Abstract
Objective
Treatment options for metastatic castration resistant prostate cancer (mCRPC) have expanded rapidly in recent years. Given the significant economic burden, we sought perform a cost-effectiveness analysis (CEA) of the contemporary treatment paradigm for mCRPC.
Methods
We devised a treatment protocol consisting of sipuleucel-T, enzalutamide, abiraterone, docetaxel, radium-223, and cabazitaxel. We estimated number and length of treatments for each therapy using dosing schedules or progression free survival data from published clinical trials. We estimated treatment cost using billing data and Medicare reimbursement values and performed a CEA. Our analysis assumed US$100,000 per life year saved (LYS) as the threshold societal willingness to pay.
Results
Incremental cost-effectiveness ratios (ICER) for strategies incorporating sipuleucel-T that were not eliminated by extended dominance exceeded the societal threshold willingness-to-pay of US$100,000 per LYS, the lowest of which was sipuleucel-T + enzalutamide + abiraterone + docetaxel at US$207,714 per LYS. Enzalutamide + abiraterone + docetaxel exhibited the most favorable ICER among strategies without sipuleucel-T at US$165,460 per LYS.
Conclusion
Based on the available survival data and current costs of treatment, all treatment strategies greatly exceed a commonly assumed societal willingness-to-pay threshold of US$100,000 per LYS. Improvements in this regard can only come with a reduction in pricing, better tailoring of treatment or significant enhancements in survival with clinical use of treatment combinations or sequences.
Keywords: Metastatic prostate cancer, Costs and cost analysis, Health expenditures, Economics, Pharmaceutical
1. Introduction
Prostate cancer is the most common cancer in males in the United States and the second leading cause of cancer death among men, with 180,890 estimated new cases in 2016 and 26,120 deaths [1]. Of men diagnosed with prostate cancer, between 10% and 20% will develop metastatic castration resistant prostate cancer (mCRPC) within 5 years of diagnosis after receiving hormone ablation therapy for metastatic disease at diagnosis or disease recurrence [2]. The first chemotherapeutic agent to show a significant survival benefit for mCRPC, docetaxel, was approved in 2004 and remained the only established treatment with a survival benefit until 2010 [3]. Over the few 2 years, four new therapeutic agents have been introduced that demonstrated survival advantages in this disease setting: sipuleucel-T, cabazitaxel, abiraterone, enzalutamide, and radium-223 [4], [5], [6], [7], [8]. Each agent has been shown to have a median survival benefit between 2 and 4 months compared with control [2], [9]. In addition to treatments intended to increase overall survival, mCRPC patients typically receive androgen deprivation therapy beyond disease progression, given that mCRPC remains driven by androgen receptor signaling and historical data suggesting better outcomes with continued androgen deprivation. These patients also receive bisphosphonates or denosumab, a human monoclonal RANK-L antibody, to reduce skeletal-related events as a result of bone metastases [10], [11].
While these medications have changed treatment patterns for patients with mCRPC, increased focus on healthcare expenditures in the United States has brought the associated cost for new pharmacologic interventions for mCRPC under scrutiny [12], [13]. In 2006, US$9.9 billion was spent on prostate cancer care in the US alone [14]. It is estimated that the amount spent in 2010 was US$11.85 billion and is expected to increase to US$15.41 billion by the year 2020 [15]. Given the high cost of treating prostate cancer and the limited cost data in the setting of newly-introduced treatments for mCRPC, we sought to calculate the estimated cost of the new treatment paradigm for mCRPC and perform a cost-effectiveness analysis (CEA) using published survival data.
2. Methods
2.1. Cost and survival estimates for the analysis
Using the 2013 American Urological Association (AUA) Guidelines for mCRPC and a previously published paradigm for the treatment of mCRPC we chose a treatment scheme for a model patient with mCRPC [16], [17]. We defined mCRPC as disease progression, a rising prostate specific antigen (PSA) while on androgen ablation, in the setting of metastatic disease. The paradigm consisted of sipuleucel-T, enzalutamide, abiraterone, docetaxel, radium-223, and finally cabazitaxel. Prednisone 10 mg daily is administered during abiraterone, cabazitaxel, and docetaxel treatments and was included in our cost estimation. We excluded the use of tertiary hormone interventions in the mCRPC patient such as bicalutamide, flutamide, and ketoconazole given the lack of consensus on use and impact on survival. It was assumed that the patient would also be receiving denosumab monthly and leuprolide every 3 months for the entire length of survival.
