Abstract
Background and Objectives:
Two of the most critical factors affecting the prognosis of an avulsed tooth after replantation are extraoral dry time and the storage media in which the tooth is placed before treatment is rendered. The present study is undertaken to evaluate the periodontal ligament (PDL) cell viability after storage of teeth in different storage media, namely, coconut water, milk, and saline.
Materials and Methods:
Forty sound human premolars undergoing extraction for orthodontic purpose were selected. The teeth were allowed to lie dry on sand/mud for 30 min followed by which they were randomly divided and stored in three different media, i.e., coconut water, milk, and saline. After 45-min storage in their respective media, the root surface was then scraped for PDL tissue.
Results:
The ANOVA and Newman–Keuls post hoc procedure for statistical analysis of viable cell count under a light microscope using hemocytometer demonstrated that coconut water preserved significantly more PDL cells viable (P < 0.05) compared with milk and saline.
Conclusion:
Storage media help in preserving the viability of PDL cells when immediate replantation is not possible. This study evaluated the posttraumatic PDL cells’ viability following storage in three different storage media. Within the parameters of this study, it was found that coconut water is the most effective media for maintaining the viability of PDL.
Keywords: Avulsion, coconut water, milk, periodontal ligament cell, saline, storage media
INTRODUCTION
Dental trauma being the most demanding injuries in the early life of an individual requires both practical and conversant management by the dentist. Andreasen and Andreasen anticipated that the frequency of these traumas might ultimately outstrip the prevalence of dental caries.[1]
Avulsion (exarticulation, total luxation) is one of the intricate forms characterized by the total displacement of the tooth out of alveolar socket. Effective management of an avulsed tooth by replantation relies on the deterrence of advanced root resorption by diminishing injury to the periodontal ligament (PDL) and judicious endodontic treatment.[2]
Immediate replantation provides the highest success but may not always be feasible. PDL cell viability can be conserved by replantation of the tooth within 15–20 min subsequently or by submersing the tooth in an appropriate storage medium until replanted.[1]
A range of storage media have been proposed till date. The ideal storage medium is still a controversy as research lacks sufficient evidence.
The present study is an attempt to access the viability of the PDL cells in three different storage mediums, namely, coconut water, milk, and saline.
MATERIALS AND METHODS
Forty human premolar teeth were extracted atraumatically for this study. The extracted teeth were noncarious and periodontally sound extracted as a part of orthodontic therapy. Instantly, the extracted teeth were allowed to lie dry on sand/mud for 30 min to simulate a state of avulsion. Later, the teeth were randomly divided and stored in three different media (10 in each medium).
Group 1: Coconut water
Group 2: Milk
Group 3: Saline.
The teeth were stored for 45 min in the respective media, which would simulate the time required to carry the tooth to the dentist followed by saline rinse. The root surface was then scraped with a scalpel except the coronal 2 mm where the cells might have been damaged during extraction.
The positive control teeth (five numbers) were neither dried nor stored in any solution, but instead immediately treated with collagenase. The negative control teeth (five numbers) were dried for 5 h with no follow-up, storage solution time, and then placed in collagenase.
The cells were then collected in test tubes containing collagenase in 2.5 ml of phosphate-buffered saline which was later incubated for 30 min and centrifuged for 4 min at 1000 rpm. The supernatant was discarded and the cells were collected with sterile micropipettes and stained with 0.4% Trypan blue stain. The cells were obtained and viewed under a light microscope with an hemocytometer at ×20 magnification.
The nonviable cells were stained blue while the viable cells did not take up the stain.
The viability percentage of the cell population of each sample was obtained while applying the following mathematical equation:
(UC/TC) ×100 = %
Where,
UC: Unstained cell count (viable cells)
TC: Total cell count (stained + unstained cells).
Statistical analysis was done using the “ANOVA” and the “Newman–Keuls post hoc procedure.”
RESULTS
The random distribution of forty orthodontically extracted teeth was done within the three study groups and the positive and negative control groups. Results of this study showed that coconut water could maintain the viability of the PDL cells up to 82%, whereas milk and saline could maintain up to 59% and 15%, respectively [Table 1].
Table 1.
Mean value and standard deviation of viable periodontal ligament cell count in the study groups
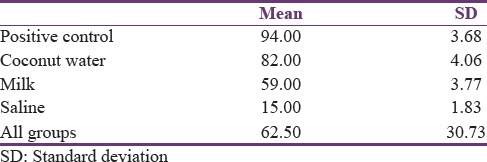
The ANOVA and Newman–Keuls post hoc procedure were used for statistical analysis of viable cell count under the light microscope using hemocytometer [Tables 2 and 3].
