Abstract
Systemic corticosterone (Cort) modulates arterial baroreflex control of both heart rate and renal sympathetic nerve activity. Because baroreceptor afferents terminate in the dorsal hindbrain (DHB), an area with dense corticosteroid receptor expression, we tested the hypothesis that prolonged activation of DHB Cort receptors increases the midpoint and reduces the gain of arterial baroreflex control of heart rate in conscious rats. Small (3–4 mg) pellets of Cort (DHB Cort) or Silastic (DHB Sham) were placed on the surface of the DHB, or Cort was administered systemically by placing a Cort pellet on the surface of the dura (Dura Cort). Baroreflex control of heart rate was determined in conscious male Sprague Dawley rats on each of 4 days after initiation of treatment. Plots of arterial pressure vs. heart rate were analyzed using a four-parameter logistic function. After 3 days of treatment, the arterial pressure midpoint for baroreflex control of heart rate was increased in DHB Cort rats (123 ± 2 mmHg) relative to both DHB Sham (108 ± 3 mmHg) and Dura Cort rats (109 ± 2 mmHg, P < 0.05). On day 4, baseline arterial pressure was greater in DHB Cort (112 ± 2 mmHg) compared with DHB Sham (105 ± 2 mmHg) and Dura Cort animals (106 ± 2 mmHg, P < 0.05), and the arterial pressure midpoint was significantly greater than mean arterial pressure in the DHB Cort group only. Also on day 4, maximum baroreflex gain was reduced in DHB Cort (2.72 ± 0.12 beats • min−1 • mmHg−1) relative to DHB Sham and Dura Cort rats (3.51 ± 0.28 and 3.37 ± 0.27 beats • min−1 • mmHg−1, P < 0.05). We conclude that Cort acts in the DHB to increase the midpoint and reduce the gain of the heart rate baroreflex function.
Keywords: corticosterone, nucleus of the solitary tract, glucocorticoid receptors, brain, stress
Elevated glucocorticoids increase the risk for cardiovascular disease (13, 20, 46, 58, 59). Accumulating evidence further indicates that altered glucocorticoid activity or sensitivity, even in the absence of elevated plasma glucocorticoid levels, may play a role in the etiology of human essential hypertension, a primary risk factor for cardiovascular disease (27, 47, 56, 57). Altered glucocorticoid activity has also been implicated in the pathogenesis of metabolic syndrome, a condition whose symptoms include obesity, elevated blood pressure, and insulin resistance or diabetes (55). Genetic factors appear to increase sensitivity to the adverse cardiovascular effects of glucocorticoids, since several recently identified polymorphisms of the human glucocorticoid receptor (GR) gene have been linked to hypertension and cardiovascular disease (25, 26, 34, 35). Complementing the data from humans, a glucocorticoid-dependent mechanism of hypertension has been demonstrated in several rat strains, including spontaneously hypertensive (18, 60) and obese Zucker rats (8). Thus multiple lines of evidence implicate glucocorticoids in the development of risk factors for cardiovascular disease. Many of these risk factors are also associated with dysregulation of autonomic function (2, 14, 36). However, the mechanisms by which glucocorticoids modulate arterial pressure regulation, particularly with respect to the effects of glucocorticoids on central pathways controlling autonomic function, remain poorly understood.
Studies in animals indicate that systemic elevations in glucocorticoids can modulate the function of the arterial baroreceptor reflex (12, 37, 40, 42–44). The arterial baroreceptor reflex is an autonomic reflex that is important for the short-term, and possibly long-term, regulation of arterial pressure (3, 29, 32, 50). Studies from our laboratory have demonstrated that systemic administration of corticosterone (Cort) modulates baroreflex control of both renal sympathetic nerve activity and heart rate to increase the arterial pressure midpoint and decreases the reflex gain independent of changes in arterial pressure in rats (37, 40). Studies from Segar et al. (42–44) demonstrate that glucocorticoids increase the midpoint and reduce the gain of the arterial baroreflex during the perinatal period. These results support the hypothesis that glucocorticoids actively modulate the baroreceptor reflex, suggesting one mechanism by which glucocorticoids may contribute to the development of cardiovascular disease.
Glucocorticoids could modulate the baroreflex by acting at one or more central nervous system sites. The contribution of the central nervous system in glucocorticoid-mediated regulation of arterial pressure has largely been neglected, due in part to the difficulties inherent in selectively manipulating central vs. peripheral glucocorticoid levels. However, utilizing a new method developed in our laboratory to selectively administer Cort to a specific area of the central nervous system, the dorsal hindbrain (DHB), we have recently demonstrated that chronic activation of DHB corticosteroid receptors increases arterial pressure, whereas blockade of DHB GRs attenuates systemic Cort-induced hypertension (38). The DHB contains a key cardiovascular regulatory site, the nucleus of the solitary tract, which receives, processes, and integrates direct input from many cardiovascular afferents, including the arterial baroreceptors, and has a high level of corticosteroid receptor expression (9, 17, 31, 38). Thus the present study was designed to test the hypothesis that selective activation of DHB Cort receptors can increase the midpoint and reduce the gain of arterial baroreflex control of heart rate. Cort or Sham pellets were implanted on the surface of the DHB of rats instrumented with indwelling arterial and venous catheters. Baroreflex control of heart rate was determined in conscious rats on each of 4 days after initiation of DHB treatment. In an additional group of rats, Cort pellets were implanted on the surface of the dura (Dura Cort) to control for any systemically mediated effects of this dose of Cort.
METHODS
Experiments were performed in 56 adult male Sprague Dawley rats purchased from Charles River Laboratories. Rats were housed in an American Association for the Accreditation of Laboratory Animal Care-accredited animal care facility on a 12:12-h light-dark cycle. Access to food and water was provided ad libitum. The Institutional Animal Care and Use Committee of the University of Missouri-Kansas City approved all procedures and protocols.
DHB pellets
Cort pellets for implantation on the DHB or dura were made from powdered Cort (minimum 92% Cort; Sigma) that was liquefied, without overheating, by gently warming the powder over a low flame (30, 38). The liquefied Cort was pipetted into a mold, or pellets were carved from larger pellets of hardened Cort. Sham pellets were made from Silastic gel (Kwik-sil; World Precision Instruments). All pellets were trimmed to produce final pellet dimensions of ~1.5 mm × 1.75 mm × 1 mm (length × width × height). These dimensions produce pellets weighing 3–4 mg. The bottom surface of each DHB pellet was coated with a thin layer of mineral oil to establish a lipophilic diffusion zone between the pellet and brain surface.
Surgery
Animals were allowed to acclimate to the animal care facility for at least 1 wk before any surgical procedure was performed, and all surgical procedures were performed using aseptic techniques. Animals were anesthetized with a combination of Domitor (metatomidine hydrochloride, 0.5 mg/kg ip; Pfizer Animal Health, Exton, PA) and Ketaset (ketamine hydrochloride, 75 mg/kg ip; Fort Dodge Animal Health, Fort Dodge, IA), and then prophylactic penicillin (Pen-Pro-G 600,000 U/kg sc; Henry Schein) was administered.
Catheters were implanted for measurement of arterial pressure and administration of drugs. For catheterization, a small incision was made over the femoral artery and vein. The vessels were isolated and cleared of tissue. An arterial catheter made of 23 g Tygon tubing with a Teflon tip was threaded in the abdominal aorta via the femoral artery for measurement of blood pressure. Two venous catheters made of PE-50 tubing pulled to a finer tip were inserted in the femoral vein for administration of drugs that were used to test baroreflex function. All catheters were tunneled under the skin to exit between the scapulae and secured in place with silk suture. The dead space in the arterial catheter was filled with sterile heparin (1,000 U/ml), and venous catheters were filled with sterile heparinized saline (20 U/ml).
