Abstract
Background:
Hemodynamics during dynamic exercise is finely regulated by some neural mechanisms. One of these mechanisms is the metabolic part of the exercise pressor reflex, i.e. the muscle metaboreflex. Hemodynamic response during the metaboreflex is characterised by the re-cruitment of the reserves in cardiac inotropism, pre-load, after-load and chronotropism. If one of these reserves is exhausted, then the cardiovascular response is achieved by recruiting one of the other re-serves, thereby indicating a remarkable plasticity of the control of circulation.
Conclusion:
In this review, the effects of a number of cardiovascular diseases – such as heart failure, heart failure with preserved ejection fraction, hypertension, type 1 and type 2 diabetes mellitus, obesi-ty and metabolic syndrome - on hemodynamics during the metaboreflex are reviewed.
Keywords: Inotropism, preload, afterload, chronotropism, systemic vascular resistance, Hemodynamics
1. INTRODUCTION: HEMODYNAMIC REGULATION DURING DYNAMIC EXERCISE IN HEALTHINESS
Physical activities such as running, cycling, and rowing require large muscle mass and produce an intense vasodilation in the working muscle due to the accumulation of metabolic by-products. Local metabolites production overwhelms the exercise-induced increase in sympathetic tone, leading to a reduction in systemic vascular resistance (SVR), a phenomenon called functional sympatholysis [1, 2]. Metabolite-induced vasodilation (i.e. SVR reduction) is a challenge for cardiovascular regulation because it would cause a drop in blood pressure. However, in healthy subjects, cardiovascular control mechanisms increase cardiac output (CO), thereby keeping mean blood pressure (MAP) at a stable level or even moderately increasing it [3-5].
This fine hemodynamic tuning is under the control of some neural mechanisms that guarantee blood supply to exercising muscle and avoid excessive MAP changes. There are at least three neural mechanisms contributing to this cardiovascular adjustment: 1) exercise pressor reflex, 2) central command, and 3) arterial baroreflex [6, 7]. The medulla oblongata contains the major nuclei that control blood pressure and the cardiovascular system and is responsible for the integration and elaboration of signals arising from these three neural mechanisms [8, 9]. It is commonly believed that the central command establishes a basal level of sympathetic activity and vagal withdrawal on the basis of the intensity of the effort and of the motor drive from the motor cortex. This basal level of autonomic activity is then adjusted on the basis of signals arising form the arterial baroreflex and from the working muscle, i.e. the exercise pressor reflex [10, 11].
In short, the cardiovascular control areas of the medulla oblongata are activated by regions of the brain responsible for motor unit recruitment which in turn set a basic level of sympathetic activation and vagal withdrawal. This autonomic modulation is then finely adjusted by the exercise pressor reflex on the basis of peripheral signals arising from mechano- and metabo- receptors (type III and IV nerve endings) within the muscle. These receptors gather information on the mechanical and metabolic status of the working muscle and reflexively adjust autonomic activity and adrenal epinephrine releases [6, 12, 13]. The autonomic response arising from the central command and the exercise pressor reflex is in turn modulated by baroreflexes, which oppose any mismatch between SVR and CO by controlling muscle vasodilation and cardiac chronotropism [7, 14-19].
Autonomic adjustments arising from the activity of central command, exercise pressor reflex and baroreflex ultimately regulate all the main hemodynamic modulators: myocardial inotropism, cardiac preload, cardiac afterload (i.e. SVR), and heart rate (HR).
In this review, we will briefly address the role played by neural feedback arising from the metabolic part of the exercise pressor reflex, the so-called muscle metaboreflex. This reflex has been demonstrated as important in the pathophysiology of exercise intolerance of several cardiovascular and metabolic diseases such as heart failure, heart failure with preserved ejection fraction, hypertension, type 1 and type 2 diabetes mellitus, obesity and metabolic syndrome.
2. MUSCLE METABOREFLEX IN CARDIOVASCULAR REGULATION DURING EXERCISE IN HEALTHINESS
Alam and Smirk [20, 21] provided the first evidence that metabolic reflex arising from skeletal muscle adjusts hemodynamics during exercise. Following this seminal research, a great bulk of evidence demonstrated that group III/IV afferents within the muscle gather information about the metabolic condition of the working muscle and are responsible for cardiovascular reflex [6, 22, 23]. This reflex was then termed muscle metaboreflex. It was later also demonstrated that mechanical changes in muscles and tendons elicit cardiovascular reflex, and this was termed mechanoreflex. These two reflexes of muscular origin together constitute the exercise pressor reflex.
Type III/IV afferents act as receptors and collect information concerning the mechanical and metabolic conditions of contracting muscles. This information is then sent to cardiovascular controlling centres located in the medulla oblongata, where the information is integrated and elaborated. In turn, the cardiovascular medullary centres organise the hemodynamic response to exercise on the basis of the mechanical and metabolic status of the working muscle.
There are many putative metabolic by-products able to activate the metaboreflex, such as lactic acid, potassium, bradykinin, arachidonic acid products, ATP, deprotonated phosphate and adenosine. Furthermore, investigations with 31P nuclear magnetic resonance spectroscopy have reported that the metaboreflex can be elicited by decrements in intramuscular pH [23-26]. It is still a matter of debate whether or not the metaboreflex is tonically activated even for mild exercise intensities or whether it is activated only when blood flow to the muscle is insufficient to warrant oxygen delivery and/or metabolites washout.
The typical hemodynamic response due to metaboreflex activation is an increase in MAP. In healthiness, in order to reach the target MAP the metaboreflex recruits the functional reserve of all four hemodynamic modulators: myocardial contractility, cardiac preload, afterload and chronotropism [6, 16, 27-40]. Thus, from the available literature it can be gleaned that the hemodynamic response to metaboreflex activation is a highly integrated phenomenon where a complex interplay between cardiac performance, preload, afterload, and HR occurs to achieve the normal cardiovascular response to exercise.
However, in several clinical situations one or more of these variables is impaired and cannot be recruited. Situations such as myocardial systolic and diastolic dysfunctions, hypertension, metabolic diseases (type 1 and type 2 diabetes mellitus and metabolic syndrome), as well as neurological diseases (multiple sclerosis, spinal cord injured patients, etc.) have all been associated with metaboreflex malfunction and abnormal hemodynamic response [2, 31, 32, 37, 41-53].
In this review, we will briefly address the consequence of these pathologies on the cardiovascular response during metaboreflex. The aim is to provide the basic understanding of the human physiopathology of metaboreflex functioning and of its dysregulation due to impairment in one or more of the four hemodynamic modulators.
