Abstract
Abstract: Background: Interatrial block (IAB) is due to disruption in the Bachmann region (BR). According to whether interatrial electrical conduction is delayed or completely blocked through the BR, it can be classified as IAB of first, second or third degree. On the surface electrocardiogram, a P wave ≥ 120 ms (partial IAB) is observed or associated to the prolongation of the P wave with a biphasic (positive / negative) morphology in the inferior leads (advanced IAB). Bayes syndrome is defined as an advanced IAB associated with atrial arrhythmia, more specifically atrial fibrillation.
Objective and Conclusion: The purpose of this review is to describe the latest evidence about an entity considered an anatomical and electrical substrate with its own name, which may be a predictor of su-praventricular arrhythmia and cardioembolic cerebrovascular accidents, as well as the role of new imag-ing techniques, such as echocardiographic strain and cardiac magnetic resonance imaging, in character-izing atrial alterations associated with this syndrome and generally in the study of anatomy and atrial function.
Keywords: Bayes syndrome, interatrial block, atrial remodeling, atrial fibrosis, atrial fibrillation, echocardiography, speckle-tracking echocardiography, cardiac magnetic resonance, Bachmann region, interatrial conduction delay
1. INTRODUCTION
The theory of a possible specialized atrial conduction tissue was first described by Lewis et al. in 1914. The first case of interatrial block (IAB) was described by Bachmann in 1941 [1], who recognized the importance of the P wave width on the electrocardiogram, around 25 years after describing the anatomy of the Bachmann's region [2]. Dr. Bayés de Luna was the first to provide an adequate description of the interatrial conduction blockade in 1979, classifying them into interatrial and intra-auricular blocks, and suggested separating IAB into two categories: a) partial, where there would be a delay in the conduction of the Bachmann's region but most of the right-to-left conduction still occurs at an auricular ceiling level; and b) advanced where there is a total block of Bachmann's region and the conduction towards the left atrium (LA) occurs from the lower part of the right atrium with a caudocranial retrograde direction mainly through the coronary sinus, and to a lesser extent through the fossa ovallis [3]. In recognition of the work in the comprehension of the IAB [4] and the association of these with supraventricular arrhythmias, this association was named Bayés Syndrome [5].
Atrial fibrosis increases the size of the extracellular matrix [6], which can affect inter and intra-auricular conduction generating different degrees of blockade [7-10]. This fibrosis can be detected by imaging techniques [11], so these imaging techniques could predict and help identifying the etiologic substrate of patients with Bayés Syndrome.
The objective of this review is to update this underdiagnosed entity and demonstrate its importance in daily clinical practice as well as to demonstrate the utility of imaging techniques in the diagnosis of Bayés Syndrome.
2. METHODS
A non-systematic review of the literature was performed, using PubMed as the main search engine. The keywords used were interatrial block, interatrial conduction, interatrial conduction delay, Bachmann region; atrial fibrillation, atrial remodelling, speckle-tracking echocardiography, Bayés Syndrome and cardiac magnetic resonance. The articles included in this review were selected by the authors as the most significant.
3. ANATOMICAL AND ELECTRICAL CONSIDERATIONS
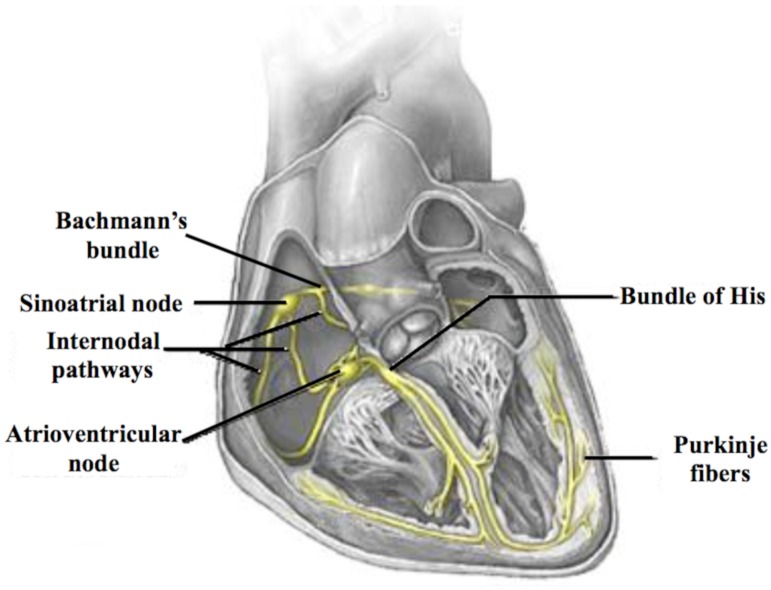
The cardiac conduction system begins at the sinoatrial node level, which is considered to be the pacemaker responsible for the initiation of action potentials, which are led from this area of the right atrium through three different internodal pathways to the atrioventricular node. Although they are not really pathways, but rather bundles that allow faster internodal conduction and are the so-called anterior conduction bundles or Bachmann's region, middle or Wenckebach bundle and posterior or Thorel's bundle (Fig. 1) [12]. As mentioned above, most of the atrial conduction occurs through the Bachmann's region and to a lesser extent through the coronary sinus (10-15% of the impulses) or the fossa ovallis (5-10% of the impulses) [13] and even through fibres subsequently located in the vicinity of the right pulmonary veins.
Fig. (1).
Conduction of the action potentials from their generation at the sinoatrial node through the right atrium to the atrioventricular node via the three internodal pathways. The transmission of the impulse from the right atrium to the left atrium occurs, mostly, through the Bachmann's region.
Bachmann's region is a stripe-like nerve structure containing myocardial fibres that cross from the subepicardium and from the end of the terminal ridge, in front of the superior vena cava, and connects them at the level of the upper interauricular groove. The upper edge is somewhat longer than the lower edge, giving it a trapezoidal shape [14]. The Bachmann's region is considered to be an ultra-fast pathway of interauricular activation, which can reach a conduction velocity of approximately 177 cm/s [15, 16]. The effective refractory period of the Bachmann's region is also longer than the rest of the right and left atrial (LA) myocardium, so that the bundle can be blocked especially when the neighbouring atrial tissue is still ready to be activated, which could represent a potential substrate for the generation of atrial arrhythmias by re-entry phenomenon, but also by automatism (in the case of flutter and atrial fibrillation (AF)).
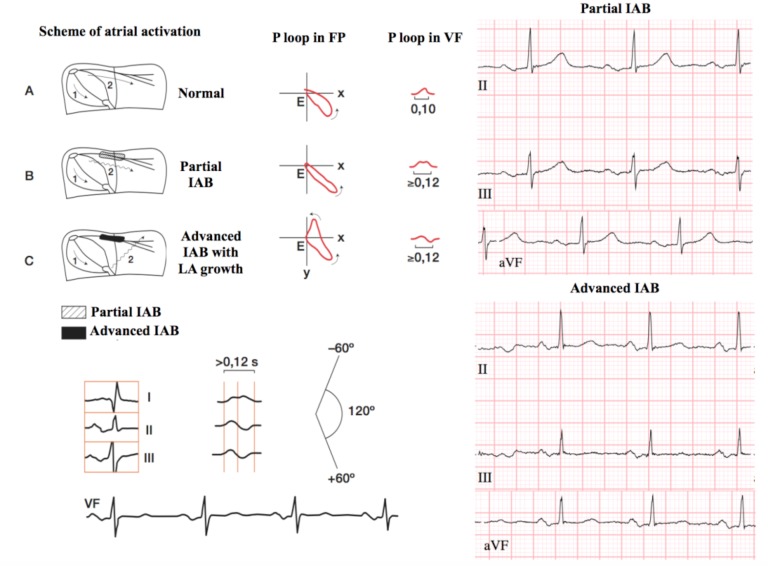
The IAB is caused by conduction damage in the Bachmann's region. The classification adopted in the 2012 Consensus document divides the IAB into the first (partial) IAB, where the electrical impulse is conducted with right atrial delay to the left but following the normal path of propagation. From the electrocardiographic point of view, a bimodal P wave of duration greater than or equal to 120 ms can be observed, especially in the inferior side derivations (II, III, aVF); second-degree IAB (atrial aberrancy), where, similarly to blockages occurring at the level of atrioventricular conduction, sinoatrial union or intraventricular level, a transient appearance block may occur at the atrial level that could be related with changes or not in heart rate, and even after the compensatory pauses generated by extrasystoles (Chung's phenomenon) [17]. Electrocardiography is represented by the temporal appearance of a first or third degree IAB [18, 19], constituting a true atrial aberrancy; and finally, in third grade IAB (advanced). In this latter type of block, the electrical impulse is blocked at the level of Bachmann's region and the depolarization of the LA occurs caudally-cranially from the lower right atrium (coronary sinus and, to a lesser extent, fossa ovallis). The electrocardiographic pattern of this disorder results in a P wave greater than or equal to 120 ms with biphasic type morphology +/- in the inferior (II, III and AVF) leads (Fig. 2).