We estimated length of treatment for each therapy using standard dosing schedules or survival data from randomized controlled trials (RCTs) if a standard dosing regimen was not defined. Cost data were obtained from the pharmaceutical provider for our institution, Besse Medical© (Amerisource Bergen Specialty Group, Frisco, TX, USA). CPT® codes for therapy administration on an outpatient basis were chosen based on common practice within our institution, and Medicare reimbursement values (MRV) were obtained through our institutional billing department. Data on survival benefit for each treatment were obtained from published RCTs. Our cost estimation does not include the price of pain medication, radiation therapy for bone metastases, or other palliative therapies such as mitoxantrone.
Overall cost for each treatment was established by multiplying the estimated treatment length or number of cycles by the unit cost obtained from Besse Medical©, the pharmaceutical supplier for our institution. If the medication was administered in office, an additional 6% was added to the total for office administration. For medications coded for injection or chemotherapy infusion, MRVs for the corresponding CPT® codes (96372 and 96365, respectively) were multiplied by the estimated number of treatments and added to the pharmaceutical cost. For medications prescribed orally, the monthly cost of the medication was multiplied by the estimated number of months of treatment received in the clinical trial. Survival benefits were assumed to be additive. We used the median placebo treatment survival time published in a sipuleucel-T phase III RCT (21.7 months) to which we added the median survival benefits of sipuleucel-T (4.1 months), docetaxel (2.4 months), cabazitaxel (2.4 months), abiraterone (3.9 months), radium-223 (3.6 months), and enzalutamide (3.7 months) to estimate the overall survival and length of time patients would receive denosumab and leuprolide treatments (41.8 months) [3], [4], [5], [6], [7], [8], [18].
2.2. CEA
We used our cost estimation and published survival data to perform a CEA of various combinations of drugs from a 6-drug treatment paradigm given in the following order: sipuleucel-T, enzalutamide, abiraterone, docetaxel, radium-223, and cabazitaxel. The CEA also included the cost of denosumab and leuprolide. Enzalutamide and abiraterone are administered before cytotoxic chemotherapy in our model given evidence showing increased efficacy in chemotherapy-naïve patients. Abiraterone is administered before cytotoxic chemotherapy given its recent approval in the chemotherapy-naïve patient [19], [20]. Given that the abiraterone pre-chemotherapy randomized trial was unblinded after a second planned interim analysis demonstrated a progression-free survival benefit with abiraterone, and patients receiving placebo were offered abiraterone, the survival benefit in the pre-chemotherapy setting remains unclear. Thus, we used the survival benefit from the post-chemotherapy phase III RCT in the CEA model. Similarly, the post-chemotherapy survival for enzalutamide was used given the crossover of patients from placebo to treatment after interim analyses were conducted [21].
As we hypothesized that few, if any, treatment options would meet the societal willingness to pay threshold of US$100,000, we conducted a second CEA using a paradigm that excluded sipuleucel-T, as it is the costliest intervention. Due to a paucity of published and standardized quality of life data for the current therapeutic approaches, we were unable to calculate quality-adjusted life years saved for any of our cost-effectiveness models.
Assuming an optimistic additive survival for the available treatments, we have created a best-case scenario in which a hypothetical patient would achieve maximum median survival benefits from all available therapies. We used a societal threshold willingness to pay of US$100,000 per life year saved (LYS) based on the rates that society is currently willing to pay for relatively high cost medical interventions such as renal dialysis for end stage renal disease and lung-volume reduction surgery [21]. Using TreeAge Pro, Version 2011(c) (TreeAge Software, Williamstown, MA, USA) we conducted a standard incremental CEA. Incremental cost-effectiveness ratios (ICER) in USD per LYS were used to determine the cost-effectiveness of each treatment step in the chosen paradigm. After arranging treatment strategies in order of effectiveness, we calculated ICERs for each treatment alternative by dividing the incremental cost (ΔC) by the incremental effect (ΔE), in years of survival, based on phase III clinical trial data.
When comparing sequentially more effective treatments, an alternative can be eliminated from consideration if it is found to be dominated. Dominance can be established in two situations: if a treatment is less effective and more costly than the previous alternative or if a treatment's ICER is higher than that of the next more effective alternative, known as extended dominance [22]. When dominance or extended dominance was identified for a given regimen, we conducted subsequent analyses without the dominated treatment regimen to establish the relative cost-effectiveness of the various approaches to treating mCRPC. Control arm survival data from the sipuleucel-T trial were used as the standard of care intervention in our CEA model that evaluated sipuleucel-T regimens, and the docetaxel phase III RCT control arm was used as the standard of care for models that without sipuleucel-T.
