Abstract
Chronic stress causes elevations in glucocorticoid secretion and also increases the incidence of hypertension and other manifestations of cardiovascular disease. The extent to which the elevated glucocorticoids mediate the stress-associated increase in cardiovascular disease risk is unknown. Chronically elevated glucocorticoids can cause hypertension by acting in the periphery, but their effects within the brain on blood pressure regulation remain largely unexplored. We developed a method to produce selective chronic increases in the endogenous glucocorticoid corticosterone or the glucocorticoid receptor antagonist mifepristone within the hindbrain region, which includes a key cardiovascular regulatory area, the nucleus of the solitary tract (NTS). Experiments were performed in male Sprague–Dawley, Wistar–Kyoto (WKY) and borderline hypertensive rats (BHR). The results indicate that elevated exogenous corticosterone can act within the hindbrain to enhance the arterial pressure response to novel restraint stress and to reduce the gain and increase the mid-point of the arterial baroreflex. Basal levels of endogenous corticosterone have no effect on the arterial pressure response to stress in normotensive rats but enhance this response in BHR. Chronic stress-induced increases in baseline corticosterone enhance the arterial pressure response to stress in BHR but attenuate the adaptation of the response in WKY rats. Furthermore, an elevated corticosterone concentration within the hindbrain is necessary but not sufficient to cause glucocorticoid-induced hypertension. The effects of corticosterone within the hindbrain on blood pressure regulation are mediated in part by the glucocortiocid receptor, but are also likely to involve mineralocorticoid receptor-mediated effects and NTS catecholaminergic neurons. These data support the hypothesis that elevated glucocorticoids acting within the brain probably contribute to the adverse effects of stress on cardiovascular health in susceptible people.
The adverse effects of chronically high levels of glucocorticoids on cardiovascular health are established, while recent evidence reveals that even slight increases in endogenous glucocorticoids are associated with a high incidence of hypertension and other cardiovascular disease risk factors (Rossi et al. 2000; Magiakou et al. 2006; Walker, 2007). Similar detrimental effects of glucocorticoids can result from intracellular amplification of glucocorticoid actions and allelic variations in the glucocorticoid receptor (GR; Walker, 2007). Multiple peripheral actions of glucocorticoids contribute to these adverse effects (Magiakou et al. 2006), but central nervous system actions of glucocorticoids on blood pressure regulation and cardiovascular function remain enigmatic. Previous studies have primarily investigated the effects of intracerebroventricular administration of glucocorticoid agonists and antagonists; however, no unified understanding of the central actions of glucocorticoids has emerged (Takahashi et al. 1983; Gomez-Sanchez et al. 1990; van den Berg et al. 1990; Tonolo et al. 1993; van Acker et al. 2001). Possible explanations for the seemingly inconsistent actions of centrally administered steroids include opposing actions in different brain regions and suppression of endogenous glucocorticoid concentrations via centrally mediated feed-back inhibition of the hypothalamic–pituitary–adrenal (HPA) axis (Dallman et al. 2006). To circumvent these concerns with intracerebroventricular steroid delivery, we developed a method to deliver corticosterone (Cort; a naturally occurring glucocorticoid) locally to the hindbrain region that includes the nucleus of the solitary tract (NTS) in rats (Scheuer et al. 2004). We focused on this brain region for several reasons. First, the NTS is an essential component of the central neuronal network that controls blood pressure. Second, the NTS contains high levels of the glucocorticoid receptor (Fuxe et al. 1985; Guyenet, 2006). Third, Geerling and co-workers performed a series of elegant studies demonstrating that both mineralocorticoid receptor (MR) and 11β-hydroxysteroid dehydrogenase 2 (11βHSD2) are expressed by some neurons in the NTS (Geerling et al. 2006a,b; Gomez-Sanchez, 2010). However, these studies also revealed that 11βHSD2 is absent in some MR-expressing neurons within the NTS, so Cort is the predicted endogenous ligand at those NTS MRs. Finally, the NTS is not reported to be a site of feedback inhibition of the HPA axis by Cort. Localized increases in Cort or the glucocorticoid receptor antagonist mifepristone (Mif) were achieved by implanting small (3–4 mg) pellets of Cort on the surface of the dorsal hindbrain with one-third of the pellet caudal to calamus scriptorius (Scheuer et al. 2004). The pellets were made by placing powdered steroid in a beaker and melting it over the low flame of a bunsen burner. Care was taken to remove the steroid as soon as it melted so that it would not overheat, and if the heated steroid was discoloured it was discarded. The melted steroid was poured into a Silastic® mold and allowed to harden, and then the pellets were removed. A mineral oil layer applied to the bottom surface of the pellet prior to implantation allowed the steroid to diffuse freely from the pellet into the brain. The first series of experiments used pellets made entirely of either Cort or Mif (Scheuer et al. 2004, 2007; Bechtold & Scheuer, 2006; Bechtold et al. 2009).