Table 2.
Comparison of four groups by ANOVA

Table 3.
Pair-wise comparison by Newman–Keuls post hoc test

DISCUSSION
Avulsion is a traumatic injury described by the complete displacement of the tooth from its socket, with damage to the periodontium. Though the damage to the periodontium is inevitable, preserving the viability of the PDL cells is critical. The part of the PDL attached to the alveolar wall stays vital and does not require treatment, whereas the one attached to the tooth recounts to the repair after reimplantation.[3]
The PDL vitality on the root surfaces urges the prospect of reinsertion of PDL fibers with the alveolar ones.[4]
The prognosis of replantation rests on the presence of viable cells in the PDL and on the proliferation to the injured zones of the root. This can be accomplished by instant replantation or through the storage of the tooth in a suitable milieu for future replantation.
When immediate replantation is not feasible, the tooth should be preserved in a medium that maintains PDL cell viability until conclusive treatment can be consummated.[5]
In 1994, the American Association of Endodontics guidelines for the Treatment of the Avulsed Permanent Tooth recommended Hank's Balanced Salt Solution (HBSS) as the appropriate transport medium for the avulsed tooth which could not be replanted instantly. In unavailability of HBSS, milk is the second best, trailed by saline, saliva (buccal vestibule), and water.[6]
Ideal storage medium
The perfect storage media should preserve cell vitality, adherence, and mitogenic and clonogenic abilities, and be readily available and accessible at the site of trauma.[6,7]
The container which transports an avulsed tooth must be strong and easy to handle, nontoxic, seepage proof, with soft internal surface, sterile, and must shield the tooth during transport, with easy path of removal without damage.[3]
The ability of the media to conserve cell viability is higher when it is nearer to physiological conditions, i.e., osmolality of 300 mOsm/kg and pH 7.0.[8]
Periodontal ligament
Normal PDL cells bear osmolality of 320 mOsm/Kg and pH of 7.2.[3]
The ideal growth of cells is observed amid pH 7.2–7.4, but higher survival rates are found between pH 6.6–7.8.[9]
The study ensured the highest safety during transportation, for each time the root is touched, the cells of the PDL perish.[3]
The survival theory of PDL cell contains deterrence of protein synthesis in the bacterial cell, promoting the action of fibroblasts and healing of connective tissue leading to early recovery.[10]
To have an ideal physiological osmolality is a significant trait for a transport medium for maintaining PDL cell viability. Hypotonic solutions are known to cause irreversible cell membrane damage compared to hypertonic solutions.[11]
Dry storage
Soder et al. proved that cells of PDL are sensitive to critical dry extra-alveolar period where severe cell damage occurs within 60 min. Subsequently, no viable cells could be reaped after 120 min from the root surface.[12] It was established that there was no substantial variance in the number of viable PDL cells with 30 min and no dry time.
In the current study, a 30-min dry time was chosen, a critical time at which the maximum damage was inflicted to PDL cells.
Currently, the American Association of Endodontists’ guidelines for treating avulsed teeth agree to the drop-off period being at 60 min of dry time. Instead of immediate replantation, the avulsed tooth is placed in fluoride after 60 min, due to the presumption that not enough viable PDL cells remain to heal the tooth.[13]
Saline
Saline offers an osmolality of 280 mOsm/kg, and though compatible with PDL cells, it is deficient of essential nutrients such as magnesium, calcium, and glucose, required for normal metabolism of PDL,[3] being the reason for low viable cell count after a 45-min storage time in the present study.
Tap water, saliva, and saline are not suggested for their hypotonic properties of high incidence of bacterial contamination leading to rapid cell death in PDL.[14]
Milk
Milk is considered a better medium than saliva in respect to the number of viable cells, cell size, and ability to recover.[15] Milk provides 3 h of vitality for PDL cells which is ample for the patient to reach the dental office for replantation.[1,16]
The presence of nutritional substances such as amino acids, carbohydrates, and vitamins makes milk more promising.[3]
A 12-h storage in milk provided 50% viability to cells, accentuating the affirmative outcome of milk on human PDL.[15] Milk, though better than water and saliva as a storage medium, does not reconstitute the lost cellular metabolites or maintain morphological integrity of the PDL cells.[17] Milk does not restore the normal morphology nor does it differentiate and mitose, instead only averts cell death.[18]
In this study, parallel to other studies, milk was found to be a suitable storage medium for a period of 1 hour.[6,18,19] When compared to their storage for more than 6 hours.[19]
Coconut water
The electrolyte composition of coconut water has a close Similarity to intracellular fluid than extracellular plasma. It is used as a blood plasma substitute as it is sterile, does not produce heat, does not destroy red blood cells, and is readily accepted by the body. The experimental teeth in this study were directly placed inside the coconut so that the organoleptic and nutritional substances are not lost from the coconut water.
