Abstract
Aim:
The aim of this work was to determine the frequency of oral changes in diabetic patients and to study the relationship between periodontal disease and diabetes mellitus.
Materials and Methods:
The study sample consisted of 440 known diabetic patients between the age group of 20–80 years, of which 212 were males and 228 were females. One hundred and six patients were below 40 years, 138 patients between 41 and 50 years, 97 in 51–60 years, and 99 above 60 years of age. Data were statistically analyzed by Student's t-test.
Results:
Nearly 57% of the patients showed a Russell's Periodontal Index score of 2–4.9, which suggested an established periodontal disease. Risk factors for the people above the age of 40 years to develop diabetes were 76%.
Conclusion:
The frequency of oral manifestations in diabetic patients was significantly high, hence showing a relationship of gingival and periodontal diseases with diabetes mellitus.
Keywords: Histological changes, hyposalivation, periodontal disease
INTRODUCTION
Duncan defined metabolism as “the sum of total tissue activity regulated by the availability, utilization, and disposal of protein, fat, carbohydrate, vitamins, minerals, water, and the influences which the endocrines exert on these processes.” Alteration from these normal metabolic processes constitute disturbances of metabolism.[1]
Diabetes mellitus is one of the major chronic health problems facing the world today and the most common among endocrine and metabolic disorders and is ranked 7th among the leading causes of death.[2]
Diabetes is a clinical syndrome characterized by hyperglycemia due to absolute (or) relative deficiency of insulin, and the lack of insulin affects the metabolism of carbohydrate, protein, and fat and causes a significant disturbance of water and electrolyte homeostasis.[3] Prevalence of diabetes is 1%–2% and 50% of diabetic patients are usually undiagnosed. The incidence rate seems to be rapidly rising these days and the ratio between type I and type II diabetic patients is 1:3.
Several oral manifestations have been reported to occur in diabetic patients. They are periodontal disease, xerostomia, dental caries, cheilosis, angular cheilitis, burning mouth sensation, taste alteration, candidiasis, and lichen planus. These changes, however, are not specific, but the most striking changes are the reduction in defense mechanisms and increased susceptibility to infections leading to destructive periodontal disease.[4]
The purpose of this present study is to determine the oral changes caused by diabetes mellitus.
MATERIALS AND METHODS
The study sample consisted of 440 known diabetic patients, concentrating mainly on the oral changes caused by diabetes. Out of these 440 patients, 212 were males and 228 were females [Table 1]. All the patients were between the age of 20–80 years sample size was selected according to random sampling [Table 2].
Table 1.
Age distribution

Table 2.
Male/female ratio

The patients were selected from Sree Balaji Medical College and Hospital, Chennai. All patients were from different socioeconomic status and standards [Tables 3 and 4].
Table 3.
Type of diabetes

Table 4.
Family history

Patients were examined with the following materials:
Plane mouth mirror
Dental probe
William's periodontal probe
Tweezers
Gloves
Mouth mask.
The patients were examined and the observations were recorded according to the format shown below:
Name, age, sex, OPD no, date, serial no, address, past medical history, family history, drug history, and personal history.
General physical examination
Vital signs - temperature, pulse, respiratory rate, and blood pressure.
Examination of oral cavity
Tongue, gingiva, buccal mucosa, labial mucosa, floor of the mouth, palate, calculus, periodontal pockets, and gingival bleeding.
Examination of teeth
Number, mobility, sensitivity, dental caries, attrition, abrasion, erosion, and missing teeth.
Russell's periodontal index was considered to examine the periodontal status of the patients.
This was given by Russell A. L in the year 1956. The following are the criteria of Russell's index.
“0” – Negative - There is no overt inflammation in the investing tissues nor loss of function due to destruction of supporting tissues.
“1” – Mild gingivitis - Overt area of inflammation in the free gingiva does not circumscribe the tooth.
