Abstract
Context:
Surveys have reported color fading as the most frequent reasons patients given for disliking their prostheses.
Aim:
The aim of the study is to compare the color variation between two maxillofacial silicone elastomers after subjecting them to extraoral aging conditions.
Subjects and Methods:
A total of 80 samples were made from M511 Maxillofacial Rubber (Part A: Part B = 10:1) and Z004 Platinum Silicone Rubber (Part A: Part B = 1:1) and divided into two main Groups A and B (40 each). These main groups were then subdivided into five subgroups (A1B1, A2B2, A3B3, A4B4, and A5B5) (n = 8); outdoor weathering, acidic perspiration, sebum (for 6 months), and neutral soap and disinfectant (for 30 h), respectively. Baseline L*a*b* values were recorded. The samples were subjected to the extraoral aging conditions, and the L* a*b* values were recorded after the aging period using a spectrophotometer.
Statistical Analysis:
The intergroup comparison was done by Kruskal–Wallis test, whereas the intragroup comparison was done by Mann–Whitney test.
Results
All groups exhibited visually detectable, mean color differences that ranged from 3.06–5.21, except for A4B4. There was no statistical significance between the two materials when subjected to extraoral aging conditions.
Conclusions:
Visually perceptible and clinically unacceptable color changes occur when exposed to various extraoral aging conditions except for neutral soap solution immersion, for which values of Δ E* were clinically acceptable (ΔE < 3). It can be said for all practical purposes, clinically, the choice between M511 Maxillofacial Rubber (Part A: Part B = 10:1) and Z004 Platinum Silicone Rubber (Part A: Part B = 1:1) would yield more or less the same results, with unacceptable norms in terms of color stability under extraoral aging conditions.
Keywords: Color stability, disinfection, facial secretions, maxillofacial silicone elastomer, outdoor weathering, spectrophotometer
INTRODUCTION
Considering the psychosocial pressures for facially disfigured patients, there is an increasing need to improve the present materials that are in use and to synthesize new elastomers, specifically for use as a facial prosthetic material. There is no facial prosthetic material so far that meets all the ideal requirements but there have been improvements in the past few decades, and silicone rubbers have been established as the current state-of-art material. Patients tend to consider color as the most important parameter in the evaluation of facial prostheses but unfortunately, color change which occurs in a short period of time is one of the most common reasons for need of a new prosthesis.[1]
Surveys have reported color fading as the most frequent reasons patients given for disliking their prostheses. The deterioration is mainly caused by environmental exposure to ultraviolet (UV) light, air pollution, and changes in humidity and temperature. Many factors play a role in altering the physical properties, and the color stability of the prosthesis material. Some of them are handling the prosthesis during cleaning and the application of adhesives and cosmetic additives.[2] Apart from this, finished facial prostheses rest on living human skin for extended periods and may absorb perspiration and sebum. These absorbed secretions may cause changes in the elastomer structure which are degradative, resulting in ultimate deterioration of the prosthesis. Regardless of the type of elastomer used in the fabrication of a facial prosthesis, its service life is usually 6 months to 2 years, with an average time of 10–12 months.[2,3]
Sweeney et al.[4,5] in 1972 reported an extensive study of the physical properties of the maxillofacial materials. A proposed specification of the maxillofacial material was also presented. Cantor et al.[6] reported on methods for evaluating prosthetic materials. A polyvinyl chloride, a plasticized poly (methyl methacrylate), and silicones were evaluated. Reflectance spectrophotometry was used to evaluate the color of maxillofacial elastomers. By studying the color of the human skin and pigmented maxillofacial materials, they suggested that isometrically matched maxillofacial materials could be developed that would match the color of the human skin.
Thereafter, researchers like Filié Haddad et al.,[1] Lemon et al.,[2] Polyzois[3] et al., and Goiato et al.[7] published their studies in the literature regarding the color stability of the maxillofacial silicone. However, the materials used, the parameters tested, and the aging conditions they were subjected to were different.
As a further contribution to the forgoing studies, this in vitro study evaluated and compared the color stability of two commercially available maxillofacial silicone elastomers after subjecting them to extraoral aging conditions like; outdoor weathering, skin secretions, and disinfectant solutions.