3. Results
3.1. Cost
Based on our calculations, the overall length of survival for patients receiving the new treatment paradigm for mCRPC is 41.8 months, including a treatment survival benefit of 20.1 months. Table 1 illustrates the method of cost estimation utilized as well as our results, described here. The cost of monthly administration of denosumab for 41.8 months and including the 6% in-office administration fee and MRV for CPT® code 96372 was estimated to be US$71,499.65 [5]. The cost of leuprolide administered every 3 months for 41.8 months using the same formula was estimated to be US$2477.46. We included the cost of daily prednisone 10 mg tablets, US$12.00 per month, in the overall cost of abiraterone, docetaxel, and cabazitaxel treatments.
Table 1.
Estimated cost and overall survival benefit for current treatments for metastatic castration resistant prostate cancer (mCRPC).
| Drug/CPT code | Unit cost (US$) | Average length/cycles of Tx | Cost average length/cycles of Tx (US$) | Office administration | Total cost (US$) | Survival benefit |
|---|---|---|---|---|---|---|
| Sipuleucel-T | 31,000.00 | 3 cycles | 93,000.00 | Yes (+6%) | 98,860.25 | 4.1 mo |
| CPT: 96365 | 88.13 | 264.39 | ||||
| Enzalutamide | 7450.00 | 8.3 mo | 61,835.00 | No | 61,835.00 | 3.7 mo |
| Abiraterone | 5390.00 | 8 mo | 43,120.00 | No | 43,216.00 | 3.9 mo |
| Prednisone (10 mg daily) | 0.40 | 8 mo | 96.00 | |||
| Docetaxel | 1515.62 | 9.5 cycles | 14,398.39 | Yes (+6%) | 16,235.26 | 2.4 mo* |
| CPT: 96365 | 88.13 | 837.24 | ||||
| Prednisone (10 mg daily) | 0.40 | 7.1 mo | 85.50 | |||
| Radium-223 | 11,500.00 | 6 treatments | 69,528.78 | Yes (+6%) | 73,700.51 | 3.6 mo |
| CPT: 96365 | 88.13 | |||||
| Cabazitaxel | 7773.00 | 6 cycles | 46,638.00 | Yes (+6%) | 50,038.79 | 2.4 mo* |
| CPT: 96365 | 88.13 | 528.78 | ||||
| Prednisone (10 mg daily) | 0.40 | 3.5 mo | 42.00 | |||
| Leuprolide (3 mo depot) | 141.75 | 41.8 mo** | 1970.32 | Yes (+6%) | 2,477.46 | |
| CPT: 96372 | 27.98 | 387.06 | ||||
| Denosumab | 1587.30 | 41.8 mo** | 66,349.14 | Yes (+6%) | 71,499.65 | |
| CPT: 96372 | 27.98 | 1169.56 | ||||
| Total | 417,862.92 | 20.1 mo |
*As compared to mitoxantrone. **Estimated by adding survival benefits of chemotherapy, radium-223, abiraterone, and enzalutamide to overall survival after sipuleucel-T therapy. CPT, current procedural terminology; mo, month.
Sipuleucel-T is administered in a standard regimen of three cycles. Using CPT® code 96365 and adding the 6% in-office administration fee, the total cost of sipuleucel-T treatment is estimated to be US$98,860 [5]. Abiraterone was administered for an average 8 months in a phase III RCT [6]. The monthly cost of abiraterone is US$5390, and the estimated cost for a course of treatment with abiraterone is US$43,216. In a phase III RCT, docetaxel was administered for an average of 9.5 cycles [3]. Using this treatment length, the cost of docetaxel treatment was estimated to be US$16,235. Of note, this is the only pharmaceutical in this paradigm currently available in generic formulation that has been shown to have a survival benefit. Radium-223 is infused in a series of six treatments, each of which costs US$11,500. After addition of the associated CPT® code and the 6% in-office administration fee, the overall cost of radium-223 is estimated to be US$73,701 [4]. In the phase III RCT, enzalutamide was administered for a mean treatment length of 8.3 months at US$7450 per month for a total treatment cost of US$61,835 [8]. Cabazitaxel was administered for an average of six cycles and, with the addition of a 6% in-office fee and MRV for CPT® code 96365, costs an estimated US$50,039 [7]. The overall cost of treatments administered for mCRPC in a model patient receiving all approved treatments US$417,862.92, or US$20,789.20 per month of survival advantage.