After catheterization, rats were prepared for implantation of a DHB or Dura pellet according to the methods previously described in detail (38). Briefly, rats were placed in a stereotaxic head frame with the head ventroflexed to allow access to the DHB between the atlas and occipital bone. A medial skin incision was made over the atlantooccipital area, and the skin and overlying muscle were retracted. Next, an incision was made in the dura to expose the dorsal surface of the hindbrain. After removal of the pia from the brain surface, a Sham or Cort pellet was carefully placed on the brain surface with approximately one-third of the pellet extending caudal to calamus scriptorius. The pellet was secured in place with a small drop of surgical glue (Vet Bond; Henry Schein) that was applied to the caudal portion of the pellet, and the exposed surface of the pellet was coated with a small amount of Silastic gel (Kwik-sil; World Precision Instruments) to limit diffusion of the pellet contents in the cerebrospinal fluid. The opening in the dura was sealed with a small patch of nitrocellulose adhered in place with a drop of surgical glue, and the skin was sutured closed. Dura Cort pellets were implanted on the surface of the dura at the same position where the dura was cut for implantation of DHB pellets. A Cort pellet was glued to the Dura, but no Silastic gel was applied. After completion of all surgical procedures, 6–8 ml of saline were administered subcutaneously to replace lost fluid, and Antisedan (atipamezole hydrochloride, 1 mg/kg ip) was administered to reverse the effects of Domitor. Animals were placed in clean cages, and their recovery was closely monitored.
Experimental protocol
Animals were allowed to recover overnight before the experimental protocol was initiated. All experiments were performed on conscious freely moving animals in their home cages. Drugs administered intravenously were dissolved in sterile isotonic saline. Experiments included in the study were performed in the following three groups of rats: DHB Sham (n = 15), DHB Cort (n = 18), and Dura Cort (n = 23). Five rats (2 DHB Sham, 2 DHB Cort, and 1 Dura Cort) were excluded from the data analysis because their morning baseline Cort concentrations were >10 µg/dl, which indicated they might have been stressed (33). Removal of these animals did not alter the outcome and conclusions of the study.
Baseline data and baroreflex testing
On the day of an experiment, rats were brought to the laboratory in the morning in their home cages. The arterial catheter was connected to a pressure transducer, and the venous lines were filled with phenylephrine and nitroprusside. Arterial pressure and heart rate were recorded continuously for 3 h. After this 3-h period, baroreflex control of heart rate was determined. First phenylephrine (200 µg/ml) was administered intravenously using an infusion pump (model A99; Razel Scientific Instruments, Stamford, CT) to increase arterial pressure by 50–60 mmHg over a 1- to 2-min interval. After arterial pressure and heart rate returned to baseline values, nitroprusside (500 µg/ml) was infused via the other venous catheter to decrease arterial pressure 40–50 mmHg over a 1- to 2-min interval.
Adrenal weight and plasma Cort concentration
Systemic exogenous Cort administration can produce feedback inhibition-induced decreases in adrenal gland weight (1). Therefore, at the termination of experiments, the rats were killed with an overdose of anesthesia (inhaled isoflurane), and the adrenal glands were removed, cleared of excess tissue, and weighed. Adrenal weights were normalized to body weight for statistical analysis. In some animals, blood samples were taken between 5:00 and 6:00 pm on the evening of the 3rd day and/or between 8:00 and 9:00 am on the morning of the 4th day of treatment to determine plasma Cort concentration. Blood (200 µl) was drawn from the arterial catheter and added to tubes containing 10 µl of heparin (1,000 U/ml). Samples were centrifuged at 4°C and 3,000 g for 15 min. The plasma was stored at −20°C until being assayed with a commercially available RIA kit (I125 RIA kit; ICN Biomedicals, Costa Mesa, CA).
Data acquisition and analysis
For the measurement of blood pressure, the arterial catheter was attached to a pressure transducer (Maxxim Medical, Athens, TX) that was connected to a bridge amplifier (World Precision Instruments, Sarasota, FL). Analog-to-digital signal processing was performed by a MacLab (ADInstruments) and a Macintosh computer. Heart rate and mean arterial pressure were calculated on-line by the MacLab software. Baseline arterial pressure and heart rate were determined by averaging the data during the final 60 min of the 3-h baseline recording period.
For the baroreflex function curves, heart rate values were first averaged into 1-mmHg mean arterial pressure bins. The data were then analyzed using a sigmoid logistic function curve according to the equation, heart rate = P4 + P1/{1 + exp[P2(mean arterial pressure − P3)]}, where P1 is the range of heart rate, P2 is the coefficient to calculate the gain as a function of pressure, P3 is the pressure at the midrange of the curve, and P4 is minimum value for heart rate (22). The best-fit curve was calculated using SigmaPlot software (SPSS). The fit of the curve to the data was estimated by calculating an r2 value for each curve. Maximum gain was calculated from the first derivative of the logistic function curve formula above. The r2 values ranged from 0.91 to 0.99. Parameter values were averaged for each group and used to generate curves for graphical presentation.
Statistical analysis
Because of the technical difficulty of the experiments and intermittent catheter malfunctions, it was not possible to determine baroreflex function for each animal on every day. Therefore, a separate between-subjects analysis was performed for each day of treatment, and values for mean arterial pressure, heart rate, body weight, adrenal weight, plasma Cort, and baroreflex parameters were analyzed by one-way ANOVA. Statistical significance was accepted at P < 0.05. When the overall ANOVA detected a significant difference between groups, Duncan’s new multiple-range test was used for post hoc analysis. “P” values are provided for the results of the overall ANOVA. Data are presented as means ± SE.
RESULTS
The rats weighed an average of 366 ± 7 g (n = 55) on the day of surgery to implant DHB or Dura pellets. There were no differences in body weight between the three groups on any day (data not shown).
Because of the trophic effects of ACTH on the adrenal gland, adrenal weight is a highly sensitive measure of chronic changes in circulating ACTH levels (41). Because exogenous systemic glucocorticoids inhibit ACTH secretion, adrenal weight is also a sensitive measure of exogenous systemic glucocorticoid concentration (1). In the present study, no differences in adrenal weights between DHB Sham (191 ± 10 mg/kg, n = 11), Dura Cort (189 ± 6 mg/kg, n = 23), or DHB Cort (199 ± 9 mg/kg, n = 16) rats were observed (P = 0.69). Additionally, plasma Cort values in the morning (DHB Sham 2.6 ± 0.3 µg/dl, n = 10; DHB Cort 2.9 ± 0.5 µg/dl, n = 17; Dura Cort 3.3 ± 0.4 µg/dl, n = 17; P = 0.48) and evening (DHB Sham 17.8 ± 3.9 µg/dl, n = 8; DHB Cort 13.5 ± 3.5 µg/dl, n = 9; Dura Cort 10.9 ± 2.8 µg/dl, n = 12; P = 0.36) were not different between groups. These results indicate that the amount of Cort from the DHB Cort pellet that diffused centrally beyond the DHB or entered the systemic circulation did not exceed normal physiological Cort concentrations. This allowed the hypothalamic-pituitary-adrenal axis to adjust endogenous Cort secretion and maintain normal circulating Cort concentrations (1). Furthermore, any reduction in endogenous hypothalamic-pituitary-adrenal axis activity must have been minimal, since a physiologically relevant reduction in ACTH secretion would have resulted in a decrease in adrenal weight (1).