SUBJECTS AND METHODS
M511 Maxillofacial rubber (Part A: Part B = 10:1) (Cosmesil series material, Principality Medical Ltd., South Wales, UK, Lot number Part A– 11M and Part B– 11I) and Z004 Platinum silicone rubber (Part A: Part B = 1:1) (Technovent Ltd., Newport, UK, Lot number-12C) were used for the study. Both the silicones are room temperature vulcanizing silicones. They were selected on the basis of being the two most commonly used silicone materials in India for both clinical and research purposes.
The sample size for the study was determined by fixing the probability of type I error at 5% and that of type II error at 20%. Thus, the power of the study was 80%.
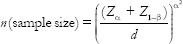
Where Zα= 1.96, Z1−β= 0.842 (for 80% power)
σ (Standard deviation [SD]) = 2.82,
d (minimum expected difference between 2 groups) = 4.82
(σ and d were taken from the parent article).[19]
Hence,
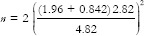
n = 5.37 ≈ 6
Taking into consideration, the experimental errors that would occur during the study, the sample size was taken to be as 8 per group.
A precise stainless steel mold with a depression measuring 30 mm in diameter and 3 mm in thickness was customized[7] [Figure 1a]. Ten polyvinyl siloxane (3M ESPE, soft putty, Lott No: MMM14043031, Bengaluru - 560 100, Karnataka, India) putty discs were obtained using the mold and were invested in a large maxillofacial flask. After the stone was set, the VPS putty samples were peeled off the mold leaving behind accurate and sharp disc-shaped depressions measuring 30 mm in diameter and 3 mm in thickness [Figure 1b].
Figure 1.
(a) Mold used for duplication of specimens with addition silicone putty impression material. (b) Mixed maxillofacial silicone packed in the customized mold used in the study
A standardized procedure was followed for the staining procedure for the specimens. Intrinsic stains were extensively used to mimic the average Indian medium skin tone.[8] The maxillofacial silicone was mixed and cured according to the manufacturer's instructions.
After the specimens were retrieved, they were finished and polished using silicone finishing burs (course and fine) followed by sandpapering using 1200 grit sandpaper discs. Baseline readings of the color were recorded before subjecting them to various extraoral aging conditions.
A total of 80 specimens were selected and divided into Group A (Cosmesil 10:1) and Group B (Technovent 1:1). Further, each group was divided into five subgroups depending on the extraoral aging conditions, they were subjected to as follows: A1B1 (outdoor weathering), A2B2 (acidic perspiration), A3B3 (simulated sebum solution), A4B4 (neutral soap solution), and A5B5 (disinfection solution).
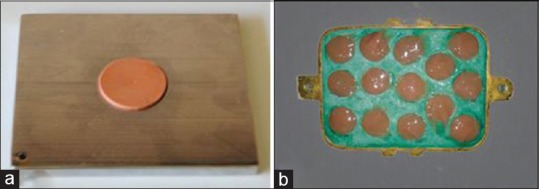
For the subgroup A1B1, the specimens were mounted by means of a stainless steel ligature wire on an untreated plywood backed exposure rack [Figure 2], approximately 45° from the horizontal to avoid standing water, and maximize the amount of sunlight on the specimens.[2] The whole assembly was placed on the roof for 6 months.
Figure 2.