Validation of the experimental approach
Initial validation of the dorsal hindbrain (DHB) pellets employed the measurement of plasma Cort concentration coupled with immuohistochemistry for the GR performed with an antibody that has a higher affinity for the ligand-bound GR compared with the unoccupied GR (Scheuer et al. 2004). Ligand-bound GR rapidly translocates from the cell cytoplasm to the nucleus, and the immunohistochemistry experiments indicated that the 100% Cort or Mif pellets delivered the steroids in sufficient quantities to cause nuclear translocation of the receptor within the NTS. In contrast, nuclear localization of the GR was not detected in the ventral hindbrain or forebrain (hippocampus) following 4 days of pellet treatment. Subsequent studies following 6 or 8 weeks of treatment produced similar results (Bechtold et al. 2009). Baseline plasma Cort concentrations were not altered by DHB Cort treatment in adrenal-intact rats (Scheuer et al. 2004; Bechtold & Scheuer, 2006). Adrenalectomized rats exhibited a small increase (about 1.5 µg dl−1) in baseline plasma Cort following treatment with 100% DHB Cort, but a similar increase in plasma Cort was measured in rats treated with the same dose of Cort administered subcutaneously (Scheuer et al. 2007). The plasma Cort response to 10 min of restraint stress tended to be augmented by DHB Cort treatment, but the effect did not reach statistical significance (Scheuer et al. 2007). Together, these data indicated that the DHB 100% Cort pellets produced a localized increase in DHB Cort concentrations within the NTS without altering plasma Cort concentration in adrenal-intact rats. Unexpectedly, chronic blockade of GR with DHB Mif pellets increased midday and evening plasma Cort concentrations, while suppressing the increase in plasma Cort in response to restraint stress (Scheuer et al. 2004; Bechtold et al. 2009). Thus, endogenous Cort may act within the DHB to inhibit the HPA axis in resting conditions, but to promote the HPA axis response to psychological stress. The mechanisms accounting for this are unknown; however, it is possible that Cort can act within the NTS to influence the recruitment of limbic system neurons that mediate the HPA axis response to stress (Jankord & Herman, 2008).
