Publisher's Note: There is an Inside Blood Commentary on this article in this issue.
Key Points
In vivo–generated thrombin and plasmin do not contribute to complement activation in nonhuman primates.
Bacteria and lipopolysaccharide are the main drivers of in vivo complement activation in E coli sepsis in baboons.
Abstract
Sepsis concurrently activates both coagulation and complement systems. Although complement activation by bacteria is well documented, work in mice and in vitro suggests that coagulation proteases can directly cleave complement proteins. We aimed to determine whether generation of coagulation proteases in vivo can activate the complement cascade in 2 highly coagulopathic models. We compared temporal changes in activation biomarkers of coagulation (thrombin-antithrombin [TAT]), fibrinolysis (plasmin-antiplasmin [PAP]), and complement (C3b, C5a, C5b-9) in baboons infused with factor Xa (FXa) and phospholipids (FXa/phosphatidylcholine-phosphatidylserine [PCPS]) vs LD100 Escherichia coli. We found that, albeit with different timing, both FXa/PCPS and E coli infusion led to robust thrombin and plasmin generation. Conversely, only E coli challenge activated the complement system, reaching a maximum at 2 hours postchallenge during the peaks of lipopolysaccharide and bacteremia but not of TAT and PAP. Despite inducing a strong burst of thrombin and plasmin, FXa/PCPS infusion did not produce measurable levels of complement activation in vivo. Similarly, ex vivo incubation of baboon serum with thrombin, plasmin, or FXa did not show noticeable complement cleavage unless supraphysiologic amounts of enzymes were used. Our results suggest that in vivo–generated thrombin and plasmin do not directly activate the complement in nonhuman primates.
Introduction
Complement and hemostasis play essential roles in innate immunity, protecting the host against bacterial invasion and dissemination.1 These evolutionarily conserved systems are composed of serine proteases, which are activated by invading pathogens or pathogen-associated molecular patterns.2 Bacterial sepsis triggers robust activation of the complement, coagulation, and fibrinolytic systems, which all contribute to disseminated intravascular coagulation, organ damage, and death.1 Numerous reports suggest close direct or indirect interactions between these systems during the hematologic response to injury.3 Complement and coagulation have synergistic effects such that the activation of one augments the activation of the other.1 Although some of the data supporting the activation of complement by coagulation or fibrinolytic proteases, including thrombin and plasmin, have been derived from in vivo studies in mice,4,5 human data are mostly based on in vitro assays with purified proteins, serum, or plasma.5-7
Limited in vivo information from relevant primate models has left lingering questions about the physiological significance and implications of this proposed crosstalk in humans. To test the in vivo role of thrombin and plasmin on complement activation in primates, we used plasma samples from baboons infused with factor Xa (FXa) and phosphatidylcholine-phosphatidylserine (PCPS) vesicles (FXa/PCPS) that trigger a robust burst of thrombin and subsequent plasmin generation in vivo.8,9 For comparison, we used plasma from baboons infused with LD100 Escherichia coli10 that leads to activation of the complement,11,12 coagulation, and fibrinolytic systems.13,14
We found that bacteria and lipopolysaccharide (LPS) were the main activators of the complement system in E coli sepsis. In contrast, FXa/PCPS-induced in vivo generation of thrombin and plasmin, even if in large enough amounts to deplete most of the fibrinogen/fibrin, minimally affected complement activation.
Study design
Baboon model of E coli sepsis and FXa/PCPS infusion
Plasma samples were derived from studies approved by the Institutional Animal Care and Use Committee of the Oklahoma Medical Research Foundation. Papio anubis baboons were IV infused with a single bolus of FXa/PCPS (36.6 pmol/kg body weight FXa and 56.3 nmol/kg body weight PCPS)9 or LD100 E coli (1 × 1010 colony-forming units [CFUs]/kg to 3 × 1010 CFU/kg).11,15
Baboon blood samples and physiological parameters were collected before (T0) and at the indicated times after challenge with FXa/PCPS or E coli. Detailed descriptions of the methods are presented in the supplemental Methods (available on the Blood Web site).