3.2. Cost-effectiveness
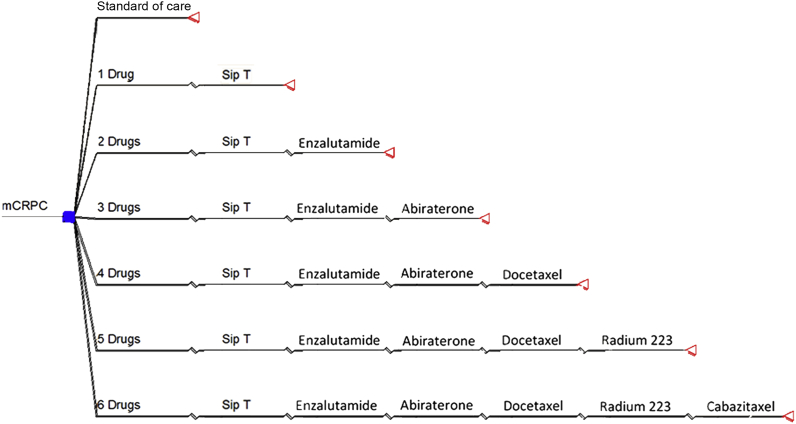
The CEA decision tree used in our initial analysis is seen in Fig. 1. The results from this analysis are presented in Table 2. Our initial CEA eliminated sipuleucel-T, sipuleucel-T + enzalutamide, and sipuleucel-T + enzalutamide + abiraterone treatment regimens from further consideration by extended dominance, meaning that sipuleucel-T alone and in combination with enzalutamide or enzalutamide and abiraterone had ICERs that exceeded the ratios of more effective alternative treatment regimens (US$312,109/LYS, US$220,594/LYS, and US$151,876/LYS, respectively). After eliminating these three treatment regimens from consideration we recalculated the ICER for the three remaining treatment options. The 4-drug, 5-drug, and 6-drug regimens were associated with ICERs of US$207,714, US$266,907, and US$271,435 per LYS, respectively, all exceeding the societal willingness to pay threshold of US$100,000/LYS.
Figure 1.
Decision tree used to calculate the incremental cost-effectiveness of the various drug regimens. The cost-effectiveness of each regimen was evaluated relative to each other and to standard of care (the control arm from the sipuleucel-T randomized controlled trial). The square node on the far left represents a decision point, depicting the choice among the drug regimens and standard of care, or no treatment. The triangles represent outcome points, which capture both the cost and survival associated with the preceding branch. The sequential therapies are separated by inflections in the tree branch.
Table 2.
Cost-effectiveness results with sipuleucel-T in a 6-drug model.
| Treatment | Cost (US$) (C) | Effect (LYS) (E) | Incremental cost (US$) (ΔC) | Incremental effect (LYS) (ΔE) | ICER (US$/LYS) (ΔC/ΔE) |
|---|---|---|---|---|---|
| Standard of care (Control arm Sip-T trial) | 38,456 | 1.81 | |||
| Sip-T | 144,524 | 2.15 | 106,117 | 0.34 | 312,109 (extended dominance) |
| Sip-T + Enzal | 212,908 | 2.46 | 68,384 | 0.31 | 220,594 (extended dominance) |
| Sip-T + Enzal + Abir | 263,027 | 2.79 | 50,119 | 0.33 | 151,876 (extended dominance) |
| SipT + Enzal + Abir + Doc | 283,510 | 2.99 | 245,103 | 1.18 | 207,714 |
| Sip-T + Enzal + Abir + Doc + Rad | 363,582 | 3.29 | 80,072 | 0.30 | 266,907 |
| Sip-T + Enzal + Abir + Doc + Rad + Cabazi | 417,869 | 3.49 | 54,287 | 0.20 | 271,435 |
Abir, abiraterone; Cabazi, cabazitaxel; Doc, docetaxel; Enzal, enzalutamide; LYS, life-years saved; Rad, radium-223; Sip-T, sipuleucel-T.
