Abstract
Background
Knowledge of the impact of the gut microbiome on conditions other than Clostridium difficile infection has been rapidly increasing, and the potential usefulness of fecal microbiota transplantation (FMT) in these indications is being explored. The need to exclude donors with an increased risk of these diseases has left uncertainties regarding the cost and feasibility of donor screening. The aim of this study was to compare our experience to other donor-screening programs and report the costs associated with establishing a donor-screening program, for the treatment of metabolic syndrome-related conditions.
Methods
Forty-six potential donors (PDs) had their medical histories and physical examinations undertaken by a physician. Blood, stool, and urine were screened for 31 viral, bacterial, fungal, and protozoan agents in addition to biochemical characteristics. The price of advertising, doctor’s visits and diagnostic tests were calculated to determine the cost of finding a donor.
Results
Of the PDs screened, 5 of 46 passed the history, examination, blood, stool, and urine tests. The most common reasons for exclusion included a body mass index >25 or the detection of Blastocystis hominis, Dientamoeba fragilis, or Helicobacter pylori. Four of five eligible donors had subsequent travel or illness that contraindicated donation, so only 1 of 46 PDs was suitable. The total cost for finding a single suitable donor was $15190 US dollars. This screening was performed in Canada, and costs in the United States would be substantially higher.
Conclusions
New potential therapeutic uses for FMT have created a demand for stricter exclusion criteria for donors. This study illustrates that screening many individuals to find a donor and the subsequent associated costs may make central processing and shipment a more reasonable alternative.
Keywords: fecal microbiota transplant, metabolic syndrome, stool bank
Fecal microbiota transplantation (FMT) has been most commonly used to treat recurrent Clostridium difficile infections [1]. The impact of the gut microbiome on many other conditions, and therefore the list of other potential indications for FMT, has been rapidly increasing [2]. Many aspects of human health, such as metabolism, autoimmune disease [3], and even mental health [4], are hypothesized to be impacted by the microbiome, and FMTs are undergoing trials to assess their efficacy in many of these conditions [2].
The concerns regarding potential transmission of pathogenic organisms and thus the need for extensive pretransplant donor-screening have been well known. To our knowledge, there have been no reports of an infectious disease being transmitted through a screened FMT donor, although there has been a report of possible cytomegalovirus transmission in an FMT from a nonscreened donor [5]. However, the actual number of potential transmissible agents screened for has been a subject of practice variation, and recently some authorities have released guidance documents to recommend minimum screening criteria [6]. A small number of clinical exclusion criteria for donation, such as recent antibiotic use, have been used for many years to improve the chances of success in prevention of recurrent C difficile [7]. However, data on the myriad of potential diseases associated with the gut microbiome has steadily increased the number of conditions for which stool donation may carry a risk of transmission. The need to exclude donors with evidence of these conditions or even evidence of being at increased risk of having a microbiome associated with these conditions (such as having a family history of the disease, because the microbiome can be similar among family members that live together [8]) has left uncertainty regarding the cost and feasibility of donor screening. Therefore, we reviewed our experience in recently establishing a new donor-screening program for FMTs for metabolic syndrome-related diseases and compared it to other groups’ screening processes and outcomes.
METHODS
Donor Recruitment
Potential donors (PDs) were recruited through a hospital workplace, a university monthly newsletter, as well as by word of mouth among staff at St. Joseph’s Health Care (a teaching hospital with over 4000 employees, 2000 residents and fellows, 1100 physicians, and 1000 healthcare student placements), Western University (with over 28000 students, 2400 employees, and 1400 faculty members) and Lawson Health Research Institute (a hospital-based research facility with over 1500 principal investigators, researchers, technicians, support staff, and trainees distributed among 10 sites) in London, Ontario, Canada. Recruitment took place from March 2015 to July 2016. The recruitment materials used listed a small number of the donor screening exclusion criteria including body mass index (BMI) >25, abnormal metabolic profile, recent antibiotic use, family history of diabetes or coronary disease, and any known transmissible agent. Potential donors who expressed interest in being screened contacted the research coordinator to obtain more information on the screening process, to schedule screening, and to provide written informed consent. A list of the full screening procedure and exclusion criteria was provided to PDs before screening was scheduled, and those who believed that they would qualify proceeded to have a full history and examination by a physician.
