Abstract
Over 1%–15% of the population worldwide is affected by nephrolithiasis, which remains the most common and costly disease that urologists manage today. Identification of at-risk individuals remains a theoretical and technological challenge. The search for monogenic causes of stone disease has been largely unfruitful and a technological challenge; however, several candidate genes have been implicated in the development of nephrolithiasis. In this review, we will review current data on the genetic inheritance of stone disease, as well as investigate the evolving role of genetic analysis and counseling in the management of nephrolithiasis.
Keywords: Genetics, Hypercalciuria, Nephrolithiasis, Urolithiasis, Calcium sensing receptor, Cystinuria, Medullary sponge kidney, Autosomal dominant polcystic kidney disease, Uric acid nephrolithiasis, Hypercalciuria
1. Introduction
Over 1%–15% of the population worldwide is affected by nephrolithiasis, which remains the most common and costly disease that urologists manage today. Identification of at-risk individuals remains a theoretical and technological challenge, as most patients who develop stone disease and subject to multiple environmental, geographic, and dietary exposures that could confound or increase the risk of developing stone disease. Establishing a genetic background for nephrolithiasis would better allow providers to determine which individuals would require rigorous evaluation and screening, and may potentially alter the future of the management of stone disease. In this review, we will summarize the current data on the genetic inheritance of stone disease based upon urinary metabolic markers, and will also investigate implications of this research for the future management of nephrolithiasis.
2. Genetics of hypercalciuria
Approximately 75% of all stones are calcium-containing stones. Many theories have been discussed regarding which patients may be predisposed to calcium nephrolithiasis, with promising data discovered regarding specific mutations in different populations.
Primary hypercalciuria is a disorder characterized by altered calcium transport in the intestine, kidney, and bone with a 24-h calcium excretion exceeding 0.1 mmol/kg body in a morning urine collection. This phenomenon is typically seen in 40%–50% of adult patients and 10% of pediatric patients with nephrolithiasis. Current epidemiologic studies suggest that approximately 20% of patients with idiopathic hypercalciuria will report some family history of nephrolithiasis [1].
A strong case was previously made for autosomal dominant inheritance as the primary form of inheritance in idiopathic hypercalciuria. In several twin studies, monozygotic twins were shown to have a significantly higher concordance for hypercalciuria than dizygotic twins [2]. Moore et al. [3] had also observed primary hypercalciuria in 43% of all first degree relatives and 36% of all relatives of individuals with hypercalciuria in nine families, suggestive of an autosomal dominant pattern of inheritance, a finding corroborated by several other investigators [3], [4], [5], [6]. Nicolaidou et al. [7] studied 40 children with hypercalciuria and 47.5% had a hypercalciuric first degree relative, 52.5% had sporadic disease.
However, critical epidemiologic analyses have found shortcomings in the theory of monogenic inheritance. In their landmark investigation, Resnick et al. [8] demonstrated that polygenic inheritance would be a more likely explanation. After interviewing 137 stone-forming patients, the author demonstrated that males were far more likely to develop nephrolithiasis; however, upon examining the possibility of X-linked, autosomal dominant, and autosomal recessive inheritance, he was unable to demonstrate a consistent relationship in his observations. He subsequently demonstrated that the risk of developing nephrolithiasis was significantly higher in the younger sibling of a patient diagnosed by calcium oxalate nephrolithiasis, there by establishing a polygenic means of inheritance. Overall, at least two or more gene loci were likely the culprit in all affected patients. Environmental factors, including dietary and geographic exposures, likely also played a role. In a twin study in Vietnam, Goldfarb et al. [9] later demonstrated that 56% of kidney stone susceptibility was attributable to genetic inheritance, while the other 44% could be explained by environmental factors. In several other studies, a codominant major gene model was found to account for heritability in over 50% of patients [10], [11].
Since the advent of these discoveries, several genes have been implicated in the pathogenesis of hypercalciuria. We will review both animal and in vivo studies of these genetic aberrations.
2.1. Calcium sensing receptor
The calcium-sensing receptor (CaSR) is a plasma membrane G-protein coupled receptor found in the apical membrane of the nephron, the parathyroid gland, bone, intestine, and C-cells of the thyroid. Its function in the nephron is complex, and it is found in the proximal convoluted tubule, the thick ascending limb of the loop of Henle, the distal convoluted tubule, and the collecting duct [12]. It is regulated by extracellular calcium levels and controls parathyroid hormone (PTH) secretion and renal tubular calcium reabsorption. While its most commonly reported affiliation is with familial hypocalciuric hypercalcemia, it has also been implicated in heritable diseases of hypercalciuria and hypocalcemia.
Several single nucleotide polymorphisms (SNPs) have been examined in this patient population. One of the most studied polymorphisms involves the presence of two glycine residues at position 990, in place of one alanine seen in non-hypercalciuric patients. Replacement of Gly990 has been affiliated with hypercalciuria [13]. In one large retrospective review, 359 French-Canadian siblings with idiopathic hypercalciuria were found to have three SNPs on chromosome 3q13.3–21: Ala986Ser, Arg990Gly, Gln1011Glu (C-terminal, exon 7) [10]. Patients with primary hyperthyroidism were shown to more commonly express Arg990Gly polymorphisms, leading to higher calcium excretion rates.
Other polymorphisms identified include the Ala986Ser SNP and multiple SNPs within introns 1 and 4 found in the Chinese from Taiwan province and Italian populations, respectively [12].
2.2. Bicarbonate sensitive adenylate cyclase
The human soluble bicarbonate-sensitive adenylate cyclase (sAC) was identified as a highly significant locus involved in three families with hypercalciuria and nephrolithiasis. In a study of three families with absorptive hypercalciuria and nephrolithiasis, they were found to have linkage between a locus on the chromosome 1q23.3–24 and the absorptive hypercalciuria phenotype affiliated with the bicarbonate sensitive adenylate cyclase [14]. Of note, patients with severe hypercalciuria with an sAC mutation are more likely to have fasting hypercalciuria and osteopenia.
2.3. Vitamin D receptor polymorphisms
The vitamin D receptor (VDR) has been extensively investigated as a potential source of resorptive hypercalciuria. Its lithogenic potential has been demonstrated in rat models in which overexpression on intestinal epithelium increased hypercalciuria in stone-forming rats [15]. Most commonly, the BsmI, Fok1, ApaI, and Taq1 polymorphisms are reliably associated with disruptions in vitamin D signaling aberrancies and calcium homeostasis. In the sentinel study on this mutation, six Indian families with hypercalciuric stone forming members, microsatellite markers of mutations on chromosome 12q12-14 were found to be associated with VDR dysregulation [16]. In a meta-analysis of the available data on VDR polymorphisms from multiple small-scale studies in Italy, Turkey, India, and Japan, the Fok1, TaqI, and ApaI gene polymorphisms were associated with a risk of nephrolithiasis in Asian patients, but not in Caucasian patients [15].