Ex vivo baboon serum assays
Baboon serum samples were incubated for 90 minutes at 37°C with increasing concentrations of (1) human thrombin (5 or 20 μg/mL [3230 National Institutes of Health (NIH) U/mg]; Enzyme Research Laboratories), (2) human plasmin (5 or 20 μg/mL [7.5 U/mg]; Sigma Aldrich), or (3) human FXa (0.04, 0.4, 4, 40, or 100 μg/mL human FXa [100 U/mg]; Enzyme Research Laboratories). The method is detailed in the supplemental Methods.
Results and discussion
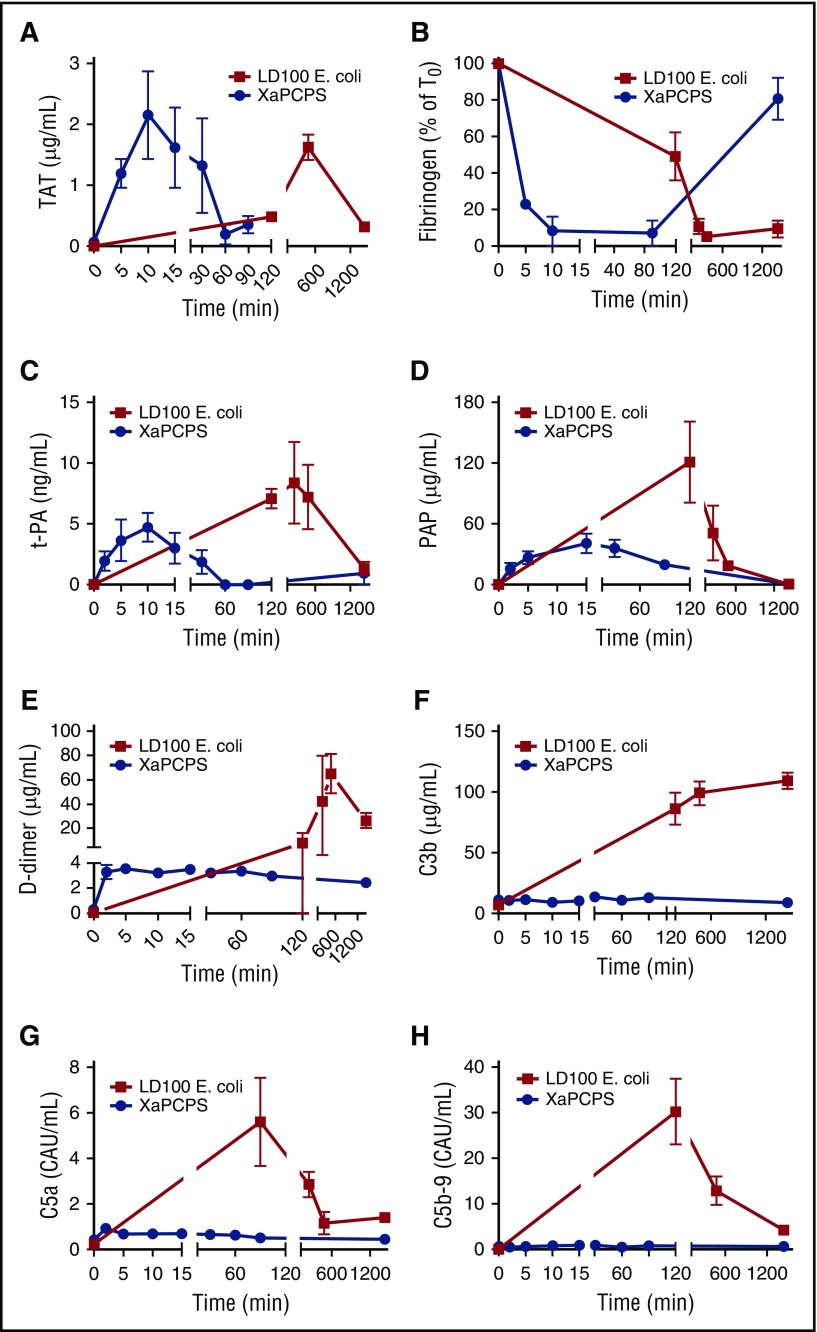
Although temporally different, both FXa/PCPS and E coli infusion induced robust thrombin generation as evidenced by thrombin-antithrombin (TAT) levels in plasma (Figure 1A). Although FXa/PCPS infusion led to maximum levels of thrombin at 10 minutes postchallenge, E coli led to a delayed response, where TAT levels peaked 8 hours after the challenge. Similarly, FXa/PCPS infusion consumed 90% of fibrinogen within 10 minutes whereas the E coli challenge consumed 90% of fibrinogen after 8 hours postinfusion (Figure 1B). Whereas both challenges activated the coagulation to the extent of almost full depletion of plasma fibrinogen, FXa/PCPS infusion generated a brief acute response whereas the procoagulant response to E coli was spread over a larger time frame.
Figure 1.
Time-course activation of coagulation, fibrinolysis, and complement pathways during Xa/PCPS vs E coli challenge. (A) TAT, (B) fibrinogen, (C) tissue plasminogen activator (t-PA), (D) plasmin-antiplasmin (PAP) complexes, (E) d-dimer, (F) C3b, (G) C5a, and (H) C5b-9 levels in baboon plasma following LD100 E coli (▪; n = 3) or FXa/PCPS infusion (●; n = 3).