Gopikrishna et al. demonstrated that coconut water was equivalent to HBSS in maintaining PDL cell viability at a storage time of 30–120 min.[20]
Gopikrishna et al. associated the efficiency of coconut water with propolis, HBSS, and milk in maintaining the viability of PDL cells on replicated avulsed teeth using collagenase-dispase assay. They reported that coconut water has considerably more viable PDL cells when compared to propolis, HBSS, or milk.[21]
Conservation of viability of cells may be due to the nutrients present in coconut water, such as proteins, amino acids, vitamins, and minerals, which nourish the cells and maintain viability.
Collagenase
Collagenase and dispase enzymes disrupt the extracellular matrix to release cells without extreme disruption and destruction of their own membrane.[22]
In this current study, to minimize the exposure of cells to active trypsin and to preserve maximum cell viability, the root surface was treated with collagenase. This procedure permitted prompt cell retrieval and preserved extreme cellular integrity, as was validated by the positive control samples.[10,22] This technique is more descriptive of the definite clinical situation because cells are not exposed to lengthy handling times to govern their viability status.
Trypan blue
The Trypan Blue exclusion staining technique was quick which distinctively differentiated vital from nonvital cells. However, the health of the vital cells and proliferation cannot be evaluated from this technique.[22,23]
In principle, only the cells with a damaged membrane allow the Trypan blue dye to pass through the membrane into cytoplasm.[24] This is based on the fact that negatively charged chromophore present on the cell membrane does not take up the stain unless the membrane is damaged. Hence, the cells which reject the dye are viable.
CONCLUSION
It is fundamental to have a storage medium easily available, for more avulsed teeth could be reimplanted, with better prognosis. Along with maintaining the viability of the PDL cells, it should be readily available near the accident site.
Coconut water is easily available in most tropical countries. It also maintains the viability of cells for longer periods of times, as shown in various studies. Nontropical countries could use milk which is available worldwide and “known to be the Gold standard” as a storage medium. However, in case of unavailability of any media, saline could be used as it is better than carrying the avulsed tooth in dry conditions.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Trope M. Clinical management of the avulsed tooth. Dent Clin North Am. 1995;39:93–112. [PubMed] [Google Scholar]
- 2.Andreasen JO, Andreasen FM, Andreasen L. Textbook and Color Atlas of Traumatic Injuries to the Teeth. 3rd edition. Munksgaard, Copenhagen (Denmark): 1994. [Google Scholar]
- 3.Gomes MC, Westphalen VP, Westphalen FH, Xavier da Silva Neto U, Fariniuk LF, Carneiro E. Study of storage media for avulsed teeth. Braz J Dent Traumatol. 2009;1:69–76. [Google Scholar]
- 4.Flores MT, Andersson L, Andreasen JO, Bakland LK, Malmgren B, Barnett F, et al. Guidelines for the management of traumatic dental injuries. II. Avulsion of permanent teeth. Dent Traumatol. 2007;23:130–6. doi: 10.1111/j.1600-9657.2007.00605.x. [DOI] [PubMed] [Google Scholar]
- 5.Harkacz OM, Sr, Carnes DL, Jr, Walker WA., 3rd Determination of periodontal ligament cell viability in the oral rehydration fluid Gatorade and milks of varying fat content. J Endod. 1997;23:687–90. doi: 10.1016/S0099-2399(97)80402-5. [DOI] [PubMed] [Google Scholar]
- 6.Huang SC, Remeikis NA, Daniel JC. Effects of long-term exposure of human periodontal ligament cells to milk and other solutions. J Endod. 1996;22:30–3. doi: 10.1016/S0099-2399(96)80233-0. [DOI] [PubMed] [Google Scholar]
- 7.Blomlöf L, Otteskog P. Viability of human periodontal ligament cells after storage in milk or saliva. Scand J Dent Res. 1980;88:436–40. doi: 10.1111/j.1600-0722.1980.tb01250.x. [DOI] [PubMed] [Google Scholar]