2.4. Diacylglycerol kinase
Diacylglycerol kinase (DGKH) is responsible for regulation of transplasmalemmal calcium ion influx through regulation of diacylglycerol (DAG) and the protein kinase C pathway. One SNP, labeled rs4142110, was initially identified in the Japanese population as a potential candidate gene in calcium oxalate nephrolithiasis [17]. In one study from Beijing, China, 507 patients treated for calcium oxalate disease and 500 control subjects underwent 24-h urine collection, which revealed that patients with a DGKH polymorphism had significantly increased hypercalciuria [18]. Certain subtypes were also noted to have hypercalcemia.
2.5. Transient receptor potential vanilloid member protein 5,6
Transient receptor potential vanilloid member protein 5 (TRPV5) is an apical membrane epithelial Ca2+ channel in the distal convoluted tubule. In a knockout mutation mouse model, knockout mice demonstrated severe hypercalciuria, accompanied by increased absorption from gut and bone [19]. In vivo studies in mice and genotyping of humans with hypercalciuria and nephrolithiasis have demonstrated that TRPV6 mutations have a similar effect in humans [20], [21].
3. Other genetic metabolic causes of stone disease
3.1. Hypocitraturia
Citrate is a known inhibitor of stone formation. It directly inhibits spontaneous nucleation of calcium oxalate by combining with calcium ion to form a nondissociable but soluble complex, leading to less free calcium to bind with oxalate [22]. It also hinders calcium oxalate and calcium phosphate crystal growth, agglomeration, and aggregation. Thus, a deficiency of citrate in the renal tubules can lead to calcium stone formation, as hypocitraturia (urinary citrate <320 mg/day) is involved in 20%–60% of calcium nephrolithiasis. Hypocitraturia can occur in distal renal tubular acidosis, in the presence of carbonic anhydrase inhibitors, and in certain genetic deficiencies.
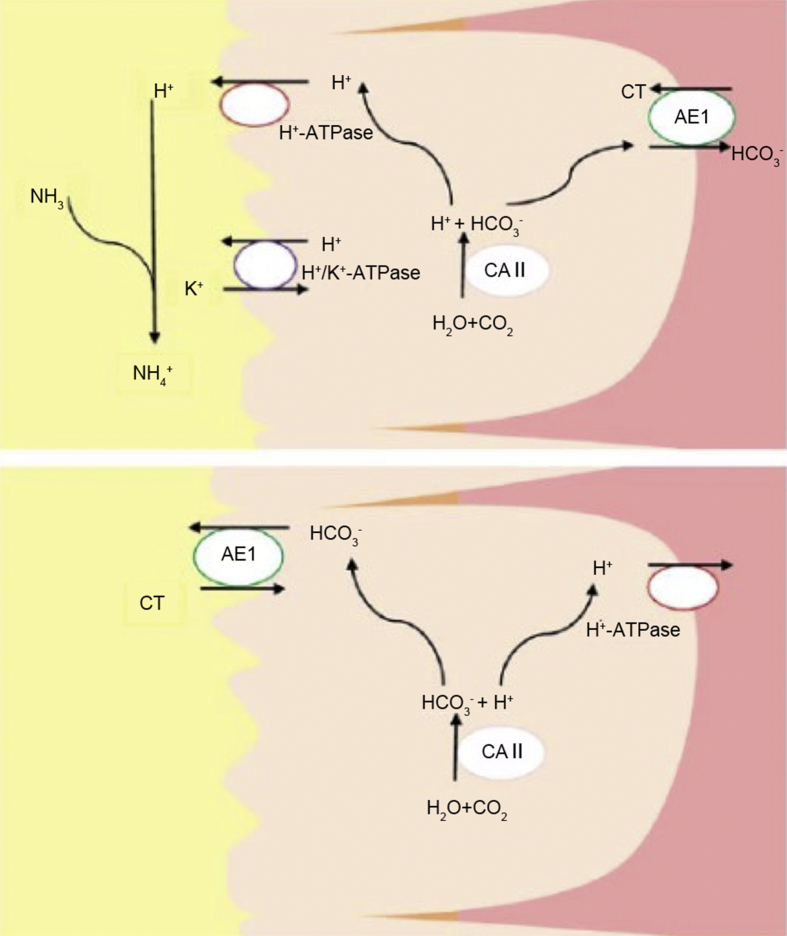
Distal renal tubular acidosis, as the name implies, is a condition in which metabolic acidosis results from impaired hydrogen ion secretion in the distal nephron through several different mechanisms. Chronic metabolic acidosis can lead to increased reabsorption of citrate in the proximal tubules, leading to hypocitraturia [22]. Certain mutations have been identified that can result in inherited forms of distal renal tubular acidosis (RTA). These mutations affect the transporter proteins in the α-intercalated cells in the distal tubule such as the B1 and A4 subunits of H+-ATPase and anion exchanger 1 (AE1), and the cytosolic enzyme carbonic anhydrase II (CAII) (Fig. 1) [23]. The gene mutations in the H+ATPase genes (ATP6V0A4 and ATPV1B1) lead to an inability of the ATP-dependent pump to secrete H+ into the lumen and thus an inability to acidify urine. It leads to an autosomal recessive distal RTA and can be associated with sensorineural hearing impairment. Mutations in the AE1 gene (which is part of the SLC4 family) present in an autosomal dominant fashion and are not generally accompanied with hearing defects. AE1 proteins exchange intracellular bicarbonate ions () for extracellular chloride ions (Cl−). CAII deficiency presents in an autosomal recessive pattern and a mixed proximal-distal RTA, with a slight predominance for distal RTA. Carbonic anhydrase is an enzyme that catalyzes the reversible hydration of CO2 to and H+ in the cytosol in the proximal collecting tubule epithelium. The H+ produced in this mechanism is secreted into the lumen. CAII deficiency thus leads to the inability to produce the H+ and inability to acidify urine. CAII deficiency is associated with osteopetrosis and cerebral calcification.
Figure 1.
Collecting tubule acid-base transporters involved in H+ secretion and -reabsorption by α-intercalated cells (A) and β-intercalated cells (B). AE1, anion exchanger 1; CAⅡ, carbonic anhydrase Ⅱ; CT, collecting tubule.
In distal RTA, the aims of treatment to correct the metabolic acidosis with alkali therapy and prevent of formation kidney stones and nephrocalcinosis, which can ultimately lead to renal failure. A mixture of sodium and potassium salts is advised (sodium bicarbonate and potassium citrate). Adults can generally be treated with 1–2 mmol/kg of sodium bicarbonate, whereas children may require as much as 4–8 mmol/kg/day due to their higher fixed urinary pH and higher bone turnover which generates a higher acid load [24]. Potassium citrate can be used to treat the hypokalemia associated with distal RTA, as well as correcting the hypocitraturia.