The second CEA evaluated treatment regimens without sipuleucel-T. Results from the analysis are found in Table 3. As previously described, placebo and treatment survival data from the docetaxel phase III clinical trial were used in this model for the standard of care. The 1-drug (enzalutamide) and 2-drug (enzalutamide + abiraterone) strategies were eliminated by reason of extended dominance due to ICERs of US$220,594/LYS and US$151,876/LYS, respectively. Compared to Standard of Care, the 3-drug (enzalutamide + abiraterone + docetaxel), 4-drug (enzalutamide + abiraterone + docetaxel + radium-223), and 5-drug (enzalutamide + abiraterone + docetaxel + radium-223 + cabazitaxel) regimens were associated with ICERs of US$165,460, US$266,907, and US$271,435 per LYS, respectively. Although the 3-drug regimen had the lowest ICER, all exceeded the assumed societal threshold willingness-to-pay of US$100,000 per LYS.
Table 3.
Cost-effectiveness results without sipuleucel-T in a 5-drug model.
| Treatment | Cost (US$) (C) | Effect (LYS) (E) | Incremental cost (US$) (ΔC) | Incremental effect (LYS) (ΔE) | ICER (US$/LYS) (ΔC/ΔE) |
|---|---|---|---|---|---|
| Standard of care (Control arm doc trial) | 33,494 | 1.58 | |||
| Enzal | 101,878 | 1.89 | 68,384 | 0.31 | 220,594 (extended dominance) |
| Enzal + Abir | 151,997 | 2.22 | 50,119 | 0.33 | 151,876 (extended dominance) |
| Enzal + Abir + Doc | 172,480 | 2.42 | 138,986 | 0.84 | 165,460 |
| Enzal + Abir + Doc + Rad | 252,552 | 2.72 | 80,072 | 0.30 | 266,907 |
| Enzal + Abir + Doc + Rad + Cabazi | 306,839 | 2.92 | 54,287 | 0.20 | 271,435 |
Abir, abiraterone; Cabazi, cabazitaxel; Doc, docetaxel; Enzal, enzalutamide; LYS, life-years saved; Rad, radium-223; Sip-T, sipuleucel-T.
4. Discussion
While the recent advent of several new pharmaceutical approaches to treating mCRPC has expanded therapeutic options for at risk patients, it has also significantly increased the potential economic burden of the disease. As these treatments have been introduced to the market and approved for treatment of a broader mCRPC population, public concern has grown regarding the impact of the cost related to these pharmaceuticals [13], [23]. Sipuleucel-T, the most expensive intervention available to date, has borne the brunt of this discussion. However, our analysis suggests that the most cost-effective treatment regimens currently available may include sipuleucel-T in combination with enzulutamide, abiraterone and docetaxel.
Few studies have examined the cost of mCRPC or terminal care for prostate cancer, and most were published before the availability of the new treatments [24], [25], [26]. While we are unable to compare these costs to our current model, these data offers a frame of reference to evaluate the changing costs in the management of patients with mCRPC today. Using SEER Medicare claims from 1991 to 2003, data representing patients treated before the approval of docetaxel in 2004, the cost of prostate cancer was divided into three time periods: initial, continuing, and terminal care, with terminal care being defined as the last year of life. By comparing prostate cancer patients to a group of non-cancer patients, the average cost for long-term care of prostate cancer was found to be US$18,168 for all stages and US$24,996 for metastatic stage IV disease compared to non-cancer patients [24]. Similarly, a retrospective analysis of 2056 patients diagnosed prostate cancer in a Midwestern United States health system between 1995 and 2000 found that the cost of care was significantly higher in the 6 months following metastasis compared to their pre-metastasis healthcare costs for the same time period (US$30,171 vs. US$20,690, p = 0.006) [25]. In a generalized linear model controlling for baseline covariates, patients who progressed to metastatic disease accrued a mean healthcare cost of US$92,523 over the course of treatment, significantly more than non-metastatic patients with a mean of US$58,036 (p = 0.0001).
Our analysis is limited to the cost of pharmaceutical treatments and the administration of those treatments, not taking into consideration the cost of treating other sequelae of CRPC such as pain, spinal cord compression, or palliative care. However, the cost of metastases and hospitalization for patients with CRPC has previously been described in patients mainly treated before the introduction of docetaxel. An estimate for the cost of skeletal-related events (SRE) in patients with prostate cancer bone metastases was created by examining health insurance claims between 2000 and 2005 and identifying patients who experienced pathologic fractures, radiation therapy, bone therapy, or spinal cord compression. It was found that the mean 1-year cost of treating patients who experience one skeletal related event was US$8484 and US$26,384 for patients with more than one SRE [27]. In a more modern cohort consisting of patients who received palliative radiation treatment for bone metastases between 2008 and 2009, total radiation costs for prostate cancer patients was US$7553 (95%CI US$6749 – US$8357) per radiation episode of care [28]. The impact of palliative treatments on overall cost may be reduced today given the benefits associated with the use of denosumab and other treatments in delaying SREs and pain palliation. However, our cost model does not incorporate these costs and thus may be an underestimate of actual charges for patients with CRPC and metastatic disease today. It is safe to assume that the cost of palliative care, pain control, and treatment of SRE would increase our estimate of healthcare costs for mCRPC. This is an important consideration as these costs are not uniform among treatment strategies and, if large enough, could significantly revise the results of our CEA.