3. MUSCLE METABOREFLEX AND THE RESERVE IN MYOCARDIAL CONTRACTILITY
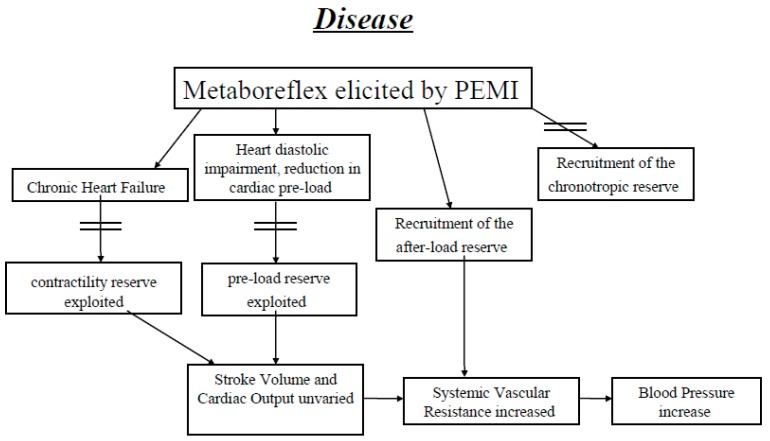
Relevant evidence has been provided in support of the hypothesis that activation of the metaboreflex recruits myocardial inotropism. Most of this evidence is derived from studies conducted in the animal setting, whereas less information is available for humans [16, 30, 39, 40, 54-56]. Experiments in animals, as well as in humans, indicate that in healthiness cardiac performance is enhanced during metaboreflex due to sympathetic tone augmentation; that is, the cardiac reserve of inotropism is recruited to support stroke volume (SV) and CO to achieve the target MAP during metaboreflex, whereas an insignificant role appears to be played by vasoconstriction, i.e. by SVR increments [16, 29, 30, 33, 39, 40, 56]. On the contrary, when inotropism cannot be enhanced, such as during chronic heart failure (CHF), this hemodynamic scenario is subverted. Data from the O’Leary laboratory, where canine CHF models were utilised, have shown that the limited capacity to enhance ventricular performance contributes to the inability to increase CO. This leads to an increase in SVR as the main mechanism by which the target MAP was reached during metaboreflex [49, 55, 57, 58]. Similar results have been obtained in humans suffering from CHF. In these patients, Crisafulli and co-workers [40] found a MAP response not different from that of normal individuals. However, CHF patients had an exaggerated increase in SVR without any increment in cardiac contractility, SV and CO during metaboreflex. The authors concluded that CHF patients were still able to increase MAP, but that there was a functional shift from a flow-mediated (i.e. CO-increase) to a vasoconstriction-mediated hemodynamic mechanism [41].
Th authors speculated that the exhausted contractility reserve of CHF patients was responsible for the phenomenon, although what caused such hemodynamic alterations was not totally understood because the limited pre-load reserve (i.e. the Frank-Starling mechanism) was also probably exhausted in these patients. It is worth noting that, despite the inability to increase SV, the metaboreflex in these patients was still able to elicit a MAP response similar than that of normal individuals, although the sustained SVR was harmful for exercise performance and probably contributed to early fatigue during exertion. The authors also speculated that when cardiac performance cannot be enhanced, the cardiovascular apparatus responds to exercise and metaboreflex by excessively increasing SVR in an attempt to achieve the target MAP.
A remarkable phenomenon was that in CHF patients who received a heart transplant, which ameliorated their heart functions and exercise capacity, an improvement in cardiovascular response to muscle metaboreflex activation was also observed [59]. The improved exercise capacity reported after heart transplant was accompanied by a gradual reduction in vasoconstriction in response to muscle metaboreflex. It took about 6 months to reverse this exaggerated vasoconstriction. As previously explained, a functional shift from flow-mediated to vasoconstriction-mediated mechanism is a hallmark of hemodynamic dysregulation in CHF patients during metaboreflex [41]. This finding suggested that, following heart transplant, the cardiovascular dysregulation present before heart transplant tended to normalise, but not immediately after transplant, i.e. after that cardiac function was improved. Thus, other factors are probably responsible for hemodynamic dysregulation during exercise in CHF patients. In particular, the muscle hypothesis of CHF postulates that a change in periphery rather than in left ventricular performance itself may limit exercise capacity. The muscle hypothesis suggests that alterations in skeletal muscle morphology, metabolism and blood flow may render the metaboreflex hyperactive because of skeletal muscle alterations [42, 43]. Results from the quoted study of heart transplant patients appear to confirm this hypothesis.
In summary, during muscle metaboreflex an increase in myocardial inotropism takes place because of sympathetic activation. The recruitment of cardiac performance sustains SV and CO and limits vasoconstriction. When there is no reserve in cardiac inotropism available, then the hemodynamic response to metaboreflex activation relies on a vasoconstriction-mediated rather than a flow-mediated mechanism.
4. MUSCLE METABOREFLEX, VENOUS RETURN, HEART DIASTOLIC FUNCTION AND THE RESERVE IN CARDIAC PRELOAD
Reports from experiments in the animal as well as in the human setting suggest that metaboreflex is able to induce a sympathetic-mediated venoconstriction and splanchnic vasoconstriction which together increase ventricular filling pressure thereby enhancing venous return [31, 35, 40, 56, 60]. The enhanced venous return results in blood volume “centralisation”, which supports SV and CO during metaboreflex. Impairments in venous return and diastolic capacity of the heart lead to a reduction in the SV response during metaboreflex and cause a shift from a flow-mediated to a vasoconstriction-mediated mechanism to reach the target blood pressure level [31, 37, 61].
In patients with spinal cord injury (SCI), Crisafulli and co-workers [31] reported a dysregulated hemodynamic response in comparison with controls during metaboreflex. In detail, SCI patients showed a blunted blood pressure response compared with non-injured individuals. Moreover, while in normal subjects the blood pressure response mainly relied on a flow-mediated mechanism (i.e. CO increment), SCI subjects were unable to increase CO because of the diminished capacity to increase SV. Moreover, the authors found that the blunted CO response was not counterbalanced by a proportional increment in SVR, which was insufficient to compensate for the reduced CO. Therefore, in SCI patients there are at least two different hemodynamic impairments during metaboreflex: 1) reduced capacity to centralise blood volume and enhance cardiac preload, and 2) reduced sympathetic-mediated arteriolar vasoconstriction. Both phenomena explain the lower MAP increments in response to the metaboreflex of these patients. The authors claimed that the described hemodynamic dysregulation was the consequence of the altered control over the cardiovascular system due to the loss of innervation below the level of the spinal lesion.
Interestingly, in a subsequent paper, the same group observed that in SCI patients a period of training (one year) could successfully improve hemodynamics, with increased MAP and CO during metaboreflex activation [62]. The authors were able to detect enhancements in ventricular filling rate and end-diastolic volume after training, which were probably the consequence of improved capacity to vasoconstrict the venous beds, which in turn reduced venous pooling and increased venous return. The authors speculated that this cardiovascular adaptation to training was possibly the consequence of a combination of an enhanced diastolic function of the heart, an augmented level of circulating catecholamines and of a more efficient myogenic response to transmural pressure at venous level.