Donor Screening
PDs’ written consents were obtained, and medical histories and examinations were conducted by a physician. A summary of questions asked during the history and exam is detailed in Table 1. If the PD passed the initial screening criteria, they were subjected to stool, urine, and blood testing for transmissible diseases and other health markers. Donor screening practices of our program are outlined in Table 1. Laboratory screening was performed as per the Health Canada guidance document “Fecal microbiota therapy used in the treatment of Clostridium difficile infection not responsive to standard therapies” [6].
Table 1.
Comparison of Donor Exclusion Criteria Between Groupsa
| Exclusion Criteria and Tests Performed | Craven et al |
Kazerouni et al [9] | Paramsothy et al [10] | Costello et al [11] | Tariq et al [12] |
|---|---|---|---|---|---|
| History/Examination | |||||
| Between 18 and 65 years of age | X | X | X | X | X |
| Any medications | X | X | X | X | |
| Antibiotics, antifungals, or antivirals in the last 3 months | X | X | X | X | X |
| Probiotics in the last 3 months | X | X | X | ||
| Hospitalization in the last 3 months | X | X | |||
| Travel to high-risk areas of infectious diarrhea in the last 3 months | X | X | X | X | X |
| Acute diarrhea within the past 6 months | X | ||||
| Tattoo or body piercing in the last 6 months | X | X | X | X | X |
| Known HIV or viral hepatitis exposure in the last 12 months | X | X | X | X | X |
| High-risk sexual behavior | X | X | X | X | X |
| Illicit drug use | X | X | X | X | X |
| Incarceration or a history of incarceration | X | X | X | X | |
| Household members with active GI infection | X | X | |||
| Chronic constipation | X | ||||
| Any gastrointestinal disorder | X | X | X | X | X |
| Overweight (BMI >25) | X | X | |||
| Obese (BMI >30) | X | X | X | ||
| Hypertension | X | X | X | X | |
| Type 2 diabetes | X | X | X | X | |
| Insulin sensitivity | X | X | X | X | |
| Hyperlipidemia | X | X | X | X | X |
| Atherosclerosis | X | X | X | ||
| Malnutrition (BMI <18) | X | X | X | ||
| Autoimmune disease | X | X | X | X | X |
| Atopic disease | X | X | X | ||
| Psychiatric history | X | X | X | X | |
| Infection with HIV, syphilis, hepatitis B or C | X | X | X | X | X |
| Malignancy | X | X | X | X | X |
| Chronic pain syndromes, neurologic or neurodevelopmental disorders | X | X | X | X | |
| History of major gastrointestinal surgery | X | X | X | ||
| Any kind of liver disease | X | ||||
| Alcoholic intake >10 g/day women and >20 g/ day men | X | ||||
| Family history of colorectal carcinoma | X | X | X | X | |
| Family history of diabetes | X | ||||
| Family history of early onset coronary disease, gastrointestinal or liver disease | X | X | |||
| Stool Tests | |||||
| Ova, cysts, and parasites | X | X | X | X | X |
| Microscopy and culture | X | X | X | X | X |
| Rotavirus | X | X | X | ||
| Norovirus | X | X | X | X | |
| Adenovirus | X | X | X | ||
| Clostridium difficile toxin | X | X | X | X | X |
| Vancomycin-resistant Enterococcus screen | X | X | X | X | |
| Carbapenem-resistant Enterobacteriaceae | X | X | |||
| Extended spectrum β-lactamase-producing Enterobacteriaceae | X | X | |||
| Fecal Giardia antigen | X | X | X | X | |
| Fecal Cryptosporidium antigen | X | X | X | X | |
| Isospora | X | ||||
| Cyclospora | X | ||||
| Microsporidia | X | X | X | ||
| Blood Tests | |||||
| Complete blood count | X | X | X | X | |
| Electrolytes, urea, and creatinine | X | X | X | ||
| Liver function tests | X | X | X | X | |
| Erythrocyte sedimentation rate | X | X | |||
| C-reactive protein | X | X | X | ||
| Fasting lipids and blood sugar level | X | X | |||
| Anti-TTG antibody for celiac disease | X | ||||
| Antinuclear antibody | X | ||||
| HIV type 1 and 2 | X | X | X | X | X |
| Hepatitis A virus IgM | X | X | X | X | X |