There are several limitations to our cost analysis. First this cost estimate is based on a yet-to-be established sequence of therapies; while the order does correlate with the recent AUA guidelines on treatment of mCRPC, there have been limited published reports on patterns of care for mCRPC patients since the introduction of these medications. In reality patients may not receive all these treatments for a variety of reasons, including disease progression and personal choice. In the sipuleucel-T phase III trial almost 50% of patients chose to forego docetaxel therapy after progressing [3]. A recent study following patients taking enzulutamide or abiraterone as first-line therapy for mCRPC noted less than 20% of patients who progressed took the other drug and less than 10% were treated with docetaxel [29]. Patterns like these would reduce total costs, though have no impact on enhanced survival. More importantly, this calculation assumes that each patient receives each medication in sequence to experience the average survival benefit for each intervention and that the survival benefits for current treatments have an additive effect, which is unknown given that each of these approved medications was studied individually and not in sequence or combination. To date, there are no data describing the length of drug therapy or survival benefits following sequential treatments; such data depending on outcome could improve or worsen the ICER of a given regimen. Indeed, there is preliminary experience sequencing abiraterone and enzulutamide noting that whichever drug follows the other is less likely to have a response which in turn is shorter than if utilized first [30], [31], [32], [33]. In addition the cost of medication administration and distribution in Manhattan, New York may not translate to other areas of the country, as Medicare reimbursement values and distribution costs vary between regions. Finally, our analysis compares costs of drug treatment to society's willingness to pay US$100,000 per LYS. There is no mandate for such an exercise in the US at the present time and is nearly meant to highlight the cost-effectiveness of these therapies. As a society we come to grips with the high cost of delivering healthcare in the environment of healthcare reform such considerations may become a factor in the decision making process as they do in UK.
There are however some potential ways to enhance cost-effectiveness of these medications by using them more selectively. However, in a follow-up analysis of the phase III sipuleucel-T versus placebo trial, both treated and control patients were divided into PSA quartiles demonstrating the marked improvement in survival the earlier the treatment is given, 13 months for the lowest quartile versus 3 months for the highest. Not surprisingly, patients with lower PSA (<22 ng/mL) had better performance status, fewer bone lesions and less advanced disease by other markers of disease extent and its consequences [34]. Thus there are clinical predictors to target patients who will gain the best outcome from sipuleucel-T treatment; no such data are available for the other therapies at the present time. In a similar vein investigators have noted the presence of androgen receptor splice variants in circulating tumor cells predict resistance to both enzulutamide and abiraterone [35]. In turn such mutations are not related to resistance to docetaxel, which demonstrated superiority to enzultualimide or abiraterone in patients with splice variants in terms of progression free survival [36]. While not yet a commercially available test utilizing presence of splice variants in circulating tumor cells to taylor treatment could save up to US$150 million per year [37].
5. Conclusion
The estimated cost of a modern treatment paradigm for mCRPC with an overall survival benefit of 20.1 months is US$417,862.92, or US$20,789.20 per additional month of survival. Our CEA shows that all currently available treatment options exceed the commonly used societal willingness to pay threshold of US$100,000 per LYS. It must be noted that, with the exception of docetaxel, all of the recently approved therapies are at early points in the life cycle of pharmaceuticals. With experience utilizing these treatment options in addition to more specific patient selection, it is possible that improved survival will be reported and market competition will reduce the cost.
Conflicts of interest
The authors declare no conflict of interest.
Footnotes
Peer review under responsibility of Second Military Medical University.