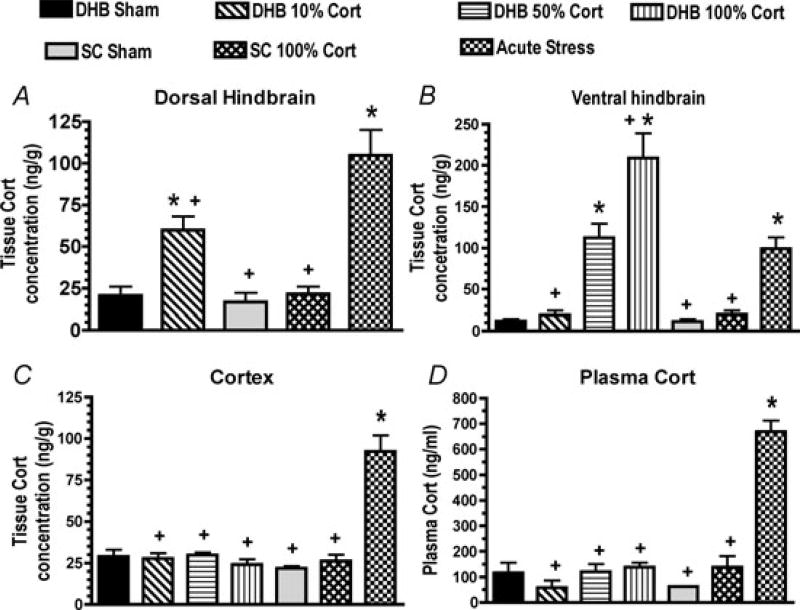
We performed subsequent experiments in 45 male Sprague–Dawley rats to measure tissue Cort concentrations within the DHB, ventral hindbrain and forebrain 6 days following implantation of DHB pellets made of 0, 10, 50 or 100% Cort (n = 6 per group). Rats with subcutaneous implantation of 0 and 100% Cort pellets (n = 6 per group) served as a negative control, while rats subjected to 60 min of restraint stress served as a positive control (n = 9). All studies were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication no. 85-23, revised 1996) and were approved by the Institutional Animal Care and Use Committee of the University of Florida. Brain tissues were harvested and processed for the extraction of Cort as was previously described for the measurement of tissue Mif concentration (Bechtold et al. 2009). Briefly, 6 days after surgery the rats were brought to the procedure room and allowed to rest quietly in their home cage for 1 h (except for the positive control group). Then they were deeply anaesthetized with isoflurane (5% in oxygen) and rapidly decapitated. The brains were removed from all rats and rapidly frozen, and trunk blood was collected for the measurement of plasma Cort. The blood was combined with heparin and then centrifuged, and the plasma samples and brain tissues were stored at −80°C. Brain tissue Cort was measured in samples of brain tissue obtained from the dorsal hindbrain, the ventral hindbrain and the cerebral cortex with average weights of 30 ± 1, 23 ± 1 and 30 ± 1 mg, respectively. The tissue samples were homogenized in 300 µl of ethanol–saline (1:1) and transferred to glass tubes containing 3 ml of methylene chloride. The samples were vortexed for 30 min then centrifuged at 1500 g for 20 min at 4°C. The organic phase was pipetted off and placed in a clean tube. The remaining aqueous layer and pellet were extracted a second time with 2 ml of methylene chloride; the pellet and aqueous phase were discarded, and the methylene chloride extract from each sample was combined with the first methylene chloride extract and dried under vacuum. The samples were reconstituted in 1 ml of extraction buffer (Oxford Biomedical Research, Oxford, MI, USA, product no. EA 66) and allowed to sit overnight. It was noted after the first few assays that some samples contained precipitate that correlated with spurious assay results, so the remaining samples were centrifuged prior to assay. Samples (50 µl aliquots) were assayed in duplicate or triplicate using an enzyme immunoassay (Oxford Biomedical Research, product no. EA 66). The extraction recovery was estimated at 93 ± 3% by adding trace amounts of tritiated Cort to some samples. The spurious results (i.e. values that were several standard deviations from the group mean) were removed from the final results. Plasma Cort was measured using MP Biomedicals (Pasadena, CA, USA) rat double antibody 125I-RIA kits. Statistical analysis was performed with analysis of variance, and Duncan’s test was used for post hoc comparisons. The results provided in Fig. 1 indicate that only the 10% pellet produced physiological increases in tissue Cort within the DHB. Higher doses delivered supraphysiological amounts of Cort to the NTS and also produced measurable increases in ventral hindbrain tissue Cort concentrations. No pellets produced detectable increases in forebrain Cort concentrations. The DHB 10% Cort pellets are now used to produce physiologically relevant localized increases in DHB Cort. We have obtained similar results after 3 weeks of pellet treatment, suggesting that relatively constant steroid delivery can be achieved using this method of pellet implantation. The combined approaches of immunohistochemistry and tissue steroid measurements strongly support the conclusion that steroids binding to receptors located within the DHB mediate the effects of the 10% DHB pellets. However, we cannot entirely rule out the possibility that the steroids are being transported to and acting at receptors in other regions of the brain.