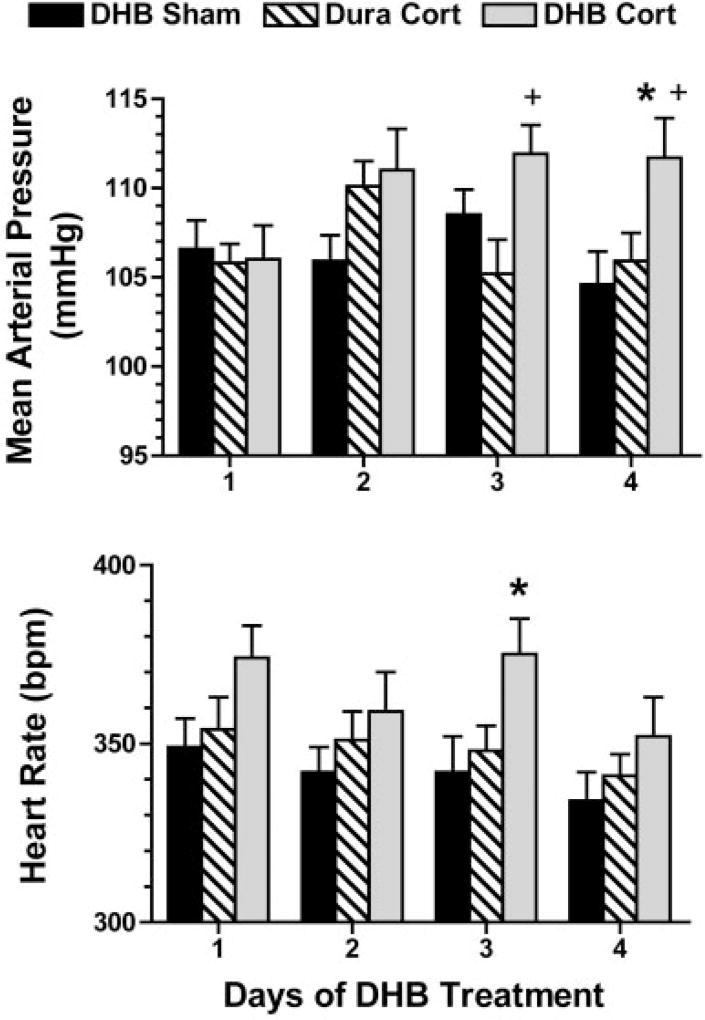
The number of rats per group that were used for baroreflex testing on each day is listed in Table 1. DHB Cort treatment significantly increased baseline mean arterial pressure (112 ± 2 mmHg, P = 0.03) by day 4 of treatment compared with DHB Sham (105 ± 2 mmHg) and Dura Cort (106 ± 2 mmHg) treatments (Fig. 1, top). Mean arterial pressure in DHB Cort rats was also significantly higher relative to Dura Cort rats on day 3 (P = 0.02). There were no significant differences in mean arterial pressure between DHB Sham and Dura Cort rats on any of the 4 days of treatment, indicating the effect of Cort to increase mean arterial pressure on day 4 was specific to the centrally administered Cort and not because of systemic diffusion of the Cort. Baseline heart rate was not different between groups on days 1 (P = 0.11), 2 (P = 0.26), or 4 (P = 0.10). On day 3, baseline heart rate (Fig. 1, bottom) was significantly higher in DHB Cort rats (375 ± 10 beats/min; P = 0.04) compared with DHB Sham (342 ± 10 beats/min) but not Dura Cort (347 ± 7 beats per min) rats.
Table 1.
Average baroreflex parameters for heart rate minimum (P1), range (P4), and maximum (P1 + P4)
| Day 1 | Day 2 | Day 3 | Day 4 | |
|---|---|---|---|---|
| P1: Heart rate range, beats/min | ||||
| DHB sham | 229 ± 13 | 217 ± 15 | 200 ± 19 | 178 ± 16 |
| Dura Cort | 199 ± 11 | 202 ± 14 | 206 ± 9 | 179 ± 9 |
| DHB Cort | 231 ± 15 | 224 ± 15 | 189 ± 13 | 178 ± 10 |
| P value | P = 0.14 | P = 0.58 | P = 0.70 | P = 0.99 |
| P4: Heart rate minimum, beats/min | ||||
| DHB sham | 233 ± 11 | 224 ± 13 | 229 ± 12 | 220 ± 13 |
| Dura Cort | 254 ± 12 | 225 ± 15 | 239 ± 7 | 243 ± 9 |
| DHB Cort | 237 ± 12 | 233 ± 15 | 256 ± 10 | 234 ± 15 |
| P value | P = 0.23 | P = 0.89 | P = 0.15 | P = 0.39 |
| P1 + P4: Heart rate maximum, beats/min | ||||
| DHB sham | 462 ± 12 | 441 ± 14 | 429 ± 17 | 398 ± 10 |
| Dura Cort | 455 ± 14 | 426 ± 10 | 445 ± 11 | 422 ± 10 |
| DHB Cort | 467 ± 12 | 457 ± 14 | 445 ± 16 | 412 ± 11 |
| P value | P = 0.78 | P = 0.31 | P = 0.69 | P = 0.25 |
| No. of animals/group | ||||
| DHB sham | 12 | 14 | 13 | 11 |
| Dura Cort | 13 | 12 | 15 | 11 |
| DHB Cort | 15 | 10 | 12 | 13 |
Fig. 1.
Mean arterial pressure (top) and heart rate (bottom) measured for 4 days in rats treated with dorsal hindbrain (DHB) or Dura pellets. P < 0.05 relative to DHB Sham (*) and relative to Dura Cort (+).
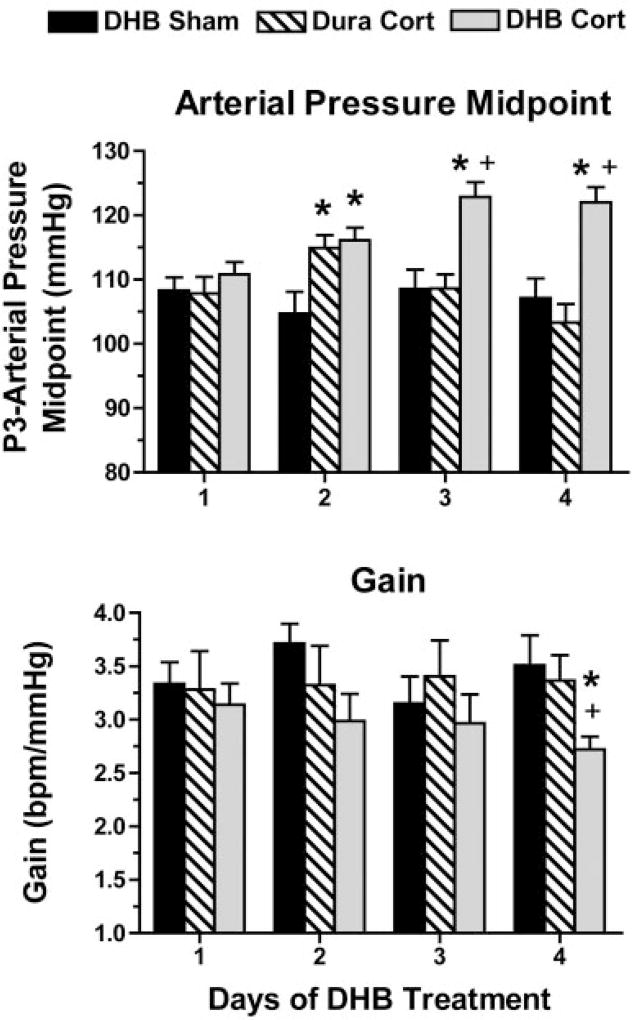
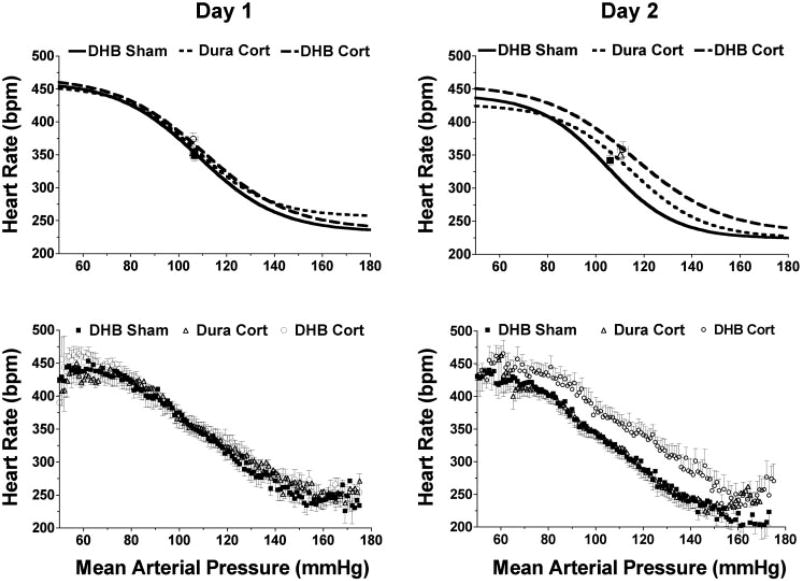
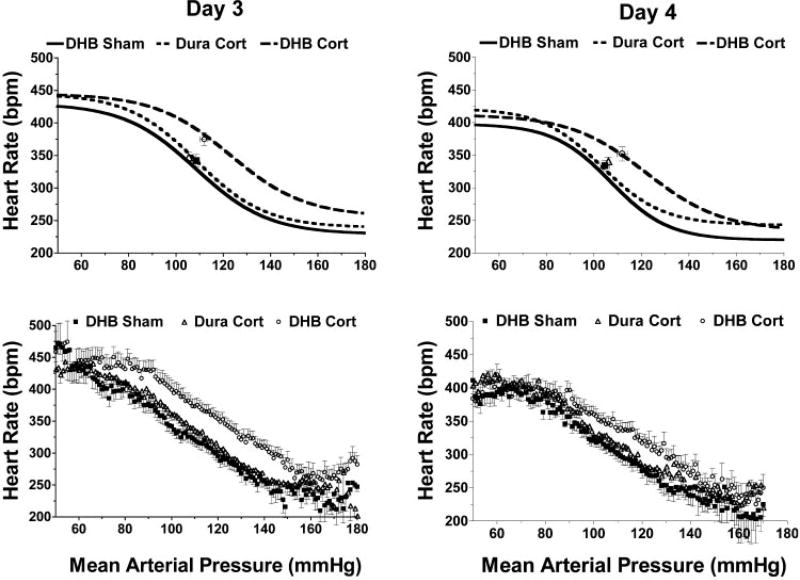
Average parameter values for the arterial pressure midpoint (P3) and maximum gain (calculated from P2) are provided in Fig. 2. Baroreflex function is shown using curves calculated from the average parameter values (Figs. 3 and 4, top) and curves of averaged raw data (Figs. 3 and 4, bottom). Resting values for mean arterial pressure and heart rate are also plotted in Figs. 3 and 4. The mean arterial pressure midpoint was significantly increased in DHB Cort rats starting on day 2 (P = 0.02) relative to DHB Sham rats and was increased on days 3 (P < 0.001) and 4 (P < 0.001) compared with both DHB Sham and Dura Cort rats (Fig. 2, top). Maximum baroreflex gain (Fig. 2, bottom) was significantly lower (P < 0.05) in DHB Cort-treated rats on day 4 relative to DHB Sham- and Dura Cort-treated rats. No significant differences in the other baroreflex parameters (heart rate range, maximum or minimum) were detected between the three groups on the 4 days of treatment (Table 1).