Another genetic mutation associated with hypocitraturia has been found in the SLC13 family of genes. SLC13 transporters regulate plasma, urinary, and tissue levels of metabolites of the citric acid cycle (citrate, succinate, α-ketoglutarate) and can be found in different types of organs [25]. They are sodium-coupled transporters that include sodium dicarboxylate 1 (NaDC1), sodium dicarboxylate 3 (NaDC3), and sodium citrate transporter (NaCT). NaDC1 is primarily found on apical membranes of the renal proximal tubules as well intestinal cells, and is involved in regulating urinary citrate. Metabolic acidosis stimulates NaCD1-mediated citrate transport which results in decreased tubular citrate levels. Acidosis also increases the expression of SLC13A2 gene and NaDC1 protein expression, which leads to hypocitraturia. The connection between the genetics of the NaDC1 transporter and kidney stone formation was studied by Okamoto et al. [26] in 2006. A PCR restriction fragment length polymorphism analysis was performed on the I550V polymorphism of NaCD1 in recurrent calcium stone formers and age-matched controls. Subjects with BB (homozygous for digested Bcl-I allele) exhibited lower urinary citrate excretion than subjects with the bb genotype. The authors inferred that the B allele of the I550V polymorphism may contribute to the hypocitraturia in recurrent stone formers. The NaDC3 transporter has a wider tissue distribution, while the NaCT transporter located primarily in the liver and the brain.
3.2. Hypomagnesemia
Familial hypomagnesemia with hypercalciuria and nephrocalcinosis (FHHNC) is a monogenic autosomal recessive syndrome that can be found in some calcium stone forming patients [27]. It results from mutations in genes that encode Claudin proteins. Claudins are major components of tight junctions and regulate free diffusion of solutes in the paracellular pathway. Claudin-16, encoded by CLDN16, is selectively expressed at the tight junctions of renal epithelial cells of the thick ascending limb of the loop of Henle and regulate paracellular excretion of magnesium and calcium [28]. Mutation in CLDN16 can lead to renal Ca2+ and Mg2+ wasting (causing nephrocalcinosis) as well as intracellular retention of these divalent cations [28]. Claudin-19, encoded by CLDN19, has also been implicated with similar clinical findings. Patients with mutations in CLDN19 present with hypomagnesemia, nephrocalcinosis, renal failure, and severe ocular abnormalities as CLDN19 is expressed in the distal renal convoluted tubule as well as the retina [29]. This mutation is associated with near blindness.
The treatment for FHHNC involves magnesium supplementation, and sometimes calcium and vitamin D supplementation. Occasionally, thiazide diuretics may be needed to aid in the reduction of hypercalciuria [27]. Unfortunately, despite treatment, the median age at which patients develop end-stage renal disease (ESRD) is 14.5 years.
3.3. Hyperoxaluria
Primary hyperoxaluria (PH) is an autosomal recessive disorder of glyoxylate metabolism. It is a rare disorder that leads to overproduction of oxalate which gets deposited as calcium oxalate in various organs, especially the kidney [30]. There are three main types of PH, each with a different enzymatic defect.
Type 1 PH accounts for 80% of PH cases [31]. Its incidence is 1 in every 120,000 births worldwide, and is more common in countries such as Kuwait and Tunisia [32]. The enzyme affected is alanine: glyoxylate aminotransferase (AGT), which is found in liver peroxisomes [33]. AGT is encoded by the AGXT gene on chromosome 2p37.3 [34]. At least 146 mutations have been discovered within the AGXT gene, predominantly single-nucleotide substitutions (75%) [35]. Deficiency in AGT leads to increased synthesis and urinary excretion of oxalate, and eventual deposition of insoluble calcium oxalate in the kidneys and urinary tract. In a vicious cycle, deposition of oxalate leads to progressive renal functional impairment (decreased glomerular filtration rate (GFR)), which leads to more deposition of calcium oxalate. The median age of initial symptoms is 5–6 years old, and patients progress to ESRD by the third decade of life [33]. Once GFR falls below 30 mL/min per 1.73 m2, plasma oxalate levels increase. And when plasma oxalate exceeds 30 mmol/L, calcium oxalate becomes deposited into tissues other than the kidney including the retina, myocardium, blood vessels, bone, and skin—a phenomenon called systemic oxalosis [34]. Clinical manifestations include diminished visual acuity, cardiac conduction defects, poor peripheral circulation, bone and joint pain, and peripheral gangrene.
Diagnosis is obtained in a stepwise approach, starting from clinical findings, to metabolic screening, and confirmed with genetic testing. A high suspicion of PH based on clinical findings should trigger metabolic screening including measuring urinary oxalate and glycolate excretion—both of which will be high. The diagnosis of PH type 1 is confirmed by molecular genetic testing demonstrating a mutation of the AGXT gene [35]. Unfortunately, diagnosis is often delayed and a significant number of patients will be diagnosed with PH when they have already progressed to ESRD. If genetic testing is not available, liver biopsy will confirm the diagnosis.
Treatment initially involves aggressive hydration and medical management. Large fluid intake is essential (>3 L/day per 1.73 m2) to decrease tubular oxalate concentration [34]. Although patients are advised to limit oxalate intake, most of the oxalate involved in the disease process is endogenous, so dietary restriction often does not help. Pyridoxal phosphate, an essential cofactor of AGT that converts glyoxylate to glycine, may be effective in a certain subgroup of PH patients [34]. Citrate supplementation helps reduce urinary calcium oxalate saturation by binding to calcium [34]. Ultimately, the most optimal and definitive treatment is combined liver and kidney transplantation for patients with progressive kidney disease [36]. The transplanted liver provides the missing enzyme, thus correcting the rate of oxalate production.
Primary hyperoxaluria type 2 is typically milder than PH type 1 and accounts for about 10% of PH cases. It is caused by deficiencies in the activity of the glyoxylate reductate/hydroxypyruvate reductase (GRHPR) cytosolic enzyme, which results in the increased amounts of glyoxylate and hydroxypyruvate, which are converted to oxalate and l-glyceric acid by lactate dehydrogenase [34]. The GRHPR enzyme, although mostly expressed in the liver, has a much wider tissue distribution than AGT [37]. Type 2 PH is also an autosomal recessive disorder caused by mutations in the GRHPR gene located on chromosome 9p11. Thirty different mutations have been discovered, including deletions, insertions, missense, and nonsense mutations—the most common being a deletion of a single base pair in exon 2 [38].
The clinical manifestations of PH type 2 is similar to that of PH type 1, but stone formation and nephrocalcinosis is less severe as well as better renal function preservation over time. The main difference from PH type 1 is that patients with PH type 2 generally do not develop ESRD [34]. Patients with PH type 2 primarily present with recurrent calcium oxalate urolithiasis. Diagnosis is established with metabolic screening for elevated urinary oxalate as well as elevated urinary l-glycerate (>28 mmol/mol creatinine) [39]. Similar to type 1 disease, definitive diagnosis is made by molecular genetic testing for mutations in the GRHPR gene, or liver biopsy that reveals decreased GRHPR activity. Medical management is similar to that of type 1 PH, except pyridoxine therapy will not reduce oxalate production. Similarly, liver transplantation is not an established treatment method for PH type 2 since the enzymatic defect is not specific to the liver as it is in type 1 [30].