Figure 1. Tissue corticosterone (Cort) concentrations in the dorsal hindbrain (DHB; A), ventral hindbrain (B) and cortex (C) and plasma Cort concentration (D) in rats treated for 6 days with DHB sham, 10, 50 or 100% Cort pellets, sham or 100% Cort subcutaneous (s.c.) pellets or subjected to restraint stress just prior to killing (acute stress).
Values for the DHB for the 50 and 100% DHB Cort treatment groups were above the high standard in the assay and are not shown. *P < 0.05 compared with the DHB Sham group. +P < 0.05 compared with acute stress.
Effects of DHB Cort on blood pressure regulation
Glucocorticoids could act within the DHB to influence one or more components of the integrated mechanisms controlling blood pressure. Our studies have focused on three such components: baseline blood pressure, arterial baroreflex function and blood pressure responses to stress (Scheuer et al. 2004, 2007; Bechtold & Scheuer, 2006; Bechtold et al. 2009). Chronic systemic Cort treatment increases baseline arterial pressure, so experiments were performed to determine the role of the dorsal hindbrain in mediating Cort-induced hypertension (Scheuer & Bechtold, 2001). In rats with hypertension resulting from systemic glucocorticoid treatment, blockade of GR with DHB Mif treatment reduced resting arterial pressure (Scheuer et al. 2004). However, the resting arterial pressure was not reduced in control rats or in normotensive rats with mild, chronic stress-induced elevations (2–3 µg dl−1) in baseline plasma Cort concentrations, and treatment with exogenous DHB Cort produced only a small and inconsistent increase in baseline arterial pressure (Scheuer et al. 2004, 2007; Bechtold & Scheuer, 2006; Bechtold et al. 2009). Therefore, elevated Cort concentrations within the hindbrain appear to be necessary but not sufficient to mediate systemic Cort-induced hypertension.
Reduced baroreflex sensitivity is associated with alterations in autonomic balance and increased risk of cardiovascular disease, including cardiac arrhythmias and hypertension (Hohnloser et al. 1994; La Rovere et al. 2001; Lanfranchi & Somers, 2002; Izzo et al. 2008), and is an independent predictor of death after myocardial infarction (La Rovere et al. 2001). Chronic elevations in systemic Cort also increase the mid-point and reduce the gain of the arterial baroreflex function, while adrenalectomy can prevent baroreflex resetting (Gardiner & Bennett, 1983; Scheuer & Mifflin, 2001). Since the NTS is a key nucleus within the brain pathways mediating this reflex (Guyenet, 2006), experiments were performed to determine whether Cort acts within this region to modulate baroreflex function. Treatment with DHB Cort (100%) pellets reduced the gain and increased the mid-point of arterial baroreflex control of heart rate. The increase in the mid-point was in part independent of the increase in baseline pressure, indicating that elevated Cort within the hindbrain can cause active resetting of the arterial baroreceptor reflex. Baroreflex function testing has not yet been performed in rats treated with 10% DHB Cort pellets.