| Hepatitis B virus surface antigen, hepatitis B virus core antibody (IgM and IgG), hepatitis B virus surface antibody | X | X | X | X | X |
| Hepatitis C virus antibody | X | X | X | X | X |
| Human T-cell lymphotropic virus 1 and 2 | X | X | X | X | |
| Epstein-Barr virus IgM | X | X | |||
| Cytomegalovirus IgM | X | X | |||
| Strongyloides stercoralis, Entamoeba histolytica, Helicobacter pylori serology | X | X | X | ||
| H pylori | X | X | X | ||
| Treponema pallidum screening cascade | X | X | X | ||
| Listeria | X | ||||
| Nasal Swab | |||||
| Methicillin-resistant Staphylococcus aureus | X | X | |||
| Urine Tests | |||||
| Gonorrhea and chlamydia | X | ||||
Abbreviations: BMI, body mass index; GI, gastrointestinal; HIV, human immunodeficiency virus; Ig, immunoglobulin; TTG, tissue transglutaminase.
aScreening procedures of Kazerouni et al [9] are based on the OpenBiome safety guidelines [13].
In addition to testing for transmissible agents, laboratory screening included screening for metabolic abnormalities (including HbA1C and fasting lipids), celiac disease (using anti-tissue transglutaminase antibodies), liver function tests, and urinalysis. Potential donors were required to have the following: a healthy weight (BMI 18–25), no underlying conditions, a normal metabolic profile (no hypertension, normal fasting lipid profile), no history of injection drug use, no new sexual partners (within the last 3 months), no ongoing or recent use of any prescription or over-the-counter mediations (including antidiarrheal drugs, mineral oil, bismuth, magnesium, or kaolin), a maximum alcohol intake of <10 g/day in women and <20 g/day in men, no recent antibiotic use (within 3 months), and no recent hospitalizations (within 3 months). Sceening also included no personal or family history of the following: diabetes, coronary disease or metabolic disease (hypertension, hyperlipidemia, diabetes, insulin insensitivity, atherosclerosis), or gastrointestinal, liver, or biliary disease (including gastroesophageal reflux, peptic ulcer disease, celiac disease, inflammatory bowel disease, Crohn’s disease, ulcerative colitis, microscopic colitis, or motility disorders). Those with previous surgery to the intestine, liver, or gallbladder (except remote appendectomy) were also excluded. Any history of malignancy removed the PD from donation consideration.
Potential donors were screened for the following transmissible agents in blood: human immunodeficiency virus type 1 and 2, human T-cell lymphotropic virus 1 and 2, hepatitis A, hepatitis B, hepatitis C, Helicobacter pylori, syphilis, Stronglyoides, schistosomiasis, amebiasis, cytomegalovirus (immunoglobulin [Ig]M), adenovirus, and Epstein-Barr virus (IgM). Stool was analyzed for the detection of Shigella, Salmonella, Yersinia, Campylobacter, Escherichia coli 0157-H7, Plesiomonas, Aeomonas, Listeria, Shiga toxins, ova, parasites, microsporidia, C difficile, rotavirus, and norovirus. Pharyngeal and rectal swabs were assessed for gonococcal and chlamydia culture, and urine was assessed for the presence of gonorrhea and chlamydia by nucleic acid amplification tests. Nasal and rectal swabs were obtained to detect the presence of methicillin-resistant Staphylococcus aureus, and the rectal swab was also assessed for the presence of vancomycin-resistant enterococci, extended spectrum β-lactamase-producing Enterobacteriaceae, and carbapenem-resistant Enterobacteriaceae. If PDs had a history of travel to endemic areas, additional testing was performed for Chagas disease, malaria, and babesiosis. If PDs traveled to Zika-endemic regions in the last 3 months, they were excluded. There were no PDs that had traveled to endemic areas, and the additional testing was not performed. All laboratory test results were returned within 3 weeks’ time, and PDs were asked to not engage in high-risk behavior after their screening took place.