Fig. 2.
Mean arterial pressure midpoint (top) and maximum baroreflex gain (bottom) on each day after DHB or Dura treatment. P < 0.05 relative to DHB Sham (*) and relative to Dura Cort (+).
Fig. 3.
Average baroreflex function curves [calculated curves (top) and averaged raw data (bottom)] for rats on days 1 and 2 after treatment with DHB or Dura pellets. bpm, Beats/min.
Fig. 4.
Average baroreflex function curves [calculated curves (top) and averaged raw data (bottom)] for rats on days 3 and 4 after treatment with DHB or Dura pellets.
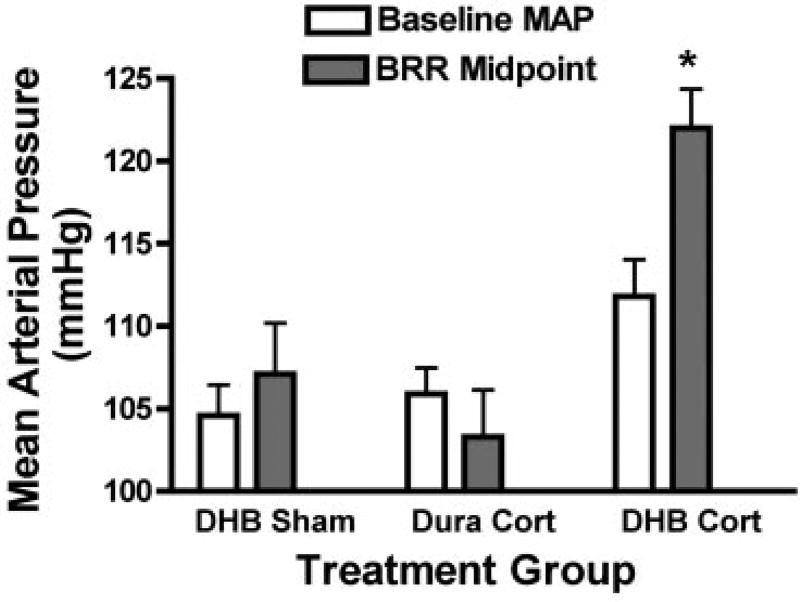
The mean arterial pressure midpoint of the baroreflex function can increase passively as a function of increased arterial pressure (3). However, the magnitude of this passive resetting of the midpoint is normally less than the increase in mean arterial pressure, and does not exceed the increase in mean arterial pressure (11). Figure 5 shows that, by day 4, the mean arterial pressure midpoint was significantly higher than the baseline mean arterial pressure only in the DHB Cort group (P < 0.01). These results, along with the fact that the significant increase in the baroreflex arterial pressure midpoint preceded the increase in baseline arterial pressure in the DHB Cort rats, suggest that DHB Cort produced active resetting of the midpoint that was not solely dependent on the increase in baseline arterial pressure.
Fig. 5.
Comparison of baseline mean arterial pressure (MAP) and baroreceptor reflex (BRR) arterial pressure midpoint for each group on day 4. *P < 0.05 for a within-group difference between the 2 variables.
DISCUSSION
Previous studies from this and other laboratories have demonstrated that systemic glucocorticoid administration can increase the arterial pressure midpoint and decrease the gain of baroreflex control of heart rate and renal sympathetic nerve activity (37, 40, 42–44). However, the sites of action of Cort to modulate baroreflex function have not been determined. Here we report that chronic Cort administration selectively to the DHB increased the baroreflex arterial pressure midpoint by day 3 and decreased the baroreflex gain by day 4 of treatment (Fig. 2). Therefore, we conclude that Cort acts in the DHB to produce changes in baroreflex function similar to changes produced by systemic elevations in Cort.
The present study used a previously validated method to selectively deliver Cort to the DHB (38). In both the present and previous studies, baseline mean arterial pressure increased in DHB Cort rats by day 4 of treatment relative to both DHB Sham and Dura Cort rats (Fig. 1 and Ref. 38). In the present study, baseline heart rate was elevated on day 3 in DHB Cort rats, but only relative to DHB Sham rats, whereas in the previous study we reported an increase in DHB Cort rats relative to both Dura Cort and DHB Sham rats on day 4. We have previously observed a variable effect of Cort on baseline heart rate (37, 40).