Enhanced blood pressure and neuroendocrine responses to acute stress predict future hypertension and cardiovascular disease (Matthews et al. 1993; Moseley & Linden, 2006; Wirtz et al. 2006; Deter et al. 2007; Wright et al. 2007; Izzo et al. 2008). We determined that DHB Cort (100%) treatment increased the arterial pressure and plasma glucose responses to novel restraint stress in male Sprague–Dawley rats, while treatment with the same dose of Cort systemically had no effect on the blood pressure response to stress (Scheuer et al. 2007). The DHB 10% Cort also enhanced the arterial pressure response to stress, confirming that physiological levels of exogenous Cort can act within the DHB to increase the arterial pressure response to novel stress (Looney et al. 2008). Additional experiments were performed to determine whether endogenous Cort could alter the arterial pressure response to novel (acute) or chronic stress (Bechtold et al. 2009). These experiments were performed in offspring of female spontaneously hypertensive rats and male Wistar–Kyoto (WKY) rats. These borderline hypertensive rats (BHR) have a genetically based increase in stress reactivity (Sanders & Lawler, 1992; Fuchs et al. 1998). Wistar–Kyoto rats were also studied. Rats were either restrained for 1–2 hours per day for 5–7 days per week for approximately 4 weeks or restrained only once, on the final day of the experiment (Bechtold et al. 2009). Dorsal hindbrain GRs were blocked by implantation of a 100% DHB Mif pellet. Control rats were treated with either DHB sham or subcutaneous Mif pellets. Wistar–Kyoto rats subjected to repeated restraint showed substantial adaptation of the arterial pressure response to stress, whereas nominal adaptation was observed in BHR. Chronic blockade of DHB GR attenuated the blood pressure response to restraint in both acutely and chronically stressed BHR. In sharp contrast, DHB GR blockade had no effect on the blood pressure response to acute stress in WKY rats and partially blocked the adaptation to repeated stress. The mechanisms accounting for the opposing effects of endogenous glucocorticoids in BHR compared with WKY rats are currently unknown. However, variable sensitivity to glucocorticoids is implicated in the diversity of psychological stress susceptibility and resilience in humans (DeRijk & de Kloet, 2005; Feder et al. 2009). Our data suggest that differential actions of glucocorticoids could also contribute to variations in cardiovascular stress reactivity and susceptibility observed in humans.
Potential mechanisms
We are currently performing studies to investigate the mechanisms of action of glucocorticoids within NTS neurons. Preliminary data suggest that NTS catecholaminergic neurons that express dopamine-β-hydroxylase (DβH) play an important role in mediating the effects of dorsal hindbrain Cort on stress reactivity (Dong et al. 2008). The data we have collected thus far indicate that GRs mediate some of the observed effects of Cort, but it is critical to determine the role of MRs, especially considering that high physiological levels of Cort could overwhelm the capacity of 11βHSD2 to degrade the steroid, leading to MR activation by Cort. Other issues of ligand specificity for the MR (see Gomez-Sanchez, 2010) also influence the effects of Cort within the NTS.
Perspectives
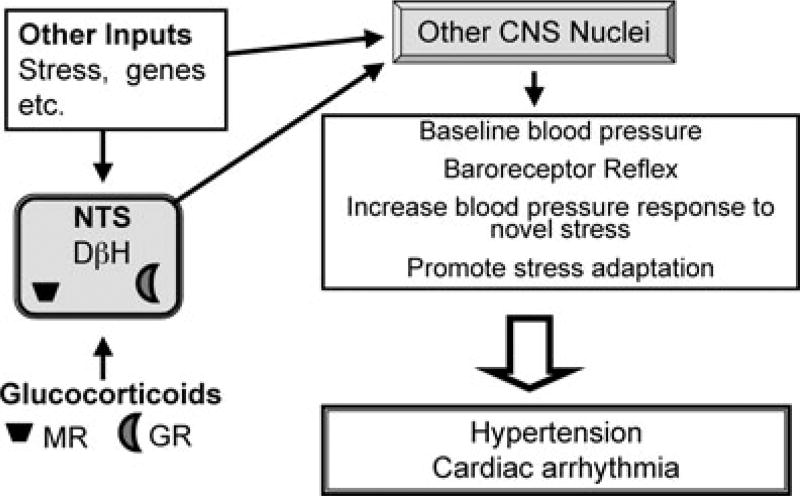
Chronic stress has the following effects: (i) increased cardiovascular disease risk; (ii) attenuation of the baroreceptor reflex; (iii) enhanced blood pressure and neuroendocrine responses to acute novel stress; (iv) attenuation of blood pressure and neuroendocrine responses to a repeated stressor; and (v) can increase baseline blood pressure (Vrijkotte et al. 2000; Lucini et al. 2005; Izzo et al. 2008; Ulrich-Lai & Herman, 2009). These mechanisms work in concert to help the organism survive the stress and prepare for the next threat. Unfortunately, long-term activation of the stress circuitry can cause multiple health problems, including an increased risk for cardiovascular disease. The studies described above indicate that Cort acts within the DHB to help mediate these effects of chronic stress. The data support the hypothesis that elevated glucocorticoids acting within the NTS probably contribute to the adverse effects of stress on cardiovascular health in susceptible people (Fig. 2).
