Abstract
Anti-angiogenic therapy with bevacizumab, an inhibitor of vascular endothelial growth factor, is commonly used in metastatic colorectal cancer and is rarely associated with gastrointestinal perforation, perforation being more frequent in the primary tumor site or at the anastomotic level. We present the case of a 64-year-old male with stage IV rectal adenocarcinoma who was on palliative chemotherapy with FOLFOX and bevacizumab. After the 4th chemotherapy cycle, our patient started fever and epigastric pain. He was hemodynamically stable, and signs of peritoneal irritation were absent. There were no alterations in the abdominal X-ray, and C-reactive protein was markedly elevated. A CT scan revealed a de novo thickness in the gastric antrum. Upper digestive endoscopy showed an ulcerated 40-mm lesion in the angulus, with a 20-mm orifice communicating with an exsudative cavity revested by the omentum. A conservative approach was decided including fasting, broad-spectrum intravenous antibiotics, and proton-pump inhibitors. Subsequent gastroduodenal series showed no contrast extravasation, allowing the resumption of oral nutrition. Esophagogastroduodenoscopy after 8 weeks showed perforation closure. Biopsies did not show neoplastic cells or Heliobacter pylori infection. Although the success in the conservative management of perforation allowing the maintenance of palliative chemotherapy (without bevacizumab), the patient died after 4 months due to liver failure. The reported case shows an uncommon endoscopic finding due to a rare complication of anti-angiogenic therapy. Additionally, it reminds clinicians that a history of gastroduodenal ulcers should be actively sought before starting anti-angiogenic treatment and that suspicion for perforation should be high in these cases.
Keywords: Gastric ulcer, Colorectal cancer, Cancer, Stomach, Gastrointestinal tract
Resumo
A terapêutica anti-angiogénica com bevacizumab, um inibidor do fator de crescimento do endotélio vascular (VEGF), é frequentemente utilizada no cancro colo-retal metastático. Associa-se raramente a perfuração gastrointestinal, que é mais frequente no local do tumor primário e na anastomose. Apresentamos o caso de um doente de 64 anos, sexo masculino, com adenocarcinoma do reto estadio IV sob quimioterapia com FOLFOX e bevacizumab. Após o 4° ciclo de quimioterapia, o doente recorreu ao Serviço de Urgência com febre e dor epigástrica; à observação, verificou-se estabilidade hemodinâmica e ausência de sinais de irritação peritoneal. A proteína C-reativa estava marcadamente elevada, não havendo alterações na radiografia simples do abdómen. Realizada TC, que mostrou um espessamento de novo no antro gástrico. A endoscopia digestiva alta realizada mostrou uma lesão ulcerada com 40mm na incisura angular, com um orifício de 20mm comunicando com loca exsudativa revestida pelo omento. Foi decidida abordagem conservadora que incluiu jejum, antibióticos endovenosos de largo espetro e inibidor da bomba de protões. Posteriormente realizado trânsito gastro-duodenal que não mostrou extravasamento do contraste, permitindo a reintrodução de alimentação oral. A endoscopia de controlo após 8 semanas mostrou encerramento completo da perfuração, não tendo as biopsias revelado alterações neoplásicas ou infeção Helicobacter pylori. Apesar do sucesso da terapêutica conservadora, permitindo continuação de quimioterapia sem bevacizumab, o doente faleceu após 4 meses devido a insuficiência hepática. O caso reportado mostra um achado endoscópico raro, devido a complicação rara da terapêutica anti-angiogénica. Além disso, relembra os clínicos da importância de pesquisar ativamente antecedentes de úlceras gastroduodenais antes do início da terapêutica anti-angiogénica e de que o índice de suspeição de perfuração deve ser elevado em doentes sob esta terapêutica.
Palavras Chave: Úlcera gástrica, Cancro colo-retal, Cancro, Estômago, Tubo digestivo
Introduction
Anti-angiogenic therapy with bevacizumab, an inhibitor of vascular endothelial growth-factor, is commonly used in metastatic colorectal cancer and other malignancies and is associated with an increased risk of gastrointestinal perforation (relative risk 2.14, 95% CI 1.19–3.5) [1]. Perforation is a rare dose-dependent adverse event occurring in approximately 0.9–1.1% of the patients [1, 2], is more frequent in the primary tumor or at the anastomotic level [3, 4], and is associated with a high mortality rate of around 21.7% [1]. Pathological mechanisms include transmural tumor necrosis and gastrointestinal wall ischemia [5].