Samples subjected to outdoor weathering
For the subgroup A2B2, the specimens were immersed in simulated acidic perspiration (pH 5.5) for 6 months, which was prepared according to the International Organization for Standardization specification 105-E04:87, part E04: color fastness to perspiration by adding L-Histidine monohydrochloride monohydrate-0.5 g (Spectrochem Pvt Ltd; Mumbai, India), sodium chloride-5 g (Spectrochem Pvt Ltd; Mumbai, India), and sodium dihydrogen orthophosphate dehydrate-2.2 g (Nice chemicals Pvt Ltd; Kochi-24) per liter of distilled water.[9]
For the subgroup A3B3, simulated sebum solution was prepared as described by Hatamleh et al.[9] by mixing palmitic acid-10% (Nice chemicals Pvt Ltd; Kochi-24), glycerine tripalmitate-2% (Nice chemicals Pvt Ltd; Kochi-24), and linoleic acid-88% (Nice chemicals Pvt Ltd; Kochi-24), and the specimens were stored in it for 6 months. The specimens of subgroup A4B4 and A5B5 were immersed in neutral soap solution (Johnson and Johnson, Brain bees solution Pvt Ltd., Pune, India-411045) and disinfectant solution (Fittydent denture cleansing tablets, Dr. Reddy's Laboratories Ltd, Hyderabad, India-500034), respectively, for 30 h.[1]
The conditioning periods were selected to simulate silicone prosthesis in service for 18–24 months. Considering that each day patients wear their prosthesis for 8–12 h, during which it is expected to be exposed atleast 6 h of daylight, normal environmental conditions, and continuous sebum and perspiration, whereas the prosthesis is on the defect site. In addition, before sleeping, patients spend an average of 5 min cleaning their prostheses. Therefore, 1 month of service equals 180 h of daylight aging, storage in sebum or acidic solutions, and 150 min of storage in cleansing solutions.[9]
Color differences of each specimen were measured using CM-3310d spectrophotometer (MINOLTA) using the Commission Internationale de I’Eclairage (CIE) L*a*b* system [Figure 3a and b] to measure the color alteration, as established by the CIE.[10] L*, a*, and b* values of each specimen after immersion at each specified time interval (T0, T30h, and T6 m) was measured and the mean was calculated. Color difference ΔE was calculated from the mean ΔL*, Δa*, and Δb* values for each specimen with the formula.[11,12,13]
Figure 3.
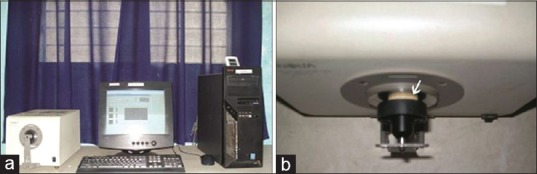
(a) CM-3310d Spectrophotometer (MINOLTA) used in the study. (b) Color testing of the specimens using CM-3310d spectrophotometer
ΔE = (ΔL*2+ Δa*2+ Δb*2)½
Where ΔL*, Δa*, and Δb* are the differences in L*, a*, and b* values before (T0) and after immersion at each time interval (T30 h and T6 m).
RESULTS
The values obtained did not follow a normal curve/Gaussian curve, and hence nonparametric tests were employed. The intergroup comparison was done by Kruskal–Wallis test, whereas the intragroup comparison was done by Mann–Whitney test. Minitab 16 Softonic Statistical software was used for the statistical analysis of the data obtained. Values of ΔE* ≤3 were considered clinically acceptable. Values more than 3 reflected unacceptable color change clinically as suggested by Fontes et al.[14] Any ΔE value more than 3 was considered to be a visually perceptible color change from the baseline reading.
The results showing the mean difference of mean ΔE*, SD, and P value of Group A and B are presented in Table 1. Intergroup comparison using Kruskal–Wallis test showed all the subgroups displaying statistically significant color differences (ΔE* ≤3) except for the subgroup A4B4 (neutral soap solution). However, the highest ΔE* value for group A was shown by the subgroup A1 - outdoor weathering (ΔE*5.04, P < 0.01) and in Group B, by subgroup B5 - disinfectant solution (ΔE*5.20, P < 0.01).
Table 1.
Mean difference of ΔE, standard deviation, and P values of Group A and Group B at different time intervals (intergroup comparison using Kruskal-Wallis test)
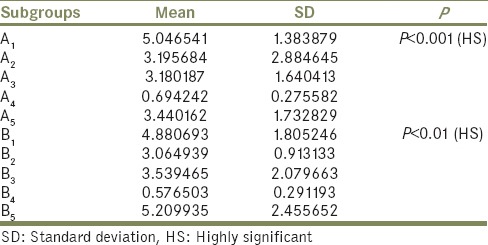
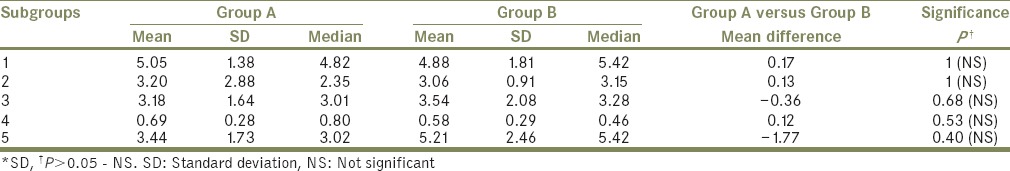
Table 2 compares the mean ΔE*, median, and SD between Group A and B and depicts their mean differences and P values after applying “Mann–Whitney” test. Thus, it can be interpreted that both materials (A and B) are most color stable when immersed in neutral soap solution and least color stable when subjected to outdoor weathering (Group A) and disinfectant solution (Group B). It can also be interpreted that there is no statistically significant difference in the color stability of the two materials tested [Graph 1].