Type 3 PH is also an autosomal recessive disease and accounts for 5% of patients with PH. It is caused by a mutation in the 4-hydroxy-2-oxoglutarrate aldolase (HOGA1) gene on chromosome 10q24.2 that encodes the mitochondrial HOGA1 enzyme [40]. HOGA1 is expressed in the kidney and the liver, and catalyzes the cleavage of 4-hydroxy-2-oxoglutarate (HOG) to pyruvate and glyoxylate. Defects in the HOGA1 enzyme are associated with calcium oxalate stone formation, although in a milder form than type 1 or type 2. Although patients with type 3 PH present at an early age, they do not develop ESRD. Thus, treatment is generally supportive, and patients do not require liver or kidney transplantation or even hemodialysis [34].
3.4. CFEX mutation (SLC26A6)
The chloride-formate exchange transporter, CFEX (or the human analogue SLC2A6) also seems to play a role in oxalate metabolism. In the proximal tubule, chloride is transported through the apical membrane through several transport proteins such as the chloride-formate exchange transporter and the chloride-oxalate exchange transporter. CFEX expression was found not only capable of mediating chloride-formate exchange, but also chloride-oxalate exchange [41]. Studies in CFEX-null mice showed that CFEX plays a role in mediating oxalate-dependent sodium chloride absorption in the proximal tubule. CFEX-null mice developed hyperoxaluria and higher incidence calcium oxalate stones when compared to normal mice [42]. These studies also showed that CFEX regulates oxalate secretion in the small intestine, limiting the net absorption of oxalate. Further studies are needed to elucidate the potential therapeutic role of CFEX in hyperoxaluria.
3.5. Uric acid nephrolithiasis
Uric acid stone formation is primarily due to low urine pH. However, there are some genetic defects that can predispose patients to uric acid urolithiasis. Renal hypouricemia (RHUC) is a rare heterogeneous inherited disorder that is caused by impaired tubular uric acid transport and results in acute kidney injury and kidney stone formation [43]. Two types have been described—with genetic defects in SLC22A12 and SLC2A9, respectively.
In the majority of patients with RHUC, the underlying defect is caused by a mutation in SLC22A12, which encodes the urate-anion exchanger URAT1 in the luminal membrane of the proximal tubular cells [44]. URAT1 is accountable for the majority of urate reabsorption in the proximal tubules. Patients with homozygous mutations for SLC22A12 typically have serum uric acid levels below 10 mg/L and a partial uric acid reabsorption defect.
Renal hypouricemia has also been found in patients with mutations in the SLC2A9 gene, which encodes the urate transporter GLUT9 on the basolateral membrane of the proximal tubular cell. Loss-of-function mutations in GLUT9 can cause severe hypouricemia in homozygous individuals and are associated with kidney stone formation and acute kidney injury [45]. As GLUT9 has been shown to be a regulator of serum uric acid, it has been identified as a potential therapeutic target for gout and cardiovascular disease.
The uric acid urolithiasis in renal hypouricemia is a result of increased urate clearance and increase in the ratio of urate to creatinine clearance. As in other forms of uric acid nephrolithiasis, treatment involves hydration and urine alkalization. Case reports have noted successful treatment of these patients with urine alkalization [46].
4. Heritable diseases affiliated with nephrolithiasis
4.1. Dent's disease
Dent's disease is an X-lined recessive proximal tubule disorder associated with mutations of the proximal tubule chloride-proton exchanger. Phenotypically, these patients are found to have nephrocalcinosis, hypercalciuria, low molecular weight proteinuria, and nephrolithiasis. In some cases, patients may present with a partial Fanconi syndrome or Batter syndrome. Dent's disease 1 is largely related to mutations in CLCN5, a chloride-potassium exchanger, which has been documented in 60% of patients with the disease. The OCRL1 mutation has been found in 15% of patients, encodes a phosphatidylinositol biphosphate 5-phosphatase is associated with an increased risk of extra-renal manifestations including developmental delay, hypotonia and cataracts. Given its significant differences from Dent's disease, this is also known as Dent's disease 2, or Lowe's syndrome. In many cases, treating the hypercalciuria is the most direct approach to prevention of kidney stone disease. Thiazide diuretics and high citrate diets have been investigated in this patient population and have been shown to help preserve renal function and prevent nephrolithiasis [47], [48].
4.2. Lesch–Nyhan disease
The 26–27 region of the X chromosome encodes the HPRT1 gene; approximately 615 cases involving different types of missense mutations, substitutions, non-sense mutations, and nonsense mutations have been recorded. These mutations have been implicated in the development of hyperuricemia leading to Lesch–Nyhan disease, an X-linked recessive disorder in which patients develop several neurobehavioral defects and recurrent uric acid nephrolithiasis. Certain mutations have been associated with less severe phenotypic variants, including Lowe's disease. DNA assays involving administration of radiolabelled hypoxanthine or guanine can detect subtle differences between phenotypically severe Lesch–Nyhan disease from milder phenotypes. Erythrocyte assays analyzing hypoxanthine-guanine phosphoribosyltransferase (HGprt) and phosphoribosyl pyrophosphate (PRPP) with radiolabelled hypoxanthine or guanine may also be used to diagnose Lesch–Nyhan disease and its variants [49]. Current research on which mutations are consistently responsible for the most severe phenotypes is ongoing [50], [51], [52]. Further, separate compensatory mechanisms may allow for continued purine synthesis outside the HGprt pathway and may reduce the severity of the disease [53], [54], [55].
4.3. Cystinuria
Cystinuria, a disease involving mutations of the subunits responsible for amino acid transport in the proximal tubule, remains one of the most challenging stone diseases to treat. Previous classifications focused on the cysteine excretion pattern of the patient's parents; however, Dello Strologo et al. [56] developed a new classification based on the mutations of the affected genes. The molecular basis of cystinuria has been extensively studied and over 200 mutations identified, largely in the SLC3A1 and SLC7A9 genes. These genes encode the two subunits of the amino acid transporter.
Type A cystinuria involves mutations of the SLC3A1 gene, which is found on Chromosome 2 [57]. This encodes the heavy subunit of the amino acid transporter, and inheritance of disease in this patient population is autosomal recessive with 100% penetrance; carriers have normal excretion patterns and normal risk of nephrolithiasis [58]. This remains the most common form of the disease and comprises 45%–64% of the patients with cystinuria [58].
