the high level of PD-L1 expression on tumor-infiltrating immune cells rather than tumor cells that are associated with higher response rates and improved OS [3], [50]. Despite this controversy, one of the PD-L1 biomarker tests (the Ventana PD-L1 assay) has been approved by the US FDA as a companion diagnostic for PD-L1 positivity in mUC patients undergoing treatment with atezolizumab [3], [50], [59], [60]. Finally, gene mutations may accumulate over time to create neoantigens that can be recognized by cytotoxic T-cells performing immune surveillance. Indeed, molecular subtypes of UC identified by The Cancer Genome Atlas (TCGA) analysis are associated with different levels of CD8+ (cytotoxic T-cell) effector genes as well as PD-L1 expression on immune cells or tumor cells. These difference scan also be a factor in determining patient responses to the immune checkpoint inhibitors [50].
Due to the dynamic feature of the host immune response to tumors and comprehensive regulation by multiple immune checkpoints, it may be difficult to identify treatment candidates and make disease management decisions with a single biomarker. Further studies on the tumor microenvironment may help us distinguish between an immunogenic tumor microenvironment and a non-immunogenic tumor microenvironment [18].
6. Combination immune therapy
Immune checkpoint inhibitors combined with other immunotherapy or conventional treatment are the development direction of the future. PD-1 and CTLA-4 play complementary roles to down-regulate adaptive immunity. Preclinical studies have demonstrated that combined blockade of PD-1 and CTLA-4 may induce non-redundant antitumor activity compared with monotherapy of each [61], [62], [63]. Therefore, a phase I trial of advanced melanoma (NCT01024231) was conducted. The results revealed that concurrent therapy with ipilimumab and nivolumab was well tolerated and provided distinct clinical activity compared with published data on monotherapy with either agent alone. Interestingly, there was no significant difference of ORR between PD-L1 positive and PD-L1 negative tumors [42]. The preliminary results of an ongoing phase I trial showed similar results of this combination in patients with mRCC [64]. The phase III study of first-line ipilimumab and nivolumab compared to sunitinib monotherapy in mRCC (NCT02231749) has completed accrual, and clinical results are pending. A separate phase III trial is currently randomizing patients with previously untreated bladder cancer to either the combination of tremelimumab-durvalumab or chemotherapy (gemcitabine with either carboplatin or cisplatin) (NCT02516241).
Conventional therapeutic strategies may also have synergistic functions with checkpoint inhibitors by inducing tumor cell death and releasing tumor-related antigens to initiate T-cell activation. The synergistic anti-tumor effect of dual blockade of VEGFR2 and PD-1 was detected in a murine colon cancer model, and there was no increased toxicity [65]. Preliminary analysis from a phase I trial of mRCC also demonstrated encouraging efficacy and manageable toxicity of nivolumab plus sunitinib or pazopanib [66]. Many other clinical trials are underway to evaluate the combination of immune checkpoint inhibitors with other targeted therapy or chemotherapy. Table 1 lists a series of ongoing trials in RCC and UC.
Table 1.
Summary of ongoing clinical trials with immune checkpoint inhibitors combined with other therapies in RCC or UC.
| Identifier | Phase | Cohort | Combinations | Primary endpoints | Estimated enrollment |
|---|---|---|---|---|---|
| NCT02210117 | Pilot | RCC | Nivolumab vs. nivolumab + bevacizumab vs. nivolumab + ipilimumab | Safety and tolerability | 60 |
| NCT02133742 | Ⅰ | RCC | Pembrolizumab + axitinib | DLT | 60 |
| NCT02348008 | Ⅰ,Ⅱ | RCC | Pembrolizumab + bevacizumab | DLT | 61 |
| NCT02599779 | Ⅱ | RCC | Pembrolizumab + SBRT | PFS | 35 |
| NCT02724878 | Ⅱ | Non-clear cell RCC | Atezolizumab + bevacizumab | ORR | 40 |
| NCT02420821 | Ⅲ | RCC | Atezolizumab + bevacizumab vs. sunitinib | PFS and OS | 830 |
| NCT01441765 | Ⅱ | RCC | CT-011 vs. CT-011 with DC/RCC fusion vaccine | Toxicity and response rate | 44 |
| NCT02437370 | Ⅰ | UC | Pembrolizumab + docetaxel vs. pembrolizumab + gemcitabine | Safety and tolerability | 38 |
| NCT01524991 | Ⅱ | UC | Gemcitabine, cisplatin + ipilimumab | 1-year OS | 36 |
| NCT02619253 | Ⅰ,Ⅱ | RCC and UC | Pembrolizumab + vorinostat | MTD | 42 |
| NCT02496208 | Ⅰ | RCC, UC | Nivolumab + cabozantinib vs. nivolumab + cabozantinib + ipilimumab | DLT | 66 |
DLT, dose limited toxicity; MTD, maximum tolerated dose; ORR, overall response rate; OS, overall survival; PFS, progression free survival; RCC, renal cell carcinoma; UC, urothelial carcinoma.