In the present study, baseline arterial pressure and baroreflex function values were largely similar between the two control groups (i.e., the DHB Sham and Dura Cort groups) with two exceptions. First, the baroreflex midpoint was higher in the Dura Cort group on day 2 relative to the DHB Sham group (Fig. 2). Second, baseline arterial pressure was higher in the DHB Cort group on day 3 relative to the Dura Cort, but not the DHB Sham group (Fig. 1). Therefore, in the case of the baroreflex arterial pressure midpoint, the Dura Cort effect was transiently similar to the DHB Cort effect. In contrast, baseline arterial pressure values in the Dura Cort group were similar to values in the DHB Sham group but were slightly lower and thus significantly different from the DHB Cort group. The DHB Sham and Dura Cort control groups differ in two key ways. First, the Dura Cort surgery is less invasive since the Dura is not penetrated. It is possible that this could account for the slightly lower arterial pressure in the Dura Cort relative to DHB Sham rats on day 3, but this does not seem likely since this was not the case on day 2. Second, the Dura Cort group is exposed to a small amount of exogenous systemic Cort, whereas the DHB Sham group is not. It is possible that there is an initial transient spike in plasma Cort from the Dura pellet that could account for the increase in the baroreflex midpoint on day 2 in Dura Cort relative to DHB Sham rats. We did not measure plasma Cort on day 2. By the morning of day 4, there were no differences in plasma Cort concentration among the treatment groups, and the effects of Dura Cort and DHB Sham treatments were the same for all measured parameters. Therefore, even if the Dura Cort transiently increased the baroreflex midpoint, the effects of DHB Cort on the baroreflex midpoint observed on days 3 and 4 are because of a specific effect of DHB Cort.
Comparison with studies using central administration of glucocorticoids
Several previous studies have investigated the contribution of centrally acting glucocorticoids to baseline arterial pressure regulation using acute microinjection and chronic infusion of receptor agonists or antagonists in the cerebral ventricals (15, 49, 51, 53, 54). In the brain, Cort binds to two primary receptor subtypes: the low-affinity but selective GR and the mineralocorticoid receptor (MR), which binds both glucocorticoids and mineralocorticoids with high affinity (10). In those studies, intracerebroventricular injection of doses of Cort large enough to act through both MRs and GRs increased baseline arterial pressure (49, 54), whereas lower doses expected to bind primarily to MRs did not (15, 54). In seeming contradiction to those results, infusion or injection of selective GR agonists produced small delayed reductions in arterial pressure, whereas a GR antagonist increased arterial pressure (15, 54). However, those studies measured blood pressure by tail cuff, a method that requires restraining and sometimes anesthetizing the rats. In fact, one group subsequently repeated their study using radiotelemetry and reported that the GR antagonist-induced increase in arterial pressure they previously observed was because of confounding effects of the tail cuff measurement protocol (53). Gomez-Sanchez et al. (15) reported that intracerebrovascular infusion of Cort attenuated the hypertensive effects of intracerebrovascular aldosterone, which binds only to the MR. The authors conclude that Cort can act centrally to antagonize MR-mediated increases in blood pressure. However, the effect of intracerebrovascular Cort to lower arterial pressure could have been because of inhibition of endogenous systemic Cort secretion by activation of central inhibitory pathways, leading to attenuated peripheral vasoconstriction (1, 52). Thus previous studies using intracerebrovascular administration of Cort are generally in agreement with our results in that higher doses of Cort, expected to activate both MRs and GRs, act centrally to produce small increases in arterial pressure. Some differences between our results and those of previous studies could be because of the route of administration of Cort. Although previous studies delivered agonists and antagonists globally to the brain by intracerebrovascular administration, we delivered Cort selectively to the DHB. In our studies, central administration of Cort did not change plasma Cort concentration. Furthermore, Cort may have divergent effects on arterial pressure in different areas of the central nervous system. Thus our results extend previous findings by demonstrating that administration of Cort selectively to the DHB produces a small increase in arterial pressure. None of these previous studies investigated the effects of centrally acting Cort on baroreflex function. We have demonstrated for the first time that central administration of Cort produces changes in baroreflex function similar to those observed with systemic elevations in Cort.
In a previous study, we reported that increases in arterial pressure produced by elevations in systemic Cort are attenuated by selective antagonism of GRs in the DHB (38). We have also reported that systemic administration of the GR antagonist Mifepristone reverses effects of systemic Cort to modulate baroreflex control of heart rate and renal sympathetic nerve activity (37, 40). However, we have not determined if the effects of Cort in the DHB on baroreflex function are mediated by MRs, GRs, or by a combined action of both receptors. An important role for the MR is supported by recent data demonstrating that intracerebrovascular infusion of aldosterone reduced the gain of the baroreflex function in Dahl Salt-sensitive rats (4). Cooperative effects of simultaneous MR and GR occupation on angiotensin receptor expression and feedback inhibition of the hypothalamic pituitary adrenal axis have been described by others and could be important for glucocorticoid effects on central cardiovascular regulation (5, 45). Furthermore, interactions between MRs and GRs on central cardiovascular regulation could explain some of the inconsistencies in the results of previous studies.
Evidence for pressure-independent baroreflex resetting by glucocorticoids
Resetting of the baroreceptor reflex results in a shift of the pressure-nerve activity or -heart rate relationship along the pressure (x) axis, leading to a change in the midpoint of the reflex function curve (3). With pressure-dependent resetting, increases or decreases in arterial pressure are followed by directionally similar changes in the baroreflex midpoint such that resting arterial pressure is maintained near the midpoint, which is the most sensitive portion of the sigmoid function curve (11). In the study by Dorward et al. (11), passive resetting was estimated at 70%, that is, the midpoint increased 7 mmHg for each 10-mmHg increase in mean arterial pressure. Even if passive resetting equaled 100%, the increase in the midpoint would not exceed the increase in mean arterial pressure. With pressure-independent or active resetting, the midpoint changes independently of changes in arterial pressure (6). Several studies in adult rats using systemic Cort administration indicate that glucocorticoids can produce pressure-independent baroreflex resetting (12, 37, 40). First, we reported that the effects of Cort to modulate baroreflex control of renal sympathetic nerve activity could be reversed with administration of the GR antagonist Mifepristone, even when the elevated baseline arterial pressure was maintained by phenylephrine infusion (40). In a second study, we reported that Cort increased the midpoint and reduced the gain of baroreflex control of heart rate even though baseline arterial pressure was not elevated (37). Gardiner and Bennett (12) demonstrated that, in the absence of glucocorticoids, the baroreceptor reflex failed to reset, suggesting that glucocorticoids are required for normal resetting. In the present study, DHB Cort produced an increase in the baroreflex midpoint (Fig. 2, top) by day 3 of treatment before the significant increase in baseline mean arterial pressure observed on day 4 of treatment. We did not measure mean arterial pressure and baroreflex function before and after DHB or Dura treatment in the same rats. Hence, to assess the degree of baroreflex resetting (11), we compared the relationship between mean arterial pressure and baroreflex midpoint for each group on the final day of the study. Mean arterial pressure and baroreflex midpoint were not different in the two control groups, as would be expected with passive adjustment of baroreflex function to prevailing mean arterial pressure (Fig. 5). In contrast, in the DHB Cort group, the midpoint was significantly greater than mean arterial pressure on day 4. The previous and present data, considered together, suggest that the increase in baroreflex midpoint produced by DHB Cort was the result of, in part, pressure-independent baroreflex resetting.