The importance of venous return is also supported by a recent study conducted in healthy individuals taking a venous-dilating agent (i.e. sublingual isosorbide dinatrate) [33]. In this investigation, an impaired SV response during metaboreflex activation was found because of a combination of shortening in diastolic time, as a consequence of tachycardia, and a reduction in the ability to propel blood volumes towards the heart because of venous dilation. Both phenomena together concurred in reducing cardiac preload and limited the possibility of recruiting the Frank-Starling mechanism during metaboreflex.
Furthermore, it should be highlighted that, along with venous return, myocardial diastolic functions are important for normal hemodynamics during metaboreflex. Recent investigations in elderly but otherwise healthy subjects [37] indicated that with ageing, a reduction in early diastolic mitral inflow velocity took place during metaboreflex in comparison with young individuals. At the same time, the atrial contribution to ventricular filling increased with age. This was the consequence of impaired myocardial relaxation and increased ventricular stiffness in aged individuals. The authors claimed that their results supported the concept that one aspect of the ageing heart is that cardiac compliance decreases. They further pointed out that impairment in diastolic functions in the elderly caused a reduction in SV and CO during metaboreflex, thereby shifting from a flow-mediated to a vasoconstriction-mediated mechanism for reaching the target MAP. On the contrary, in young healthy subjects the main mechanism through which the blood pressure response was achieved was a flow-mediated (i.e. CO-mediated) mechanism obtained by means of preload increment. Hence, adequate ventricular compliance and cardiac filling are essential for normal hemodynamics during metaboreflex.
To further support the notion that the reserve in cardiac preload and the possibility of recruiting the Frank-Starling mechanism are important during metaboreflex, there is a very recent investigation in patients with heart failure with preserved ejection fraction (HFpEF) [61]. In this study, the authors discovered that diastolic dysfunction impaired hemodynamics during metaboreflex by precluding the possibility of recruiting the preload reserve. Thus, the Frank-Starling mechanism was not available and SV and CO could not increase. The authors were able to demonstrate that patients with HFpEF did not show any increment in end-diastolic volume and ventricular filling rate during metaboreflex, whereas these parameters were enhanced in a control group. The result was that, in HFpEF patients, SV could not increase but actually decreased. This was in sharp contrast to what was observed in healthy control patients, where SV increased. The impaired diastolic function of HFpEF patients caused a functional shifting from a flow-mediated (i.e. CO-increase) to an SVR-mediated mechanism (i.e. vasoconstriction) through which the target blood pressure response was obtained during metaboreflex.
Collectively, all these data support the notion that in humans the preload reserve and the heart diastolic functions are as important as the inotropic reserve for having normal hemodynamics during metaboreflex activation. If there is inadequate ventricular compliance and/or an impaired cardiac filling, then CO cannot be sustained via the Frank-Starling mechanism and the target MAP is reached by means of exaggerated arteriolar vasoconstriction, i.e. SVR increase. This is in line with the concept that, in healthy individuals, the hemodynamic response to metaboreflex relies mainly on a flow-mediated mechanism rather than on vasoconstriction [16, 28, 54, 55]. In contrast, if the functional reserve in SV and CO cannot be elicited, then SVR increment becomes the pivotal mechanism through which the target blood pressure is achieved during metaboreflex [41-43].
5. MUSCLE METABOREFLEX AND THE RESERVE IN CARDIAC AFTERLOAD (SYSTEMIC VASCULAR RESISTANCE)
As previously stated, together with inotropic and preload reserves, the afterload reserve in healthiness is also recruited to reach the target MAP during metaboreflex. However, several clues in animals, as well as in humans, suggest that arteriolar constriction (i.e. SVR increment) is not the main mechanism through which metaboreflex operates to increase blood pressure. Rather, the reserve in vasoconstriction is recruited whenever one or more of the other functional reserves (i.e. inotropism and preload) are impaired and can no longer be recruited.
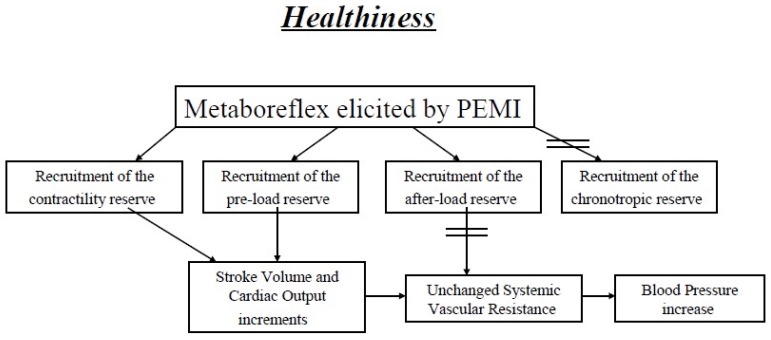
In animal models of metaboreflex activation it has been reported that cardiovascular response to metaboreflex relies mainly on CO during mild exercise, whereas peripheral vasoconstriction becomes more important as exercise intensity rises [60, 63-65]. It was then suggested that if the inotropic reserve of the heart is not fully exploited, as during mild exercise, then the subsequent metaboreflex-induced MAP response relies mainly on CO. Contrarily, when the inotropic reserve is fully utilised, as during strenuous efforts, then metaboreflex relies mainly on peripheral vasoconstriction. Hence, it is likely that the degree of recruitment of the SVR reserve (i.e. the degree of vasoconstriction) depends on the possibility of increasing CO which, in turn, depends on the availability of contractility and preload reserve. Similar findings have been reported in healthy humans [29, 30, 34], thereby indicating that the cardiovascular apparatus operates with remarkable plasticity to regulate cardiovascular response to metaboreflex and that, in the presence of an available cardiac reserve in terms of inotropism and preload, the flow-increased mechanism is preferentially used rather than peripheral vasoconstriction (Fig. 1).
Fig. (1).
Normal hemodynamic modulation during metaboreflex activated by the post-exercise muscle ischemia (PEMI) method. In healthiness, during PEMI-elicited metaboreflex the functional reserves of three of the four hemodynamic modulators (inotropism, preload and afterload) are recruited, whereas the reserve in chronotropism cannot be investigated. The target blood pressure level is achieved mainly by inotropism and preload adjustments, while little role is played by the reserve in afterload.