Figure 2. Hypothesis Model.
The diagram illustrates the overall hypothesis that glucocorticoids released during chronic stress act on glucocorticoid receptors (GR) and possibly mineralocorticoid receptors (MR) within the hindbrain to modulate neuronal output of the nucleus of the solitary tract (NTS). The altered NTS neuronal output in turn modulates the activity of other central nervous system (CNS) nuclei that control neuronal and hormonal mediators of cardiovascular function, resulting in an overall increased incidence of hypertension, cardiac arrhythmia and other manifestations of cardiovascular disease in susceptible individuals. (DβH, dopamine-β-hydroxylase.)
Acknowledgments
I would like to thank Dr Maureen Keller-Wood for her invaluable assistance in developing and validating the methods for measurement of tissue corticosterone concentrations. This study was supported by grant R01 HL 076807 from the National Heart, Lung and Blood Institute.
References
- Bechtold AG, Patel G, Hochhaus G, Scheuer DA. Chronic blockade of hindbrain glucocorticoid receptors reduces blood pressure responses to novel stress and attenuates adaptation to repeated stress. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1445–R1454. doi: 10.1152/ajpregu.00095.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtold AG, Scheuer DA. Glucocorticoids act in the dorsal hindbrain to modulate baroreflex control of heart rate. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1003–R1011. doi: 10.1152/ajpregu.00345.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman MF, Pecoraro NC, La Fleur SE, Warne JP, Ginsberg AB, Akana SF, Laugero KC, Houshyar H, Strack AM, Bhatnagar S, Bell ME. Glucocorticoids, chronic stress, and obesity. Prog Brain Res. 2006;153:75–105. doi: 10.1016/S0079-6123(06)53004-3. [DOI] [PubMed] [Google Scholar]
- DeRijk R, de Kloet ER. Corticosteroid receptor genetic polymorphisms and stress responsivity. Endocrine. 2005;28:263–270. doi: 10.1385/ENDO:28:3:263. [DOI] [PubMed] [Google Scholar]
- Deter H-C, Bleecher A, Weber CS. Cardiovascular reactivity of patients with essential and renal hypertension in an emotion-triggering interview. Behav Med. 2007;32:117–125. doi: 10.3200/BMED.32.4.117-125. [DOI] [PubMed] [Google Scholar]
- Dong Y, Looney B, Daubert D, Su Y, Scheuer DA. Lesioning NTS catecholaminergic neurons augments the arterial pressure response to acute stress; 62nd High Blood Pressure Research Conference Abstracts; 2008. p. LB051. [Google Scholar]
- Feder A, Nestler EJ, Charney DS. Psychobiology and molecular genetics of resilience. Nat Rev Neurosci. 2009;10:446–457. doi: 10.1038/nrn2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs LC, Hoque AM, Clarke NL. Vascular and hemodynamic effects of behavioral stress in borderline hypertensive and Wistar-Kyoto rats. Am J Physiol Regul Integr Comp Physiol. 1998;274:R375–R382. doi: 10.1152/ajpregu.1998.274.2.R375. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Harfstrand A, Agnati LF, Yu ZY, Cintra A, Wikstrom AC, Okret S, Cantoni E, Gustafsson J-A. Immunocytochemical studies on the localization of glucocorticoid receptor immunoreactive nerve cells in the lower brainstem and spinal cord of the male rat using a monoclonal antibody against rat liver glucocorticoid receptor. Neurosci Lett. 1985;60:1–6. doi: 10.1016/0304-3940(85)90372-6. [DOI] [PubMed] [Google Scholar]
- Gardiner SM, Bennett T. Post-adrenalectomy hypotension in rats; absence of baroreflex resetting or effects of naloxone. Clin Sci. 1983;64:371–376. doi: 10.1042/cs0640371. [DOI] [PubMed] [Google Scholar]