Study limitations
In the present study, we used a validated method to chronically administer Cort to the DHB, a key cardiovascular control area of the brain (30, 31, 38). Limitations of this model have been discussed previously (38). This model results in delivery of Cort to an area of the brain primarily restricted to the structures within the DHB, with very limited diffusion to other brain sites. The structures within the DHB that are important for autonomic regulation and express GRs are the nucleus of the solitary tract and the dorsal motor nucleus of the vagus (7, 28, 48). However, the preganglionic parasympathetic efferents originating from the dorsal motor nucleus primarily affect gastrointestinal and pancreatic function, whereas cardiac vagal preganglionic fibers originate primarily in the more ventral nucleus ambiguous (28). Therefore, the most likely site of action of the DHB Cort to modulate baroreflex control of heart rate is the nucleus of the solitary tract. However, other sites of action of Cort within the DHB cannot be completely excluded with the techniques employed in the present experiments. Moreover, there are presumably other central sites where glucocorticoids can act to regulate arterial pressure and autonomic function.
The exact dose of Cort being delivered to the tissue in the DHB is unknown; however, we have some evidence from our previous study that physiological levels of Cort can act in the DHB to modulate cardiovascular regulation (38). In that study, we treated some rats with systemic Cort to produce an increase in plasma Cort within the physiological range. Systemic Cort increased arterial pressure by ~15 mmHg, and blockade of the DHB GRs with Mifepristone attenuated this increase in pressure. Furthermore, DHB Mifepristone increased peak plasma Cort concentrations, presumably because of attenuation of feedback inhibition of the hypothalamic-pituitary-adrenal axis. Thus blockade of DHB Cort receptors antagonized effects of physiological levels of systemic Cort. Furthermore, DHB Cort had no effect on feedback regulation of endogenous Cort secretion, suggesting that levels in the DHB were within the physiological range. However, only direct measurement of tissue Cort levels will determine the dose of Cort that is delivered to the brain from the DHB pellet.
In the present study, animals were given limited recovery time after surgery before experiments were initiated to examine the time course of DHB Cort effects on baroreflex function. However, baseline plasma Cort, mean arterial pressure, and heart rate were comparable to those we have observed in conscious animals with longer recovery periods (37). Baroreflex parameter values measured in this study were also similar to those we have previously reported in conscious rats with longer recovery periods (37), and there was little variability in the gain and midpoint of the baroreflex function over the course of treatment in DHB Sham rats (Fig. 2). Overall, these results indicate the brief recovery period is unlikely to have affected the outcome and conclusions of the study. The current study was designed to examine initial changes in baroreflex function and arterial pressure. Future studies will be needed to determine long-term effects of DHB Cort on arterial pressure regulation.
Perspectives
Glucocorticoids are elevated in a number of conditions, including chronic stress, and are commonly administered to patients to treat autoimmune and inflammatory conditions (41). Elevated glucocorticoids can produce clinical conditions that increase cardiovascular disease risk, including hypertension, diabetes, obesity, and depression (13, 20, 41, 46, 58, 59). These conditions are also characterized by aberrant regulation of the autonomic nervous system, including altered baroreflex function (2, 14, 16, 36). In the present study, we demonstrated that elevated DHB Cort reduced the gain and increased the midpoint of the baroreceptor reflex. In humans, reduced baroreflex gain is specifically associated with alterations in autonomic balance and increased risk of cardiovascular disease, including cardiac arrhythmias (19, 21, 23), and is an independent predictor of death after myocardial infarction (24). A direct relationship between oral steroid use and increased risk of cardiac arrhythmia has recently been reported in humans (20). In a study of effects of chronic systemic glucocorticoid treatment on myocardial ischemia, Scheuer and Mifflin (39) observed incidences of severe arrhythmia in Cort-treated, but not sham treated, rats during ischemia or reperfusion. Therefore, the effect of DHB glucocorticoids to alter baroreflex gain could contribute to their pathophysiological effects on the cardiovascular system. The effects of DHB Cort to increase the baroreflex midpoint and reduce the reflex gain could also contribute to the development or maintenance of hypertension, although the role of the baroreflex in long-term regulation of arterial pressure is controversial (3, 29, 32, 50). In summary, the results of the present study improve our understanding of the mechanisms that account for the increased cardiovascular disease risk imposed by elevated glucocorticoids and emphasize the importance of understanding the nature and mechanisms of glucocorticoid effects on central control of autonomic function.
Acknowledgments
We thank Kathy Vernon for excellent technical assistance.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-076807.
References
- 1.Akana SF, Scribner KA, Bradbury MJ, Strack AM, Walker CD, Dallman MF. Feedback sensitivity of the rat hypothalamo-pituitary-adrenal axis and its capacity to adjust to exogenous corticosterone. Endocrinology. 1992;131:585–594. doi: 10.1210/endo.131.2.1322275. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez GE, Davy BM, Ballard TP, Beske SD, Davy KP. Weight loss increases cardiovagal baroreflex function in obese young and older men. Am J Physiol Endocrinol Metab. 2005;289:E665–E669. doi: 10.1152/ajpendo.00487.2004. [DOI] [PubMed] [Google Scholar]
- 3.Barrett CJ, Malpas SC. Problems, possibilities, and pitfalls in studying the arterial baroreflexes’ influence over long-term control of blood pressure. Am J Physiol Regul Integr Comp Physiol. 2005;288:R837–R845. doi: 10.1152/ajpregu.00456.2004. [DOI] [PubMed] [Google Scholar]
- 4.Bing SH, Wang HL, Leenen FHH. Chronic central infusion of aldoseterone leads to sympathetic hyperactivity and hypertension in Dahl S but not Dahl R rats. Am J Physiol Heart Circ Physiol. 2005;288:H517–H524. doi: 10.1152/ajpheart.00651.2004. [DOI] [PubMed] [Google Scholar]
- 5.Bradbury MJ, Akana SF, Dallman MF. Roles of type I and II corticosteroid receptors in regulation of basal activity in the hypothalamo-pituitary-adrenal axis during the diurnal trough and peak: evidence for a nonadditive effect of combined receptor occupation. Endocrinology. 1994;134:1286–1296. doi: 10.1210/endo.134.3.8119168. [DOI] [PubMed] [Google Scholar]