“2” – Gingivitis - Inflammation completely circumscribes the tooth, but there is no apparent break in the epithelial attachment.
“6” – Gingivitis with pocket formation - The epithelial attachment has been broken and there is a pocket formation. There is no interference with normal masticatory function. The tooth is firm in its socket and has not drifted.
“8” – Advanced destruction with loss of masticatory function. The tooth may be loose, may have drifted, or may be depressible in its socket.
Clinical conditions and periodontal index scores
Clinically normal supportive tissues → 0–0.2
Simple gingivitis → 0.3–0.9
Beginning of destructive periodontal disease → 1.0–1.9
Established periodontal disease → 2.0–4.9
Terminal disease → 5.0–8.0.
Materials used for examination and biopsy
Local anesthetic solution
Syringe - 25-gauge needle (1 inch long)
Scissors
Bard-Parker handles
Blades no. 12 and 15
Tissue forceps
10% formalin solution
Sterile cotton
Gloves
Mouth mask.
Preparation of the patient
Consent of the patients was obtained and appointment for biopsy was given. Diabetes mellitus was brought into control by a physician and the physician's consent was obtained to take the biopsy.
Method of obtaining biopsy
The patient was made to sit on a dental chair and local anesthetic solution was given at the specified site. The lidocaine 0.2% was injected around the site of biopsy, to obtain accurate histopathological features. Then, using Bard-Parker blade, a small portion of the buccal mucosa was cut and removed using tissue forceps. The biopsied area was sutured and the tissue was then fixed in 10% formalin solution. The tissue was processed by the usual procedures to obtain 5 μ paraffin sections. The sections were stained with hematoxylin and eosin (H and E) method.
Hematoxylin and eosin method
The routine H and E staining was done as follows:
Remove paraffin wax with xylene for 5 min
Treat with absolute alcohol, two changes - 1 min each
Wash in water for 5 min
Stain in Harris hematoxylin for 1–2 min
Wash in water for 3 min
Differentiate in 1% acid alcohol - one dip
Immediate water wash
Stain in 1% aqueous eosin solution - 1–2 dips
Dehydrate in two changes of acetone - 10 dips each
Clear in two changes of xylene - 10 dips each.
The stained sections were observed under the light microscope and the histopathological features were recorded.
Smear
Smears were taken from the oral cavity and were alcohol fixed and stained by Periodic acid–Schiff method.
Procedure
Washed in running water – 2 min
1% periodic acid – 5 min
Washed in running water for 5 min
Stained in Schiff's reagent for 30 min
Washed in running water for 2 min
Stained in hematoxylin for 1 min
Washed in running water for 2 min
Mounted in DPX mountant.
Smears were viewed under the microscope for the presence of hyphal forms and yeast cells.
Method for determining hyposalivation
Timothy S Miles followed the questionnaire method for determining the condition called hyposalivation; the questionnaire was as follows;
Does your mouth feel dry? (during a meal)
Is there any difficulty in swallowing food?
Do you sip liquids while swallowing dry foods?
Do you think that the amount of saliva is less, and have you noticed it?
RESULTS AND OBSERVATION
Among 440 patients, 212 were males and 228 were females; of these patients, 106 were of 1–14, 138 in 41–50, 97 in 51–60, and 99 in 60 and above years of age.
From the above reading shown, it is evident that majority of the diabetic patients examined during the course of the study belonged to age group of 41–50 years.
The periodontal examination was computed in all cases according to periodontal index of Russell. A mouth mirror and a periodontal probe were used under good lighting. By Russell's method, the periodontal status of each tooth was evaluated as follows: 0–2 → pocket formation and severe gingivitis and 6–8 were of severe gingivitis, pocket formation, and alveolar bone destruction.