Fig. (2).
Normal interatrial conduction (A), partial interatrial block (B) and advanced (C) with electrocardiographic tracings.
The advanced IAB is a clinically important and under recognized entity and has been shown to be associated with the development of supraventricular arrhythmias [20]. It is often associated with LA growth. The duration of the P wave itself is more a criterion of IAB than of LA growth, which helps in the diagnosis of the latter when it is associated with other electrocardiographic criteria. LA growth causes prolonged interatrial conduction time due to the elongation of the fibres composing the Bachmann's region [21]. This delay is due to the increase in the distance that the action potential has to travel rather than a blocking of the Bachmann's region. In the lower leads, biphasic P waves can be observed but without the terminal negative deflection observed in the third degree IAB. In the presence of advanced atrial growth, P wave in V1 has a marked negative component that is higher than the positive (+/-). This situation does not occur in the presence of IAB without LA growth [22].
Prevalence of IAB is difficult to establish, due in part to the fact that it is an underdiagnosed entity and because there are discrepancies in the data published to date, which is mainly due to three factors: 1) studies published before the consensus of 2012 showed a cut off point for the 110 ms wave instead of the correct 120 ms, 2) many studies used only the II lead of the electrocardiogram, or that derivation where P-wave had greater amplitude, instead of making a correct measurement so that we must choose the derivation where P-wave starts and the derivation where P ends as a last measurement, which does not have to correspond to the derivation that was chosen for the start of the P-wave and 3) P-wave measurement methods vary from one study to another (automatic, manual or semiautomatic). The prevalence depends mainly on age and associated cardiac pathology, showing figures of around 5.4% in people under 20 years of age up to 60% in patients over 50 years of age [23-27]. This increase in the prevalence is probably due to the increase in the degree of fibrosis and fatty infiltration that occurs with aging and this can impair the conduction at the auricular level [7, 8, 28]. With aging, it not only increases the age of the population but also the prevalence of cardiovascular disease increases, greatly affecting the heart and, specifically, the specialized cardiac conduction system [29]. Because of the improvement in cardiovascular therapeutics, the survival of patients increases, so that a large percentage of them may develop an IAB during the course of their disease [30].
The fact that the prevalence of IAB increases with age [24, 31-36], as with AF59-60, is likely to be related to the degree of atrial fibrosis [37].
Within the elderly population, it has been shown that the frequency of IAB increases in the different age groups, so that in septuagenarians the prevalence of this disorder is around 40% [27, 38] and about 50% in octogenarians [36]. In the ARIC (Atherosclerosis Risk in Communities) study, there was an incidence, in the general population, of IAB of 2.3 cases per 1000 people-year [39, 40].
As with AF and IAB, the presence of atrial origin extrasystoles is increased with age and atrial extrasystoles at a number greater than 100/day has been shown to be associated with an increased risk of AF [41]. It has also been shown that the incidence of this type of extra-systole is greater in patients with IAB than in those who present a normal P wave duration, and they are also more frequent in patients with advanced IAB when compared with patients with partial IAB [27].
It has been observed that the prevalence of IAB also depends on the underlying cardiac pathology, so a prevalence of around 1% has been reported in patients with structural disease including various valvular heart diseases [39]. It has been shown that in patients with mitral insufficiency, there are higher rates of IAB and AF compared to those that show a P wave of normal duration [27].
Most recently, Alenxander B et al. showed that an effective cardiac resynchronization therapy (CRT) –biventricular pacing of at least 92% over the course of a minimum of 1 year- facilitates a positive reverse atrial electrical remodelling, manifested as a reduce P wave duration and this would help to decrease the percentage of patients with advanced IAB [42].
Previous studies have demonstrated a higher prevalence of advanced IAB in patients with heart failure and coronary artery disease of ischemic origin. Sadiq et al. [43], found the presence of advanced IAB in up to 38% of patients with heart failure undergoing resynchronization therapy. Álvarez-García et al. [44], have described the presence of advanced IAB in 17% of patients admitted for heart failure and maintained sinus rhythm. He found that in this type of patients the presence of partial IAB was extremely high, around 64%, compared with 18% who presented normal duration of the P wave. In patients who were to undergo coronary revascularization surgery with a mean age of 65 years, Conde et al. [45] have shown an IAB prevalence of 36% (22% partial and 14% advanced).
Alexander B et al. showed association between extent coronary artery disease and higher incidence of IAB and they comment in this article that fibrosis is the end result of a variety of cardiac process, including ischemia, which share common molecular profibrotic pathways and this could be result in impaired atrial conduction and, generate, thus, IAB. Also, in the same way, they showed higher prevalence of new-onset atrial fibrillation in patients with IAB after non-ST-segment elevation myocardial infarction (NSTEMI) [46].
4. IAB and supraventricular arrhythmias
IAB has been consistently associated with supraventricular arrhythmias, especially with AF [20, 47]. Bayés de Luna et al. showed a higher incidence of supraventricular tachyarrhythmias in the group of patients with advanced IAB and LA growth over those patients who only showed LA growth or LA growth with partial IAB [20]. A progression of paroxysmal to permanent AF has been demonstrated in patients with a longer P wave duration [45].
The mechanism by which supraventricular tachyarrhythmias can be induced in the context of IAB has been demonstrated following Rensma et al. study, which showed that changes in the wavelength of the atrial impulse were inversely proportional to the onset of tachyarrhythmias [48, 49]. In the same study, it was observed that the ectopic beats had a shorter wavelength depending on the degree of prematurity. Given the context of a fixed IAB and retrograde conduction, variations in the refractory period and the conduction velocity of the atrial myocardium constitute a possible substrate for the induction and maintenance of supraventricular arrhythmias.
5. IAB and other associations
LA growth associated with poor contractile capacity in the setting of an IAB predisposes to thrombus formation and subsequent thromboembolism [50, 51]. Other studies have corroborated the association of IAB with the presence of neurological events of ischemic origin [52, 53].
Apiyasawat et al. showed a higher incidence of IAB in patients with evidence of myocardial ischemia undergoing stress echocardiography [54].
Patients with sleep apnea-hypopnea syndrome show a higher prevalence of arrhythmias, especially supraventricular arrhythmias [55]. More recently, there has been a statistically significant association between duration and dispersion [56] of the P wave of the IAB with apnea severity based on the apnea-hypopnea index [57-59]. This could be the result of atrial remodelling caused by the pathophysiological mechanisms of this syndrome such as pulmonary hypertension, systemic hypertension, volume overload at the atrial level, etc. acting as a substrate for the generation and maintenance of supraventricular arrhythmias.
Delayed atrial conduction may also be associated with other etiologies such as systemic inflammatory diseases or infectious diseases [60]. It has been shown that the atrial tissue of diabetic patients undergoes a persistent oxidative stress process when compared to non-diabetic patients, which may play a potential role in delaying atrial conduction [61]. In addition, there are diseases that can directly affect Bachmann's region such as ostium secundum atrial septal defect, interatrial septal lymphoma, septal hypertrophy or amyloidosis that may prolong the duration of interatrial conduction [62-64]. A fixed and dynamic elongation of the fibres responsible for atrial conduction in situations of hypervolemia and cardiac valve diseases can damage the atrial conduction system and generate IAB.
6. IAB AND IMAGE TECHNIQUES
The Bachmann's region may be affected by the fibrosis process, which is a process not circumscribed to this region, but broader and in which the LA is involved. It is for this reason that the determination of the degree of atrial fibrosis with current imaging techniques, fundamentally transthoracic echocardiography (TTE) and cardiac magnetic resonance (CMR), will allow the characterization of the patient affected by IAB and Bayés Syndrome.
Atrial fibrosis could be defined as a significant increase in the presence of collagen fibres in atrial myocardial tissue. The myocardium is a complex tissue composed of several cell types, some of them are myocardial tissue cells (cardiomyocytes, fibroblasts, endothelial cells and smooth muscle cells) but there are also cells that migrate to the myocardium, such as lymphocytes, plasma cells, mast cells or macrophages.
These cells that migrate to the myocardium interact with their own cells both in physiological conditions and in pathological conditions [65, 66]. Of all of them, fibroblasts represent the largest proportion of myocardial cells, approximately two-thirds [67]. Under disease conditions, myofibroblasts exist in the myocardial interstice and, together with fibroblasts, they are involved in the synthesis and excessive deposition of extracellular matrix [68-70], although it has subsequently been demonstrated that this process of fibrinogenesis is mainly responsible for the fibroblasts of the atrial myocardium rather than myofibroblasts [6].
Globally, the extracellular matrix constitutes a complex network that is arranged around the different cell types, providing a mechanical support not only to the cellular component, but also to the blood vessels and nerve fibres. Thus, distributing the mechanical forces throughout the entire myocardial tissue allowing the transmission of mechanical and chemical signals to the cells and electrically separating the atria from the ventricles, facilitates the adequate transmission of the electrical impulse between the two atria [71]. Therefore, the increase in the size of the extracellular matrix, at the level of the atrial interstice, would favour the slowing of the impulse and with this the appearance of different degrees of IAB.