References
- 1.American Cancer Society . American Cancer Society; Atlanta: 2016. Cancer facts & figures. [Google Scholar]
- 2.Kirby M., Hirst C., Crawford E.D. Characterising the castration-resistant prostate cancer population: a systematic review. Int J Clin Pract. 2011;65:1180–1192. doi: 10.1111/j.1742-1241.2011.02799.x. [DOI] [PubMed] [Google Scholar]
- 3.Tannock I.F., de Wit R., Berry W.R., Horti J., Pluzanska A., Chi K.N. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 4.Parker C., Nilsson S., Heinrich D., Helle S.I., O'Sullivan J.M., Fosså S.D. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–223. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 5.Kantoff P.W., Higano C.S., Shore N.D., Berger E.R., Small E.J., Penson D.F. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 6.de Bono J.S., Logothetis C.J., Molina A., Fizazi K., North S., Chu L. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Bono J.S., Oudard S., Ozguroglu M., Hansen S., Machiels J.P., Kocak I. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 8.Scher H.I., Fizazi K., Saad F., Taplin M.E., Sternberg C.N., Miller K. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 9.Smaletz O., Scher H.I., Small E.J., Verbel D.A., McMillan A., Regan K. Nomogram for overall survival of patients with progressive metastatic prostate cancer after castration. J Clin Oncol. 2002;20:3972–3982. doi: 10.1200/JCO.2002.11.021. [DOI] [PubMed] [Google Scholar]
- 10.Fizazi K., Carducci M., Smith M., Damião R., Brown J., Karsh L. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377:813–822. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mottet N., Bellmunt J., Bolla M., Joniau S., Mason M., Matveev V. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2011;59:572–583. doi: 10.1016/j.eururo.2011.01.025. [DOI] [PubMed] [Google Scholar]
- 12.Petrylak D.P. Haymarket Media Inc; New York, NY: 2011. Examining the pharmacoeconomics of advanced prostate cancer. Renal and Urology News; pp. 38–42. [Google Scholar]
- 13.Pollack A. The New York Times Company; New York, NY: 2011. New drugs fight prostate cancer, but at high cost. New York Times. [Google Scholar]
- 14.Cancer trends progress report – 2009/2010 update – costs of cancer care. Available from URL: http://progressreport.cancer.gov/doc_detail.asp?pid=1&did=2009&chid=95&coid=926&mid= [Accessed 21 December 2011].
- 15.Mariotto A.B., Yabroff K.R., Shao Y., Feuer E.J., Brown M.L. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103:117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yap T.A., Zivi A., Omlin A., de Bono J.S. The changing therapeutic landscape of castration-resistant prostate cancer. Nat Rev Clin Oncol. 2011;8:597–610. doi: 10.1038/nrclinonc.2011.117. [DOI] [PubMed] [Google Scholar]
- 17.Cookson MS, Roth BJ, Dahm P, Engstrom C, Freedland SJ, Hussain M, et al. Castration-resistant prostate cancer: AUA guideline. Available from URL: http://www.auanet.org/education/guidelines/castration-resistant-prostate-cancer.cfm [Accessed 8 May 2013]. [DOI] [PubMed]
- 18.Sheikh N.A., Petrylak D., Kantoff P.W., Dela Rosa C., Stewart F.P., Kuan L.Y. Sipuleucel-T immune parameters correlate with survival: an analysis of the randomized phase 3 clinical trials in men with castration-resistant prostate cancer. Cancer Immunol Immunother. 2013;62:137–147. doi: 10.1007/s00262-012-1317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryan C.J., Smith M.R., de Bono J.S., Molina A., Logothetis C.J., de Souza P. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–148. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beer T.M., Armstrong A.J., Rathkopf D.E., Loriot Y., Sternberg C.N., Higano C.S. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424–443. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neumann P.J., Rosen A.B., Weinstein M.C. Medicare and cost-effectiveness analysis. N Engl J Med. 2005;353:1516–1522. doi: 10.1056/NEJMsb050564. [DOI] [PubMed] [Google Scholar]
- 22.Karlsson G., Johannesson M. The decision rules of cost-effectiveness analysis. Pharmacoeconomics. 1996;9:113–120. doi: 10.2165/00019053-199609020-00003. [DOI] [PubMed] [Google Scholar]