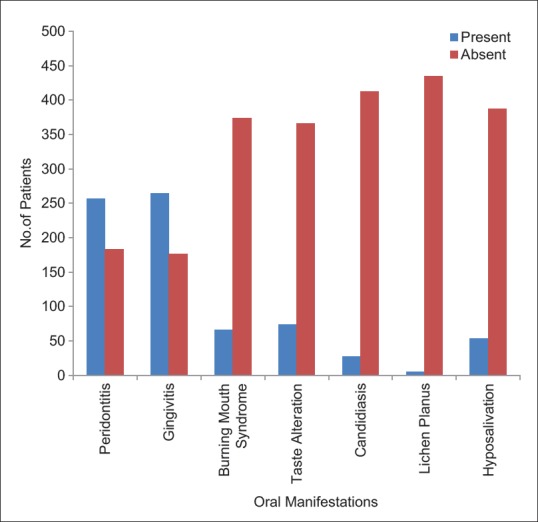
Majority of the patients (257) had a periodontal index ranging from 4 to 6 indicating severe gingivitis with pocket formation. One hundred and eighty-three patients and a periodontal index ranging from 2 to 4 and 81 patients had an index ranging from 0 to 2. Fifty-seven patients showed mobility of teeth indicating a periodontal index of 6–8 [Figure 1a and b].
Figure 1.
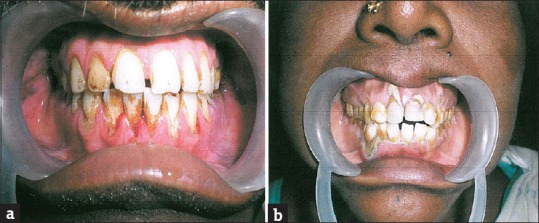
(a and b) Chronic generalized periodontitis
Data recorded showed that majority of diabetic patients examined during the study belonged to the age group of 41–50 years.
A comparison was made between the age of patients and the periodontal index. Most of the patients suffering from periodontal disease belonged to the age group of 60 years and above.
The burning mouth syndrome was observed in 15% of the diabetic patients, according to the clinical symptoms.
The association of taste alteration in diabetic patients was studied and about 16.83% patients showed the symptoms of the taste alteration.
Hyposalivation was observed in 53 (12.05%) diabetic patients which was studied by the clinical symptoms.
Significant changes were also observed on the tongue and candidiasis was seen in 27 (6.14%) of the diabetic patients. The tongue appeared smooth and glistening red [Figure 2a-c]. The patients also complained of a severe burning sensation and some complained of taste alteration.
Figure 2.
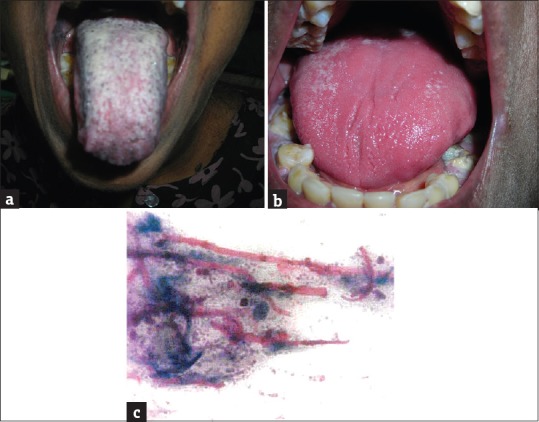
(a) Candidiasis - pseudomembranous type, (b) candidiasis - erythematous type, (c) PAS stained smear showing candidal hyphae
Lichen planus was observed in 5 (1.14%) diabetic patients [Figure 3a and b].
Figure 3.