Studies conducted on canine models of CHF have shown that the limited capacity to enhance ventricular performance contributes to the inability to increase CO and that this causes excessive vasoconstriction and increases in SVR during metaboreflex [55, 57, 58]. These early findings in animals were confirmed in humans suffering from CHF [41], where activation of the metaboreflex produced an increase in blood pressure similar to that observed in healthy individuals but with a completely different hemodynamic adjustment. Indeed, in CHF patients, the rise in MAP was the result of a sympathetic-mediated increase in SVR, whereas in normal controls it was the result of CO elevation, thus confirming observations made in dog models of CHF [41]. The authors concluded that in CHF contractility could not increase during metaboreflex and that this dysfunction led to a reduction in SV and CO. Thus, in humans suffering from CHF, there was a functional shift from a flow-mediated to a peripheral-mediated mechanism (i.e. arteriolar constriction) to reach the target MAP. However, the authors pointed out that the limited capacity to increase SV could also be due to an exhausted reserve of cardiac preload in CHF [41]. To date and to the best of our knowledge, no one has assessed the importance of diastolic impairment in the abnormal response to the metaboreflex of CHF patients, although a recent investigation underscored a similar hemodynamic scenario in patients suffering from HFpEF [61]. It is to be noted that results in humans were in good accordance with the notion that CHF causes exaggerated vasoconstriction in response to metaboreflex which, contrary to metabolic vasodilatation due to functional sympatholysis, also vasoconstricts the working muscle and does not allow improvement in muscle perfusion [66, 67]. This fact may contribute to the early exertional fatigue often reported by these patients [42, 43]. Therefore, CHF may be considered a disease that disrupts the normal plasticity of circulatory response to metaboreflex and to exercise, constituting an integrated response involving the recruitment of the functional reserves in myocardial contractility and preload, which are exhausted in these patients.
While the exaggerated SVR increments are well described in CHF, in the recent past several investigations have reported that augmented vasoconstriction in response to metaboreflex can also be responsible for abnormal hemodynamics in other cardiovascular diseases. In particular, although not unanimously, several recent papers have reported that excessive sympathetic activity occurs during metaboreflex in hypertension [46, 51-53, 68, 69]. This may lead to detrimental effects, such as elevated SVR, which in turn causes excessive vasoconstriction and elevated blood pressure. Likewise, scientific evidence shows elevated MAP response and exaggerated sympathetic tone in response to metaboreflex in some metabolic pathologies such as obesity, metabolic syndrome and type 2 diabetes mellitus [47, 70, 71].
Moreover, as previously shown, results in the elderly but otherwise healthy subjects indicated that ageing impairs heart diastolic functions and caused a functional shift from a flow-mediated (i.e. CO increase) to a vasoconstriction-mediated (i.e. SVR increase) mechanism through which the target MAP is achieved during metaboreflex [37]. This occurrence is in line with the concept that ageing results in cardiovascular stiffening, with progressive alterations of measures of ventricular filling and relaxation, and that healthy older adults show impaired vasodilatory response [72, 73]. Furthermore, this finding supports the concept that, whenever the inotropic and/or the preload reserve are unavailable, then mechanisms controlling hemodynamics recruit the reserve in afterload, i.e. arteriolar vasoconstriction.
An excessive increase in SVR during metaboreflex was also recently discovered in a study of patients with multiple sclerosis [44, 45]. The authors speculated that this exaggerated SVR response was probably the consequence of the physical deconditioning of these patients, which caused a shift in fibre type composition from type I to a greater proportion of type II fibres, thereby inducing a more pronounced production of metabolic by-products in the muscle, which in turn caused a more robust metaboreflex engagement. However, this hemodynamic scenario did not change after a 6-month programme of physical intervention which was able to increase physical capacity in these patients [45]. This finding seems to contradict the concept that deconditioning is the main cause of the excessive vasoconstriction in the arteriolar bed of patients suffering from multiple sclerosis. Thus, further research is necessary in order to better understand which mechanism leads to exaggerated SVR increments during metaboreflex in these patients.
While most of the clinical situations studied were characterised by an increase in SVR response to metaboreflex, less research has been conducted in pathologies which possibly lead to a reduced capacity to increase afterload. A peculiar hemodynamic scenario was observed in young patients with type 1 diabetes mellitus (DM1) [32]. These patients were found to have a blunted MAP response during metaboreflex recruitment compared with healthy controls. In detail, they showed a reduced capacity to vasoconstrict the arteriolar bed which was compensated by a higher SV response and a more evident CO during metaboreflex. Hence, cardiovascular regulation was somewhat altered in these patients. The authors hypothesised that some form of autonomic failure was present in DM1 patients even in the absence of an overt autonomic neuropathy. One explanation for this phenomenon was that repeated episodes of overt or sub-clinical hypoglycemia may have reduced the capacity to activate the sympathetic tone, thereby leading to autonomic failure, a phenomenon already reported in DM1 patients [32, 74, 75]. This impaired hemodynamics was well tolerated by these patients probably because they were young. However, the authors suggested that this condition could progressively deteriorate and progressively lead to symptomatic manifestations of sympathetic deficit.
It should be stressed that, to the best of our knowledge, there is no research dealing with metaboreflex in patients suffering from sympathetic deficits such as pure autonomic dysfunction, Parkinson's disease, etc.
6. METABOREFLEX AND THE CHRONOTROPIC RESERVE
Most of our understanding of the HR response in humans during metaboreflex is based on investigation conducted with the post-exercise muscle ischemia (PEMI) method. There is a general consensus that HR does not take part in the hemodynamic response during PEMI. This is because during PEMI an increment in the parasympathetic tone takes place due to two distinct phenomena: intense arterial baroreflex stimulation because of sustained blood pressure and withdrawal of the central command on cessation of exercise [12, 14-16, 29, 30, 35, 76, 77]. The consequence is that, in the PEMI setting, the parasympathetic tone rises and obscures the effect of the metaboreflex-induced sustained sympathetic tone on the sinus node. Thus, concomitant sustained parasympathetic and sympathetic tones occur and this explains the lack of HR response [12, 15, 78]. It should however be noted that a significant inter-subject variability has been reported and that tachycardia may be present in some subjects [79]. Different HR behaviour is on the other hand present if metaboreflex is evoked by inducing muscle ischemia during exercise. In this condition, HR does increase [34], thus suggesting that muscle ischemia during muscle contraction is capable of eliciting chronotropic stimulation and that the lack of HR response during PEMI is actually to be ascribed to the high parasympathetic tone which takes place in this setting (Fig. 2).
Fig. (2).
If cardiac contractility and or preload reserves are exhausted during PEMI, the possibility of having a flow-mediated blood pressure increase is precluded. The hemodynamic consequence is that the target blood pressure is reached by recruiting the reserve in cardiac afterload (i.e. systemic vascular resistance increase). In this case, systemic vascular elevation becomes the main mechanism through which cardiovascular reflexes operate to adjust hemodynamics.