Type B cystinuria is related to mutations of the SLC7A9 gene on Chromosome 19, which encodes the light subunit of the amino acid transporter [59], [60]. Inheritance in this population has been autosomal recessive or autosomal dominant with incomplete penetrance. Carriers have an increased risk of cystinuria and up to 18% increased risk of nephrolithiasis [58].
In rare instances, patients may have mutations in both subunits of the transporter with a later onset of disease manifestations. This subtype has been named cystinuria type AB and is found in 1.2%–4% of patients with cystinuria [59].
The mainstays of treatment in this population revolve around stone prevention, including Thiols, dietary changes, and urinary alkalinization with potassium citrate [57].
4.4. Medullary sponge kidney
Medullary sponge kidney (MSK) is an autosomal dominant inheritable disease characterized by nephrocalcinosis, nephrolithiasis, renal colic, hematuria, urinary tract infection (UTI), hyperparathyroidism, and renal insufficiency. Approximately 3%–5% of patients with nephrolithiasis have MSK, and there are ongoing investigations into the role of the RET gene and glial cell line-derived neurotrophic factor (GDNF) in the development of MSK [61]. Diagnosis is made by contrast imaging. The incidence of stone formation has been estimated to be around 12% [62]. Current theories behind stone formation include urinary stasis, type II renal tubular acidosis (RTA), and hypercalciuria. The incidence of stone disease in this population has been highly variable in the literature. In a retrospective review of 100 patients with MSK and nephrolithiasis, the frequency of hypocitraturia and hyperoxaluria were on par with that of the general population, while no patients carried a diagnosis of RTA. The authors ultimately concluded that the stone forming mechanisms in this population were idiopathic and not related to the underlying genetic abnormality leading to the development of kidney stones [63].
4.5. Autosomal dominant polycystic kidney disease
The incidence of nephrolithiasis in patients with autosomal dominant polycystic kidney disease (ADPKD) approaches 20%–36% [64]. The development of nephrolithiasis is ascribed to corticomedullary architectural disruption related to cyst growth [64]. Increased numbers of cysts and larger cyst sizes are also associated with an increased likelihood of recurrent nephrolithiasis in these populations [65]. The genetic anomalies related to ADPKD are a result of mutations in the PKD1 and PKD2 genes (polycystin–1 and polycystin–2), which lead to a dedifferentiation of tubular epithelium within the nephron. Most frequently, urinalyses in these patients will reveal hypercitraturia, with a lesser incidence of hyperoxaluria, hyperuricosuria, and hypercalciuria. Patients will also often have a lower urinary pH. In one mouse model, the Pkd-1 haploinsufficiency was associated with hyperuricosuria, which may ultimately reveal why this population is found to develop uric acid calculi [66].
5. Non-metabolic diseases
5.1. Osteopontin
A multifunctional glycosylated phosphoprotein has been found to be involved in stimulating deposition and adhesion of crystals in the early stages of calcium oxalate stone formation. It is also expressed in high volume in the distal tubule cells of stone-forming rats [67]. The OPN gene encodes a complex protein involved in inflammation, cell survival, and crystalline interaction. The mechanism for lithogenesis remains unclear. Two SNPs, named novel 2 and novel 3 in the promoter region of OPN are associated with increased risk of nephrolithiasis [68].
5.2. Tamm–Horsfall
Tamm–Horsfall protein is made in the thick ascending limb of the loop of Henle and released to the urine by proteolytic cleavage. It is believed to play a key role in prevention of stone formation. Mutations in Tamm–Horsfall protein may also work synergistically with OPN. The most common SNP is the uromodulin (UMOD) mutation. UMOD knockout mice have been shown to be prone to Escherichia coli UTI and calcium oxalate nephrolithiasis. Overexcretion of Tamm-Horsfall protein (THP) has also been demonstrated in patients with recurrent calcium nephrolithiasis [69].
5.3. Pyrophosphate defects
Inorganic pyrophosphate inhibits crystalline deposition and growth by binding to the surface of calcium phosphate crystals. A mutation in its main transporter, ANKH, leads to hypermineralizing disorders. The most common disorder affiliated with altered ANKH expression is cranio-metaphyseal dysplasia and calcium pyrophosphate dehydrate deposition disease, or CCAL2. Mutations in ANKH may predispose patients to nephrolithiasis in other settings as well [70].
6. Future directions
In an attempt to further characterize general candidate genes for nephrolithiasis, a series was conducted on 206 patients in Thailand to perform whole genome SNP genotyping using DNA microarray. Four candidate genes and one haplotype known as PAQR6 were noted to be significantly more prevalent in the population with nephrolithiasis, however the authors were unable to detect which SNP in each gene was responsible for the increased disease prevalence [71].
Future investigations into identification of other candidate genes will focus on patient's metabolic profiles, urine composition, and analysis of gut microbiomes. Genome wide association studies will also play a role in identifying potential candidate genes, such as the sequencing of NLP1 [72], and will open the door for confirmatory studies in animal and human studies. In the future, genomics will play a crucial role in both the early identification and appropriate treatment of patients with kidney stone disease.
Conflicts of interest
The authors declare no conflict of interest.
Footnotes
Peer review under responsibility of Second Military Medical University.