- 23.Pollack A. The New York Times Company; New York: 2012. New drug for prostate cancer gets F.D.A. Nod. The New York Times. [Google Scholar]
- 24.Stokes M.E., Black L., Benedict A., Roehrborn C.G., Albertsen P. Long-term medical-care costs related to prostate cancer: estimates from linked SEER-Medicare data. Prostate Cancer Prostatic Dis. 2010;13:278–284. doi: 10.1038/pcan.2010.5. [DOI] [PubMed] [Google Scholar]
- 25.Penson D.F., Moul J.W., Evans C.P., Doyle J.J., Gandhi S., Lamerato L. The economic burden of metastatic and prostate specific antigen progression in patients with prostate cancer: findings from a retrospective analysis of health plan data. J Urol. 2004;171:2250–2254. doi: 10.1097/01.ju.0000127732.63726.4c. [DOI] [PubMed] [Google Scholar]
- 26.Piper N.Y., Kusada L., Lance R., Foley J., Moul J., Seay T. Adenocarcinoma of the prostate: an expensive way to die. Prostate Cancer Prostatic Dis. 2002;5:164–166. doi: 10.1038/sj.pcan.4500565. [DOI] [PubMed] [Google Scholar]
- 27.Lage M.J., Barber B.L., Harrison D.J., Jun S. The cost of treating skeletal-related events in patients with prostate cancer. Am J Manag Care. 2008;14:317–322. [PubMed] [Google Scholar]
- 28.Hess G., Barlev A., Chung K., Hill J.W., Fonseca E. Cost of palliative radiation to the bone for patients with bone metastases secondary to breast or prostate cancer. Radiat Oncol. 2012;7:168. doi: 10.1186/1748-717X-7-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malangone-Monaco E., Foley K., Varker H., Wilson K.L., McKenzie S., Ellis L. Prescribing patterns of oral antineoplastic therapies observed in the treatment of patients with advanced prostate cancer between 2012 and 2014: results of an oncology EMR analysis. Clin Ther. 2016;38:1817–1824. doi: 10.1016/j.clinthera.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Noonan K.L., North S., Bitting R.L., Armstrong A.J., Ellard S.L., Chi K.N. Clinical activity of abiraterone acetate in patients with metastatic castration-resistant prostate cancer progressing after enzalutamide. Ann Oncol. 2013;24:1802–1807. doi: 10.1093/annonc/mdt138. [DOI] [PubMed] [Google Scholar]
- 31.Badrising S., van der Noort V., van Oort I.M., van den Berg H.P., Los M., Hamberg P. Clinical activity and tolerability of enzalutamide (MDV3100) in patients with metastatic, castration-resistant prostate cancer who progress after docetaxel and abiraterone treatment. Cancer. 2014;120:968–975. doi: 10.1002/cncr.28518. [DOI] [PubMed] [Google Scholar]
- 32.Schrader A.J., Boegemann M., Ohlmann C.H., Schnoeller T.J., Krabbe L.M., Hajili T. Enzalutamide in castration-resistant prostate cancer patients progressing after docetaxel and abiraterone. Eur Urol. 2014;65:30–36. doi: 10.1016/j.eururo.2013.06.042. [DOI] [PubMed] [Google Scholar]
- 33.Loriot Y., Bianchini D., Ileana E., Sandhu S., Patrikidou A., Pezaro C. Antitumour activity of abiraterone acetate against metastatic castration-resistant prostate cancer progressing after docetaxel and enzalutamide (MDV3100) Ann Oncol. 2013;24:1807–1812. doi: 10.1093/annonc/mdt136. [DOI] [PubMed] [Google Scholar]
- 34.Schellhammer P.F., Chodak G., Whitmore J.B., Sims R., Frohlich M.W., Kantoff P.W. Lower baseline prostate-specific antigen is associated with a greater overall survival benefit from sipuleucel-T in the Immunotherapy for Prostate Adenocarcinoma Treatment (IMPACT) trial. Urology. 2013;81:1297–1302. doi: 10.1016/j.urology.2013.01.061. [DOI] [PubMed] [Google Scholar]
- 35.Antonarakis E.S., Lu C., Wang H., Luber B., Nakazawa M., Roeser J.C. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371:1028–1038. doi: 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Antonarakis E.S., Lu C., Luber B., Wang H., Chen Y., Nakazawa M. Androgen receptor splice variant 7 and efficacy of taxane chemotherapy in patients with metastatic castration-resistant prostate cancer. JAMA Oncol. 2015;1:582–591. doi: 10.1001/jamaoncol.2015.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Markowski M.C., Frick K.D., Eshleman J.R., Luo J., Antonarakis E.S. Cost-savings analysis of AR-V7 testing in patients with metastatic castration-resistant prostate cancer eligible for treatment with abiraterone or enzalutamide. Prostate. 2016 doi: 10.1002/pros.23232. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]