Hence, studies conducted with PEMI can explore the reserve of only three of the four hemodynamic modulators: inotropism, preload, and afterload, whereas the reserve in chronotropism cannot be investigated. In this regard, it would be useful to conduct a study in humans with artificial pace-makers to verify which hemodynamic strategy is adopted when HR is fixed and cannot increase during metaboreflex activated during exercise by inducing muscle ischemia. To the best of our knowledge, to date no one has conducted such a study.
CONCLUSION AND FUTURE DIRECTIONS
Collectively, data obtained in humans during metaboreflex elicited by means of the PEMI method indicate that hemodynamic regulation is characterised by a remarkable level of plasticity. A high level of integration of cardiac performance, preload and afterload exists. Indeed, when cardiac contractility cannot be enhanced, the possibility to increase SV and CO is precluded. As a consequence, the target blood pressure is reached by recruiting the reserve of cardiac afterload (i.e. arteriolar vasoconstriction). Likewise, if the reserve in cardiac preload is exhausted and/or if venous return is impaired, then SVR elevation is the main mechanism through which the cardiovascular reflexes operate to adjust hemodynamics. Hence, impairments in the capacity to increase SV and CO are compensated by exaggerated arteriolar constriction.
It seems that in healthy subjects the preferred cardiovascular adjustment is a flow-mediated (i.e. CO-mediated) mechanism obtained by recruiting the inotropic and preload reserves whereas, when these reserves can no longer be used, SVR increment becomes pivotal in hemodynamic response during PEMI. In a clinical perspective, PEMI may be routinely employed to evaluate patients with cardiovascular, metabolic and nervous problems. However, to date, there are no standard references of normality. Therefore, studies aimed at defining normal hemodynamics during PEMI, characterising abnormal responses and verifying whether hemodynamics abnormalities can be reverted are claimed. Little is known about the relevance of metaboreflex impairments for patients’ prognosis. Association between the depletion of peripheral muscle mass and metaboreflex overactivity and exercise limitation in patients with CHF have been observed. It appears that progression of the CHF syndrome is related to peripheral muscle maladaptive changes, with altered autonomic reflex control [43, 50, 80]. Importantly, it also appears that physical training can partially reverse this condition [43, 80]. However, studies dealing with the effects of training on hemodynamic response during the PEMI test are very scarce and are limited to CHF.
Finally, the PEMI method is usually applied in patients in limited muscle mass and after exercise at mild intensity (after handgrip at 30% of maximum). This avoids excessive stresses in the cardiovascular system of these patients, thereby allowing its use even in individuals with compromised circulation. However, the reverse of the coin is that we do not know much about hemodynamic dysregulation after exercise at higher intensities.
CONSENT FOR PUBLICATION
Not applicable.
ACKNOWLEDGEMENTS
This study was supported by the University of Cagliari and the Italian Ministry of Education, Universities and Research.
CONFLICT OF INTEREST
The author declares no conflict of interest, financial or otherwise.
REFERENCES
- 1.Ichinose M., Maeda S., Kondo N., Nishiyasu T. Blood pressure regulation II: what happens when one system must serve two masters: oxygen delivery and pressure regulation? Eur. J. Appl. Physiol. 2014;114:451–465. doi: 10.1007/s00421-013-2691-y. [DOI] [PubMed] [Google Scholar]
- 2.Murphy M.N., Mizumo M., Mitchell J.H., Smith S.A. Cardiovascular regulation by skeletal muscle reflexes in health and disease. Am. J. Physiol. Heart Circ. Physiol. 2011;301:H1191–H1204. doi: 10.1152/ajpheart.00208.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewis S.F., Taylor R.M., Graham R.M., Pettinger W.A., Schutte J.E., Blomqvist C.G. Cardiovascular responses to exercise as functions of absolute and relative work load. J. Appl. Physiol. 1983;54:1314–1323. doi: 10.1152/jappl.1983.54.5.1314. [DOI] [PubMed] [Google Scholar]
- 4.Crisafulli A., Tocco F., Pittau G., et al. Detection of lactate threshold by including haemodynamic and oxygen extraction data. Physiol. Meas. 2006;27:85–97. doi: 10.1088/0967-3334/27/1/008. [DOI] [PubMed] [Google Scholar]
- 5.Higginbotham M.B., Morris K.G., Williams R.S., McHale P.A., Coleman R.E., Cobb F.R. Regulation of stroke volume during submaximal and maximal upright exercise in normal man. Circ. Res. 1986;58:281–291. doi: 10.1161/01.res.58.2.281. [DOI] [PubMed] [Google Scholar]
- 6.Nóbrega ACL, O'Leary DS, Silva BM, Marongiu E, Piepoli MF, Crisafulli A. Neural regulation of cardiovascular response to exercise: role of central command and peripheral afferents. 2014 doi: 10.1155/2014/478965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crisafulli A, Marongiu E, Ogho S. Cardiovascular reflexes activity and their interaction during exercise. 2015 doi: 10.1155/2015/394183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green A.L., Paterson D.J. Identification of the neurocircuitry controlling cardiovascular function in humans using functional neurosurgery: implications for exercise control. Exp. Physiol. 2008;93:1022–1028. doi: 10.1113/expphysiol.2007.039461. [DOI] [PubMed] [Google Scholar]
- 9.Guyenet P.G. The sympathetic control of blood pressure. Nat. Rev. Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 10.Strange S., Secher N.H., Pawelczyk J.A., et al. Neural control of cardiovascular responses and of ventilation during dynamic exercise in man. J. Physiol. 1993;470:693–704. doi: 10.1113/jphysiol.1993.sp019883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rowell L.B., O’Leary D.S. Reflex control of the circulation during exercise: chemoreflexes and mechanoreflexes. J. Appl. Physiol. 1990;69:407–418. doi: 10.1152/jappl.1990.69.2.407. [DOI] [PubMed] [Google Scholar]