Table 2.
“Mean,” “standard deviation,” and “median” for Group A and Group B along with their mean difference and “P values” after applying Mann-Whitney test
Graph 1.
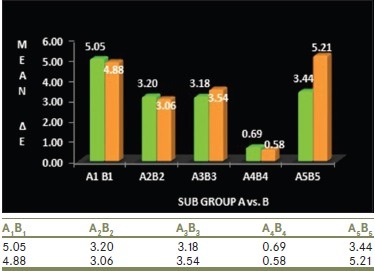
Comparison of mean ΔE values of Group A and Group B specimens at different time intervals
DISCUSSION
Maxillofacial prostheses require frequent replacement because of rapid discoloration in a service environment or degradation of the physical and dynamic properties of the base elastomer or both.[15] The important criteria for maxillofacial materials were described by Chalian, Drane, and Standish[16] in 1971 as ease of application and retention, color stability, durability, lack of toxicity, strong peripheries, translucency, ease of cleaning, light weight, ease of fabrication, and physical and chemical inertness.
Color stability is the property a material has, of retaining color for a period of time in a certain environment. Two of the color systems used to assess the chromatic differences are the Munsell color system and the CIE L*a*b* color system. Cantor et al.[6] reported methods for evaluating prosthetic facial materials. The authors investigated the esthetics of the materials and color matching of skin and facial materials using reflectance spectrophotometry. Since then, reflectance spectrophotometry and color and optical density have been used to evaluate the color stability.
The ADA recommends the use of CIE L*a*b* system, which quantifies the color alterations using a mathematical equation expressed by ΔE* and obtained with the variation of three coefficients (L*a* and b*) where,
L* = Color luminosity (ranging from 0-black to 100-white)
a* = Ranges from 90 to 70 and represent the greenness on the positive axis and redness on the negative axis
b* = Ranges from 80 to 100 and represents yellowness (positive b*) and blueness (negative b*).
ΔE = ([ΔL]2 + [Δa]2 + [Δb]2)1/2
The samples were subjected to outdoor weathering from the month of February to July 2013. The maximum temperature that was recorded during this time period was 39°C in the month of April and May, and the lowest temperature recorded was 14°C in the month of February. The highest amount of rainfall that was recorded was 86 mm in the month of May. However, the average rainfall recorded during this time period was 39.3 mm. The above data were collected form a website named www.accuweather.com.
There was a highly statistically significant color change that was noted in the specimens before and after outdoor weathering irrespective of the material being used similar to the studies conducted by Lemon et al.,[2] Polyzois[3] Haug et al.,[18] and Hatamleh et al.[19] This significant color change can be attributed to the presence of UV light irradiation present in the solar radiation which may have enhanced cross-linking, along with accelerated interaction of the fatty acids with silicone, breaking down the chain bonds, and decomposing the elastomer as suggested by Hatamleh et al.[19]
There was a highly statistically significant color change that was noted in the specimens before and after immersion in acidic perspiration irrespective of the material being used. This is in accordance with the in vitro studies conducted by Polyzois et al.[10] and Hatamleh et al.[19] This significant color change can be attributed to the catalytic effect of the acidic environment on the cross-linking reaction, which leads to the formation of additional polymer network in the silicone.
The specimens showed a highly statistically significant color change when exposed to simulated sebum solution similar to the studies conducted by Polyzois et al.[10] and Hatamleh et al.[19,20] This can be related to the fact that sebum fatty acids tend to interact with silicone, breaking chain bonds, and decomposing the elastomer.