Our donor screening methods were compared to 4 other programs, which have published their full screening methods and acceptance rates to illustrate the inconsistencies in screening PDs (Table 1). The acceptance rates of these programs were contrasted with our own to determine what effect expanded screening programs had on overall donor enrollment (Table 2). The cost of the screening program was estimated by itemizing costs with all values given in US dollars (USD).
Table 2.
A Comparison of Various Donor Screening Program’s Acceptance Rates
| Study | Sample Size | Proportion who Pass History/ Exam | Pass Stool Test | Pass Blood Test | Overall Acceptance |
|---|---|---|---|---|---|
| Craven et al | 46 | 50% (23/46) | 61% (14/23) | 57% (13/23) | 11% (5/46) |
| Kazerouni et al [9] | 77 | 35% (27/77) | 44% (12/27) | 100% (12/12) | 16% (12/77) |
| Paramsothy et al [10] | 116 | 25% (29/116) | 48% (14/29) | 97 % (28/29) | 10% (12/116) |
| Costello et al [11] | 44 | 50% (22/44) | 68% (15/22) | 93% (14/15) | 37% (14/44) |
| Tariq et al [12] | 21 | 43% (9/21) | 78% (7/9) | 89% (8/9) | 24% (5/21) |
RESULTS
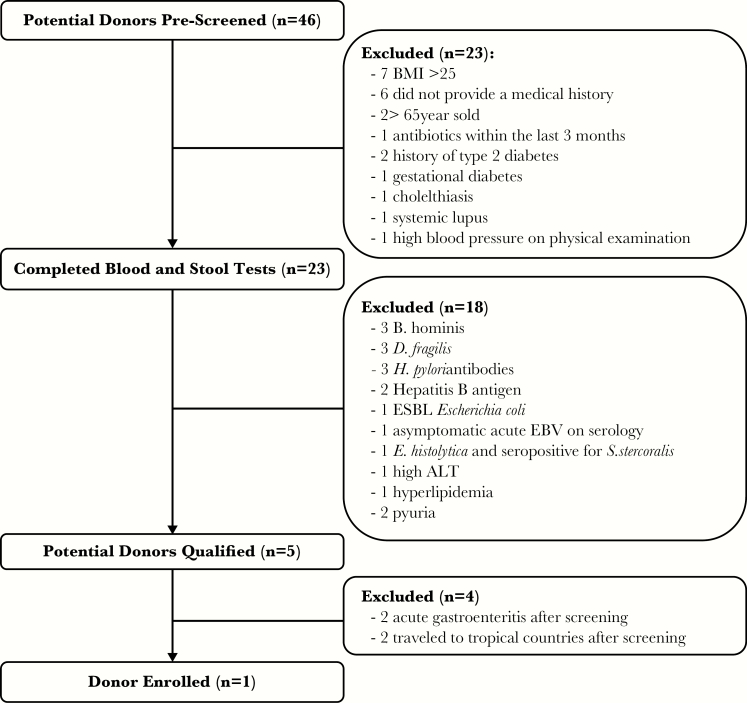
Potential donors were screened as per the methods mentioned previously. Forty-six PDs were screened in total (Figure 1) including 25 females and 21 males aged 20–73 years (median, 35 years). Of the 46 PDs that were screened, 23 passed the history and examination by a physician. The most common reasons for exclusion included having a BMI >25 and not providing a medical history. Of those 23 participants, 5 passed all blood, urine, stool, and pharyngeal/rectal swab screening. The most common reasons for excluding PDs after blood and stool testing were positive tests for Blastocystis hominis, Dientamoeba fragilis, and H pylori. Of those 5, 2 were subsequently excluded after they had acute gastroenteritis, and another 2 were excluded because they traveled to tropical countries after screening. One of these PDs also moved out of the region after screening. Therefore, after full screening of 46 potential volunteers, only 1 donor was able to donate on a regular basis for the program.