Atrial fibrosis generates an increase in the rigidity of the cardiac wall, resulting in a deterioration of the cardiac function and, consequently, affecting the conduction of the nerve impulse [72], thus increasing the risk of IAB development.
In animal models, atrial fibrosis has been shown to cause slowing of the conduction of the nerve impulse due to an impaired coupling between the myocytes, leading to a greater heterogeneity [73], and may favour the appearance of different degrees of IAB.
7. Echocardiography
LA can be assessed anatomically and functionally by TTE. More recently, new techniques such as speckle tracking echocardiography have been added, thus assessing the deformity of the atrial wall, facilitating the knowledge not only of anatomical changes but also of functional changes, which, in turn, are related to atrial fibrosis observed in patients with IAB.
The size of the LA is a consistent predictor of cardiovascular morbidity and mortality. The anteroposterior diameter measured in M-Mode or in 2D mode is not a useful enough tool to represent the true dimension of LA, hence it is recommended to measure its volume following both the ellipsoid and Simpson method in the 4 and 2-chambers views [74]. The LA passive volume assessment includes the measurement of the volume prior to atrial contraction, the minimum LA volume measured with the closure of the mitral valve at end diastole and the maximum LA volume that is measured just before the opening of the mitral valve at end systole. However, volume assessment in the LA active phase is obtained by measuring atrial reservoir volume (calculated as the difference between LA maximum and minimum volumes), the volume during the passive emptying phase (calculated as the difference between atrial volume and volume before atrial contraction) and volume during atrial contraction (calculated as the difference between the pre-contraction volume and the minimum volume).
Tissue Doppler Imaging (TDI) was the first echocardiographic technique used to assess atrial function. However, it is limited by its low reproducibility, because it is angle-dependent, because of the presence of artefacts in the signal and because it is unable to obtain information about the most curved portion of the atrium at the auricular ceiling. TDI is widely independent of the potential translational effects generated by adjacent myocardial segments. The techniques of strain, strain rate and speckle tracking may, however, overcome these limitations [72, 75, 76] and are capable of adequately quantifying regional myocardial function. In addition, because of atrial fibrosis and consequent reduction of atrial compliance, it damages the atrial reservoir function, these new techniques allow the detection of this damage, even before the onset of atrial dilation occurs.
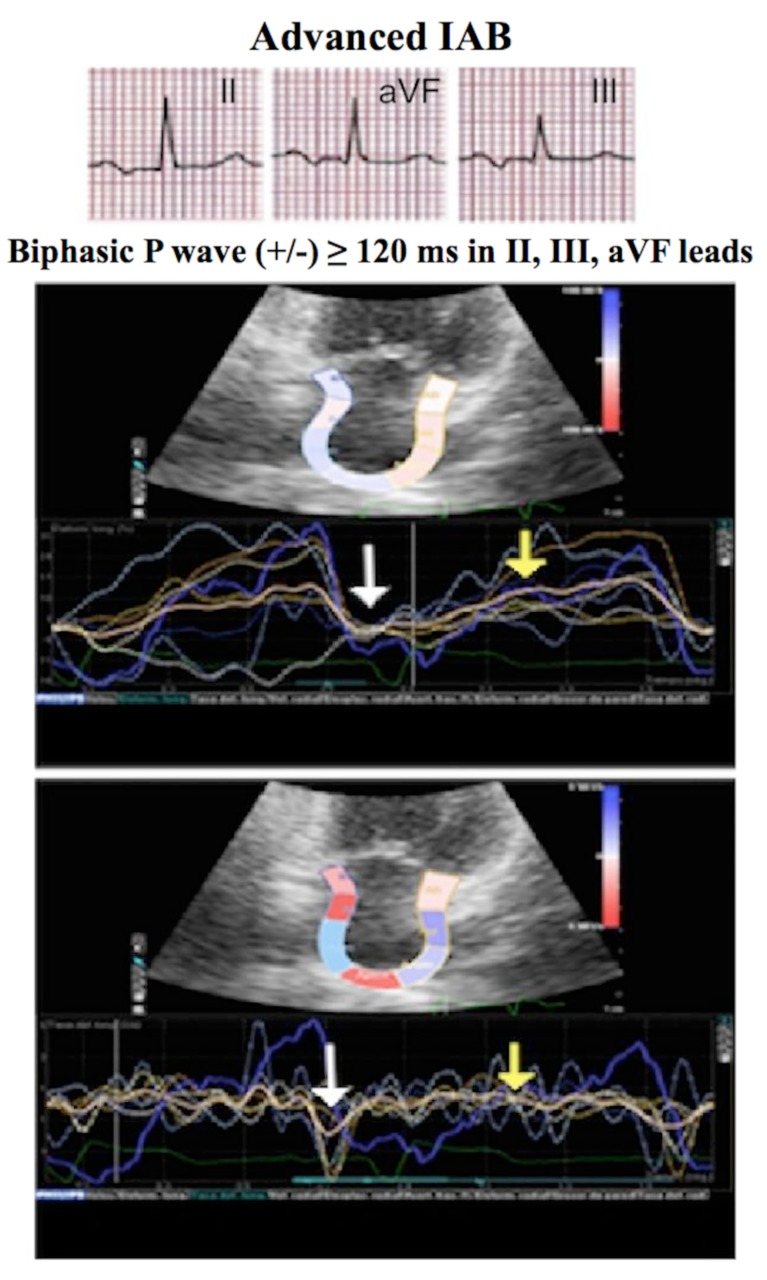
The dilation of LA has been one of the first objectives that were proposed to assess the LA function and the alteration of its structure, which could predict the onset of supraventricular arrhythmias so that a higher atrial volume would associate a greater tendency to degenerate in supraventricular arrhythmias, especially AF in elderly patients [77]. In particular, the first independent parameter associated with the risk of AF was the difference between the maximum and the lowest LA volume [78]. However, these studies showed wide interoperative variability and a low-moderate predictive capacity (Fig. 3).
Fig. (3).
Electrocardiogram, strain and strain rate of a patient with advanced IAB. In patients with advanced IAB there is a decrease in strain and strain rate in the contraction (white arrow) and reservoir (yellow arrow) phases as well as dispersion at the confluence of the deformity peaks between the different segments.
Atrial fibrosis is generated as a consequence of a complex process among factors such as age, different structural heart diseases, LA growth that occurs in diabetic patients, hypertensive patients, patients with heart failure or with mitral valvulopathy, electrical alterations, endothelial dysfunction, atrial inflammation, diverse neurohormonal alterations or genetic predisposition [79] and, all this, induces a slow and progressive process of auricular remodelling [80] which can condition the appearance of electrical disorders between the muscular fibres as dispersion of the conduction favouring, thus, the phenomenon of re-entry and the perpetuation of arrhythmias [81].
Transthoracic tissue Doppler echocardiography technique can be assess the atrial electromechanical delay (EMD) and P-wave dispersion. Both processes are damaged in various pathological inflammatory conditions and, given this, we could establish a correlation between Bayés Syndrome because these inflammatory conditions, also, can be etiologies of this syndrome.
EMD has been defined as the temporal interval between the onset of cardiac electrical activity and myocardial contraction [82]. It can be easily quantified by transthoracic tissue Doppler echocardiopgraphy, so that atrial EMD is defined as temporal interval between onset P wave on ECG and onset of tele-diastolic wave (A’) (expression of LA mechanical activation). Quintana et al. [83] evaluated LA electromechanical coupling through tissue velocity echocardiography by determining several intervals:
Temporal interval between onset P-wave on surface ECG to the onset atrial contraction (A’-wave) from the lateral mitral annulus (lateral PA).
Temporal interval between onset P-wave to the onset atrial contraction (A’-wave) from the septal mitral annulus (septal PA).
Temporal interval between onset P-wave to the onset atrial contraction (A’-wave) from right ventricular tricuspid annulus (tricuspid PA).
Give these intervals, we can define [72]:
Inter-atrial EMD as the difference between lateral PA and tricuspid PA (lateral PA – tricuspid PA).
Intra-atrial EMD as the difference between septal PA and tricuspid PA (septal PA – tricuspid PA).
Intra-left atrial EMD as the difference between lateral PA and septal PA (lateral PA – septal PA).
Also, we obtain other temporal intervals that are interesting to identify patients with IAB, such as [84, 85]:
Atrial-Ventricular (AV) time interval, measure from the onset of the atrial contraction (A’-wave), when the tissue velocity curve leaves the baseline, to its termination at the point returns to the baseline, at the onset of ventricular isovolumic contraction (IVC). Thus, the AV interval corresponds to atrial contraction time. With TDI onset of systole is characterized by motion of the myocardium toward the apex, resulting in third, positive tissue velocity curve. This curve has 2 peaks: the first, close to the onset of systole and of low amplitude, corresponded to the IVC, and the second, of higher velocity and longer duration (S), represents the ventricular myocardial motion during ejection.