- 6.Brooks VL, Ell KR, Wright RM. Pressure-independent resetting produced by chronic infusion of angiotensin II in rabbits. Am J Physiol Heart Circ Physiol. 1993;265:H1275–H1282. doi: 10.1152/ajpheart.1993.265.4.H1275. [DOI] [PubMed] [Google Scholar]
- 7.Cintra A, Zoli M, Rosen L, Agnati F, Okret S, Wikstrom AC, Gustafsson JA, Fuxe K. Mapping and computer assisted morphometry and microdensitometry of glucocorticoid receptor immunoreactive neurons and glial cells in the rat central nervous system. Neuroscience. 1994;62:843–897. doi: 10.1016/0306-4522(94)90481-2. [DOI] [PubMed] [Google Scholar]
- 8.Clapham JC, Turner NC. Effects of the glucocorticoid II receptor antagonist mifepristone on hypertension in the obese Zucker Rat. J Pharmacol Exp Ther. 1997;282:1503–1508. [PubMed] [Google Scholar]
- 9.de Kloet ER, van Acker SA, Sibug RM, Oitzl MS, Meijer OC, Rahmouni K, de Jong W. Brain mineralocorticoid receptors and centrally regulated functions. Kidney Int. 2000;57:1329–1336. doi: 10.1046/j.1523-1755.2000.00971.x. [DOI] [PubMed] [Google Scholar]
- 10.de Kloet RE. Brain corticosteroid receptor balance and homeostatic control. Front Neuroendocrinol. 1991;12:95–164. [Google Scholar]
- 11.Dorward PK, Andresen MC, Burke SL, Oliver JR, Korner PI. Rapid resetting of the aortic baroreceptors in the rabbit and its implication for short-term and longer term reflex control. Circ Res. 1982;50:428–439. doi: 10.1161/01.res.50.3.428. [DOI] [PubMed] [Google Scholar]
- 12.Gardiner SM, Bennett T. Post-adrenalectomy hypotension in rats: absence of baroreflex resetting or effects of naloxone. Clin Sci (Colch) 1983;64:371–376. doi: 10.1042/cs0640371. [DOI] [PubMed] [Google Scholar]
- 13.Girod JP, Brotman DJ. Does altered glucocorticoid homeostasis increase cardiovascular disease risk? Cardiovasc Res. 2004;64:217–226. doi: 10.1016/j.cardiores.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein DS. Arterial baroreflex sensitivity, plasma catecholamines, and pressor responsiveness in essential hypertension. Circulation. 1983;68:234–240. doi: 10.1161/01.cir.68.2.234. [DOI] [PubMed] [Google Scholar]
- 15.Gomez-Sanchez EP, Venkataraman MT, Thwaites D, Fort C. ICV infusion of corticosterone antagonizes ICV-aldosterone hypertension. Am J Physiol Endocrinol Metab. 1990;258:E649–E653. doi: 10.1152/ajpendo.1990.258.4.E649. [DOI] [PubMed] [Google Scholar]
- 16.Grippo AJ, Moffitt JA, Johnson AK. Cardiovascular alterations and autonomic imbalance in an experimental model of depression. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1333–R1341. doi: 10.1152/ajpregu.00614.2001. [DOI] [PubMed] [Google Scholar]
- 17.Harfstrand A, Fuxe K, Cintra A, Agnati LF, Zini I, Wikstrom AC, Okret S, Yu ZY, Goldstein M, Steinbusch H, Verhofstad A, Gustafsson JA. Glucocorticoid receptor immunoreactivity in monoaminergic neurons of rat brain. Proc Natl Acad Sci USA. 1986;83:9779–9783. doi: 10.1073/pnas.83.24.9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hashimoto K, Makino S, Hirasawa R, Takao T, Sugawara M, Murakami K, Ono K, Ota Z. Abnormalities in the hypothalamo-pituitary-adrenal axis in spontaneously hypertensive rats during development of hypertension. Endocrinology. 1989;125:1161–1167. doi: 10.1210/endo-125-3-1161. [DOI] [PubMed] [Google Scholar]
- 19.Hohnloser SH, Klingenheben T, van de Loo A, Hablawetz E, Just H, Schwartz PJ. Reflex versus tonic vagal activity as a prognostic parameter in patients with sustained ventricular tachycardia or ventricular fibrillation. Circulation. 1994;89:1068–1073. doi: 10.1161/01.cir.89.3.1068. [DOI] [PubMed] [Google Scholar]
- 20.Huerta C, Lanes SF, Rodriguez LAG. Respiratory medications and the risk of cardiac arrhythmias. Epidemiology. 2005;16:360–366. doi: 10.1097/01.ede.0000158743.90664.a7. [DOI] [PubMed] [Google Scholar]
- 21.Izzo JI, Black HR. Hypertension Primer. 3. Philadelphia, PA: Lippincott Williams & Wilkens; 2003. [Google Scholar]
- 22.Kent BB, Drane JW, Blumenstein B, Manning JW. A mathematical model to assess changes in the baroreceptor reflex. Cardiology. 1972;57:295–310. doi: 10.1159/000169528. [DOI] [PubMed] [Google Scholar]
- 23.Lanfranchi PA, Somers VK. Arterial baroreflex function and cardiovascular variability: interactions and implications. Am J Physiol Regul Integr Comp Physiol. 2002;283:R815–R826. doi: 10.1152/ajpregu.00051.2002. [DOI] [PubMed] [Google Scholar]
- 24.La Rovere MT, Pinna GD, Hohnloser SH, Marcus FI, Mortara A, Nohara R, Bigger JTJ, Camm AJ, Schwartz PJ. Baroreflex sensitivity and heart rate variability in the identification of patients at risk for life-threatening arrhythmias: implications for clinical trials. Circulation. 2001;103:2072–2076. doi: 10.1161/01.cir.103.16.2072. [DOI] [PubMed] [Google Scholar]
- 25.Lin RCY, Wang XL, Morris BJ. Association of coronary artery disease with glucocorticoid receptor N363S variant. Hypertension. 2003;41:404–407. doi: 10.1161/01.HYP.0000055342.40301.DC. [DOI] [PubMed] [Google Scholar]
- 26.Lin RCY, Wang YSW, Morris BJ. Association and linkage analyses of glucocorticoid receptor gene markers in essential hypertension. Hypertension. 1999;34:1186–1192. doi: 10.1161/01.hyp.34.6.1186. [DOI] [PubMed] [Google Scholar]
- 27.Litchfield WR, Hunt SC, Jeunemaitire X, Fisher ND, Hopkins PN, Williams RR, Corvol P, Williams GH. Increased urinary free cortisol: a potential intermediate phenotype of essential hypertension. Hypertension. 1998;31:569–574. doi: 10.1161/01.hyp.31.2.569. [DOI] [PubMed] [Google Scholar]
- 28.Loewy AD, Spyer KM. Vagal preganglionic neurons. In: Loewy AD, Spyer KM, editors. Central Regulation of Autonomic Function. New York: Oxford Univ; 1990. pp. 68–87. [Google Scholar]
- 29.Lohmeier TE, Hildebrandt DA, Warren S, May PJ, Cunningham JT. Recent insights into the interactions between the baroreflex and kidneys in hypertension. Am J Physiol Regul Integr Comp Physiol. 2005;288:R828–R836. doi: 10.1152/ajpregu.00591.2004. [DOI] [PubMed] [Google Scholar]
- 30.Meyer JS, Micco DJ, Stephenson BS, Krey LC, McEwen BS. Subcutaneous implantation method for chronic glucocorticoid replacement therapy. Physiol Behav. 1979;22:867–870. doi: 10.1016/0031-9384(79)90330-5. [DOI] [PubMed] [Google Scholar]
- 31.Mifflin SW, Felder RB. Synaptic mechanisms regulating cardiovascular afferent inputs to solitary tract nucleus. Am J Physiol Heart Circ Physiol. 1990;259:H653–H661. doi: 10.1152/ajpheart.1990.259.3.H653. [DOI] [PubMed] [Google Scholar]
- 32.Osborn JW, Jacob F, Guzman P. A neural set point for the long-term control of arterial pressure: beyond the arterial baroreceptor reflex. Am J Physiol Regul Integr Comp Physiol. 2005;288:R846–R855. doi: 10.1152/ajpregu.00474.2004. [DOI] [PubMed] [Google Scholar]