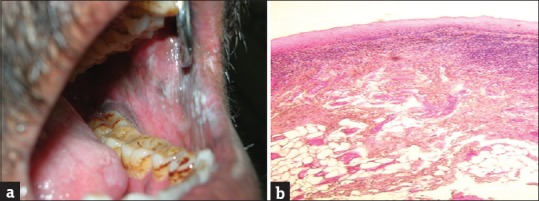
(a) Lichen planus, (b) lichen planus - histopathology
DISCUSSION
Diabetes mellitus is one of the major chronic health problems facing the world today and the most common among endocrine and metabolic disorders and is ranked 7th among the leading causes of death.[2]
Diabetes is a clinical syndrome characterized by hyperglycemia due to absolute (or) relative deficiency of insulin [Figure 4], and the lack of insulin affects the metabolism of carbohydrate, protein, and fat and causes a significant disturbance of water and electrolyte homeostasis.[3]
Figure 4.
Oral manifestations in diabetes patients
Majority of the patients were under medication, which clearly indicates the increasing awareness among people about the fatal outcome of this disease and the necessity of proper treatment and diet restrictions, but the number of diabetics, in general, continues to increase every year, due to increasing population and inheritance of genes which precipitate the diseases.[5]
This study was undertaken to investigate the effects of diabetes mellitus on oral environment.
Various oral changes caused by diabetes mellitus in our study were:
Periodontitis
Gingivitis
Hyposalivation
Taste alteration
Burning mouth syndrome
Candidiasis
Lichen planus.
We examined the oral cavity of 440 patients during the course of the study, and out of them, 212 were males and 228 were females with respective percentages of 48.18% and 51.82%.
The maximum number of patients examined belonged to the age group of above 40 years and there was an increasing incidence of diabetes after the age of 40 years.
The relationship between diabetes mellitus and periodontal diseases has been studied extensively with variation in results.
In our study, we observed an increased incidence of periodontal diseases among majority of the diabetic patients and found that periodontal disease was predominantly seen in patients ranging from 60 years and above, which was followed by patients ranging in age from 51 to 60 years, indicating increasing incidence of periodontal diseases with age.
There was an increased incidence of gingivitis seen in patients below 40 years of age followed by the age group ranging above 60 years.
The increased involvement of periodontium in diabetics was in accordance with the review by Mealey and Oates, who stated that periodontal disease risk is three times greater in diabetic patients, than in the general population.[6]
In our study, periodontitis was seen in 58.41% diabetic patients. This increased incidence of periodontitis was in correlation with the studies by Stegeman and Kinane et al., where they demonstrated that diabetics were at a greater risk of developing periodontal disease.[7,8]
However, in contradiction to our results, Kinane et al. in his review concluded that the duration of diabetes does not influence the periodontal severity.[8]
Out of the 440 patients examined, gingivitis was observed in 60% cases. This study was similar to that demonstrated by Mattout et al. where an elevated gingival index was seen in association with diabetics.[9]
In our study, hyposalivation was seen in 53 patients (12.05%) with majority of the patients having a periodontal disease, thus revealing a strong correlation between decreased salivary flow rate which creates a favorable environment for the growth of organisms and initiation of periodontal disease.
This finding was similar to the studies of Ship, who demonstrated that diabetics had a decreased salivary flow rate,[10] but this finding was in contradiction to the studies of Quirino et al. (68.6%) where they demonstrated an increased incidence of hyposalivation in diabetics,[11] whereas Miralles et al. suggested that there were no differences between the diabetics and healthy subjects in case of salivary flow.
In the present study, burning mouth syndrome was observed in 15% patients. This finding was in close relation to that demonstrated by Quirino et al. where burning mouth syndrome was observed in 19.3% and 11.5% patients, respectively,[11] but this was also in contradiction to the study by Gibson et al. where he demonstrated 36.8% patients with burning mouth syndrome.[12]
Taste alteration was found in 16.8% patients of our study. This finding is similar to that demonstrated by Gibson et al. where he observed 20.7% patients with taste alteration but was not in accordance with the study by Quirino et al. where 36.5% patients demonstrated the symptom.[11,12]
Our present study showed 5 patients (1.14%) with lichen planus which was found to be in accordance with the study conducted by Maria Albrecht et al., in which 1% of diabetics showed oral lichen planus. This finding was also demonstrated in the studies of Gibson et al. and Ship.[10,12]
In the present study, 6.14% patients showed candidiasis as one of the manifestations of diabetes which was similar to the studies of Gibson et al. and Ship.[10,12]
This was in contradiction to the study by Quirino et al. where he demonstrated that diabetics had no significant mucosal lesion when compared to the controls.[11]
CONCLUSION
Diabetes mellitus is a clinical syndrome characterized by hyperglycemia due to absolute or relative deficiency of insulin and the lack of insulin that affects the metabolism of carbohydrate, protein, and fat and causes a significant disturbance of water and electrolyte homeostasis.