- Geerling JC, Engeland WC, Kawata M, Loewy AD. Aldosterone target neurons in the nucleus tractus solitarius drive sodium appetite. J Neurosci. 2006a;26:411–417. doi: 10.1523/JNEUROSCI.3115-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerling JC, Kawata M, Loewy AD. Aldosterone-sensitive neurons in the rat central nervous system. J Comp Neurol. 2006b;494:515–527. doi: 10.1002/cne.20808. [DOI] [PubMed] [Google Scholar]
- Gomez-Sanchez EP. The mammalian mineralocorticoid receptor: tying down a promiscuous receptor. Exp Physiol. 2010;95 doi: 10.1113/expphysiol.2008.045914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Sanchez EP, Venkataraman MT, Thwaites D, Fort C. ICV infusion of corticosterone antagonizes ICV-aldosterone hypertension. Am J Physiol Endocrinol Metab. 1990;258:E649–E653. doi: 10.1152/ajpendo.1990.258.4.E649. [DOI] [PubMed] [Google Scholar]
- Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- Hohnloser SH, Klingenheben T, van de Loo A, Hablawetz E, Just H, Schwartz PJ. Reflex versus tonic vagal activity as a prognostic parameter in patients with sustained ventricular tachycardia or ventricular fibrillation. Circulation. 1994;89:1068–1073. doi: 10.1161/01.cir.89.3.1068. [DOI] [PubMed] [Google Scholar]
- Izzo JLJ, Sica DA, Black HR, editors. Hypertension Primer. Lippincott Williams & Wilkins; Philadelphia: 2008. [Google Scholar]
- Jankord R, Herman JP. Limbic regulation of hypothalamo-pituitary-adrenocortical function during acute and chronic stress. Ann N Y Acad Sci. 2008;1148:64–73. doi: 10.1196/annals.1410.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rovere MT, Pinna GD, Hohnloser SH, Marcus FI, Mortara A, Nohara R, Bigger JTJ, Camm AJ, Schwartz PJ. Baroreflex sensitivity and heart rate variability in the identification of patients at risk for life-threatening arrhythmias: implications for clinical trials. Circulation. 2001;103:2072–2076. doi: 10.1161/01.cir.103.16.2072. [DOI] [PubMed] [Google Scholar]
- Lanfranchi PA, Somers VK. Arterial baroreflex function and cardiovascular variability: interactions and implications. Am J Physiol Regul Integr Comp Physiol. 2002;283:R815–R826. doi: 10.1152/ajpregu.00051.2002. [DOI] [PubMed] [Google Scholar]
- Looney B, Daubert DL, Su Y, Scheuer DA. Low doses of corticosterone act in the dorsal hindbrain to enhance the arterial pressure response to both acute and repeated stress. FASEB J. 2008;22:1171–1174. [Google Scholar]
- Lucini D, Di Fede G, Parati G, Pagani M. Impact of chronic psychosocial stress on autonomic cardiovascular regulation in otherwise healthy subjects. Hypertension. 2005;46:1201–1206. doi: 10.1161/01.HYP.0000185147.32385.4b. [DOI] [PubMed] [Google Scholar]
- Magiakou MA, Smyrnaki P, Chrousos GP. Hypertension in Cushing’s syndrome. Best Pract Res Clin Endocrinol Metab. 2006;20:467–482. doi: 10.1016/j.beem.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Woodall KL, Allen MT. Cardiovascular reactivity to stress predicts future blood pressure status. Hypertension. 1993;22:479–485. doi: 10.1161/01.hyp.22.4.479. [DOI] [PubMed] [Google Scholar]
- Moseley JV, Linden W. Prediciting blood pressure and heart rate change with cardiovascular reactivity and recovery: results from 3-year and 10-year follow up. Psychosom Med. 2006;68:833–844. doi: 10.1097/01.psy.0000238453.11324.d5. [DOI] [PubMed] [Google Scholar]
- Rossi R, Tauchmanova L, Luciano A, Di Martino M, Battista C, Del Viscovo L, Nuzzo V, Lombardi G. Subclinical Cushing’s syndrome in patients with adrenal incidentaloma: clinical and biochemical features. J Clin Endocrinol Metab. 2000;85:1440–1448. doi: 10.1210/jcem.85.4.6515. [DOI] [PubMed] [Google Scholar]