Figure 1.
Potential donor screening outcomes using expanded donor screening methods. ALT, alanine aminotransferase; BMI, body mass index; EBV, Epstein-Barr virus; ESBL, extended spectrum β-lactamase.
The cost of a full work-up (including history, examination, blood, stool, and urine screening, and administration) at our center was approximately $440 USD per person, and those that were excluded after the history and examination cost $150 USD per person (Table 3). The cost of the history, examination, and administration was based on the fee of a 1-hour doctor’s appointment and 3 hours of the research coordinator’s time. The costs of laboratory tests performed in hospital were provided by a laboratory manager at St. Joseph’s Health Care, London, Ontario, Canada (where the tests were performed) in October 2016. The exchange rate on June 20, 2017 of Canadian to USD (0.75) was used to convert the cost of donor screening to USD. Of the 46 donors who were screened, 23 had the full work-up and 23 were excluded before full screening took place. The total for patients who had full screening was approximately $10120 USD, and the total for patients with only a doctor’s visit was $3450 USD. Eighteen PDs had abnormal laboratory results, and they had a follow-up appointment with a physician adding an additional $1620 USD in costs. To find a single donor, approximately $15190 USD was spent. If the same testing were to be completed in the United States it would cost approximately $3770 USD per PD for laboratory tests alone (Table 3).
Table 3.
Cost of Screening a Single Donor for Fecal Transplanta
| Screening Tests | Cost per Person in Canada (USD) | Cost per Person in United States (USD) |
|---|---|---|
| Stool Tests | ||
| Ova, cysts, and parasites | $21.00 | $138.84 |
| Microscopy and culture | $13.50 | NAb |
| Rotavirus | NAc | $97.94 |
| Norovirus | NAc | $108.70 |
| Adenovirus | NAc | $78.02 |
| Clostridium difficile toxin GDH and toxin | $30 | $100.09 |
| Vancomycin-resistant Enterococcus | $2.63 | NAb |
| Fecal Giardia antigen | $10.88 | $88.15 |
| Fecal Cryptosporidium antigen | $10.88 | $86.10 |
| Helicobacter pylori | NAc | $169.69 |
| Extended spectrum β-lactamase-producing Enterobacteriaceae | NAc | $77.49 |
| Microsporidia | NAc | $72.11 |
| Blood Tests | ||
| Complete blood count | $6.20 | $42.18 |
| Electrolytes | $7.77 | $39.69 |
| Urea | $1.94 | $49.04 |
| Creatinine | $1.94 | $46.64 |
| Alanine aminotransferase | $1.94 | $19.03 |
| Alkaline phosphatase | $1.94 | $49.04 |
| Total bilirubin | $1.94 | $19.03 |
| Albumin | $1.94 | $41.16 |
| Fasting lipids | $10.48 | $147.35 |
| HbA1c | NAc | $71.39 |
| Glucose | $1.94 | $34.80 |
| Anti-TTG antibody (for celiac disease) | $11.95 | $156.68 |
| HIV type 1 and 2 | NAc | $110.85 |
| Hepatitis A virus IgM | $15.00 | $96.86 |
| Hepatitis B virus surface antigen | $17.25 | $398.44 |
| Hepatitis B virus core antibody IgM and IgG | $14.42 | $107.63d |
| Hepatitis B virus surface antibody | $15.46 | $52.28 |
| Hepatitis C virus antibody | $15.14 | $66.73 |
| Human T-cell lymphotropic virus 1 and 2 | NAc | $110.85 |
| Epstein-Barr virus IgM | $26.25 | $75.34 |
| Cytomegalovirus IgM | $30.00 | $76.41 |
| Strongyloides stercoralis serology | NAc | $145.29 |
| Entamoeba histolytic serology | NAc | $96.86 |
| H pylori serology | NAc | NAb |
| Treponema pallidum screening cascade | NAc | $75.78 |
| Listeria | NAc | $127.00 |
| Swabs | ||
| Gonorrhea | NAc | $150.68 |