References
- 1.Curhan G.C., Willet W.C., Rimm E.B., Stamper M.J. Family history and risk of kidney stones. J Am Soc Nephrol. 1997;8:1568–1573. doi: 10.1681/ASN.V8101568. [DOI] [PubMed] [Google Scholar]
- 2.Stechman M.J., Loy N.Y., Thakker R.V. Genetics of hypercalciuric nephrolithiasis: renal stone disease. Ann N Y Acad Sci. 2007;1116:461–484. doi: 10.1196/annals.1402.030. [DOI] [PubMed] [Google Scholar]
- 3.Moore E.S., Coe F.L., McMann B.J., Favus M.J. Idiopathic hypercalciuria in children: prevalence and metabolic characteristics. J Ped. 1978;92:906–910. doi: 10.1016/s0022-3476(78)80358-8. [DOI] [PubMed] [Google Scholar]
- 4.Lerolle N., Lantz B., Paillard F., Gattegno B., Flahault A., Ronco P. Risk factors for nephrolithiasis in patients with familial idiopathic hypercalciuria. Am J Med. 2002;113:99–103. doi: 10.1016/s0002-9343(02)01152-x. [DOI] [PubMed] [Google Scholar]
- 5.Harangi F., Mehes K. Family investigations in indiopathic hypercalciuria. Eur J Pediatr. 1993;152:64–68. doi: 10.1007/BF02072519. [DOI] [PubMed] [Google Scholar]
- 6.Mehes K., Szelid Z. Autosomal dominant inheritance of hypercalciuria. Eur J Pediatr. 1980;133:239–242. doi: 10.1007/BF00496083. [DOI] [PubMed] [Google Scholar]
- 7.Nicolaidou P., Themeli S., Karpathios T., Georgouli H., Athanassaki K., Xaidara A. Family pattern of idiopathic hypercalciuria and its subtypes. J Urol. 1996;155:1042–1044. [PubMed] [Google Scholar]
- 8.Resnick M., Pridgen D.B., Goodman H.O. Genetic predisposition to formation of calcium oxalate renal calculi. N Engl J Med. 1968;278:1313–1318. doi: 10.1056/NEJM196806132782403. [DOI] [PubMed] [Google Scholar]
- 9.Goldfarb D.S., Fischer M.E., Keich Y., Goldberg J. A twin study of genetic and dietary influences on nephrolithiasis: a report from the Vietnam Era Twin (VET) Registry. Kidney Int. 2005;67:1053–1061. doi: 10.1111/j.1523-1755.2005.00170.x. [DOI] [PubMed] [Google Scholar]
- 10.Loredo-Osti J.C., Roslin N.M., Tessier J., Fujiwara T.M., Morgan K., Bonnardeaux A. Segregation of urine calcium excretion in families ascertained for nephrolithiasis: evidence for a major gene. Kidney Int. 2005;68:966–971. doi: 10.1111/j.1523-1755.2005.00490.x. [DOI] [PubMed] [Google Scholar]
- 11.Hunter D., De Lange M., Snieder H., MacGregor A.J., Swaminathan R., Thakker R.V. Genetic contribution to bone metabolism, calcium excretion, and vitamin D and parathyroid hormone regulation. J Bone Min Res. 2001;16:371–378. doi: 10.1359/jbmr.2001.16.2.371. [DOI] [PubMed] [Google Scholar]
- 12.Berisoglu H., Suleyman S., Alper O., Emin O. Calcium-sensing receptor gene polymorphisms in patients with calcium urolithiasis: a systematic review. Ren Fail. 2014;36:1187–1192. doi: 10.3109/0886022X.2014.937673. [DOI] [PubMed] [Google Scholar]
- 13.Vezzoli G., Terranegra A., Arcidiacono T., Biasion R., Coviello D., Syren M.L. R990G polymorphism of calcium-sensing receptor does produce a gain-of-function and predispose to primary hypercalciuria. Kidney Int. 2007;71:1155–1162. doi: 10.1038/sj.ki.5002156. [DOI] [PubMed] [Google Scholar]
- 14.Reed B.Y., Heller H.J., Gitomer W.L., Pak C.Y. Mapping a gene defect in absorptive hypercalciuria to chromosome 1q23.3-q24. J Clin Endorcinol Metab. 1999;84:3907–3913. doi: 10.1210/jcem.84.11.6155. [DOI] [PubMed] [Google Scholar]
- 15.Zhou T.B., Jiang Z.P., Li A.H., Jul L. Association of vitamin D receptor Bsml (rs1544410), Fok1 (rs2228570), TaqI (rs731236), and Apal (rs 7975232) gene polymorphism with the nephrolithiasis susceptibility. J Recept Signal Transduct Res. 2015;35:107–114. doi: 10.3109/10799893.2014.936459. [DOI] [PubMed] [Google Scholar]
- 16.Mittal R.D., Mishra D.K., Srivastava P., Manchanda P., Bid H.K., Kappor R. Polymorphisms in the vitamin D receptor and the androgen receptor gene associated with the risk of urolithiasis. Indian J Clin Biochem. 2010;25:119–126. doi: 10.1007/s12291-010-0023-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Urabe Y., Tanikawa C., Takahashi A., Okada Y., Morizono T., Tsunoda T. A genome-wide association study of nephrolithiasis in the Japanese population identifies novel susceptible Loci at 5q35.3, 7p14.3, and 13q14.1. PLoS Genet. 2012;8:e1002541. doi: 10.1371/journal.pgen.1002541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu Y., Zeng G., Mai Z., Ou L. Association study of DGKH gene polymorphisms with calcium oxalate stone in Chinese population. Urolithiasis. 2014;42:379–385. doi: 10.1007/s00240-014-0692-x. [DOI] [PubMed] [Google Scholar]
- 19.Loh N.Y., Bentley L., Dimke H., Verkaart S., Tammaro P., Gorvin C.M. Autosomal dominant hypercalciuria in a mouse model due to a mutation of the epithelial calcium channel, TRPV5. PLoS One. 2013;8:e55412. doi: 10.1371/journal.pone.0055412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki Y., Pasch A., Bonny O., Mohaupt M.G., Hediger M.A., Frey F.J. Gain-of-function haplotype in the epithelial calcium channel TRPV6 is a risk factor for renal calcium stone formation. Hum Mol Genet. 2008;17:1613–1618. doi: 10.1093/hmg/ddn048. [DOI] [PubMed] [Google Scholar]
- 21.Bianco S.D., Peng J.B., Takanaga H., Suzuki Y., Crescenzi A., Kos C.H. Marked disturbance of calcium homeostasis in mice with targeted disruption of the Trpv6 calcium channel gene. J Bone Min Res. 2007;22:274–285. doi: 10.1359/jbmr.061110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakhaee K., Maalouf N.M., Sinnott B. Clinical review. Kidney stones 2012: pathogenesis, diagnosis, and management. J Clin Endocrinol Metab. 2012;97:1847–1860. doi: 10.1210/jc.2011-3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Batlle D., Haque S.K. Genetic causes and mechanisms of distal renal tubular acidosis. Nephrol Dial Transpl. 2012;27:3691–3704. doi: 10.1093/ndt/gfs442. [DOI] [PubMed] [Google Scholar]
- 24.Soriano J.R. Renal tubular acidosis: the clinical entity. J Am Soc Nephrol. 2002;13:2160–2170. doi: 10.1097/01.asn.0000023430.92674.e5. [DOI] [PubMed] [Google Scholar]
- 25.Pajor A.M. Sodium-coupled dicarboxylate and citrate transporters from the SLC13 family. Pflugers Arch. 2014;466:119–130. doi: 10.1007/s00424-013-1369-y. [DOI] [PubMed] [Google Scholar]