- 33.Ottenweller JE, Servatius RJ, Natelson BH. Repeated stress persistently elevates morning, but not evening, plasma corticosterone levels in male rats. Physiol Behav. 1994;55:337–340. doi: 10.1016/0031-9384(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 34.Rosmond R. The glucocorticoid receptor gene and its association to metabolic syndrome. Obesity Res. 2002;10:1078–1086. doi: 10.1038/oby.2002.146. [DOI] [PubMed] [Google Scholar]
- 35.Rosmond R, Chagnon YC, Holm G, Chagnon M, Perusse L, Lindell K, Carlsson B, Bouchard C, Bjorntorp P. A glucocorticoid receptor gene marker is associated with abdominal obesity, leptin and dysregulation of the hypothalamic-pituitary-adrenal axis. Obesity Res. 2000;8:211–218. doi: 10.1038/oby.2000.24. [DOI] [PubMed] [Google Scholar]
- 36.Sanya EO, Brown CM, Dutsch M, Zikeli U, Neundorfer B, Hilz MJ. Impaired cardiovagal and vasomotor responses to baroreceptor stimulation in type II diabetes mellitus. Eur J Clin Invest. 2003;33:582–588. doi: 10.1046/j.1365-2362.2003.01170.x. [DOI] [PubMed] [Google Scholar]
- 37.Scheuer DA, Bechtold AG. Glucocorticoids modulate baroreflex control of heart rate in conscious normotensive rats. Am J Physiol Regul Integr Comp Physiol. 2002;282:R475–R483. doi: 10.1152/ajpregu.00300.2001. [DOI] [PubMed] [Google Scholar]
- 38.Scheuer DA, Bechtold AG, Shank SS, Akana SF. Glucocorticoids act in the dorsal hindbrain to increase arterial pressure. Am J Physiol Heart Circ Physiol. 2004;286:H458–H467. doi: 10.1152/ajpheart.00824.2003. [DOI] [PubMed] [Google Scholar]
- 39.Scheuer DA, Mifflin SW. Chronic corticosterone treatment increases myocardial infarct size in rats with ischemia reperfusion injury. Am J Physiol Regul Integr Comp Physiol. 1997;272:R2017–R2024. doi: 10.1152/ajpregu.1997.272.6.R2017. [DOI] [PubMed] [Google Scholar]
- 40.Scheuer DA, Mifflin SW. Glucocorticoids modulate baroreflex control of renal sympathetic nerve activity. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1440–R1449. doi: 10.1152/ajpregu.2001.280.5.R1440. [DOI] [PubMed] [Google Scholar]
- 41.Schimmer BP, Parker KL. Adrenocorticotropic hormone; adrenocortical steroids and their synthetic analogs; inhibitors of the synthesis and actions of adrenocortical hormones. In: Hardman JG, Limbird LE, Gilman AG, editors. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 10. New York: McGraw-Hill; 2001. pp. 1649–1677. [Google Scholar]
- 42.Segar JL, Bedell KA, Smith OJ. Glucocorticoid modulation of cardiovascular and autonomic function in preterm lambs: role of ANG II. Am J Physiol Regul Integr Comp Physiol. 2001;280:R646–R654. doi: 10.1152/ajpregu.2001.280.3.R646. [DOI] [PubMed] [Google Scholar]
- 43.Segar JL, Lumbers ER, Nuyt AM, Smith OJ, Robillard JE. Effect of antenatal glucocorticoids on sympathetic nerve activity at birth in preterm sheep. Am J Physiol Regul Integr Comp Physiol. 1998;274:R160–R167. doi: 10.1152/ajpregu.1998.274.1.R160. [DOI] [PubMed] [Google Scholar]
- 44.Segar JL, Van Natta T, Smith OJ. Effects of fetal ovine adrenalectomy on sympathetic and baroreflex responses at birth. Am J Physiol Regul Integr Comp Physiol. 2002;283:R460–R467. doi: 10.1152/ajpregu.00056.2002. [DOI] [PubMed] [Google Scholar]
- 45.Shelat SG, King JL, Flanagan-Cato LM, Fluharty SJ. Mineralocorticoids and glucocorticoids cooperatively increase salt intake and angiotensin II receptor binding in rat brain. Neuroendocrinology. 1999;69:339–351. doi: 10.1159/000054436. [DOI] [PubMed] [Google Scholar]
- 46.Sholter DE, Armstrong PW. Adverse effects of corticosteroids on the cardiovascular system. Can J Cardiol. 2000;16:505–511. [PubMed] [Google Scholar]
- 47.Soro A, Ingram MC, Tonolo G, Glorioso N, Fraser R. Mildly raised corticosterone excretion rates in patients with essential hypertension. J Hum Hypertens. 1995;9:391–393. [PubMed] [Google Scholar]
- 48.Sun M-K. Central neural organization and control of sympathetic nervous system in mammals. Prog Neurobiol. 1995;47:157–233. doi: 10.1016/0301-0082(95)00026-8. [DOI] [PubMed] [Google Scholar]
- 49.Takahashi H, Takeda K, Ashizawa H, Inoue A, Yoneda S, Yoshimura M, Ijichi H. Centrally induced cardiovascular and sympathetic responses to hydrocortisone in rats. Am J Physiol Heart Circ Physiol. 1983;245:H1013–H1018. doi: 10.1152/ajpheart.1983.245.6.H1013. [DOI] [PubMed] [Google Scholar]
- 50.Thrasher TN. Baroreceptors, baroreceptor unloading, and the long-term control of blood pressure. Am J Physiol Regul Integr Comp Physiol. 2005;288:R819–R827. doi: 10.1152/ajpregu.00813.2004. [DOI] [PubMed] [Google Scholar]
- 51.Tonolo G, Soro A, Madeddu P, Troffa C, Melis MG, Patteri G, Pinna Parpaglia P, Sabino G, Maioli M, Glorioso N. Effect of chronic intracerebroventricular dexamethasone on blood pressure in normotensive rats. Am J Physiol Endocrinol Metab. 1993;264:E843–E847. doi: 10.1152/ajpendo.1993.264.6.E843. [DOI] [PubMed] [Google Scholar]
- 52.Ullian ME. The role of corticosteroids in the regulation of vascular tone. Cardiovasc Res. 1999;41:55–64. doi: 10.1016/s0008-6363(98)00230-2. [DOI] [PubMed] [Google Scholar]
- 53.van Acker SA, Fluttert MF, Sibug RM, de Kloet ER. Intracerebroventricular administration of a glucocorticoid receptor antagonist enhances the cardiovascular responses to brief restraint. Eur J Pharmacol. 2001;430:87–91. doi: 10.1016/s0014-2999(01)01341-3. [DOI] [PubMed] [Google Scholar]
- 54.van den Berg DTWM, De Kloet ER, van Dijken HH, de Jong W. Differential central effects of mineralocorticoid and glucocorticoid agonists and antagonists on blood pressure. Endocrinology. 1990;126:118–124. doi: 10.1210/endo-126-1-118. [DOI] [PubMed] [Google Scholar]
- 55.Wake DJ, Walker BR. 11 Beta-hydroxysteroid dehydrogenase type 1 in obesity and the metabolic syndrome. Mol Cell Endocrinol. 2004;215:44–54. doi: 10.1016/j.mce.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 56.Walker B, Stewart PM, Shackleton CH, Padfield PL, Edwards CRW. Deficient inactivation of cortisol by 11-beta-hydroxysteroid dehydrogenase in essential hypertension. Clin Endocrinol (Oxf) 1993;39:221–227. doi: 10.1111/j.1365-2265.1993.tb01778.x. [DOI] [PubMed] [Google Scholar]
- 57.Walker BR, Best R, Shackleton CHL, Padfield PL, Edwards CRW. Increased vasoconstrictor sensitivity to glucocorticoids in essential hypertension. Hypertension. 1996;27:190–196. doi: 10.1161/01.hyp.27.2.190. [DOI] [PubMed] [Google Scholar]
- 58.Wei L, MacDonald TM, Walker BR. Taking glucocorticoids by prescription is associated with subsequent cardiovascular disease. Ann Intern Med. 2004;141:764–770. doi: 10.7326/0003-4819-141-10-200411160-00007. [DOI] [PubMed] [Google Scholar]
- 59.Whitworth JA, Mangos GJ, Kelley JJ. Cushing, cortisol and cardiovascular disease. Hypertension. 2000;36:912–916. doi: 10.1161/01.hyp.36.5.912. [DOI] [PubMed] [Google Scholar]
- 60.Yagil Y, Levin M, Krakoff LR. Effect of glucocorticoid deficiency on arterial pressure in conscious spontaneously hypertensive rats. Hypertension. 1989;2:99–104. doi: 10.1093/ajh/2.2.99. [DOI] [PubMed] [Google Scholar]