7. Other immune checkpoint molecules
The therapies and checkpoint targets described above are farthest along in clinical development. Nevertheless, there are other immune checkpoint molecules which have been identified in basic studies or have already moved forward into clinical investigation.
Lymphocyte-activation gene-3 (LAG-3; also known as CD223) is highly expressed on regulatory T-cells (Treg) to amplify the immunosuppressive function of Treg cells but also to inhibit CD8+ effector T-cell activity [67], [68]. The only known ligands for LAG-3 are MHC class II molecules, which are highly expressed on some epithelial cancers and tumor-infiltrating APCs. Since LAG-3 and PD-1 are typically co-expressed on anergic or exhausted T-cells, dual blockade of these two receptors may cause a synergistic reversal of tumor-specific immunosuppression. Impressive evidence from a preclinical murine study showed that Pd1−/−Lag3−/−double-knockout mice completely reject even poorly immunogenic tumors but rapidly develop severe autoimmune syndromes [69]. Hence, the balance between antitumor effects and autoimmunity should be carefully considered in such combination strategies. Recently, anti-LAG-3 agents have moved forward into clinical development to evaluate the safety and efficacy of anti-LAG-3 as either a monotherapy or in combination with anti-PD-1 agents (NCT01968109 and NCT02460224).
In addition to LAG-3, many other molecules involved in antitumor immunity may become potential target for cancer treatment, such as T-cell immunoglobulin and mucin-domain containing-3 (TIM-3; also known as HAVcr2), B and T lymphocyte attenuator (BLTA; also known as CD273) and adenosine A2a receptor (A2aR). All of these “second generation” inhibitory receptors are under basic or preclinical investigation [17], [22].
On the other hand, limited studies focus on using agonist antibodies to promote immunoactivation. CD27 is an activating transmembrane receptor expressed on not only naive and activated T cells, but also memory B cells and a subset of NK cells [70]. The therapeutic potential of CD27 was fist revealed in a study investigating the antitumor effect of an anti-CD40 agonist. With a CD70 mAb blocking the CD70/CD27 pathway, the antitumor activity of the anti-CD40 mAb was totally abolished, indicating the importance of CD27 in promoting antitumor immunity [71]. Activating the CD27 receptor is therefore a novel approach to increasing cytotoxic T-cell responses. 1F5 (varlilumab) was the first fully humanized antibody to recognize and stimulate CD27-expressing T cells, increasing the number of antigen-specific CD8+ T cells with potent antitumor activity [72]. Varlilumab has now entered clinical investigation. There are several ongoing phase I/II studies combining varlilumab and PD-1/PD-L1 blockade (nivolumab and atezolizumab, respectively) accruing advanced solid tumors (NCT02335918 and NCT02543645). Both of these studies will have a planned phase II dose expansion cohort of mRCC patients. There is also another phase I/II study specifically recruiting patients with mRCC to investigate the combination of varlilumab and sunitinib, thus combining immune activation with an anti-angiogenesis agent (NCT02386111).
As more targetable immune checkpoint targets are identified, future combination approaches are essential to improve upon the current response rates seen with immune checkpoint monotherapies.
8. Summary
The landscape of immune checkpoint inhibitors has rapidly expanded in the past 5 years. With immense interest from the medical community in immunogenic cancers such as urothelial and renal cell carcinomas, great advances in immunotherapy have culminated in the approval of atezolizumab for urothelial cancer and nivolumab for RCC. However, PD-L1 status is not a reliable predictive biomarker for treatment response, and further research is needed to find clinical characteristics of sensitivity or resistance to these immunotherapies. Clinical response rates to immunotherapy in the treatment-refractory setting remain poor, and prospective studies are needed for both combination immune therapy strategies as well as for combinations of immune and cytotoxic therapies. Several studies are currently evaluating immunotherapies in first-line therapy for metastatic disease in both mRCC and mUC. Finally, since these agents are beneficial in the metastatic setting, it is important to evaluate their utility for patients with localized disease. Ongoing research is evaluating the use of immune checkpoint therapies in earlier disease states such as adjuvant therapy to prevent metastases after definitive local treatments, as well as neoadjuvant therapy to improve local disease control.