- 26.Okamoto N., Aruga S., Matsuzaki S., Takahashi S., Matsushita K., Kitamura T. Associations between renal sodium-citrate cotransporter (hNaDC-1) gene polymorphism and urinary citrate excretion in recurrent renal calcium stone formers and normal controls. Int J Urol. 2007;14:344–349. doi: 10.1111/j.1442-2042.2007.01554.x. [DOI] [PubMed] [Google Scholar]
- 27.Seeley H.H., Loomba-Albrecht L.A., Nagel M., Butani L., Bremer A.A. Familial hypomagnesemia with hypercalciuria and nephrocalcinosis in three siblings having the same genetic lesion but different clinical presentations. World J Pediatr. 2012;8:177–180. doi: 10.1007/s12519-011-0295-3. [DOI] [PubMed] [Google Scholar]
- 28.Kausalya P.J., Amasheh S., Günzel D., Wurps H., Müller D., Fromm M. Disease-associated mutations affect intracellular traffic and paracellular Mg2+ transport function of Claudin-16. J Clin Investig. 2006;116:878–891. doi: 10.1172/JCI26323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Konrad M., Schaller A., Seelow D., Pandey A.V., Waldegger S., Lesslauer A. Mutations in the tight-junction gene claudin 19(CLDN19) are associated with renal magnesium wasting, renal failure, and severe ocular involvement. Am J Hum Genet. 2006;79:949–957. doi: 10.1086/508617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoppe B. An update on primary hyperoxaluria. Nat Rev Nephrol. 2012;8:467–475. doi: 10.1038/nrneph.2012.113. [DOI] [PubMed] [Google Scholar]
- 31.Hoppe B., Langman C.B. A United States survey on diagnosis, treatment, and outcome of primary hyperoxaluria. Pediatr Nephrol. 2003;18:986–991. doi: 10.1007/s00467-003-1234-x. [DOI] [PubMed] [Google Scholar]
- 32.Cochat P., Deloraine A., Rotily M., Olive F., Liponski I., Deries N. Epidemiology of primary hyperoxaluria type 1. Société de Néphrologie and the Société de Néphrologie Pédiatrique. Nephrol Dial Transpl. 1995;10(Suppl. 8):3–7. doi: 10.1093/ndt/10.supp8.3. [DOI] [PubMed] [Google Scholar]
- 33.Chevalier-Porst F., Rolland M.O., Cochat P., Bozon D. Maternal isodisomy of the telomeric end of chromosome 2 is responsible for a case of primary hyperoxaluria type 1. Am J Med Genet A. 2005;132A:80–83. doi: 10.1002/ajmg.a.30375. [DOI] [PubMed] [Google Scholar]
- 34.Hoppe B., Beck B.B., Milliner D.S. The primary hyperoxalurias. Kidney Int. 2009;75:1264–1271. doi: 10.1038/ki.2009.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams E.L., Acquaviva C., Amoroso A., Chevalier F., Coulter-Mackie M., Monico C.G. Primary hyperoxaluria type 1: update and additional mutation analysis of the AGXT gene. Hum Mutat. 2009;30:910–917. doi: 10.1002/humu.21021. [DOI] [PubMed] [Google Scholar]
- 36.Bergstralh E.J., Monico C.G., Lieske J.C., Herges R.M., Langman C.B., Hoppe B. IPHR Investigators. Transplantation outcomes in primary hyperoxaluria. Am J Transpl. 2010;10:2493–2501. doi: 10.1111/j.1600-6143.2010.03271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cregeen D.P., Williams E.L., Hulton S., Rumsby G. Molecular analysis of the glyoxylate reductase (GRHPR) gene and description of mutations underlying primary hyperoxaluria type 2. Hum Mutat. 2003;22:497. doi: 10.1002/humu.9200. [DOI] [PubMed] [Google Scholar]
- 38.Cochat P., Rumsby G. Primary hyperoxaluria. N Engl J Med. 2013;369:649–658. doi: 10.1056/NEJMra1301564. [DOI] [PubMed] [Google Scholar]
- 39.Chlebeck P.T., Milliner D.S., Smith L.H. Long-term prognosis in primary hyperoxaluria type II (L-glyceric aciduria) Am J Kidney Dis. 1994;23:255–259. doi: 10.1016/s0272-6386(12)80981-4. [DOI] [PubMed] [Google Scholar]
- 40.Williams E.L., Bockenhauer D., van't Hoff W.G., Johri N., Laing C., Sinha M.D. The enzyme 4-hydroxy-2-oxoglutarate aldolase is deficientin primary hyperoxaluria type 3. Nephrol Dial Transpl. 2012;27:3191–3195. doi: 10.1093/ndt/gfs039. [DOI] [PubMed] [Google Scholar]
- 41.Jiang Z., Grichtchenko, Boron W.F., Aronson P.S. Specificity of anion exchange mediated by mouse Slc26a6. J Biol Chem. 2002;277:33963–33967. doi: 10.1074/jbc.M202660200. [DOI] [PubMed] [Google Scholar]
- 42.Aronson P.S. Essential roles of CFEX-mediated Cl−-oxalate exchange in proximal tubule NaCl transport and prevention of urolithiasis. Kidney Int. 2006;70:1207–1213. doi: 10.1038/sj.ki.5001741. [DOI] [PubMed] [Google Scholar]
- 43.Mancikova A., Krylov V., Hurba O., Sebesta I., Nakamura M., Ichida K. Functional analysis of novel allelic variants in URAT1 and GLUT9 causing renal hypouricemia type 1 and 2. Clin Exp Nephrol. 2016;20:578–584. doi: 10.1007/s10157-015-1186-z. [DOI] [PubMed] [Google Scholar]
- 44.Ichida K., Hosoyamada M., Hisatome I., Enomoto A., Hikita M., Endou H. Clinical and molecular analysis of patients with renal hypouricemia in Japan-influence of URAT1 gene on urinary urate excretion. J Am Soc Nephrol. 2004;15:164–173. doi: 10.1097/01.asn.0000105320.04395.d0. [DOI] [PubMed] [Google Scholar]
- 45.Matsuo H., Chiba T., Nagamori S., Nakayama A., Domoto H., Phetdee K. Mutations in glucose transporter 9 gene SLC2A9 cause renal hypouricemia. Am J Hum Genet. 2008;83:744–751. doi: 10.1016/j.ajhg.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hisatome I., Tanaka Y., Kotake H., Kosaka H., Hirata N., Fujimoto Y. Renal hypouricemia due to enhanced tubularsecretion of urate associated with urolithiasis: successful treatment of urolithiasis by alkalization of urine K+, Na+-citrate. Nephron. 1993;65:578–582. doi: 10.1159/000187567. [DOI] [PubMed] [Google Scholar]
- 47.Claviere-Martin F., Ramos-Trujillo E., Garcia-Nieto V. Dent's disease: clinical features and molecular basis. Pediatr Nephrol. 2011;26:693–704. doi: 10.1007/s00467-010-1657-0. [DOI] [PubMed] [Google Scholar]
- 48.Cebotaru V., Kaul S., Devuyst O., Cai H., Racusen L., Guggino W.B. High citrate diet delays progression of renal insufficiency in the CIC-5 knockout mouse model of Dent's disease. Kidney Int. 2005;68:642–652. doi: 10.1111/j.1523-1755.2005.00442.x. [DOI] [PubMed] [Google Scholar]