- 12.O’Leary D.S. Autonomic mechanisms of muscle metaboreflex control of heart rate. J. Appl. Physiol. 1993;74:1748–1754. doi: 10.1152/jappl.1993.74.4.1748. [DOI] [PubMed] [Google Scholar]
- 13.Kaur J., Spranger M.D., Hammond R.L., et al. Muscle metaboreflex activation during dynamic exercise evokes epinephrine release resulting in β2-mediated vasodilation. Am. J. Physiol. 2015;308:H524–H529. doi: 10.1152/ajpheart.00648.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piepoli M., Clark A.L., Coats A.J. Muscle metaboreceptors in hemodynamic, autonomic, and ventilatory responses to exercise in men. Am. J. Physiol. 1995;269:H1428–H1436. doi: 10.1152/ajpheart.1995.269.4.H1428. [DOI] [PubMed] [Google Scholar]
- 15.Iellamo F., Pizzinelli P., Massaro M., Raimondi G., Peruzzi G., Legramante J.M. Muscle metaboreflex contribution to sinus node regulation during static exercise. Circulation. 1999;100:27–32. doi: 10.1161/01.cir.100.1.27. [DOI] [PubMed] [Google Scholar]
- 16.Crisafulli A., Scott A.C., Wensel R., et al. Muscle metaboreflex-induced increases in stroke volume. Med. Sci. Sports Exerc. 2003;35:221–228. doi: 10.1249/01.MSS.0000048639.02548.24. [DOI] [PubMed] [Google Scholar]
- 17.Fadel P.J., Ogoh S., Watenapaugh D.E., et al. Carotid baroreflex regulation of sympathetic nerve activity during dynamic exercise in humans. Am. J. Physiol. 2001;280:H1383–H1390. doi: 10.1152/ajpheart.2001.280.3.H1383. [DOI] [PubMed] [Google Scholar]
- 18.Sheriff D.D. Baroreflex resetting during exercise: mechanisms and meaning. Am. J. Physiol. 2006;290:H1406–H1407. doi: 10.1152/ajpheart.01275.2005. [DOI] [PubMed] [Google Scholar]
- 19.Raven P.B., Fadel P.J., Ogoh S. Arterial baroreflex resetting during exercise: a current perspective. Exp. Physiol. 2006;91:37–49. doi: 10.1113/expphysiol.2005.032250. [DOI] [PubMed] [Google Scholar]
- 20.Alam M., Smirk F.H. Observations in man upon a blood pressure raising reflex arising from the voluntary muscles. J. Physiol. 1937;89:372–383. doi: 10.1113/jphysiol.1937.sp003485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alam M., Smirk F.H. Observations in man on a pulse-accelerating reflex from the voluntary muscles of the legs. upon a blood pressure raising reflex arising from the voluntary muscles. J. Physiol. 1938;92:167–177. doi: 10.1113/jphysiol.1938.sp003592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coote J.H., Hilton S.M., Perez-Gonzales J.F. The reflex nature of the pressor response to muscular exercise. J. Physiol. 1971;215:789–804. doi: 10.1113/jphysiol.1971.sp009498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaufman M.P., Longhurst J.C., Rybicki K.J., Wallach J.H., Mitchell J.H. Effect of static muscular contraction on impulse activity of group III and IV afferents in cats. J. Appl. Physiol. 1983;55:105–112. doi: 10.1152/jappl.1983.55.1.105. [DOI] [PubMed] [Google Scholar]
- 24.Andreani C.M., Hill J.M., Kaufman M.P. Responses of group III and IV afferents to dynamic exercise. J. Appl. Physiol. 1997;82:1811–1817. doi: 10.1152/jappl.1997.82.6.1811. [DOI] [PubMed] [Google Scholar]
- 25.Cui J., Mascarenhas V., Moradkhan R., Blaha C., Sinoway L.I. Effects of muscle metabolites on responses of muscle sympathetic nerve activity to mechanoreceptor(s) stimulation in healthy humans. Am. J. Physiol. 2008;294:R458–R466. doi: 10.1152/ajpregu.00475.2007. [DOI] [PubMed] [Google Scholar]
- 26.Kaufman M.P., Rybicki K.J. Discharge properties of group III and IV muscle afferents: their responses to mechanical and metabolic stimuli. Circ. Res. 1987;61:160–165. [PubMed] [Google Scholar]
- 27.Amann M., Blain G.M., Proctor L.T., Sebranek J.J., Pegelow D.F., Dempsey J.A. Group III and IV muscle afferents contribute to ventilatory and cardiovascular response rhythmic exercise in humans. J. Appl. Physiol. 2010;109:966–976. doi: 10.1152/japplphysiol.00462.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amann M., Blain G.M., Proctor L.T., Sebranek J.J., Pegelow D.F., Dempsey J.A. Implications of group III and IV muscle afferents for high-intensity endurance exercise performance in humans. J. Physiol. 2011;589:5299–5309. doi: 10.1113/jphysiol.2011.213769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crisafulli A., Salis E., Pittau G., et al. Modulation of cardiac contractility by muscle metaboreflex following efforts of different intensities in humans. Am. J. Physiol. 2006;291:H3035–H3042. doi: 10.1152/ajpheart.00221.2006. [DOI] [PubMed] [Google Scholar]
- 30.Crisafulli A., Milia R., Lobina A., et al. Hemodynamic effect of metaboreflex activation in men after running above and below the velocity of the anaerobic threshold. Exp. Physiol. 2008;93:447–457. doi: 10.1113/expphysiol.2007.041863. [DOI] [PubMed] [Google Scholar]
- 31.Crisafulli A., Milia R., Vitelli S., et al. Hemodynamic responses to metaboreflex activation: Insights from spinal cord-injured humans. Eur. J. Appl. Physiol. 2009;106:525–533. doi: 10.1007/s00421-009-1045-2. [DOI] [PubMed] [Google Scholar]
- 32.Roberto S., Marongiu E., Pinna M., et al. Altered hemodynamics during muscle metaboreflex in young, type 1 diabetes patients. J. Appl. Physiol. 2012;113:1323–1331. doi: 10.1152/japplphysiol.00280.2012. [DOI] [PubMed] [Google Scholar]
- 33.Marongiu E., Piepoli M., Milia R., et al. Effects of acute vasodilation on the hemodynamic response to muscle metaboreflex. Am. J. Physiol. 2013;305:H1387–H1396. doi: 10.1152/ajpheart.00397.2013. [DOI] [PubMed] [Google Scholar]
- 34.Crisafulli A., Piras F., Filippi M., et al. Role of heart rate and stroke volume during muscle metaboreflex-induced cardiac output increase: differences between activation during and after exercise. J. Physiol. Sci. 2011;61:385–394. doi: 10.1007/s12576-011-0163-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bastos B.G., Williamson J.W., Harrelson T., Nôbrega A.C. Left ventricular volumes and hemodynamic responses to postexercise ischemia in healthy humans. Med. Sci. Sports Exerc. 2000;32:1114–1118. doi: 10.1097/00005768-200006000-00012. [DOI] [PubMed] [Google Scholar]