Conflicts of interest
The authors declare no conflict of interest.
Footnotes
Peer review under responsibility of Second Military Medical University.
References
- 1.Ferlay J., Soerjomataram I., Dikshit R., Eser S., Mathers C., Rebelo M. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Jemal A. Cancer statistics. CA Cancer J Clin. 2015;2015:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3.Powles T., Eder J.P., Fine G.D., Braiteh F.S., Loriot Y., Cruz C. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558–562. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 4.Patil S., Manola J., Elson P., Negrier S., Escudier B., Eiken T. Improvement in overall survival of patients with advanced renal cell carcinoma: prognostic factor trend analysis from an international data set of clinical trials. J Urol. 2012;188:2095–2100. doi: 10.1016/j.juro.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 5.Raman R., Vaena D. Immunotherapy in metastatic renal cell carcinoma: a comprehensive review. Biomed Res Int. 2015;2015:367354. doi: 10.1155/2015/367354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Massari F., Santoni M., Ciccarese C., Santini D., Alfieri S., Martignoni G. PD-1 blockade therapy in renal cell carcinoma: current studies and future promises. Cancer Treat Rev. 2015;41:114–121. doi: 10.1016/j.ctrv.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 7.McDermott D.F., Regan M.M., Clark J.I., Flaherty L.E., Weiss G.R., Logan T.F. Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J Clin Oncol. 2005;23:133–141. doi: 10.1200/JCO.2005.03.206. [DOI] [PubMed] [Google Scholar]
- 8.Dutcher J.P., Fisher R.I., Weiss G., Aronson F., Margolin K., Louie A. Outpatient subcutaneous interleukin-2 and interferon-alpha for metastatic renal cell cancer: five-year follow-up of the cytokine working group study. Cancer J Sci Am. 1997;3:157–162. [PubMed] [Google Scholar]
- 9.Vogelzang N.J., Lipton A., Figlin R.A. Subcutaneous interleukin-2 plus interferon alfa-2a in metastatic renal cancer: an outpatient multicenter trial. J Clin Oncol. 1993;11:1809–1816. doi: 10.1200/JCO.1993.11.9.1809. [DOI] [PubMed] [Google Scholar]
- 10.Dutcher J.P., Atkins M., Fisher R., Weiss G., Margolin K., Aronson F. Interleukin-2-based therapy for metastatic renal cell cancer: the Cytokine Working Group experience, 1989–1997. Cancer J Sci Am. 1997;3(Suppl. 1):S73–S78. [PubMed] [Google Scholar]
- 11.Negrier S., Escudier B., Lasset C., Douillard J.Y., Savary J., Chevreau C. Recombinant human interleukin-2, recombinant human interferon alfa-2a, or both in metastatic renal-cell carcinoma. Groupe Francais d'Immunotherapie. N Engl J Med. 1998;338:1272–1278. doi: 10.1056/NEJM199804303381805. [DOI] [PubMed] [Google Scholar]
- 12.Motzer R.J., Bukowski R.M. Targeted therapy for metastatic renal cell carcinoma. J Clin Oncol. 2006;24:5601–5608. doi: 10.1200/JCO.2006.08.5415. [DOI] [PubMed] [Google Scholar]
- 13.Carballido E.M., Rosenberg J.E. Optimal treatment for metastatic bladder cancer. Curr Oncol Rep. 2014;16:404. doi: 10.1007/s11912-014-0404-2. [DOI] [PubMed] [Google Scholar]
- 14.Tsujihashi H., Matsuda H., Uejima S., Akiyama T., Kurita T. Immunoresponse of tissue infiltrating lymphocytes in bladder tumors. J Urol. 1989;141:1467–1470. doi: 10.1016/s0022-5347(17)41348-6. [DOI] [PubMed] [Google Scholar]
- 15.Tsujihashi H., Matsuda H., Uejima S., Akiyama T., Kurita T. Immunocompetence of tissue infiltrating lymphocytes in bladder tumors. J Urol. 1988;140:890–894. doi: 10.1016/s0022-5347(17)41851-9. [DOI] [PubMed] [Google Scholar]
- 16.Patard J., Saint F., Velotti F., Abbou C., Chopin D. Immune response following intravesical bacillus Calmette-Guerin instillations in superficial bladder cancer: a review. Urol Res. 1998;26:155–159. doi: 10.1007/s002400050039. [DOI] [PubMed] [Google Scholar]
- 17.Carosella E.D., Ploussard G., LeMaoult J., Desgrandchamps F. A systematic review of immunotherapy in urologic cancer: evolving roles for targeting of CTLA-4, PD-1/PD-L1, and HLA-G. Eur Urol. 2015;68:267–279. doi: 10.1016/j.eururo.2015.02.032. [DOI] [PubMed] [Google Scholar]