Clinical Case
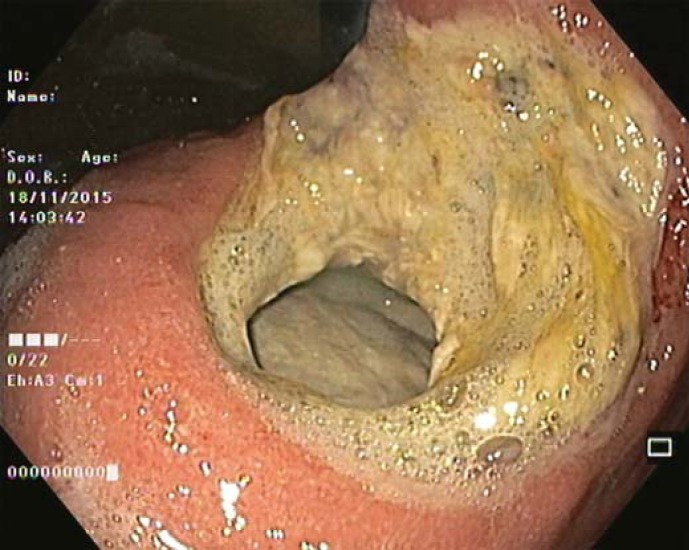
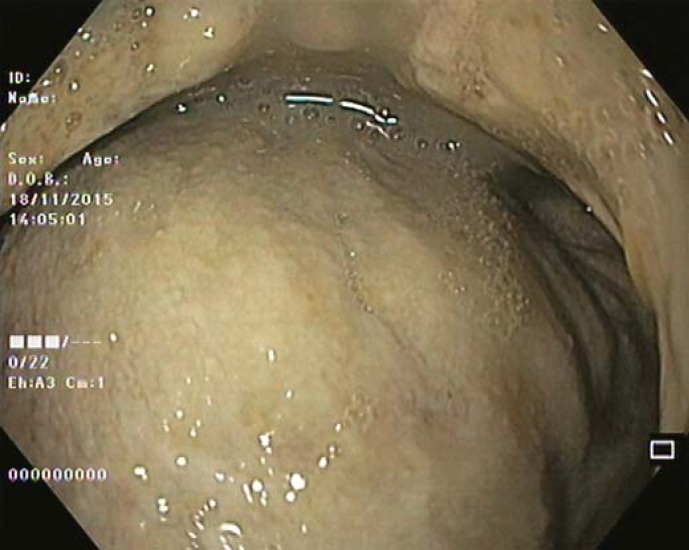
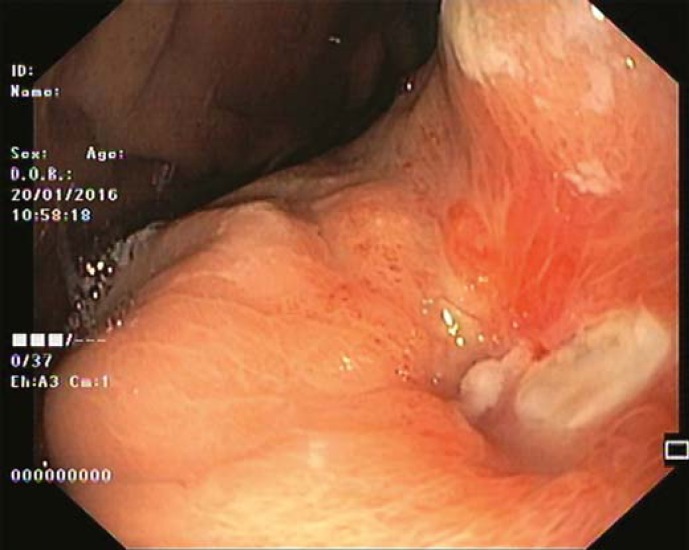
Our 64-year-old male patient had a stage IV rectal mutated KRAS adenocarcinoma (hepatic, pulmonary, adrenal, and bone metastasis) that was submitted to an anterior rectal resection due to obstruction at presentation (pT3N2aM1). Chemotherapy with FOLFOX and bevacizumab was initiated after surgery, with clinical and serological response. After the 4th chemotherapy cycle, our patient started fever and epigastric pain. He was hemodynamically stable, and signs of peritoneal irritation were absent. There were no alterations in the abdominal X-ray, and C-reactive protein was markedly elevated (265 mg/L). A CT scan was performed that revealed de novo thickness in the gastric antrum. Upper digestive endoscopy showed an ulcerated 40-mm lesion in the angulus, with a 20-mm orifice communicating with an exsudative cavity lined by the omentum (Fig. 1, 2). A conservative approach was decided including fasting, broad-spectrum intravenous antibiotics and proton-pump inhibitor. Subsequent gastroduodenal series showed no contrast extravasation, allowing the resumption of oral nutrition. Esophagogastroduodenoscopy after 8 weeks showed perforation closure, being evident a clean-base, 15-mm ulcer (Fig. 3). Biopsies of the ulcer and normal gastric mucosa did not show neoplastic cells, and Heliobacter pylori bacillus was not identified. Despite the success in the conservative management of gastric ulcer perforation allowing the maintenance of palliative chemotherapy without bevacizumab, the patient eventually died 4 months later due to hepatic metastasis progression and liver failure.
Fig. 1.
Perforated giant gastric ulcer in the incisura with a 20-mm orifice.
Fig. 2.
An exsudative cavity revested by the omentum is seen through the orifice.
Fig. 3.
After 8 weeks of proton-pump inhibitor therapy, a 15-mm, clean-base ulcer was apparent at the site of previous perforation.
Discussion