Because thrombin is a major secretagogue of t-PA, the main driver of plasmin generation in the vasculature, we tested the effect of FXa/PCPS and E coli infusion on t-PA release. We also measured (PAP as a measure of plasmin generation, and fibrinogen/fibrin degradation products and d-dimer as plasma markers of fibrinolysis. t-PA content in plasma was increased by FXa/PCPS and E coli infusion (Figure 1C). Similarly, both FXa/PCPS and E coli infusion induced robust plasmin generation (Figure 1D) and fibrin degradation (Figure 1E). Fibrinolytic activation markers reached maximum levels after 10 to 40 minutes after FXa/PCPS vs 8 hours after E coli infusion.
Despite robust plasmin and thrombin generation by both FXa/PCPS and E coli, FXa/PCPS infusion did not induce complement activation as measured by activation markers C3b, C5a, and soluble C5b-916 (Figure 1F-H). In contrast, E coli challenge induced strong complement activation with a maximum response at 120 minutes (Figure 1F-H).
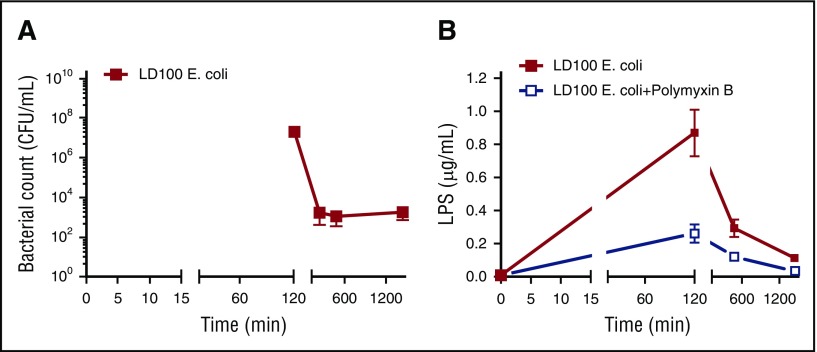
E coli and LPS are reported to activate both classical and alternative pathways of the complement system.17 Maximal amounts of complement activation markers (Figure 1F-H) coincided with the level of bacteremia (Figure 2A) and endotoxin (LPS) (Figure 2B).18 These findings suggest that bacteria and LPS are the main activators for the complement system in E coli sepsis. Importantly, we observed a temporal difference between maximal activation of complement (120 minutes; Figure 1F-H) and the peak of coagulation/fibrinolytic markers following E coli challenge (240-480 minutes; Figure 1A-E), suggesting no direct effect of coagulation proteases on complement activation.