Any pathological process impairing atrial conduction may result in re-entrant atrial arrhythmias [81]. Atrial conductions disorders are frequents in elderly subjects and/or those with structural heart diseases, mainly mitral valve diseases, hypertension, dilated cardiomyopathy [86-89].
P-wave dispersion is an ECG index believed to reflect heterogeneous atrial conduction by detecting abnormal atrial conduction with ECG leads of different orientation. The exact mechanism of P-wave dispersion is not well known, but it is thought that structural and electrophysiological changes in the atrial myocardium caused by changing the chemical composition of the proteins presents in cell membrane structure. Furthermore, extracellular protein deposition and interstitial fibrosis of myocardium can cause prolongation of P-wave dispersion by forming heterogeneity in atrial conduction velocity and atrial refractoriness [85].
From the point of clinician view, the EMD temporal intervals through TDI and Speckle-Tracking techniques can be help to identify patients at greater risk of developing IAB, and, thus, we are aware of appearance Bayes Syndrome in these special subjects.
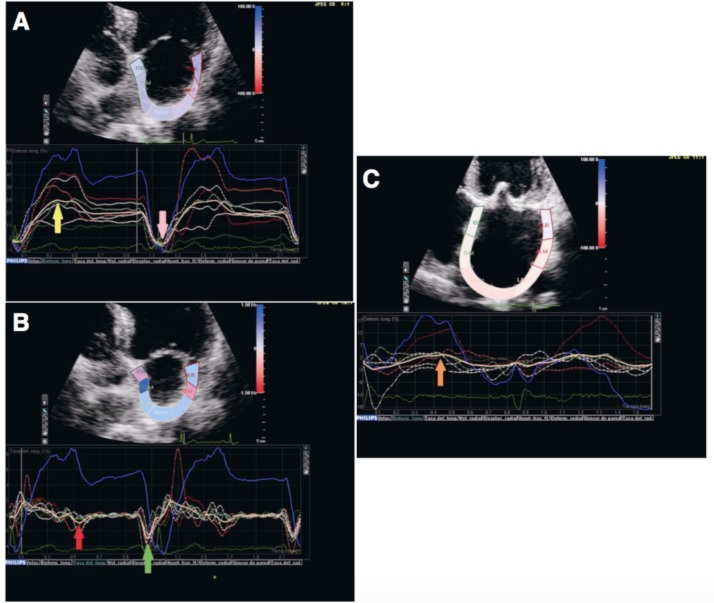
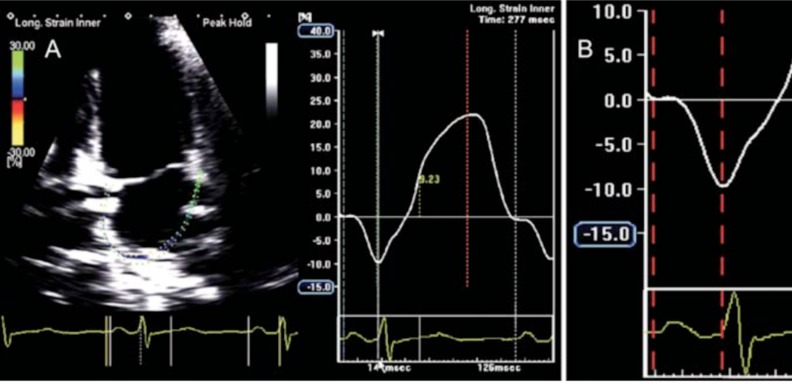
The longitudinal deformation of the LA is an excellent parameter for the evaluation of the atrial function, especially if it is evaluated when the peak of elongation or maximum compliance occurs and is represented by a positive increase of the strain or strain rate value in healthy individuals and is diminished in patients with AF and atrial fibrosis [90]. When there is sinus rhythm, the speckle-tracking technique is able to identify a positive peak strain value that is identified with the reservoir phase during ventricular systole (Fig. 4, panel A), which is related, directly, with atrial compliance, and a negative peak value related to atrial contraction (Fig. 4, panel A). The strain rate during the left ventricular diastole has two peak negative values, the first one corresponds to the passive filling phase of the left ventricle (Fig. 4, panel B) and the second with the contractile function of the LA (Fig. 4, panel B). In the case of atrial fibrosis, there is a decrease in atrial compliance, causing a deterioration of the atrial reservoir function and consequently a reduced value of the strain (Fig. 4, panel C).
Fig. (4).
A, Overall longitudinal strain at the level of the left atrium of a healthy person. In sinus rhythm, a positive peak value corresponding to the reservoir phase (yellow arrow) and a negative peak value related to atrial contraction (pink arrow) is observed. B. Overall longitudinal stratification in a healthy person. During the left ventricular diastole, two peaks can be identified, the first corresponds to early passive filling (red arrow) and the second to the pump function of the left atrium (green arrow). C. The reduction of the atrial compliance causes a deterioration of the reservoir function, with a reduction of the strain value (orange arrow).
It has recently been demonstrated by a speckle-tracking LA analysis in patients with severe mitral regurgitation who underwent valve surgery a further reduction of the value of atrial longitudinal strain directly proportional to the degree of atrial fibrosis [91], which may be related to the higher frequency of IAB observed in this type of patients. In this study the peak value of the longitudinal strain was shown as the best parameter of those that were assessed to demonstrate LA fibrosis with an area under the curve of 0.89 [92]. This study demonstrated the existence of new parameters to evaluate atrial function, based on speckle-tracking techniques, more sensitive than the methods previously considered, which were limited to assess the size and atrial function using simple TTE to detect the consequences of atrial fibrosis. Therefore, the analysis of strain and strain rates using speckle-tracking may involve a non-invasive method of measuring the degree of atrial fibrosis and identify with them the patients at higher risk of developing IAB.
LA electromechanical coupling can be non-invasively evaluated through 2D speckle-tracking echocardiography by measuring the time from onset of the P-wave to the negative peak of the global longitudinal strain curve (Fig. 5) [72].
Fig. (5).
Global left atrial (LA) longitudinal strain (A) and detail of global LA longitudinal strain during contractile phase (B) showing and electromechanical delay, measured from the onset of the P-wave to the negative peak of the curve (time interval depicted between the two red dotted lines).
Of the aforementioned and similarly to what happens in AF, although to a much lesser extent, in the case of IAB as a consequence of atrial fibrosis, part of the atrial contribution to the ventricular filling is lost. This, in the case of FA, has been demonstrated using pulsed Doppler techniques [93] by measuring the peak velocities of the early filling wave (E) and the atrial filling wave (A). Through these parameters, the percentage of atrial contribution to left ventricular filling can be calculated by dividing the velocity-time curve of the A wave, between the total diastolic velocity-time curve of the mitral pulsed Doppler in diastole. This percentage has a lower percentage numeric value in the immediacy of a satisfactory electrical cardioversion and increases as the days that remain in sinus rhythm [94] pass and improve the contractile function of LA. As mentioned previously, and from a theoretical point of view, in patients with extensive fibrosis, there is an alteration of the atrial diastolic function so that the pressures rises at the level of LA and this increases the tension exerted on LA wall, thus generating a stimulus to generate fibrosis, so that the A wave of pulsed Doppler can also be affected by altering the percentage of atrial contribution to ventricular filling, although this requires studies to corroborate it.
8. Magnetic resonance
Cardiac-Magnetic Resonance (CMR) allows an excellent evaluation not only of ventricular volumes, parietal thickness, systolic function of both ventricles or valvular function, but, through the administration of gadolinium and the so-called “sequences of delayed myocardial enhancement” allows to detect areas of fibrosis which may have prognostic value. The gadolinium behaves like a marker of the interstitial space, so that in the healthy myocardium the interstitial space is very small, due to the great compaction of the sarcomeres, accumulating little contrast. Instead, any situation that increases the interstitial space, such as fibrosis, will increase the concentration of gadolinium per unit of tissue volume.
The importance of adequately detecting myocardial fibrosis has led to the development of new imaging modalities such as the so-called T1 study of myocardial mapping. In order to obtain this type of sequence, a brief apnea of about 10 seconds is required, which allows the calculation of myocardial T1 relaxation time, which is directly related to the presence of diffuse myocardial fibrosis. This technique makes it possible to detect the presence of fibrosis even before there is evident structural damage. However, the cost, the high level of experience and the limited availability of the technique limits its use in the daily clinical setting.