| Chlamydia | NAc | $75.24 |
| MRSA | $15.00 | $159.60 |
| Carbapenem-resistant Enterobacteriaceae | NAc | $171.12 |
| Total per person (laboratory testing) | $287.39 | $3772.49 |
| Other Costs | ||
| Administrative fee | $56.25 | NA |
| Doctor’s visit | $90.00 | NA |
| Advertising | $4.88 | NA |
| Total per person | $438.52 | NA |
Abbreviations: GDH, glutamate dehydrogenase; HIV, human immunodeficiency virus; MRSA, methicillin-resistant Staphylococcus aureus; NA, not available; TTG, tissue transglutaminase; USD, United States dollar.
aCanadian costs include the fixed cost of advertising, supply costs of materials used, and time spent scheduling and screening donors. American costs include the supply costs of materials used and labor.
bNot available because same test is not offered by diagnostic service.
cNot available because cost was not publically available in Canada by the provincial public health laboratory.
dIgM only.
DISCUSSION
The demand for donors for FMTs is increasing. The criteria for donor screening among institutions are inconsistent, and recruitment of donors for FMT clinics and studies can be very difficult. Knowing the total number of people to screen to find a suitable number of donors is helpful when determining how much recruitment will need to be done. This also determines the program feasibility and costs of establishing a program. We found that of 46 volunteers screened, only 5 passed clinical and laboratory screening, and due to subsequent events only 1 of 5 was available for ongoing donation. Of the 4 donors who were successfully screened and passed all of the tests, but subsequently excluded, 2 may be available for donation at a later date after completing repeat screening because the other 2 declined repeat screening. Tariq et al [12] had a similar experience in which approximately half of their accepted donor pool were excluded after passing screening, with 1 donor becoming pregnant, 2 testing positive for Shiga toxin in stool, and 1 opted out of being a donor.
It was found that 11% of our PDs screened were eligible to be donors for FMTs. This 11% success rate is comparable to both Kazerouni et al [9] and Paramsothy et al [10] who found that 15.6% and 10.3% of PDs passed the screening process, respectively. OpenBiome, the longest standing international stool bank, currently reports that less than 3% of PDs applying to their program are accepted to be donors [13]. Costello et al [11] found higher rates of success for stool tests (68%) compared to our data and that of others [9, 10, 12]. We found that the most common reasons for exclusion in our cohort were B hominis, D fragilis, and H pylori. Both B hominis and D fragilis were also the leading reasons for exclusion found by Paramsothy et al [10]. Although common problems, the loss of successful PDs to acute gastroenteritis and travel to tropical countries seems to be uncharacteristically high in this pool of PDs. It would be very difficult to prevent these problems from occurring in the future because we have no control over the wide variety of factors that may cause acute gastroenteritis and a donor’s vacation plans. None of the PDs had plans for upcoming travel when screened; however, many were University undergraduate or graduate students and last-minute travel was a common phenomenon. If restrictions were placed on donors about where they could travel, donor retention would likely suffer.