Figure 2.
Time course of bacteremia and LPS content in blood. (A) Blood samples were collected at the indicated time points and CFUs were determined by plating on trypticase soy agar. Mean counts of bacteria were plotted semilogarithmically against time in minutes. (B) Time course of LPS content in plasma was determined by using HEK-Blue TLR4 cells.
In contrast to previous in vivo studies in mice or in vitro studies with human blood,5-7 our results demonstrate that thrombin and plasmin have no detectable effect on in vivo complement activation in baboons (Figure 1A-F). Testing the effect of proteases ex vivo using whole blood or plasma approaches is not feasible as blood anticoagulants interfere with complement activation or directly block thrombin. Because human serum was previously used as a source of complement proteins in ex vivo assays,6 we incubated baboon serum with thrombin, FXa, or plasmin (see supplemental Results). As shown in supplemental Figure 1, incubation with thrombin even at very high concentrations did not induce complement activation, whereas incubation with plasmin and FXa induced elevation of complement activation markers only at supraphysiologic doses (20 µg/mL plasmin or 40-100 µg/mL FXa, respectively). Our ex vivo data support the conclusion that cross-activation of the complement system by coagulation proteases is very low or nonexistent, and probably has no physiological implications. These results are not necessarily suggesting that baboons are somehow different from humans in this respect, as similar results were obtained when we used human serum (data not shown). We had to use baboon serum because all known anticoagulants strongly interfere with complement activation. However, we recognize that this approach has limitations, including a small consumption (<1%) of complement proteins and partial depletion of antithrombin (AT) and α2-antiplasmin during serum preparation.
Discrepancies between our and previously published data may result from differences in complement and coagulation systems between species19 or reflect differences in the experimental conditions. The bulk of published data are derived from ex vivo assays using nonphysiologic amounts of purified proteases incubated with plasma or purified complement proteins.5,6 Differently from ex vivo, both thrombin20 and plasmin21 are tightly controlled in vivo by inhibitors such as AT and α2-antiplasmin, resulting in short-lived protease activity. Moreover, thrombin inactivation in vivo is enhanced by vessel wall–associated heparan sulfates22 that strongly amplify AT activity.22 In ex vivo assays, inhibition of thrombin by AT is inefficient in the absence of heparin23 and there is no clearance involved. In contrast, TAT24 and PAP21 complexes are rapidly formed and cleared in vivo within minutes, affecting the availability of the enzymes to act on complement proteins.
We cannot fully refute that baboon clotting or fibrinolytic enzymes could cleave complement proteins in a purified system. Unfortunately, we cannot replicate experiments with purified proteins as neither baboon coagulation proteases nor complement proteins are available in purified forms. However, experiments with a high concentration of purified enzymes in the absence of natural inhibitors have limited physiological relevance.
The published in vivo data are restricted to mouse models of injury-induced thrombosis5 that do not differentiate between the effects of the proteases and tissue damage. Differently, we used a model of pure coagulopathic challenge, with no significant tissue damage. Our model has clinical relevance because nonhuman primates have the closest phylogenetic proximity to humans.
The caveat of this study is that the time frame of thrombin and plasmin generation in the 2 models is not comparable, as Xa/PCPS infusion leads to an acute burst of thrombin and plasmin, whereas in E coli sepsis, thrombin and plasmin are generated over several hours.