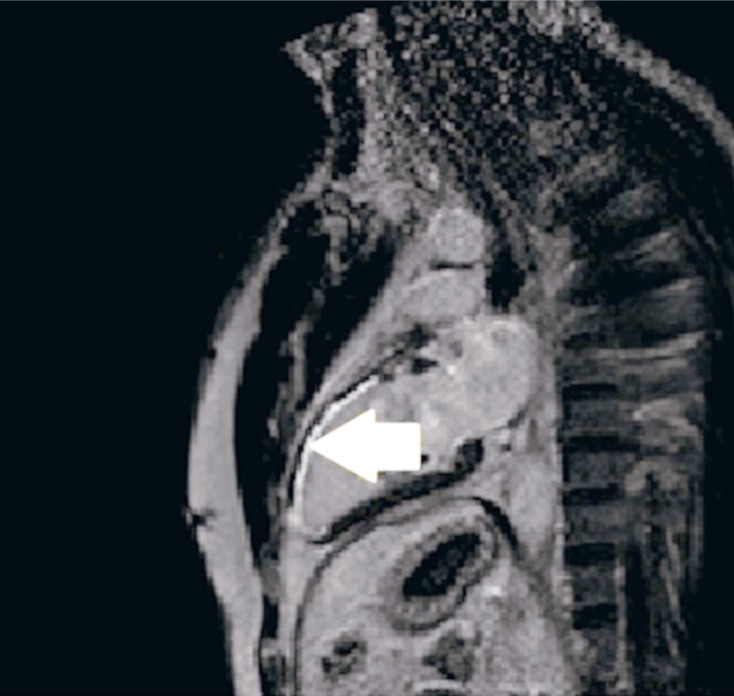
Today, one of the main indications for CMR is the identification of myocardial fibrosis by late myocardial enhancement. Myocardial fibrosis in the left ventricle is shown as a hyper-intense area, while healthy areas appear as hypo-intense or null areas (Fig. 6).
Fig. (6).
Cardiac magnetic resonance with gadolinium. It shows a hyper-intense area in the territory corresponding to the anterior descending artery with transmural affection indicating degree of fibrosis at this level.
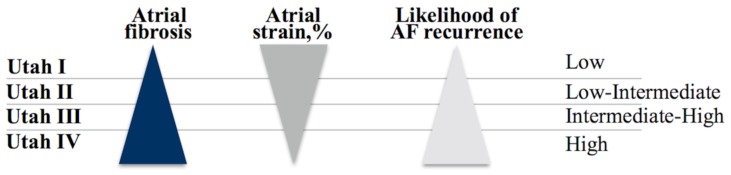
The first study of atrial evaluation by CMR consisted of measures of atrial volumes [95, 96]. Depending on the degree of fibrosis in the atrial wall, a new classification has been proposed, developed from imaging techniques such as CMR and TTE using LA strain technique, dividing patients into four large groups [97]: Utah I (less than or equal to 5% delayed enhancement in the atrial myocardium), Utah II (between 5 and 20%), Utah III (between 20 and 35%) and Utah IV (greater than 35%), so that patients with a higher degree of fibrosis, lower atrial strain, and therefore, with a higher degree of Utah, have been shown to be more likely to have persistent and/or recurrent supraventricular arrhythmias after cardiac ablation (Fig. 7). Therefore, the integration of the Utah classification and the LA strain may provide a more customised approach in daily clinical practice to identify those patients who are more susceptible to arrhythmogenic development.
Fig. (7).
The black triangle represents the degree of fibrosis according to the Utah classification. The dark grey triangle represents the degree of atrial longitudinal strain according to the Utah classification and the light grey triangle represents the probability of recurrence of atrial fibrillation according to the Utah classification.
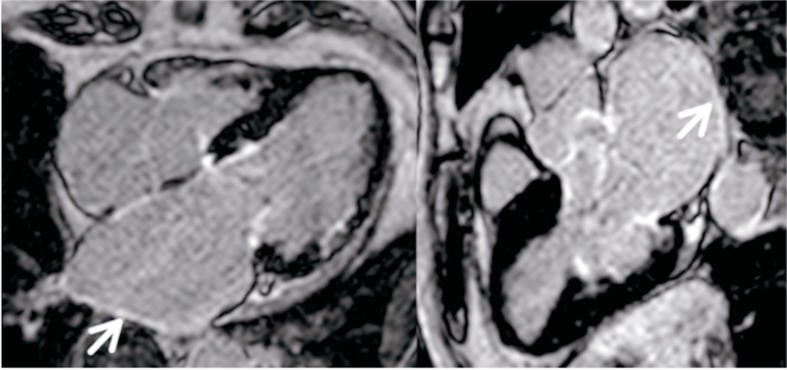
CMR has been considered as the gold-standard imaging technique for the diagnosis of fibrotic tissue at a ventricular level [98]. The two main steps to analyse atrial fibrosis by CMR are, on the one hand, the division of the anatomical structures of the atrial myocardium, which is developed manually and, therefore, consumes a significant part of the analysis time. On the other hand, the detection of areas of fibrosis inside the atrial walls. The inclusion of fatty tissue or extra-auricular tissues may lead to an erroneous diagnosis of fibrotic scarring. The assessment of atrial fibrosis by means of CMR has several associated difficulties such as atrial wall thinness (1-2 mm) and limited resolution coupled with the unpredictable shape of the LA wall. Fibrotic changes are seen as fine areas of delayed enhancement at the level of the atrial wall (Fig. 8).
Fig. (8).
Cardiac magnetic resonance image of a patient with ischemic cardiomyopathy showing left atrial dilation with late contrast uptake and hyper-intense fine areas along the atrial wall in relation to atrial fibrosis (arrows).
The main clinical application of CMR involves the non-invasive evaluation of the degree of atrial fibrosis and the prediction of the risk of recurrence of AF after ablation. A large amount of fibrosis, and therefore, gadolinium deposition in the ablation zone, represents a zone of isolation of the focus of AF and is therefore associated with a lower degree of recurrence of the arrhythmia [99].
More recently, a technique based on the T1 mapping sequence and the quantification of the extracellular volume fraction of LA has been proposed. The relaxation time T1 of the posterior wall of LA is shorter in patients with AF than in the controls. On the other hand, it is well known that there is an inverse linear relationship between left ventricular late lavage time in T1 sequences and the amount of myocardial fibrosis [100, 101], so it has been suggested that this value may be related to the degree of LA fibrosis. However, the above statement comes from preliminary studies that need further confirmation taking into account the limitations of the T1 technique in terms of safety and reproducibility.
The possibility of detecting fibrosis by means of late contrast uptake with CMR is controversial, since a study should be carried out to analyse this technique with regard to the gold technique such as the histological study, which is very difficult to perform in humans.
More recently, Benito E. et al. have shown the association between advanced IAB and atrial fibrosis in both atria detected by late gadolinium enhacement CMR, explaining the abnormal atrial activation. Overall, this publication demonstrates non-invasively method to detect atrial fibrosis and this is a key factor in explaining the ECG pattern of advanced IAB [102].
CMR, like TEE, helps to identify areas of fibrosis at the LA level that can prolong conduction at the interatrial level and allow identification of patients at increased risk of developing IAB and possible supraventricular arrhythmias.
9. Limitations
The main limitations of these imaging techniques are, in the case of the TTE, in the wide variability in cut-off points for the global strain values of LA [54], to which is added the fact that each company, with its software, uses different algorithms. There are also no validation studies to categorize atrial deformity since strain techniques were initially developed to assess ventricular function. It is more difficult to adequately assess the thin atrial wall through speckle-tracking. A possible solution to this would be the standardization of strain measurements by the different companies in charge of it [103].
As for CMR and the uptake of gadolinium delayed enhancement for the detection of fibrosis, there is a limitation that should be corroborated by anatomopathological studies, which is difficult to perform in humans. There is also poor inter- and intra-operative reproducibility when it comes to assessing fibrosis by CMR, which is partly related to the absence of standardized protocols when acquiring and processing images, since their visual evaluation is quite dependent operator, besides having a wide inter-observer variability. All this could be reduced with new software, new technological advances and improved resolution of CMR equipment that will allow more automatic processing of images. Until then it is essential to develop standardized protocols that allow a uniform assessment of the acquisition and processing of images [9].
Consent for Publication
Not applicable.
ACKNOWLEDGEMENTS
The authors thank Dr. Bayés de Luna for allowing us to use Fig. (2) in the present review.
CONFLICT OF INTEREST
The author declares no conflict of interest, financial or otherwise.