The reported case shows an uncommon endoscopic finding due to a rare complication of antiangiogenic therapy. Additionally, it reminds clinicians that a history of gastroduodenal ulcers should be actively sought before starting therapy with antiangiogenic medications in order to take preventive measures such as avoidance of nonsteroidal anti-inflammatory drugs and corticosteroids and possibly prescription of proton-pump inhibitors. Furthermore, suspicion for perforation should be high in patients with abdominal pain to timely treat the patient and stop bevacizumab. Of note, lower doses of bevacizumab (2.5 mg/kg per week) seem to decrease the risk of gastrointestinal perforation (vs. 5 mg/kg per week) [1].
Statement of Ethics
This study did not require informed consent or review/approval by the appropriate ethics committee.
Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- 1.Hapani S, Chu D, Wu S. Risk of gastrointestinal perforation in patients with cancer treated with bevacizumab: a meta-analysis. Lancet Oncol. 2009;10:559–568. doi: 10.1016/S1470-2045(09)70112-3. [DOI] [PubMed] [Google Scholar]
- 2.Qi WX, Shen Z, Tang LN, Yao Y. Bevacizumab increases the risk of gastrointestinal perforation in cancer patients: a meta-analysis with a focus on different subgroups. Eur J Clin Pharmacol. 2014;70:893–906. doi: 10.1007/s00228-014-1687-9. [DOI] [PubMed] [Google Scholar]
- 3.Hatake K, Doi T, Uetake H, Takahashi Y, Ishihara Y, Shirao K. Bevacizumab safety in Japanese patients with colorectal cancer. Jpn J Clin Oncol. 2016;46:234–240. doi: 10.1093/jjco/hyv182. [DOI] [PubMed] [Google Scholar]
- 4.Bege T, Lelong B, Viret F, Turrini O, Guiramand J, Topart D, et al. Bevacizumab-related surgical site complication despite primary tumor resection in colorectal cancer patients. Ann Surg Oncol. 2009;16:856–860. doi: 10.1245/s10434-008-0279-2. [DOI] [PubMed] [Google Scholar]
- 5.Han ES, Monk BJ. What is the risk of bowel perforation associated with bevacizumab therapy in ovarian cancer? Gynecol Oncol. 2007;105:3–6. doi: 10.1016/j.ygyno.2007.01.038. [DOI] [PubMed] [Google Scholar]