- 8.Moreira-Neto JJ, Gondim JO, Raddi MS, Pansani CA. Viability of human fibroblasts in coconut water as a storage medium. Int Endod J. 2009;42:827–30. doi: 10.1111/j.1365-2591.2009.01591.x. [DOI] [PubMed] [Google Scholar]
- 9.Olson BD, Mailhot JM, Anderson RW, Schuster GS, Weller RN. Comparison of various transport media on human periodontal ligament cell viability. J Endod. 1997;23:676–9. doi: 10.1016/S0099-2399(97)80399-8. [DOI] [PubMed] [Google Scholar]
- 10.Caglar E, Sandalli N, Kuscu OO, Durhan MA, Pisiriciler R, Caliskan EA, et al. Viability of fibroblasts in a novel probiotic storage media. Dent Traumatol. 2010;26:383–7. doi: 10.1111/j.1600-9657.2010.00914.x. [DOI] [PubMed] [Google Scholar]
- 11.Lindskog S, Blomlöf L. Influence of osmolality and composition of some storage media on human periodontal ligament cells. Acta Odontol Scand. 1982;40:435–41. doi: 10.3109/00016358209025118. [DOI] [PubMed] [Google Scholar]
- 12.Söder PO, Otteskog P, Andreasen JO, Modéer T. Effect of drying on viability of periodontal membrane. Scand J Dent Res. 1977;85:164–8. doi: 10.1111/j.1600-0722.1977.tb00549.x. [DOI] [PubMed] [Google Scholar]
- 13.Doyle DL, Dumsha TC, Sydiskis RJ. Effect of soaking in hank's balanced salt solution or milk on PDL cell viability of dry stored human teeth. Endod Dent Traumatol. 1998;14:221–4. doi: 10.1111/j.1600-9657.1998.tb00843.x. [DOI] [PubMed] [Google Scholar]
- 14.Ozan F, Polat ZA, Er K, Ozan U, Deǧer O. Effect of propolis on survival of periodontal ligament cells: New storage media for avulsed teeth. J Endod. 2007;33:570–3. doi: 10.1016/j.joen.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 15.Blomlöf L. Storage of human periodontal ligament cells in a combination of different media. J Dent Res. 1981;60:1904–6. doi: 10.1177/00220345810600111301. [DOI] [PubMed] [Google Scholar]
- 16.Pearson RM, Liewehr FR, West LA, Patton WR, McPherson JC, 3rd, Runner RR, et al. Human periodontal ligament cell viability in milk and milk substitutes. J Endod. 2003;29:184–6. doi: 10.1097/00004770-200303000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Krasner P, Person P. Preserving avulsed teeth for replantation. J Am Dent Assoc. 1992;123:80–8. doi: 10.14219/jada.archive.1992.0300. [DOI] [PubMed] [Google Scholar]
- 18.Patel S, Dumsha TC, Sydiskis RJ. Determining periodontal ligament (PDL) cell vitality from exarticulated teeth stored in saline or milk using fluorescein diacetate. Int Endod J. 1994;27:1–5. doi: 10.1111/j.1365-2591.1994.tb00220.x. [DOI] [PubMed] [Google Scholar]
- 19.Khademi AA, Atbaee A, Razavi SM, Shabanian M. Periodontal healing of replanted dog teeth stored in milk and egg albumen. Dent Traumatol. 2008;24:510–4. doi: 10.1111/j.1600-9657.2008.00648.x. [DOI] [PubMed] [Google Scholar]
- 20.Thomas T, Gopikrishna V, Kandaswamy D. Comparative evaluation of maintenance of cell viability of an experimental transport media “coconut water” with Hank's balanced salt solution and milk, for transportation of an avulsed tooth: An in vitro cell culture study. J Conserv Dent. 2008;11:22–9. doi: 10.4103/0972-0707.43414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gopikrishna V, Baweja PS, Venkateshbabu N, Thomas T, Kandaswamy D. Comparison of coconut water, propolis, HBSS, and milk on PDL cell survival. J Endod. 2008;34:587–9. doi: 10.1016/j.joen.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 22.Martin MP, Pileggi R. A quantitative analysis of propolis: A promising new storage media following avulsion. Dent Traumatol. 2004;20:85–9. doi: 10.1111/j.1600-4469.2004.00233.x. [DOI] [PubMed] [Google Scholar]
- 23.Sigalas E, Regan JD, Kramer PR, Witherspoon DE, Opperman LA. Survival of human periodontal ligament cells in media proposed for transport of avulsed teeth. Dent Traumatol. 2004;20:21–8. doi: 10.1111/j.1600-4469.2004.00219.x. [DOI] [PubMed] [Google Scholar]
- 24.Saxena P, Pant VA, Wadhwani KK, Kashyap MP, Gupta SK, Pant AB. Potential of the propolis as storage medium to preserve the viability of cultured human periodontal ligament cells: An in vitro study. Dent Traumatol. 2011;27:102–8. doi: 10.1111/j.1600-9657.2011.00974.x. [DOI] [PubMed] [Google Scholar]