The present study was carried out to determine the oral changes in diabetes mellitus patients. Four hundred and forty known diabetic patients were taken up in the study. Out of them, 212 were males and 228 were females.
The oral cavity of diabetes mellitus individuals when examined thoroughly showed several findings and among the various oral manifestations that were observed periodontitis and gingivitis exhibited a strong correlation to diabetes mellitus.
Other manifestations observed were candidiasis, hyposalivation, burning mouth syndrome, taste alteration, and lichen planus. There was a strong correlation between periodontal disease and hyposalivation.
However, additional studies may be required to evaluate
Relationship between periodontal disease and diabetes mellitus
Relationship between periodontal disease and hyposalivation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Ankita Bohra, Bhateja S. Oral aspects of metabolic disorders. J Metab Dis. 2015;4:1. [Google Scholar]
- 2.Bhutani J, Bhutani S. Worldwide burden of diabetes. Indian J Endocrinol Metab. 2014;18:868–70. doi: 10.4103/2230-8210.141388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009;32(Suppl 1):S62–7. doi: 10.2337/dc09-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vellappally S. Burning mouth syndrome: A Review of the etiopathologic factors and management. J Contemp Dent Pract. 2016;17:171–6. doi: 10.5005/jp-journals-10024-1822. [DOI] [PubMed] [Google Scholar]
- 5.Plantinga LC, Tuot DS, Powe NR. Awareness of chronic kidney disease among patients and providers. Adv Chronic Kidney Dis. 2010;17:225–36. doi: 10.1053/j.ackd.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mealey BL, Oates TW American Academy of Periodontology. Diabetes mellitus and periodontal diseases. J Periodontol. 2006;77:1289–303. doi: 10.1902/jop.2006.050459. [DOI] [PubMed] [Google Scholar]
- 7.Stegeman CA. Oral manifestations of diabetes. Home Healthc Nurse. 2005;23:233–40. doi: 10.1097/00004045-200504000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Kinane DF, Peterson M, Stathopoulou PG. Environmental and other modifying factors of the periodontal diseases. Periodontol 2000. 2006;40:107–19. doi: 10.1111/j.1600-0757.2005.00136.x. [DOI] [PubMed] [Google Scholar]
- 9.Mattout C, Bourgeois D, Bouchard P. Type 2 diabetes and periodontal indicators: Epidemiology in France 2002-2003. J Periodontal Res. 2006;41:253–8. doi: 10.1111/j.1600-0765.2006.00862.x. [DOI] [PubMed] [Google Scholar]
- 10.Ship JA. Diabetes and oral health: An overview. J Am Dent Assoc. 2003;134(Suppl 1):4S–10S. doi: 10.14219/jada.archive.2003.0367. [DOI] [PubMed] [Google Scholar]
- 11.Quirino MR, Birman EG, Paula CR. Oral manifestations of diabetes mellitus in controlled and uncontrolled patients. Braz Dent J. 1995;6:131–6. [PubMed] [Google Scholar]
- 12.Gibson J, Lamey PJ, Lewis M, Frier B. Oral manifestations of previously undiagnosed non-insulin dependent diabetes mellitus. J Oral Pathol Med. 1990;19:284–7. doi: 10.1111/j.1600-0714.1990.tb00843.x. [DOI] [PubMed] [Google Scholar]