- 49.Fairbanks L.D., Simmonds H.A., Webster D.R. Use of Intact erythrocytes in the diagnosis of inherited purine and pyrimidine disorders. J Inherit Metab Dis. 1987;10:174–186. doi: 10.1007/BF01800045. [DOI] [PubMed] [Google Scholar]
- 50.Nguyen K.V., Naviaux R.K., Paik K.K., Nyhan W.L. Lesch-Nyhan syndrome: mRNA expression of HPRT in patients with enzyme proven deficiency of HPRT and normal HPRT coding region of the DNA. Mol Genet Metab. 2012;106:498–501. doi: 10.1016/j.ymgme.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 51.Sampat R., Fu R., Larovere L.E., Torres R.J., Ceballos-Picot I., Fischbach M. Mechanisms for phenotypic variation in Lesch-Nyhan disease and its variants. Hum Genet. 2011;129:71–78. doi: 10.1007/s00439-010-0901-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O'Neill J.P., Finette B.A. Transition mutations at CpG dinucleotides are the most frequent in vivo spontaneous single-based substitution mutation in the human HPRT gene. Environ Mol Mutagen. 1998;32:188–191. doi: 10.1002/(sici)1098-2280(1998)32:2<188::aid-em16>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 53.Nyhan W.L. A disorder of uric acid metabolism and cerebral function in childhood. Arthritis Rheum. 1965;8:659–664. doi: 10.1002/art.1780080425. [DOI] [PubMed] [Google Scholar]
- 54.Rosenbloom F.M. Possible mechanism for increased purine biosynthesis de novo in Lesch Nyhan syndrome. Fed Proc. 1968;27:1063–1066. [PubMed] [Google Scholar]
- 55.Sorensen L.B. Mechanism of excessive purine biosynthesis in hypoxanthine-guanine phosphoribosyltransferase deficiency. J Clin Investig. 1970;49:968–978. doi: 10.1172/JCI106316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dello Strologo E., Pras E., Pontesilli C., Beccia E., Ricci-Barbini V., de Sanctis L. Comparison between SLC3A1 and SLC7A9 cystinuria patients and carriers: a need for a new classification. J Am Soc Nephrol. 2002;13:2547–2553. doi: 10.1097/01.asn.0000029586.17680.e5. [DOI] [PubMed] [Google Scholar]
- 57.Adreassen K.H., Pedersen K.V., Osther S.S., Jung H.U., Lildal S.K., Osther P.J. How should patients with cysteine stone disease be evaluated and treated in the twenty-first century? Urolithiasis. 2016;44:65–76. doi: 10.1007/s00240-015-0841-x. [DOI] [PubMed] [Google Scholar]
- 58.Eggermann T., Zerres K., Nunes V., Font-Llitjós M., Bisceglia L., Chatzikyriakidou A. Clinical utility gene card for: Cystinuria. Eur J Hum Genet. 2012;20 doi: 10.1038/ejhg.2011.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wong K.A., Mein R., Wass M., Flinter F., Pardy C., Bultitude M. The genetic diversity of cystinuria in a UK population of patients. BJU Int. 2015;116:109–116. doi: 10.1111/bju.12894. [DOI] [PubMed] [Google Scholar]
- 60.Barbosa M., Lopes A., Mota C., Martins E., Oliveira J., Alves S. Clinical, biochemical and molecular characterization of cystinuria in a cohort of 12 patients. Clin Genet. 2012;81:47–55. doi: 10.1111/j.1399-0004.2011.01638.x. [DOI] [PubMed] [Google Scholar]
- 61.Torregrossa R., Anglani F., Fabris A., Gozzini A., Tanini A., Del Prete D. Classification of GDNF gene sequence variations in patients with medullary sponge kidney disease. Am Soc Nephrol. 2010;5:1205–1210. doi: 10.2215/CJN.07551009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ginalski J.M., Portmann L., Jaeger P. Does medullary sponge kidney cause nephrolithiasis? AJUR Am J Roentgenol. 1990;155:299–302. doi: 10.2214/ajr.155.2.2115256. [DOI] [PubMed] [Google Scholar]
- 63.McPhail E.F., Gettman M.T., Patterson D.E., Rangel L.J., Krambeck A.E. Nephrolithiasis in medullary sponge kidney: evaluation of clinical and metabolic features. Urology. 2012;79:277–281. doi: 10.1016/j.urology.2011.07.1414. [DOI] [PubMed] [Google Scholar]
- 64.Torres V.E., Bengal R.J., Nickander K.K., Grande J.P., Low P.A. Renal concentration of alpha tocopherol: dependence on gender and lack of effect on polycystic kidney disease in Han: SPRD rats. Am J Kidney Dis. 1998;31:687–693. doi: 10.1053/ajkd.1998.v31.pm9531187. [DOI] [PubMed] [Google Scholar]
- 65.Grampas S.A., Chandhoke P.S., Fan J., Glass M.A., Townsend R., Johnson A.M. Anatomic and metabolic risk factors for nephrolithiasis in patients with autosomal dominant polycystic kidney disease. Am J Kidney Dis. 2000;36:53–57. doi: 10.1053/ajkd.2000.8266. [DOI] [PubMed] [Google Scholar]
- 66.Ferraz R.R., Fonseca J.M., Germino G.G., Onuchic L.F., Heilberg I.P. Determination of urinary lithogenic parameters in murine models orthologous to autosomal dominant polycystic kidney disease. Urolithiasis. 2014;42:301–307. doi: 10.1007/s00240-014-0664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Khan S.R., Johnson J.M., Peck A.B., Cornelius J.G., Glenton P.A. Expression of osteopontin in rat kidneys: induction during ethylene glycol induced calcium oxalate nephrolithiasis. J Urol. 2002;168:1173–1181. doi: 10.1016/S0022-5347(05)64621-6. [DOI] [PubMed] [Google Scholar]
- 68.Gao B., Yasui T., Itoh Y., Li Z., Okada A., Tozawa K. Association of osteopontin gene haplotypes with nephrolithiasis. Kidney Int. 2007;72:592–598. doi: 10.1038/sj.ki.5002345. [DOI] [PubMed] [Google Scholar]
- 69.Devyust O., Pirson Y. Genetics of hypercalciuric stone forming diseases. Kidney Int. 2007;72:1065–1072. doi: 10.1038/sj.ki.5002441. [DOI] [PubMed] [Google Scholar]
- 70.Carr G., Sayer J.A., Simmons N.L. Expression and localization of the pyrophosphate transporter, ANK, in murine kidney cells. Cell Physiol Biochem. 2007;20:507–516. doi: 10.1159/000107534. [DOI] [PubMed] [Google Scholar]
- 71.Rungroi N., Nettuwakul C., Sudtachat N., Praditsap O., Sawasdee N., Sritippayawan S. A whole genome SNP genotyping by DNA microarray and candidate gene association study for kidney stone disease. BMC Med Genet. 2014;15:50. doi: 10.1186/1471-2350-15-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wolf M.T., Zalewski I., Martin F.C., Ruf R., Müller D., Hennies H.C. Mapping a new suggestive gene locus for autosomal dominant nephrolithiasis to chromosome 9q33.2-q34.2 by total genome search for linkage. Nephrol Dial Transpl. 2005;20:909–914. doi: 10.1093/ndt/gfh754. [DOI] [PubMed] [Google Scholar]