In conclusion, because thrombin and plasmin generated after FXa/PCPS infusion did not increase complement biomarkers, our results suggest that these proteases do not contribute by themselves to complement activation in vivo. However, it is possible that chronically produced coagulation proteases could act synergistically with the bacteria to promote complement activation and immunothrombosis during bacteremic sepsis. Actually, the converse effect of complement on activation of coagulation was demonstrated, as complement inhibition in baboon models of E coli sepsis was accompanied by dampening of coagulation biomarkers.11,25 Hence, future studies to determine whether inhibition of coagulation proteases can decrease complement activation induced by sepsis are warranted.
Delineating the crosstalk between the complement and coagulation pathways is important to better understand the pathophysiology of sepsis and may help to identify new therapeutic targets.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
The authors are indebted to their colleagues Ray Rezaie, for critically reading the manuscript, and Kandice Tessneer, for editing the manuscript.
This work was supported by grants from the National Institutes of Health, National Institute of General Medical Sciences (GM097747, GM116184, GM121601, and P30GM114731 [F.L.]), and the National Institute of Allergy and Infectious Diseases (U19AI062629 [F.L.]).
Footnotes
Presented in abstract form at the 58th annual meeting of the American Society of Hematology, San Diego, CA, 2-6 December 2016.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: R.S.K., R.S., C.L. performed biochemical analysis and enzyme-linked immunosorbent assay; F.B.T. designed the research; R.S. performed animal experiments; F.L. conceived and supervised the study; R.S.K., C.L., and F.L. analyzed the results and wrote the manuscript; and all authors read and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Florea Lupu, Cardiovascular Biology Research Program, Oklahoma Medical Research Foundation, 825 NE 13th St, Oklahoma City, OK 73104; e-mail: florea-lupu@omrf.org.
References
- 1.Lupu F, Keshari RS, Lambris JD, Coggeshall KM. Crosstalk between the coagulation and complement systems in sepsis. Thromb Res. 2014;133(suppl 1):S28-S31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esmon CT. The impact of the inflammatory response on coagulation. Thromb Res. 2004;114(5-6):321-327. [DOI] [PubMed] [Google Scholar]
- 3.Markiewski MM, DeAngelis RA, Lambris JD. Complexity of complement activation in sepsis. J Cell Mol Med. 2008;12(6A):2245-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huber-Lang M, Sarma JV, Zetoune FS, et al. . Generation of C5a in the absence of C3: a new complement activation pathway. Nat Med. 2006;12(6):682-687. [DOI] [PubMed] [Google Scholar]
- 5.Foley JH, Walton BL, Aleman MM, et al. . Complement activation in arterial and venous thrombosis is mediated by plasmin. EBioMedicine. 2016;5:175-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amara U, Flierl MA, Rittirsch D, et al. . Molecular intercommunication between the complement and coagulation systems. J Immunol. 2010;185(9):5628-5636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krisinger MJ, Goebeler V, Lu Z, et al. . Thrombin generates previously unidentified C5 products that support the terminal complement activation pathway. Blood. 2012;120(8):1717-1725. [DOI] [PubMed] [Google Scholar]
- 8.Giles AR, Nesheim ME, Herring SW, Hoogendoorn H, Stump DC, Heldebrant CM. The fibrinolytic potential of the normal primate following the generation of thrombin in vivo. Thromb Haemost. 1990;63(3):476-481. [PubMed] [Google Scholar]
- 9.Taylor FB Jr, Hoogendoorn H, Chang AC, et al. . Anticoagulant and fibrinolytic activities are promoted, not retarded, in vivo after thrombin generation in the presence of a monoclonal antibody that inhibits activation of protein C. Blood. 1992;79(7):1720-1728. [PubMed] [Google Scholar]