- 18.Sharma P., Allison J.P. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 19.Noessner E., Brech D., Mendler A.N., Masouris I., Schlenker R., Prinz P.U. Intratumoral alterations of dendritic-cell differentiation and CD8+ T-cell anergy are immune escape mechanisms of clear cell renal cell carcinoma. Oncoimmunology. 2012;1:1451–1453. doi: 10.4161/onci.21356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Linsley P.S., Greene J.L., Tan P., Bradshaw J., Ledbetter J.A., Anasetti C. Coexpression and functional cooperation of CTLA-4 and CD28 on activated T lymphocytes. J Exp Med. 1992;176:1595–1604. doi: 10.1084/jem.176.6.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walunas T.L., Lenschow D.J., Bakker C.Y., Bakker C.Y., Linsley P.S., Freeman G.J. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 22.Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tivol E.A., Borriello F., Schweitzer A.N., Lynch W.P., Bluestone J.A., Sharpe A.H. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 24.Leach D.R., Krummel M.F., Allison J.P. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:22. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 25.Hodi F.S., O'Day S.J., McDermott D.F., Weber R.W., Sosman J.A., Haanen J.B. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDermott D., Haanen J., Chen T.T., Lorigan P., O'Day S., MDX010-20 Investigators Efficacy and safety of ipilimumab in metastatic melanoma patients surviving more than 2 years following treatment in a phase III trial (MDX010-20) Ann Oncol. 2013;24:2694–2698. doi: 10.1093/annonc/mdt291. [DOI] [PubMed] [Google Scholar]
- 27.Yang J.C., Hughes M., Kammula U., Royal R., Sherry R.M., Topalian S.L. Ipilimumab (Anti-CTLA4 antibody) causes regression of metastatic renal cell Cancer associated with enteritis and hypophysitis. J Immunother. 2007;30:825–830. doi: 10.1097/CJI.0b013e318156e47e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rini B.I., Stein M., Shannon P., Eddy S., Tyler A., Stephenson J.J., Jr. Phase 1 dose-escalation trial of tremelimumab plus sunitinib in patients with metastatic renal cell carcinoma. Cancer. 2011;117:758–767. doi: 10.1002/cncr.25639. [DOI] [PubMed] [Google Scholar]
- 29.Motzer R.J., Hutson T.E., Tomczak P., Michaelson M.D., Bukowski R.M., Rixe O. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 30.Carthon B.C., Wolchok J.D., Yuan J., Kamat A., Ng Tang D.S., Sun J. Preoperative CTLA-4 blockade: tolerability and immune monitoring in the setting of a presurgical clinical trial. Clin Cancer Res. 2010;16:2861–2871. doi: 10.1158/1078-0432.CCR-10-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liakou C.I., Kamat A., Tang D.N., Chen H., Sun J., Troncoso P. CTLA-4 blockade increases IFNγ-producing CD4+ ICOShi cells to shift the ratio of effector to regulatory T cells in cancer patients. Proc Natl Acad Sci. 2008;105:14987–14992. doi: 10.1073/pnas.0806075105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Powderly J.D., Gutierrez M., Wang D., Chae Y.K., Mahadevan D., Braiteh F.S. A phase 1b/2, open-label study to evaluate the safety and tolerability of MEDI6469 in combination with immune therapeutic agents or therapeutic mAbs in patients with selected advanced solid tumors or aggressive B-cell lymphomas. ASCO Meet Abstr. 2015 TPS3091. [Google Scholar]