Disinfectant solution showed a highly significant color change in the specimens as reported by Pesqueira et al.[21] This may be due to the fact that Fittydent (sodium perborate-based disinfectant) mainly acts by means of oxygen liberation mechanism. It is reasonable to think that, although they remove small stains, also cause prosthesis whitening as already cited in other studies.[1]
The deterioration of the color of the maxillofacial silicone prosthesis is not by virtue of a single factor or aging condition. Infact, it is due to the combined effect of various factors such as environmental exposure, humidity, UV radiation, air pollutants, exposure to facial secretions, and the method of disinfection. Apart from these external factors, certain internal factors such as the composition of the silicone, degree of cross-linking, mode of curing, extrinsic and intrinsic stains used; all play an important role in maintaining or degrading the color of the silicone prosthesis.
Within the limitations of this in vitro study and from the results obtained, the following can be inferred:
Immersion of the specimens in neutral soap solution produced the least color change irrespective of the material used
All the specimens produced a statistically significant color change when subjected to extraoral aging conditions except immersion in neutral soap solution irrespective of the material used
There was no statistically significant difference in the color stability of M511 Maxillofacial Rubber (Part A: Part B = 10:1), Cosmesil series material and Z004 Platinum Silicone Rubber (Part A: Part B = 1:1), Technovent Ltd that were compared in the study.
The limitations of this study include:
Evaluation of the color stability of the materials was done based on intrinsic staining only. Further studies are required for the evaluation of color stability based on extrinsic staining (with and without sealant application), addition of pigments, and flockings which also play an important role in the color stability of maxillofacial silicone elastomer
The effect of outdoor weathering on color stability is limited to the areas with tropical climatic conditions like that present in India and cannot be extrapolated worldwide
Manipulation of the maxillofacial silicone elastomer was done by mechanical hand mixing. Further, studies are required for the comparison of the color stability of silicone elastomers between mechanical hand mixing and vacuum mixing.
CONCLUSIONS
With the results of the study, it is safe to conclude that the maximum duration for which a prosthesis remains color stable is 12–18 months and needs to be changed once in every 18 months for better patient acceptance. It is difficult to extrapolate the results of this study to clinical conditions. However, the results of this study can give an insight into how different maxillofacial silicone elastomers may behave when exposed to different extraoral aging conditions, thus affecting the clinician's choice of material and the patient's concern toward the prosthesis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Filié Haddad M, Coelho Goiato M, Micheline Dos Santos D, Moreno A, Filipe D’almeida N, Alves Pesqueira A, et al. Color stability of maxillofacial silicone with nanoparticle pigment and opacifier submitted to disinfection and artificial aging. J Biomed Opt. 2011;16:095004. doi: 10.1117/1.3625401. [DOI] [PubMed] [Google Scholar]
- 2.Lemon JC, Chambers MS, Jacobsen ML, Powers JM. Color stability of facial prostheses. J Prosthet Dent. 1995;74:613–8. doi: 10.1016/s0022-3913(05)80314-2. [DOI] [PubMed] [Google Scholar]
- 3.Polyzois GL. Color stability of facial silicone prosthetic polymers after outdoor weathering. J Prosthet Dent. 1999;82:447–50. doi: 10.1016/s0022-3913(99)70032-6. [DOI] [PubMed] [Google Scholar]
- 4.Sweeney WT, Fischer TE, Castleberry DJ, Cowperthwaite GF. Evaluation of improved maxillofacial prosthetic materials. J Prosthet Dent. 1972;27:297–305. doi: 10.1016/0022-3913(72)90039-x. [DOI] [PubMed] [Google Scholar]