- 36.Ichinose M.J., Sala-Mercado J.A., Coutsos M., et al. Modulation of cardiac output alters the mechanisms of the muscle metaboreflex pressor response. Am. J. Physiol. 2009;298:H245–H250. doi: 10.1152/ajpheart.00909.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milia R., Roberto S., Mulliri G., et al. Effect of aging on hemodynamic response to metaboreflex activation. Eur. J. Appl. Physiol. 2015;115(8):1693–1703. doi: 10.1007/s00421-015-3153-5. [DOI] [PubMed] [Google Scholar]
- 38.O’Leary D.S., Augustyniak R.A. Muscle metaboreflex increases ventricular performance in conscious dogs. Am. J. Physiol. 1998;275:H220–H224. doi: 10.1152/ajpheart.1998.275.1.H220. [DOI] [PubMed] [Google Scholar]
- 39.Sala-Mercado J.A., Hammond R.L., Kim J.K., Rossi N.F., Stephenson L.W., O’Leary D.S. Muscle metaboreflex control of ventricular contractility during dynamic exercise. Am. J. Physiol. 2006;290:H751–H757. doi: 10.1152/ajpheart.00869.2005. [DOI] [PubMed] [Google Scholar]
- 40.Spranger M.D., Sala-Mercado J.A., Coutsos M., et al. Role of cardiac output vs. peripheral vasoconstriction in mediating muscle metaboreflex pressor responses: dynamic exercise vs. post-exercise muscle ischemia. Am. J. Physiol. 2013;304:R657–R663. doi: 10.1152/ajpregu.00601.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crisafulli A., Salis E., Tocco F., et al. Impaired central hemodynamic response and exaggerated vasoconstriction during muscle metaboreflex activation in heart failure patients. Am. J. Physiol. 2007;292:H2988–H2996. doi: 10.1152/ajpheart.00008.2007. [DOI] [PubMed] [Google Scholar]
- 42.Piepoli M., Dimopoulos K., Concu A., Crisafulli A. Cardiovascular and ventilatory control during exercise in chronic heart failure: role of muscle reflexes. Int. J. Cardiol. 2008;130:3–10. doi: 10.1016/j.ijcard.2008.02.030. [DOI] [PubMed] [Google Scholar]
- 43.Piepoli M.F., Crisafulli A. Pathophysiology of human heart failure: importance of skeletal muscle myopathy and reflexes. Exp. Physiol. 2014;99:609–615. doi: 10.1113/expphysiol.2013.074310. [DOI] [PubMed] [Google Scholar]
- 44.Marongiu E., Olla S., Magnani S., et al. Metaboreflex activity in multiple sclerosis patients. Eur. J. Appl. Physiol. 2015;115(12):2481–2490. doi: 10.1007/s00421-015-3271-0. [DOI] [PubMed] [Google Scholar]
- 45.Magnani S., Olla S., Pau M., et al. Effects of six months training on physical capacity and metaboreflex activity in patients with multiple sclerosis. Front. Physiol. 2016;14(7):531. doi: 10.3389/fphys.2016.00531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Delaney E.P., Greaney J.L., Edwards D.G., Rose W.C., Fadel P.J., Farquhar W.B. Exaggerated sympathetic and pressor responses to handgrip exercise in older hypertensive humans: role of the muscle metaboreflex. Am. J. Physiol. 2010;299:H1318–H1327. doi: 10.1152/ajpheart.00556.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Milia R., Velluzzi F., Roberto S., et al. Differences in hemodynamic response to metaboreflex activation between obese patients with metabolic syndrome and healthy subjects with obese phenotype. Am. J. Physiol. 2015;309:H779–H789. doi: 10.1152/ajpheart.00250.2015. [DOI] [PubMed] [Google Scholar]
- 48.Negrao C.E., Trombetta I.C., Batalha L.T., et al. Muscle metaboreflex control is diminished in normotensive obese women. Am. J. Physiol. 2001;281:H469–H475. doi: 10.1152/ajpheart.2001.281.2.H469. [DOI] [PubMed] [Google Scholar]
- 49.O’Leary D.S. Altered reflex cardiovascular control during exercise in heart failure: animal studies. Exp. Physiol. 2006;91:73–77. doi: 10.1113/expphysiol.2005.031179. [DOI] [PubMed] [Google Scholar]
- 50.Ponikowski P.P., Chua T.P., Francis D.P., Capucci A., Coats A.J., Piepoli M.F. Muscle ergoreceptor overactivity reflects deterioration in the clinical status and cardiorespiratory reflex control in chronic heart failure. Circulation. 2001;104:2324–2330. doi: 10.1161/hc4401.098491. [DOI] [PubMed] [Google Scholar]
- 51.Rondon M.U., Laterza M.C., de Matos L.D., et al. Abnormal muscle metaboreflex control of sympathetic activity in never-treated hypertensive subjects. Am. J. Hypertens. 2006;19:951–957. doi: 10.1016/j.amjhyper.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 52.Sausen M.T., Delaney E.P., Stillabower M.E., Farquhar W.B. Enhanced metaboreflex sensitivity in hypertensive humans. Eur. J. Appl. Physiol. 2009;105:351–356. doi: 10.1007/s00421-008-0910-8. [DOI] [PubMed] [Google Scholar]
- 53.Spranger M.D., Kaur J., Sala-Mercado J.A., et al. Exaggerated coronary vasoconstriction limits muscle metaboreflex-induced increases in ventricular performance in hypertension. Am. J. Physiol. 2017;312(1):H68–H79. doi: 10.1152/ajpheart.00417.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O’Leary D.S., Augustyniak R.A. Muscle metaboreflex increases ventricular performance in conscious dogs. Am. J. Physiol. 1998;275:H220–H224. doi: 10.1152/ajpheart.1998.275.1.H220. [DOI] [PubMed] [Google Scholar]
- 55.O’Leary D.S., Sala-Mercado J.A., Augustyniak R.A., Hammond R.L., Rossi N.F., Ansorge E.J. Impaired muscle metaboreflex-induced increases in ventricular function in heart failure. Am. J. Physiol. 2004;287:H2612–H2618. doi: 10.1152/ajpheart.00604.2004. [DOI] [PubMed] [Google Scholar]
- 56.Shoemaker J.K., Mattar L., Kerbeci P., Trotter S., Arbeille P., Hughson R.L. WISE 2005: stroke volume changes contribute to the pressor response during ischemic handgrip exercise in women. J. Appl. Physiol. 2005;103:228–233. doi: 10.1152/japplphysiol.01334.2006. [DOI] [PubMed] [Google Scholar]
- 57.Kim J.K., Sala-Mercado J.A., Hammond R.L., Rodriguez J., Scislo T., O’Leary D.S. Attenuated arterial baroreflex buffering of muscle metaboreflex in heart failure. Am. J. Physiol. 2005;289:H2416–H2423. doi: 10.1152/ajpheart.00654.2005. [DOI] [PubMed] [Google Scholar]