- 33.Freeman G.J., Long A.J., Iwai Y., Bourque K., Chernova T., Nishimura H. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tseng S.Y., Otsuji M., Gorski K., Huang X., Slansky J.E., Pai S.I. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J Exp Med. 2001;193:839–846. doi: 10.1084/jem.193.7.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong H., Strome S.E., Salomao D.R., Tamua H., Hirano F., Flies D.B. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 36.Iwai Y., Ishida M., Tanaka Y., Okazaki T., Honjo T., Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brahmer J.R., Drake C.G., Wollner I., Powderly J.D., Picus J., Sharfman W.H. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Topalian S.L., Hodi F.S., Brahmer J.R., Gettinger S.N., Smith D.C., McDermott D.F. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McDermott D.F., Drake C.G., Sznol M., Choueiri T.K., Powderly J.D., Smith D.C. Survival, durable response, and long-term safety in patients with previously treated advanced renal cell carcinoma receiving nivolumab. J Clin Oncol. 2015;33:2013–2020. doi: 10.1200/JCO.2014.58.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Motzer R.J., Rini B.I., McDermott D.F., Redman B.G., Kuzel T.M., Harrison M.R. Nivolumab for metastatic renal cell carcinoma: results of a randomized phase II trial. J Clin Oncol. 2015;33:1430–1437. doi: 10.1200/JCO.2014.59.0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Motzer R.J., Escudier B., McDermott D.F., George S., Hammers H.J., Srinivas S. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolchok J.D., Kluger H., Callahan M.K., Postow M.A., Rizvi N.A., Lesokhin A.M. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patnaik A., Kang S.P., Rasco D., Papadopoulos K.P., Elassaiss-Schaap J., Beeram M. Phase I study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in patients with advanced solid tumors. Clin Cancer Res. 2015;21:4286–4293. doi: 10.1158/1078-0432.CCR-14-2607. [DOI] [PubMed] [Google Scholar]
- 44.Inman B.A., Sebo T.J., Frigola X., Dong H., Bergstralh E.J., Frank I. PD-L1 (B7-H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: associations with localized stage progression. Cancer. 2007;109:1499–1505. doi: 10.1002/cncr.22588. [DOI] [PubMed] [Google Scholar]
- 45.Nakanishi J., Wada Y., Matsumoto K., Azuma M., Kikuchi K., Ueda S. Overexpression of B7-H1 (PD-L1) significantly associates with tumor grade and postoperative prognosis in human urothelial cancers. Cancer Immunol Immunother. 2007;56:1173–1182. doi: 10.1007/s00262-006-0266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Plimack E.R., Bellmunt J., Gupta S., Berger R., Montgomery R.B., Heath K. Pembrolizumab (MK-3475) for advanced urothelial cancer: updated results and biomarker analysis from KEYNOTE-012. ASCO Meet Abstr. 2015:4502. [Google Scholar]
- 47.Powderly J.D., Koeppen H., Hodi F.S., Sosman J.A., Gettinger S.N., Desai R. Biomarkers and associations with the clinical activity of PD-L1 blockade in a MPDL3280A study. ASCO Meet Abstr. 2013:3001. [Google Scholar]
- 48.McDermott D.F., Sosman J.A., Sznol M., Massard C., Gordon M.S., Hamid O. Atezolizumab, an anti–programmed death-ligand 1 antibody, in metastatic renal cell carcinoma: long-term safety, clinical activity, and immune correlates from a phase Ia study. J Clin Oncol. 2016;34:833–842. doi: 10.1200/JCO.2015.63.7421. [DOI] [PubMed] [Google Scholar]
- 49.Brahmer J.R., Tykodi S.S., Chow L.Q., Hwu W.J., Topalian S.L., Hwu P. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosenberg J.E., Hoffman-Censits J., Powles T., van der Heijden M.S., Balar A.V., Necchi A. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387:1909–1920. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ibrahim R., Stewart R., Shalabi A. PD-L1 blockade for cancer treatment: MEDI4736. Semin Oncol. 2015;42:474–483. doi: 10.1053/j.seminoncol.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 52.Heery C.R., O'Sullivan Coyne G.H., Madan R.A., Schlom J., von Heydebreck A., Cuillerot J. Phase I open-label, multiple ascending dose trial of MSB0010718C, an anti-PD-L1 monoclonal antibody, in advanced solid malignancies. ASCO Meet Abstr. 2014:3064. [Google Scholar]
- 53.Taube J.M., Anders R.A., Young G.D., Xu H., Sharma R., McMiller T.L. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4 doi: 10.1126/scitranslmed.3003689. 127ra37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thompson R.H., Kuntz S.M., Leibovich B.C., Dong H., Lohse C.M., Webster W.S. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66:3381–3385. doi: 10.1158/0008-5472.CAN-05-4303. [DOI] [PubMed] [Google Scholar]