- 5.Gupta A, Jain D. Materials used for maxillofacial prosthesis reconstruction: A literature review. J Indian Prosthodont Soc. 2003;3:11–5. [Google Scholar]
- 6.Cantor R, Webber RL, Stroud L, Ryge G. Methods for evaluating prosthetic facial materials. J Prosthet Dent. 1969;21:324–32. doi: 10.1016/0022-3913(69)90295-9. [DOI] [PubMed] [Google Scholar]
- 7.Goiato MC, Pesqueira AA, dos Santos DM, Zavanelli AC, Ribeiro Pdo P. Color stability comparison of silicone facial prostheses following disinfection. J Prosthodont. 2009;18:242–4. doi: 10.1111/j.1532-849X.2008.00411.x. [DOI] [PubMed] [Google Scholar]
- 8.Guttal SS, Patil NP, Nadiger RK, Kulkarni R. A study on reproducing silicone shade guide for maxillofacial prostheses matching indian skin color. Indian J Dent Res. 2008;19:191–5. doi: 10.4103/0970-9290.42949. [DOI] [PubMed] [Google Scholar]
- 9.Hatamleh MM, Polyzois GL, Silikas N, Watts DC. Effect of extraoral aging conditions on mechanical properties of maxillofacial silicone elastomer. J Prosthodont. 2011;20:439–46. doi: 10.1111/j.1532-849X.2011.00736.x. [DOI] [PubMed] [Google Scholar]
- 10.Polyzois GL, Tarantili PA, Frangou MJ, Andreopoulos AG. Physical properties of a silicone prosthetic elastomer stored in simulated skin secretions. J Prosthet Dent. 2000;83:572–7. doi: 10.1016/s0022-3913(00)70017-5. [DOI] [PubMed] [Google Scholar]
- 11.2nd ed. Paris: Bureau Central de la CIE; 1985. Commission Internationale de I’Eclairage – CIE. Colorimetry, Official Recommendation of the International Commission on Illumination. Publication CIE No. 15.2 (TC-1.3) [Google Scholar]
- 12.Gupta R, Parkash H, Shah N, Jain V. A spectrophotometric evaluation of color changes of various tooth colored veneering materials after exposure to commonly consumed beverages. J Indian Prosthodont Soc. 2005;5:72–8. [Google Scholar]
- 13.Singh SV, Aggarwal P. Effect of tea, coffee and turmeric solutions on the colour of denture base acrylic resin: An in vitro study. J Indian Prosthodont Soc. 2012;12:149–53. doi: 10.1007/s13191-012-0122-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fontes ST, Fernández MR, de Moura CM, Meireles SS. Color stability of a nanofill composite: Effect of different immersion media. J Appl Oral Sci. 2009;17:388–91. doi: 10.1590/S1678-77572009000500007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koran A, Yu R, Powers JM, Craig RG. Color stability of a pigmented elastomer for maxillofacial appliances. J Dent Res. 1979;58:1450–4. doi: 10.1177/00220345790580050301. [DOI] [PubMed] [Google Scholar]
- 16.Yu R, Koran A, 3rd, Craig RG. Physical properties of a pigmented silicone maxillofacial material as a function of accelerated aging. J Dent Res. 1980;59:1141–8. doi: 10.1177/00220345800590070801. [DOI] [PubMed] [Google Scholar]
- 17.Kiat-Amnuay S, Mekayarajjananonth T, Powers JM, Chambers MS, Lemon JC. Interactions of pigments and opacifiers on color stability of MDX4-4210/type A maxillofacial elastomers subjected to artificial aging. J Prosthet Dent. 2006;95:249–57. doi: 10.1016/j.prosdent.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Haug SP, Andres CJ, Moore BK. Color stability and colorant effect on maxillofacial elastomers. Part III: Weathering effect on color. J Prosthet Dent. 1999;81:431–8. doi: 10.1016/s0022-3913(99)80010-9. [DOI] [PubMed] [Google Scholar]
- 19.Hatamleh MM, Watts DC. Effect of extraoral aging conditions on color stability of maxillofacial silicone elastomer. J Prosthodont. 2010;19:536–43. doi: 10.1111/j.1532-849X.2010.00627.x. [DOI] [PubMed] [Google Scholar]
- 20.Hatamleh MM, Watts DC. Porosity and color of maxillofacial silicone elastomer. J Prosthodont. 2011;20:60–6. doi: 10.1111/j.1532-849X.2010.00652.x. [DOI] [PubMed] [Google Scholar]
- 21.Pesqueira AA, Goiato MC, dos Santos DM, Haddad MF, Ribeiro Pdo P, Coelho Sinhoreti MA, et al. Effect of disinfection and accelerated aging on color stability of colorless and pigmented facial silicone. J Prosthodont. 2011;20:305–9. doi: 10.1111/j.1532-849X.2011.00693.x. [DOI] [PubMed] [Google Scholar]