- 58.Hammond R.L., Augustyniak R.A., Rossi N.F., Churchill P.C., Lapanowsky K., O’Leary D.S. Heart failure alters the strength and mechanisms of the muscle metaboreflex. Am. J. Physiol. 2000;278:H818–H828. doi: 10.1152/ajpheart.2000.278.3.H818. [DOI] [PubMed] [Google Scholar]
- 59.Crisafulli A., Tocco F., Milia R., et al. Progressive improvement in hemodynamic response to muscle metaboreflex in heart transplant recipients. J. Appl. Physiol. 2013;114:421–427. doi: 10.1152/japplphysiol.01099.2012. [DOI] [PubMed] [Google Scholar]
- 60.Sheriff D.D., Augstyniak R.A., O’Leary D.S. Muscle chemoreflex-induced increases in right atrial pressure. Am. J. Physiol. 1998;275:H767–H775. doi: 10.1152/ajpheart.1998.275.3.H767. [DOI] [PubMed] [Google Scholar]
- 61.Roberto S., Mulliri G., Milia R., et al. Hemodynamic response to muscle reflex is abnormal in patients with heart failure with preserved ejection fraction. J. Appl. Physiol. 2017;22(2):376–385. doi: 10.1152/japplphysiol.00645.2016. [DOI] [PubMed] [Google Scholar]
- 62.Milia R, Roberto S, Marongiu E, et al. Improvement in hemodynamic responses to metaboreflex activation after one year of training in spinal cord injured humans. 2014 doi: 10.1155/2014/893468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Augustyniak R.A., Collins H.L., Ansorge E.J., Rossi N.F., O’Leary D.S. Severe exercise alters the strength and mechanisms of the muscle metaboreflex. Am. J. Physiol. 2001;280:H1645–H1652. doi: 10.1152/ajpheart.2001.280.4.H1645. [DOI] [PubMed] [Google Scholar]
- 64.Sheriff D.D., Wyss C., Rowell L., Scher A. Does inadequate oxygen delivery trigger pressor response to muscle hypoperfusion during exercise? Am. J. Physiol. 1987;253:H1199–H1207. doi: 10.1152/ajpheart.1987.253.5.H1199. [DOI] [PubMed] [Google Scholar]
- 65.Wyss C., Ardell J.L., Scher A.M., Rowell L.B. Cardiovascular responses to graded reductions in hindlimb perfusion in exercising dogs. Am. J. Physiol. 1983;245:H481–H486. doi: 10.1152/ajpheart.1983.245.3.H481. [DOI] [PubMed] [Google Scholar]
- 66.Shoemaker J.K., Kunselman A.R., Silber D.H., Sinoway L.I. Maintained exercise pressor response in heart failure. J. Appl. Physiol. 1998;85:1793–1799. doi: 10.1152/jappl.1998.85.5.1793. [DOI] [PubMed] [Google Scholar]
- 67.Sinoway L.I., Prophet S. Skeletal muscle metaboreceptors stimulation opposes peak metabolic vasodilation in humans. Circ. Res. 1990;66:1576–1584. doi: 10.1161/01.res.66.6.1576. [DOI] [PubMed] [Google Scholar]
- 68.Choi H-M., Stebbins C.L., Lee O-T., et al. Augmentation of the exercise pressor reflex in pre-hypertension: roles of the muscle metaboreflex and mechanoreflex. Appl. Physiol. Nutr. Metab. 2013;38:209–215. doi: 10.1139/apnm-2012-0143. [DOI] [PubMed] [Google Scholar]
- 69.Greaney J.L., Matthews E.L., Boggs M.E., Edwards D.G., Duncan R.L., Faquhar W.B. Exaggerated exercise pressor reflex in adult with moderately elevated systolic blood pressure: role of purinergic receptors. Am. J. Physiol. 2014;306:H132–H141. doi: 10.1152/ajpheart.00575.2013. [DOI] [PubMed] [Google Scholar]
- 70.Dipla K., Zafeiridis A., Koidou I., Geladas N., Vrabas I.S. Altered hemodynamic regulation and reflex control during exercise and recovery in obese boys. Am. J. Physiol. 2010;299:H2090–H2096. doi: 10.1152/ajpheart.00087.2010. [DOI] [PubMed] [Google Scholar]
- 71.Trombetta I.C., Batalha L.T., Rondon M.U., et al. Weight loss improves neurovascualr and muscle metaboreflex control in obesity. Am. J. Physiol. 2003;285:H974–H982. doi: 10.1152/ajpheart.01090.2002. [DOI] [PubMed] [Google Scholar]
- 72.Santulli G., Ciccarelli M., Trimarco B., Iaccarino G. Physical activity ameliorates cardiovascular health in elderly subjects: the functional role of the β adrenergic system. Front. Physiol. 2013;4:209. doi: 10.3389/fphys.2013.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Richards J.C., Crecelius A.R., Larson D.G., Luckasen G.J., Dinenno F.A. Impaired peripheral vasodilation during graded systemic hypoxia in healthy older adults: role of the sympathoadrenal system. Am. J. Physiol. 2017;312:H832–H841. doi: 10.1152/ajpheart.00794.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ahlborg G., Lundberg J.M. Exercise-induced changes in neuropeptide Y, noradrenaline and endothelin-1 levels in young people with type I diabetes. Clin. Physiol. 1996;16:645–655. doi: 10.1111/j.1475-097x.1996.tb00741.x. [DOI] [PubMed] [Google Scholar]
- 75.Bottini P., Boschetti E., Pampanelli S., et al. Contribution of autonomic neuropathy to reduced plasma adrenaline responses to hypoglycemia in IDDM. Diabetes. 1997;46:814–823. doi: 10.2337/diab.46.5.814. [DOI] [PubMed] [Google Scholar]
- 76.Iellamo F., Legramante J.M., Raimondi G., Peruzzi G. Baroreflex control of sinus node during dynamic exercise in humans: effects of central command and muscle reflexes. Am. J. Physiol. 1997;272:H1157–H1164. doi: 10.1152/ajpheart.1997.272.3.H1157. [DOI] [PubMed] [Google Scholar]
- 77.Iellamo F. Baroreflex control of heart rate during exercise: a topic of perennial conflict. J. Appl. Physiol. 2001;90:1184–1185. doi: 10.1152/jappl.2001.90.3.1184. [DOI] [PubMed] [Google Scholar]
- 78.Nishiyasu T., Nobusuke T., Morimoto K., Nishiyasu M., Yamaguchi Y., Murakami N. Enhancement of parasympathetic cardiac activity during activation of muscle metaboreflex in humans. J. Appl. Physiol. 1994;77:2778–2783. doi: 10.1152/jappl.1994.77.6.2778. [DOI] [PubMed] [Google Scholar]
- 79.Watanabe K., Ichinose M., Fujii N., Matsumoto M., Nishiyasu T. Individual differences in heart rate response to activation of the muscle metaboreflex in humans. Am. J. Physiol. 2010;299:H1708–H1714. doi: 10.1152/ajpheart.00255.2010. [DOI] [PubMed] [Google Scholar]
- 80.Piepoli M.F., Kaczmarek A., Francis D.P., et al. Reduced peripheral skeletal muscle mass and abnormal reflex physiology in chronic heart failure. Circulation. 2006;114:126–134. doi: 10.1161/CIRCULATIONAHA.105.605980. [DOI] [PubMed] [Google Scholar]