- 55.Robert C., Long G.V., Brady B., Dutriaux C., Maio M., Mortier L. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 56.Paz-Ares L., Horn L., Borghaei H., Spigel D.R., Steins M., Ready N. Phase III, randomized trial (CheckMate 057) of nivolumab (NIVO) versus docetaxel (DOC) in advanced non-squamous cell (non-SQ) non-small cell lung cancer (NSCLC) ASCO Meet Abstr. 2015 LBA109. [Google Scholar]
- 57.Herbst R.S., Gordon M.S., Fine G.D., Sosman J.A., Soria J., Hamid O. A study of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic tumors. ASCO Meet Abstr. 2013:3000. [Google Scholar]
- 58.Callea M., Genega E.M., Gupta M., Fay A.P., Song J., Carvo I. PD-L1 expression in primary clear cell renal cell carcinomas (ccRCCs) and their metastases. ASCO Meet Abstr. 2014:467. [Google Scholar]
- 59.Powles T., Nickles D., Van Allen E., Chappey C., Zou W., Kowanetz M. Immune biomarkers associated with clinical benefit from atezolizumab (MPDL3280a; anti-PD-L1) in advanced urothelial bladder cancer (UBC) J Immunother Cancer. 2015;3(Suppl. 2):83. [Google Scholar]
- 60.Herbst R.S., Soria J.C., Kowanetz M., Fine G.D., Hamid O., Gordon M.S. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Curran M.A., Montalvo W., Yagita H., Allison J.P. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 2010;107:4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Parry R.V., Chemnitz J.M., Frauwirth K.A., Lanfranco A.R., Braunstein I., Kobayashi S.V. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Selby M., Engelhardt J., Lu L.S., Quigley M., Wang C., Chen B. Antitumor activity of concurrent blockade of immune checkpoint molecules CTLA-4 and PD-1 in preclinical models. ASCO Meet Abstr. 2013:3061. [Google Scholar]
- 64.Hammers H., Plimack E.R., Infante J.R., Emstoff M., Rini B.I., McDermott D.F. Phase I study of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma (MRCC) Ann Onc. 2014;25(Suppl. 4):iv361–iv372. [Google Scholar]
- 65.Yasuda S., Sho M., Yamato I., Yoshiji H., Wakatsuki K., Nishiwada S. Simultaneous blockade of programmed death 1 and vascular endothelial growth factor receptor 2 (VEGFR2) induces synergistic anti-tumour effect in vivo. Clin Exp Immunol. 2013;172:500–506. doi: 10.1111/cei.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Amin A., Plimack E.R., Infante J.R., Ernstoff M.S., Rini B.I., McDermott D.F. Nivolumab (anti-PD-1; BMS-936558, ONO-4538) in combination with sunitinib or pazopanib in patients (pts) with metastatic renal cell carcinoma (mRCC) ASCO Meet Abstr. 2014:5010. [Google Scholar]
- 67.Grosso J.F., Kelleher C.C., Harris T.J., Maris C.H., Hipkiss E.L., De Marzo A. LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems. J Clin Invest. 2007;117:3383–3392. doi: 10.1172/JCI31184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang C.T., Workman C.J., Flies D., Pan X., Marson A.L., Zhou G. Role of LAG-3 in regulatory T cells. Immunity. 2004;21:503–513. doi: 10.1016/j.immuni.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 69.Woo S.R., Turnis M.E., Goldberg M.V., Bankoti J., Selby M., Nirschl C.J. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72:917–927. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Watts T.H. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 71.French R.R., Taraban V.Y., Crowther G.R., Rowley T.F., Gray J.C., Johnson P.W. Eradication of lymphoma by CD8 T cells following anti-CD40 monoclonal antibody therapy is critically dependent on CD27 costimulation. Blood. 2007;109:4810–4815. doi: 10.1182/blood-2006-11-057216. [DOI] [PubMed] [Google Scholar]
- 72.He L.Z., Prostak N., Thomas L.J., Vitale L., Weidlick J., Crocker A. Agonist anti-human CD27 monoclonal antibody induces T cell activation and tumor immunity in human CD27-transgenic mice. J Immunol. 2013;191:4174–4183. doi: 10.4049/jimmunol.1300409. [DOI] [PubMed] [Google Scholar]