REFERENCES
- 1.Bachmanm G. The significance of splitting of the P-wave in the electrocardiogram. Ann. Intern. Med. 1941;14(9):1702–1709. [Google Scholar]
- 2.Bachmann G. The inter-auricular time interval. Am J Physiol-Leg Content. 1916;41:309–320. [Google Scholar]
- 3.Bayés de Luna A. Bloqueo a nivel auricular. Rev. Esp. Cardiol. 1979;39:5. [PubMed] [Google Scholar]
- 4.Bayés de Luna A., Fort de Ribot R., Trilla E., et al. Electrocardiographic and vectocardiographic study of interatrial conduction disturbances with left atrial retrograde activation. J. Electrocardiol. 1985;18:1–13. doi: 10.1016/s0022-0736(85)80029-7. [DOI] [PubMed] [Google Scholar]
- 5.Conde D., Baranchuk A. Interatrial block as anatomical-electrical substrate for supraventricular arrhythmias: Bayes syndrome. Arch. Cardiol. Mex. 2014;84:32–40. doi: 10.1016/j.acmx.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Octavian I., Ionica P., Anca-María O., et al. Evaluation of cardiac microvasculature in patients with diffuse myocardial fibrosis. Rom. J. Morphol. Embryol. 2016;57(4):1351–1356. [PubMed] [Google Scholar]
- 7.Gramley F., Lorenzen J., Knackstedt C., et al. Age-related atrial fibrosis. Age (Dordr.) 2009;31:27–38. doi: 10.1007/s11357-008-9077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fleg J.L., Strait J. Age-associated changes in cardiovascular structure and function: a fertile milieu for future disease. Heart Fail. Rev. 2012;17:545–554. doi: 10.1007/s10741-011-9270-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pontecorboli G., Figueras I., Ventura R.M., et al. Use of delayed-enfacement magnetic resonance imaging for fibrosis detection in the atria: a review. Europace. 2016;155:469–473. doi: 10.1093/europace/euw053. [DOI] [PubMed] [Google Scholar]
- 10.Tereshchenko L.G. Correlation of interatrial block with left atrial enlargement and P-Wave Terminal Force. In: Baranchuck A., editor. Interatrial block and Supraventricular Arrhythmias. Clinical implications of Bayés’ syndrome. 1st ed. Cardiotext Publishing; 2016. pp. 102–103. [Google Scholar]
- 11.Lacalzada-Almeida J., García-Niebla J. How to detect atrial fibrosis. J. Geriatr. Cardiol. 2017;14(3):185–194. doi: 10.11909/j.issn.1671-5411.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conde D., Seoane L., Gysel M., Miltrione S., Bayés de Luna A., Baranchuk A. Bayes’ syndrome: the association between interatrial block and supraventricular arrhythmias. Expert Rev. Cardiovasc. Ther. 2015;13:541–550. doi: 10.1586/14779072.2015.1037283. [DOI] [PubMed] [Google Scholar]
- 13.Tapanainen J.M., Juro R., Holmqvist F., et al. Interatrial right-to-lef conduction in patients with paroxysmal atrial fibrillation. J. Interv. Card. Electrophysiol. 2009;25:117–122. doi: 10.1007/s10840-008-9359-2. [DOI] [PubMed] [Google Scholar]
- 14.Ho S.Y., Anderson R.H., Sánchez-Quintana D. Atrial structure and fibres; Morphologic bases of atrial conduction. Cardiovasc. Res. 2002;54:325–326. doi: 10.1016/s0008-6363(02)00226-2. [DOI] [PubMed] [Google Scholar]
- 15.Harrild D., Henriquez C. A computer model of normal condcution in the human atria. Circ. Res. 2000;87:E25–E36. doi: 10.1161/01.res.87.7.e25. [DOI] [PubMed] [Google Scholar]
- 16.Ariayarajah V., Ali M., Spodick D.H. Intermittent advanced atrial depolarization abnormality? Cardiology. 2008;110:68–72. doi: 10.1159/000109409. [DOI] [PubMed] [Google Scholar]
- 17.Chung D.K., Chung E.K. Post-ectopic inhibition phenomenon. W. V. Med. J. 1972;68:168–169. [PubMed] [Google Scholar]
- 18.Bayés de Luna A., Platonov P., Cosio F.G., et al. Interatrial blocks. A separate entity from left atrial enlargement: a consensus report. J. Electrocardiol. 2012;45:445–451. doi: 10.1016/j.jelectrocard.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 19.Chhabra L., Devadoss R., Chaubey V.K., Spodick D.H. Interatrial block in the modern era. Curr. Cardiol. Rev. 2014;10:181–189. doi: 10.2174/1573403X10666140514101748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bayés de Luna A., Guindo J., Vinolas X., Martínez Rubio A., Oter R., Bayes-Genis A. Third-degree inter-atrial block and supra ventricular tachyarrhythmias. Europace. 1999;1:43–46. doi: 10.1053/eupc.1998.0006. [DOI] [PubMed] [Google Scholar]
- 21.Boineau J. The prolonged P wave and intertrial block. Time to consider a broader concept and different terminology. J. Electrocardiol. 2005;38:327–329. doi: 10.1016/j.jelectrocard.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Bayés de Luna A. Textbook of clinical electrocardiography. Oxford, UK: Wiley-Blackwell; 2012. [Google Scholar]
- 23.Jairath U.C., Spodick D.H. Exceptional prevalence of intertrial block in a general hospital population. Clin. Cardiol. 2001;24:548–550. doi: 10.1002/clc.4960240805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asad N., Spodick D.H. Prevalence of intertribal block in a general hospital population. Am. J. Cardiol. 2003;91:609–610. doi: 10.1016/s0002-9149(02)03320-9. [DOI] [PubMed] [Google Scholar]
- 25.Ariyarajah V., Asad N., Tandar A., Spodick D.H. Interatrial block: pandemic prevalence, significance, and diagnosis. Chest. 2005;128:970–975. doi: 10.1378/chest.128.2.970. [DOI] [PubMed] [Google Scholar]
- 26.Gialafos E., Psaltopoulou T., Papaioannou T.G., et al. Prevalence of interatrial block in young healthy men < 35 years of age. Am. J. Cardiol. 2007;100:995–997. doi: 10.1016/j.amjcard.2007.04.041. [DOI] [PubMed] [Google Scholar]
- 27.Martinez-Selles M., Masso-van Roessel A., Álvarez-García J., et al. Interatrial block and atrial arrhythmias in centenarians: prevalence, associations, and clinical implications. Heart Rhythm. 2016;13:645–651. doi: 10.1016/j.hrthm.2015.10.034. b. [DOI] [PubMed] [Google Scholar]
- 28.Huo Y., Mitrofanova L., Orshanskaya V., Holmberg P., Holmqvist F., Platonov P.G. P-wave characteristics and histological atrial abnormality. J. Electrocardiol. 2014;2014(47):275–280. doi: 10.1016/j.jelectrocard.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 29.Rossi S., Fortunati I., Carnevali L., et al. The effect of aging on the specialized conducting system: a telemetry ECG study in rats over a 6 month period. PLoS One. 2014;9:e112697. doi: 10.1371/journal.pone.0112697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martínez-Sellés M., Lambert Rodríguez J.L., Barrios V., et al. Clinical cardiology, geriatric cardiology, heart failure, and transplantation 2015: a selection of topical issues. Rev. Esp. Cardiol. 2016;69:159–166. doi: 10.1016/j.rec.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 31.Go A.S., Hylek E.M., Philips K.A., et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 32.Martínez-Sellés M., García de la Villa B., Cruz-Jentoft A.J., et al. Centenarians and their hearts: A prospective registry with comprenhensive geriatric assessment, electro-cardiogram, echocardiography, and follow-up. Am. Heart J. 2015;169:798–805.e2. doi: 10.1016/j.ahj.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Ariyarajah V., Spodick D. Interatrial block: a prevalent, widely neglected and portentous abnormality. J. Electrocardiol. 2008;41:61–62. doi: 10.1016/j.jelectrocard.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 34.Conde D., Baranchuk A., Bayés de Luna A. Advanced interatrial block as a substrate of supraventricular tachyarrhythmias: a well recongnized syndrome. J. Electrocardiol. 2015;48:135–140. doi: 10.1016/j.jelectrocard.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 35.Barachova L., Wagner G.S. The time for naming the interatrial block Syndrome: Bayes Syndrome. J. Electrocardiol. 2015;48:133–134. doi: 10.1016/j.jelectrocard.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 36.Ninios I., Pliakos C., Ninios V., et al. Prevalence of interatrial block in a general population of elderly people. Ann. Noninvasive Electrocardiol. 2007;12:298–300. doi: 10.1111/j.1542-474X.2007.00178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fleg J.L., Strait J. Age-associated changes in cardiovascular structure and function: a fertile milieu for future disease. Heart Fail. Rev. 2012;17:545–554. doi: 10.1007/s10741-011-9270-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ariyarajah V., Puri P., Kranis M., et al. Prevalence of interatrial block in the Program of All-Inclusive Care for the Elderly (PACE). Am. J. Geriatr. Cardiol. 2006;15:174–177. doi: 10.1111/j.1076-7460.2006.04518.x. [DOI] [PubMed] [Google Scholar]