- Sanders BJ, Lawler JE. The borderline hypertensive rat (BHR) as a model for environmentally-induced hypertension: a review and update. Neurosci Biobehav Rev. 1992;16:207–217. doi: 10.1016/s0149-7634(05)80181-2. [DOI] [PubMed] [Google Scholar]
- Scheuer DA, Bechtold AG. Glucocorticoids potentiate central actions of angiotensin to increase arterial pressure. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1719–R1726. doi: 10.1152/ajpregu.2001.280.6.R1719. [DOI] [PubMed] [Google Scholar]
- Scheuer DA, Bechtold AG, Shank SS, Akana SF. Glucocorticoids act in the dorsal hindbrain to increase arterial pressure. Am J Physiol Heart Circ Physiol. 2004;286:H458–H467. doi: 10.1152/ajpheart.00824.2003. [DOI] [PubMed] [Google Scholar]
- Scheuer DA, Bechtold AG, Vernon KA. Chronic activation of dorsal hindbrain corticosteroid receptors augments the arterial pressure response to acute stress. Hypertension. 2007;49:127–133. doi: 10.1161/01.HYP.0000250088.15021.c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuer DA, Mifflin SW. Glucocorticoids modulate baroreflex control of renal sympathetic nerve activity. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1440–R1449. doi: 10.1152/ajpregu.2001.280.5.R1440. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Takeda K, Ashizawa H, Inoue A, Yoneda S, Yoshimura M, Ijichi H. Centrally induced cardiovascular and sympathetic responses to hydrocortisone in rats. Am J Physiol Heart Circ Physiol. 1983;245:H1013–H1018. doi: 10.1152/ajpheart.1983.245.6.H1013. [DOI] [PubMed] [Google Scholar]
- Tonolo G, Soro A, Madeddu P, Troffa C, Melis MG, Patteri G, Pinna Parpaglia P, Sabino G, Maioli M, Glorioso N. Effect of chronic intracerebroventricular dexamethasone on blood pressure in normotensive rats. Am J Physiol Endocrinol Metab. 1993;264:E843–E847. doi: 10.1152/ajpendo.1993.264.6.E843. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Acker SA, Fluttert MF, Sibug RM, de Kloet ER. Intracerebroventricular administration of a glucocorticoid receptor antagonist enhances the cardiovascular responses to brief restraint. Eur J Pharmacol. 2001;430:87–91. doi: 10.1016/s0014-2999(01)01341-3. [DOI] [PubMed] [Google Scholar]
- Van Den Berg DTWM, De Kloet ER, van Dijken HH, de Jong W. Differential central effects of mineralocorticoid and glucocorticoid agonists and antagonists on blood pressure. Endocrinology. 1990;126:118–124. doi: 10.1210/endo-126-1-118. [DOI] [PubMed] [Google Scholar]
- Vrijkotte TGM, van Doornen LJP, de Geus JC. Effects of work stress on ambulatory blood pressure, heart rate, and heart rate variability. Hypertension. 2000;35:880–886. doi: 10.1161/01.hyp.35.4.880. [DOI] [PubMed] [Google Scholar]
- Walker BR. Glucocorticoids and cardiovascular disease. Eur J Endocrinol. 2007;157:545–559. doi: 10.1530/EJE-07-0455. [DOI] [PubMed] [Google Scholar]
- Wirtz PH, von Kanel R, Mohiyeddini C, Emini L, Ruedisueli K, Groessbauer S, Ehlert U. Low social support and poor emotional regulation are associated with increased stress hormone reactivity to mental stress in systemic hypertension. J Clin Endocrinol Metab. 2006;91:3857–3865. doi: 10.1210/jc.2005-2586. [DOI] [PubMed] [Google Scholar]
- Wright CE, O’Donnell K, Brydon L, Wardle J, Steptoe A. Family history of cardiovascular disease is associated with cardiovascular responses to stress in healthy young men and women. Int J Psychophysiol. 2007;63:275–282. doi: 10.1016/j.ijpsycho.2006.11.005. [DOI] [PubMed] [Google Scholar]