Kazerouni et al [9] estimated the cost of screening 1 donor for their public stool bank (OpenBiome) to be $885 USD per person. We estimated the cost of this screening program at $440 USD per person in Canada, with the total cost of screening to find 1 viable donor being $15190 USD. The difference in costs is likely, at least partially, related to a lower cost for medical procedures, laboratory tests, and physician time in Canada than in the United States. For example, the cost of laboratory tests in Canada versus the United States are as follows; ova, cysts and parasites, $21.00 USD versus $138.84 USD; C difficile toxin, $30.00 USD versus $100.09 USD; and hepatitis B virus core antibody IgM, $14.42 USD versus $107.63 USD. (The pricing of Quest Diagnostics laboratory tests was used to provide costs in the United States.) Overall, the cost of the same laboratory testing in Canada versus the United States was approximately $290 and $3770, respectively. There are several tests that were provided free of charge in Canada by the provincial public health laboratory (eg, Gonorrhea, chlamydia, and HTLV 1 and 2), and this was also a contributing factor as to why the price of screening a PD in Canada was significantly lower than in the United States. No cost estimates were publically available for the laboratory tests performed by the provincial public health laboratory.
The 1 donor successfully selected will require ongoing intermittent routine rescreening every 6 months. At present, there is no consensus on the frequency of rescreening nor has it been specified in clinical guidelines [3]. Interim testing varies among programs from every month [11] to 6 months’ time. Our experience was that our 1 initially successful donor provided 20 stools before rescreening was required. At the time of this manuscript’s final submission, this donor has just developed acute gastroenteritis, and so the program is now on hold. Other common reasons for donor subsequent exclusion could include travel to the tropics, requiring antibiotics, or starting a new sexual relationship. This has now necessitated rescreening a large cohort of new volunteers.
Finally, there is data suggesting that the efficacy of FMT for different diseases with microbiome indications varies by donor characteristics that are difficult to predict. Repeated donations from different donors were required for success in a landmark C difficile therapy study [14]. Furthermore, a particular donor seemed to be especially effective in a study of FMT for ulcerative colitis [15], but whether the same donor’s microbiome would also be ideal for other indications is unknown. We included a wide range of ages (18–65) in PD screening. Although some authorities note that microbiome senescence may lead to lower efficacy with an older donor, the longer a patient has lived without complications, the more likely they may have a healthy microbiome. Ideally, multiple donors would be screened to find ones that are repeatedly successful for different diseases treated by FMT. This would dramatically increase the number of donors required for screening and increase costs to have a stool bank that specializes in treating multiple diseases.
Limitations of this study include that the sample size was small (n = 46), and the reasons for exclusion after PDs passed screening may not be generalizable for other populations, because the rates of acute gastroenteritis and tropical travel in the 5 accepted PDs were unusually high. The cost of screening a PD for FMT is underestimated because the costs of tests from the provincial public health laboratory were not made available, and these tests were performed in Canada where the cost of physician’s time and laboratory testing is significantly lower than in the United States. The strengths of this study are that it shows (1) a minimum cost estimate for clinics thinking of using FMTs and establishing their own pool of donors and (2) the difficulties that can be anticipated to establish and maintain a donor pool from a single center without significant funding.
Our data raise the concern regarding the feasibility of individual centers establishing and maintaining FMT donor pools. Reimbursement for FMT is not high enough to counter the costs of screening PDs for programs: for example, in the US, an FMT is reimbursed $76 USD through Medicare. In Ontario, there is no reimbursement whatsoever. Central banks with storage and shipment of frozen samples may be necessary to maintain programs [16]. However, to enable this, full transparency and reporting of all screening protocols will be necessary to enable clinicians and regulators to determine the acceptability and potential efficacy of biobank stools for their clinical context.
CONCLUSIONS
Novel uses for FMTs, as well as new insights on how the microbiome affects human health, have created a demand for stricter exclusion criteria for donors. Anticipating the number of PDs that must be screened to find a suitable donor can help to determine the amount of recruitment that must be done and the funds required to accomplish this. The large number of donors who require screening and the resultant cost may make the establishment of multiple local programs a nonviable goal with central processing and shipment being a more reasonable alternative.