- 39.O’Neal W.T., Zhang Z.M., Loehr L.R., et al. Electrocardiographic advanced interatrial block and atrial fibrillation risk in the general population. Am. J. Cardiol. 2016;117:1755–1759. doi: 10.1016/j.amjcard.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Neal W.T., Kamel H., Zhang Z.M., Chen L.Y., Alonso A., Soliman E.Z. Advanced interatrial block and ischemic stroke: The Atherosclerosis Risk in Communities Study. Neurology. 2016;87(4):352–356. doi: 10.1212/WNL.0000000000002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Acharya T., Tringali S., Bhullar M., et al. Frecuent atrial premature complexes and their association with risk of atrial fibrillation. Am. J. Cardiol. 2015;116(12):1852–1857. doi: 10.1016/j.amjcard.2015.09.025. [DOI] [PubMed] [Google Scholar]
- 42.Alexander BD, Sadiq F, Azimi K, et al. Reverse atrial electrical remodeling induced by cardiac resynchronization therapy. J Electrocardiol . 2017 doi: 10.1016/j.jelectrocard.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 43.Sadiq Ali F, Enriquez A, Conde D, et al. Advanced interatrial block predicts new onset atrial fibrillation in patiens with severe heart failure and cardiac resynchronization therapy. 2015 doi: 10.1111/anec.12258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alvarez-García J, Massó-van Roessel A, Vives-Borrás M, et al. Prevalence, clinical profile and short-term prognosis of interatrial block in patients admitted for worsening of heart failure. J Am Coll Cardiol . 2016 [Google Scholar]
- 45.Conde D., van Oosten E.M., Hamilton A., et al. Prevalence of interatrial block in patients undergoing coronary bypass graft surgery. Int. J. Cardiol. 2014;171:e98–e99. doi: 10.1016/j.ijcard.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 46.Alexander B., MacHaalany J., Lam B., et al. Comparison of the extent of coronary artery disease in patients with versus without interatrial block and implications for New-Onset Atrial Fibrillation. Am. J. Cardiol. 2017;119(8):1162–1165. doi: 10.1016/j.amjcard.2016.12.032. [DOI] [PubMed] [Google Scholar]
- 47.Agarwal Y.K., Aronow W.S., Levy J.A., Spodick D.H. Association of inreratrial block with development of atrial fibrillation. Am. J. Cardiol. 2003;91:882. doi: 10.1016/s0002-9149(03)00027-4. [DOI] [PubMed] [Google Scholar]
- 48.Abe Y., Fukumami M., Yamada T., et al. Prediction of transition to chronic atrial fibrillation in patients with paroxysmal atrial fibrillation by signal-averaged electrocardiography: a prospective study. Circulation. 1997;96:2612–2616. doi: 10.1161/01.cir.96.8.2612. [DOI] [PubMed] [Google Scholar]
- 49.Rensma P.L., Allesie M.A., Lammers W.J., et al. Length of excitation wave and susceptibility to reentrant atrial arrhythmias in normal conscious dogs. Circ. Res. 1988;62:395–410. doi: 10.1161/01.res.62.2.395. [DOI] [PubMed] [Google Scholar]
- 50.Ariyarajah V., Mercado K., Apiyasawat S., Spodick D.H. Correlation of left atrial size with P-wave duration on inreratrial block. Chest. 2005;128:2615–2618. doi: 10.1378/chest.128.4.2615. [DOI] [PubMed] [Google Scholar]
- 51.Goyal S.B., Spodick D.H. Electromechanical dysfunction of the left atrium associated with interatrial block. Am. Heart J. 2001;142:823–827. doi: 10.1067/mhj.2001.118110. [DOI] [PubMed] [Google Scholar]
- 52.Hughes T.M., Worrall B.B. Acute interatrial block is a distinct risk factor for ischemic stroke. Neurology. 2016;87:344–345. doi: 10.1212/WNL.0000000000002905. [DOI] [PubMed] [Google Scholar]
- 53.Arboix A., Martí L., Dorison S., Sanchez M.J. Bayés syndrome and acute cardioembolic ischemic stroke. World J. Clin. Cases. 2017;5(3):93–101. doi: 10.12998/wjcc.v5.i3.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Apiyasawat Sl., Thomas A.J., Spodick D.H. Interatrial block during exercise tolerance tests as an additional parameter for the diagnosis of ischemic heart disease. J. Electrocardiol. 2005;38(4) Suppl.:150–153. doi: 10.1016/j.jelectrocard.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 55.Gami A.S., Pressman G., Caples S.M., et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation. 2004;110:364–367. doi: 10.1161/01.CIR.0000136587.68725.8E. [DOI] [PubMed] [Google Scholar]
- 56.Baranchuk A., Parfrey B., Lim L., et al. Interatrial block in patients with obstructive sleep apnea. Cardiol. J. 2011;18:171–175. [PubMed] [Google Scholar]
- 57.Can I., Aytemir K., Demir A.U., et al. P-wave duration and dispersion in patients with obstructive sleep apnea. Int. J. Cardiol. 2009;133:e85–e89. doi: 10.1016/j.ijcard.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 58.Cagirci G., Cay S., Gulsoy K.G., et al. Tissue Doppler atrial conduction times and electrocardiogram interlead P-wave durations with varying severity of obstructive sleep apnea. J. Electrocardiol. 2011;44:478–482. doi: 10.1016/j.jelectrocard.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 59.Maeno K., Kasai T., Kasagi S., et al. Relationship between atrial conduction delay and obstructive sleep apnea. Heart Vessels. 2013;28(5):639–645. doi: 10.1007/s00380-012-0288-8. [DOI] [PubMed] [Google Scholar]
- 60.Mizuno R., Fujimoto S., Nakano H., et al. Atrial involvement in patients with progressive systemic sclerosis: relationship between ultrasonic tissue characterization of the atrium and interatrial conduction. Cardiology. 1999;91:134–139. doi: 10.1159/000006893. [DOI] [PubMed] [Google Scholar]
- 61.Anderson E.J., Kypson A.P., Rodríguez E., Anderson C.A., Lehr E.J., Neuer P.D. Substrate-specific derangements in mitochondrial metabolism and redox balance in the atrium of the type 2 diabetic human heart. J. Am. Coll. Cardiol. 2009;54:1891–1898. doi: 10.1016/j.jacc.2009.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Engelen M.A., Juergens K.U., Breithardt G., Eckardt L. Interatrial conduction delay and atrioventricular block due to primary cardiac lymphoma. J. Cardiovasc. Electrophysiol. 2005;16:926. doi: 10.1046/j.1540-8167.2005.50003.x. [DOI] [PubMed] [Google Scholar]
- 63.Thilén U., Carlson J., Platonov P.G., Havmöller R., Olsson S.B. Prolonged P wave duration in adults with secundum atrial septal defect: a marker of delayed conduction rather than increased atrial size? Europace. 2007;9(Suppl. 6):vi105–vi108. doi: 10.1093/europace/eum214. [DOI] [PubMed] [Google Scholar]
- 64.Röcken C., Peters B., Juenemann G., et al. Atrial amyloidosis: an arrhythmogenic substrate for persistent atrial fibrillation. Circulation. 2002;106:2091–2097. doi: 10.1161/01.cir.0000034511.06350.df. [DOI] [PubMed] [Google Scholar]
- 65.Fan D., Takawale A., Lee K., Kassiri Z. Cardiac fibroblasts, fibrosis and extracellular matrix remodeling in heart disease. Fibrogenesis Tissue Repair. 2012;5(1):15. doi: 10.1186/1755-1536-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Souders C.A., Bowers S.L., Baudino T.A. Cardiac fibroblast: the renaissance cell. Circ. Res. 2009;105(12):1164–1176. doi: 10.1161/CIRCRESAHA.109.209809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Camelliti P., Borg T.K., Kohl P. Structural and functional characterization of cardiac fibrosis. Cardiovasc. Res. 2005;65(1):40–51. doi: 10.1016/j.cardiores.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 68.Tomasek J.J., Gabbiani G., Hinz B., Chaponnier C., Brown R.A. Myofibroblast and mechano-regulation of connective tissue remodeling. Nat. Rev. Mol. Cell Biol. 2002;3(5):349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 69.Petrov V.V., Fagard R.H., Lijnen P.J. Stimulation of collagen production by transforming growth factor-beta1 during differentiation of cardiac fibroblasts to myofibroblasts. Hypertension. 2002;39(2):258–263. doi: 10.1161/hy0202.103268. [DOI] [PubMed] [Google Scholar]
- 70.Baum J., Duffy H.S. Fibroblast and myofibroblasts: what are we talking about? J. Cardiovasc. Pharmacol. 2011;57(4):376–379. doi: 10.1097/FJC.0b013e3182116e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Krenning G., Zeisberg E.M., Kalluri R. The origin of fibroblasts and mechanism of cardiac fibrosis. J. Cell. Physiol. 2010;225(3):631–637. doi: 10.1002/jcp.22322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Todaro M.C., Choudhuri I., Belohlavek M., et al. New echocardiographic techniques for evaluation of left atrial mechanics. Eur. Heart J. Cardiovasc. Imaging. 2012;13:973–984. doi: 10.1093/ehjci/jes174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li D., Fareh S., Leung T.K., Nattel S. Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation. 1999;100:87–95. doi: 10.1161/01.cir.100.1.87. [DOI] [PubMed] [Google Scholar]