- 10.Taylor FB Jr, Kinasewitz GT, Lupu F. Pathophysiology, staging and therapy of severe sepsis in baboon models. J Cell Mol Med. 2012;16(4):672-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silasi-Mansat R, Zhu H, Popescu NI, et al. . Complement inhibition decreases the procoagulant response and confers organ protection in a baboon model of Escherichia coli sepsis. Blood. 2010;116(6):1002-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor FB Jr, Hack E, Lupu F. Observations on complement activity in the two-stage inflammatory/hemostatic response in the baboon and human models of E. coli sepsis and endotoxemia. Adv Exp Med Biol. 2006;586:203-216. [DOI] [PubMed] [Google Scholar]
- 13.Taylor FB., Jr Staging of the pathophysiologic responses of the primate microvasculature to Escherichia coli and endotoxin: examination of the elements of the compensated response and their links to the corresponding uncompensated lethal variants. Crit Care Med. 2001;29(suppl 7):S78-S89. [DOI] [PubMed] [Google Scholar]
- 14.Zhu H, Tang Y, Ivanciu L, et al. . Temporal dynamics of gene expression in the lung in a baboon model of E. coli sepsis. BMC Genomics. 2007;8(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor FB Jr, Stearns-Kurosawa DJ, Kurosawa S, et al. . The endothelial cell protein C receptor aids in host defense against Escherichia coli sepsis. Blood. 2000;95(5):1680-1686. [PubMed] [Google Scholar]
- 16.Mollnes TE, Lea T, Harboe M, Tschopp J. Monoclonal antibodies recognizing a neoantigen of poly(C9) detect the human terminal complement complex in tissue and plasma. Scand J Immunol. 1985;22(2):183-195. [DOI] [PubMed] [Google Scholar]
- 17.Mollnes TE, Brekke OL, Fung M, et al. . Essential role of the C5a receptor in E coli-induced oxidative burst and phagocytosis revealed by a novel lepirudin-based human whole blood model of inflammation. Blood. 2002;100(5):1869-1877. [PubMed] [Google Scholar]
- 18.Creasey AA, Stevens P, Kenney J, et al. . Endotoxin and cytokine profile in plasma of baboons challenged with lethal and sublethal Escherichia coli. Circ Shock. 1991;33(2):84-91. [PubMed] [Google Scholar]
- 19.Ekdahl KN, Teramura Y, Hamad OA, et al. . Dangerous liaisons: complement, coagulation, and kallikrein/kinin cross-talk act as a linchpin in the events leading to thromboinflammation. Immunol Rev. 2016;274(1):245-269. [DOI] [PubMed] [Google Scholar]
- 20.Carlson TH. Clearance of thrombin in vivo: significance of alternative pathways. Mol Cell Biochem. 1986;71(2):97-105. [DOI] [PubMed] [Google Scholar]
- 21.Levi M, de Boer JP, Roem D, ten Cate JW, Hack CE. Plasminogen activation in vivo upon intravenous infusion of DDAVP. Quantitative assessment of plasmin-alpha 2-antiplasmin complex with a novel monoclonal antibody based radioimmunoassay. Thromb Haemost. 1992;67(1):111-116. [PubMed] [Google Scholar]
- 22.Jordan RE, Oosta GM, Gardner WT, Rosenberg RD. The kinetics of hemostatic enzyme-antithrombin interactions in the presence of low molecular weight heparin. J Biol Chem. 1980;255(21):10081-10090. [PubMed] [Google Scholar]
- 23.Rosenberg RD. Biochemistry of heparin antithrombin interactions, and the physiologic role of this natural anticoagulant mechanism. Am J Med. 1989;87(3B):2S-9S. [DOI] [PubMed] [Google Scholar]
- 24.Lollar P, Owen WG. Clearance of thrombin from circulation in rabbits by high-affinity binding sites on endothelium. Possible role in the inactivation of thrombin by antithrombin III. J Clin Invest. 1980;66(6):1222-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keshari RS, Silasi R, Popescu NI, et al. . Inhibition of complement C5 protects against organ failure and reduces mortality in a baboon model of Escherichia coli sepsis. Proc Natl Acad Sci USA. 2017;114(31):E6390-E6399. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.