Acknowledgment
We thank Kim Spearin and Shannon Schofield (St. Joseph’s Health Care in London, Ontario) for help in acquiring and calculating the costs of laboratory tests.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Kelly CR, Khoruts A, Staley C et al. . Effect of fecal microbiota transplantation on recurrence in multiply recurrent Clostridium difficile infection: a randomized trial. Ann Intern Med 2016; 165:609–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jung Lee W, Lattimer LD, Stephen S et al. . Fecal microbiota transplantation: a review of emerging indications beyond relapsing Clostridium difficile toxin colitis. Gastroenterol Hepatol 2015; 11:24–32. [PMC free article] [PubMed] [Google Scholar]
- 3. Dunne JL, Triplett EW, Gevers D et al. . The intestinal microbiome in type 1 diabetes. Clin Exp Immunol 2014; 177:30–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tillisch K, Labus J, Kilpatrick L et al. . Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology 2013; 144:1394–401, 1401.e1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hohmann EL, Ananthakrishnan AN, Deshpande V. Case Records of the Massachusetts General Hospital. Case 25-2014. A 37-year-old man with ulcerative colitis and bloody diarrhea. N Engl J Med 2014; 371:668–75. [DOI] [PubMed] [Google Scholar]
- 6. Health Canada. Fecal microbiota therpay used in the treatment of Clostridium difficile infection not responsive to conventional therapies. Guidance Document 2015. Available at: https://www.canada.ca/en/health-canada/services/drugs-health-products/public-involvement-consultations/biologics-radiopharmaceuticals- genetic-therapies/guidance-document-regulation-fecal-microbiota-therapy.html. Accessed 22 May 2017 [Google Scholar]
- 7. Aas J, Gessert CE, Bakken JS. Recurrent Clostridium difficile colitis: case series involving 18 patients treated with donor stool administered via a nasogastric tube. Clin Infect Dis 2003; 36:580–5. [DOI] [PubMed] [Google Scholar]
- 8. Song SJ, Lauber C, Costello EK et al. . Cohabitating family members share microbiota with one another and with their dogs. Elife 2013; 2:e00458. doi: 10.7554/eLife.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kazerouni A, Burgess K, Burns LJ, Wein LM. Optimal screening and donor management in a public stool bank. Microbiome 2015; 3:75. doi: 10.1186/s40168-015-0140-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Paramsothy S, Borody TJ, Lin E et al. . Donor recruitment for fecal microbiota transplantation. Inflamm Bowel Dis 2015; 21:1600–6. [DOI] [PubMed] [Google Scholar]
- 11. Costello SP, Tucker EC, La Brooy J et al. . Establishing a fecal microbiota transplant service for the treatment of Clostridium difficile infection. Clin Infect Dis 2016; 62:908–14. [DOI] [PubMed] [Google Scholar]
- 12. Tariq R, Weatherly R, Kammer P et al. . Donor screening experience for fecal microbiota transplantation in patients with recurrent C. difficile infection. J Clin Gastroenterol 2016; doi:10.1097/MCG.0000000000000768. [DOI] [PubMed] [Google Scholar]
- 13. OpenBiome. Quality and safety program. 2017. Available at: http://www.openbiome.org/safety/. Accessed 20 September 2017. [Google Scholar]
- 14. van Nood E, Vrieze A, Nieuwdorp M et al. . Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med 2013; 368:407–15. [DOI] [PubMed] [Google Scholar]
- 15. Moayyedi P, Surette MG, Kim PT et al. . Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology 2015; 149:102–109.e6. [DOI] [PubMed] [Google Scholar]
- 16. Malani PN, Rao K. Expanded evidence for frozen fecal microbiota transplantation for Clostridium difficile infection: a fresh take. JAMA 2016; 315:137–8. [DOI] [PMC free article] [PubMed] [Google Scholar]