- 74.Lang R.M., Bierig M., Devereux R.B., et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J. Am. Soc. Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 75.Blume G.G., McLeod C.J., Barnes M.E., et al. Left atrial function: Physiology, assessment, and clinical implications. Eur. J. Echocardiogr. 2011;12:421–430. doi: 10.1093/ejechocard/jeq175. [DOI] [PubMed] [Google Scholar]
- 76.Cameli M., Lisi M., Righini F., et al. Usefulness of atrial deformation analysis to predict left atrial fibrosis and endocardial thickness in patients with undergoing mitral valve operations for severe mitral regurgitation secondary to mitral valve prolapse. Am. J. Cardiol. 2013;111:595–601. doi: 10.1016/j.amjcard.2012.10.049. [DOI] [PubMed] [Google Scholar]
- 77.Tsang T.S., Barnes M.E., Bailey K.R., et al. Left atrial volume: important risk marker of incident atrial fibrillation in 1655 older men and women. Mayo Clin. Proc. 2001;76(5):467–475. doi: 10.4065/76.5.467. [DOI] [PubMed] [Google Scholar]
- 78.Fatema K., Barnes M.E., Bailey K.R., et al. Minimun vs. maximum left atrial volume study. Eur. J. Echocardiogr. 2009;10(2):282–286. doi: 10.1093/ejechocard/jen235. [DOI] [PubMed] [Google Scholar]
- 79.Calenda B.W., Fuster V., Halperin J.L., Granger C.B. Stroke risk assessment in atrial fibrillation: risk factors and markers of atrial myopathy. Nat. Rev. Cardiol. 2016;13:549–559. doi: 10.1038/nrcardio.2016.106. [DOI] [PubMed] [Google Scholar]
- 80.Allesie M.A., De Groot N.M., Houben R.P., et al. Electropathological substrate of long-standing persistent atrial fibrillation in patients with structural heart disease longitudinal dissociation. Circ Arrhythm Electrophysiol. 2010;3:606–615. doi: 10.1161/CIRCEP.109.910125. [DOI] [PubMed] [Google Scholar]
- 81.Spach M.S., Josephson M.E. Initiating reentry: the role of non-uniform anisotropy in small circuits. J. Cardiovasc. Electrophysiol. 1994;5:182–209. doi: 10.1111/j.1540-8167.1994.tb01157.x. [DOI] [PubMed] [Google Scholar]
- 82.Cui Q.Q., Zhang W., Wang H., et al. Assessment of atrial electromechanical coupling and influencial factors in non-rheumatic paroxysmal atrial fibrillation. Clin. Cardiol. 2008;31:74–78. doi: 10.1002/clc.20162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Quintana M., Lindell P., Saha S.K., et al. Assessment of atrial regional and global electromechanical function by tissue velocity echocardiography: a feasibility study on health individuals. Cardiovasc. Ultrasound. 2005;3:4. doi: 10.1186/1476-7120-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Deniz A., Sahiner I., Aytemir K., et al. Tissue Doppler Echocardiography can be useful technique to evaluate atrial conduction time. Cardiol. J. 2012;19:487–493. doi: 10.5603/cj.2012.0089. [DOI] [PubMed] [Google Scholar]
- 85.Rein A.J., O’Donnel C.P., Colan S.D., Mark G.R. Tissue velocity doppler assessment of atrial and ventricular electromechanical coupling and atrioventricular time intervals in normal subjects. Am. J. Cardiol. 2003;92:1347–1350. doi: 10.1016/j.amjcard.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 86.Pekdemir H., Cancel M., Yagmur J., et al. Assessment of atrial conduction time by tissue Doppler echocardiography and P-wave dispersión in patients with mitral annulus calcificaction. J. Electrocardiol. 2010;43:339–343. doi: 10.1016/j.jelectrocard.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 87.Pala S., Tigen K., Karaahmet T., et al. Assessment of atrial electromechanical delay by tissue Doppler echocardiography in patients with nonischemic dilated cardiomyopathy. J. Electrocardiol. 2010;43:344–350. doi: 10.1016/j.jelectrocard.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 88.Akcay A., Acar G., Suner A., et al. Effects of slow coronary artery flow on P-wave dispersion and atrial electromechanical coupling. J. Electrocardiol. 2009;42:328–333. doi: 10.1016/j.jelectrocard.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 89.Emiroglu M.Y., Bulut M., Sahin M., et al. Assessment of atrial conduction time in patients with essential hypertension. J. Electrocardiol. 2011;44:251–256. doi: 10.1016/j.jelectrocard.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 90.Vianna-Pinton R., Moreno C.A., Baxter C.M., et al. Two-dimensional speckle-tracking echocardiography of the left atrium: feasibility and regional contraction and relaxation differences in normal subjects. J. Am. Soc. Echocardiogr. 2009;22:229–305. doi: 10.1016/j.echo.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 91.Cameli M., Lisi M., Righini F.M., et al. Usefulness of atrial deformation analysis to predict lef atrial fibrosis and endocardial thickness in patients undergoing mitral valve operations for severe mitral regurgitation secondary to mitral valve prolapse. Am. J. Cardiol. 2013;111:595–601. doi: 10.1016/j.amjcard.2012.10.049. [DOI] [PubMed] [Google Scholar]
- 92.Ferguson J.J., III, Manning W.J., Come P.C. Pulsed Doppler echocardiographic determination of the time course of left ventricular filling: validation with cineangiography. Am. Heart J. 1989;117:127–132. doi: 10.1016/0002-8703(89)90666-2. [DOI] [PubMed] [Google Scholar]
- 93.Manning W.J., Leeman D.E., Gotch P.J., Come P.C. Pulsed doppler evaluation of atrial mechanical function after electrical cardioversion of atrial fibrillation. J. Am. Coll. Cardiol. 1989;13:617–623. doi: 10.1016/0735-1097(89)90602-5. [DOI] [PubMed] [Google Scholar]
- 94.Therkeisen S.K., Groenning B.A., Svendsen J.H., Jensen G.B. Atrial and ventricular volume and function in persistent and permanent atrial fibrillation, a magnetic resonance imagin study. J. Cardiovasc. Magn. Reson. 2005;7:465–473. doi: 10.1081/jcmr-200053618. [DOI] [PubMed] [Google Scholar]
- 95.Therkeisen S.K., Groenning B.A., Svendsen J.H., Jensen G.B. Atrial and ventricular volume and function evaluated by magnetic resonance imaging in patients with persistent atrial fibrillation before and after cardioversion. Am. J. Cardiol. 2006;97:1213–1219. doi: 10.1016/j.amjcard.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 96.Mahnkopf C., Badger T.J., Burgon N.S., et al. Evaluation of the left atrial substrate in patients with lone atrial fibrillation using delayed-enhanced MRI: implications for disease progression and response to catheter ablation. Heart Rhythm. 2010;7:1475–1481. doi: 10.1016/j.hrthm.2010.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim R.J., Wu E., Rafael A., et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N. Engl. J. Med. 2000;343:1445–1453. doi: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- 98.Peters D.C., Wylie J.V., Hauser T-H., et al. Detection of pulmonary vein and left atrial scar after catheter ablation with three-dimensional navigator-gated delayed enhancement MR imaging: initial experience. Radiology. 2007;243:690–695. doi: 10.1148/radiol.2433060417. [DOI] [PubMed] [Google Scholar]
- 99.Iles L., Pfluger H., Phrommintikul A., et al. Evaluation of diffuse myocardial fibrosis in heart failure with cardiac magnetic resonance contrast-enhanced T1mapping. J. Am. Coll. Cardiol. 2008;52:1574–1580. doi: 10.1016/j.jacc.2008.06.049. [DOI] [PubMed] [Google Scholar]
- 100.Sibley C.T., Noureldin R.A., Gai N., et al. T1 mapping in cardiomyopathy at cardiac MR: comparison with endomyocardial biopsy. Radiology. 2012;265:724–732. doi: 10.1148/radiol.12112721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Beinart R., Khurram I.M., Liu S., et al. Cardiac magnetic resonance T1 mapping of left atrial myocardium. Heart Rhythm. 2013;10:1325–1331. doi: 10.1016/j.hrthm.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Benito E.M., Bayés de Luna A., Baranchuk A., Lluis M. Extensive atrial fibrosis assessed by late gadolinium enhacement cardiovascular magnetic resonance associated with advanced interatrial block electrocardiogram pattern. Europace. 2017;19(3):377. doi: 10.1093/europace/euw294. [DOI] [PubMed] [Google Scholar]
- 103.Voigt J.U., Pedrizzetti G., Lysyansky P., et al. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Indusrty Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging. 2015;16:1–11. doi: 10.1093/ehjci/jeu184. [DOI] [PubMed] [Google Scholar]