Abstract
Mucociliary clearance, driven by the engine of ciliary beating, is the primary physical airway defense against inhaled pathogens and irritants. A better understanding of the regulation of ciliary beating and mucociliary transport is necessary for identifying new receptor targets to stimulate improved clearance in airway diseases, such as cystic fibrosis and chronic rhinosinusitis. In this study, we examined the protease-activated receptor (PAR)-2, a GPCR previously shown to regulate airway cell cytokine and mucus secretion, and transepithelial Cl− current. PAR-2 is activated by proteases secreted by airway neutrophils and pathogens. We cultured various airway cell lines, primary human and mouse sinonasal cells, and human bronchial cells at air–liquid interface and examined them using molecular biology, biochemistry, and live-cell imaging. We found that PAR-2 is expressed basolaterally, where it stimulates both intracellular Ca2+ release and Ca2+ influx, which activates low-level nitric oxide production, increases apical membrane Cl− permeability ∼3–5-fold, and increases ciliary beating ∼20–50%. No molecular or functional evidence of PAR-4 was observed. These data suggest a novel and previously overlooked role of PAR-2 in airway physiology, adding to our understanding of the role of this receptor in airway Ca2+ signaling and innate immunity.—McMahon, D. B., Workman, A. D., Kohanski, M. A., Carey, R. M., Freund, J. R., Hariri, B. M., Chen, B., Doghramji, L. J., Adappa, N. D., Palmer, J. N., Kennedy, D. W., Lee, R. J. Protease-activated receptor 2 activates airway apical membrane chloride permeability and increases ciliary beating.
Keywords: trypsin, GPCR, calcium signaling, respiratory epithelial physiology
Protease-activated receptors (PARs) are GPCRs expressed in many tissues, including the airway (1–3), where they may have an important role in inflammation in diseases like asthma and chronic rhinosinusitis (CRS) (4, 5). PAR-2 is highly expressed in airway tissues and airway cell lines, such as A549 and 16HBE14o− (16HBE) cells (6), and in primary nasal cells (7). The regulation of PAR-2 expression is poorly understood; PAR-2 mRNA is up-regulated in allergic rhinitis nasal samples (8), although others found no alteration of PAR-2 expression in patients with allergic fungal rhinosinusitis (9). PAR-2 has multiple known effects, including activating fluid secretion from airway submucosal glands of mouse trachea (10) and from human inferior turbinate explants (11). PAR-2 increases amphiregulin release and EGFR-driven Muc5AC production from NCI-H292 bronchial cells (12, 13) and in 16HBE cells through a calcium (Ca2+)-dependent pathway (14).
PARs are activated by proteolytic cleavage of the extracellular N terminus, which exposes a new sequence that acts as an intramolecular-tethered ligand (15). Thrombin activates PAR-1, PAR-3, and PAR-4, whereas trypsin-like proteases activate PAR-2 and PAR-4. Neutrophil elastase activates PAR-2 and stimulates Muc5AC secretion from Calu-3 and 16HBE bronchial cells (14, 16). PAR-2 may also be disarmed by some elastases and cathepsin G (17) via extracellular domain cleavage downstream of the activating site, preventing trypsin activation but not activation by peptide agonists (6). Pathogens also secrete proteases that activate PARs. PAR-2 activation in human bronchial epithelial cells exposed to Aspergillus fumigatus extract suppresses polyI:C and human rhinovirus serotype 16 signaling, possibly promoting T helper 2 inflammation (18). Alternaria fungal aspartate proteases activate PAR-2–dependent Ca2+ signaling in 16HBE cells (19) and increase granulocyte macrophage-CSF factor, IL-6, and IL-8 production in BEAS-2B and Calu-3 cells (20). Others have suggested PAR-2 is not the only mechanism of Alternaria-induced Ca2+ signaling (21). Acanthamoeba species also secrete proteases that activate PAR-2 and increase expression of CCL11, CCL17, CCL22, TSLP, and IL-25 in mice (22). Some house dust mite proteases activate PAR-2 (1–3, 23). PAR-2–deficient mice have reduced lung inflammation in response to dust mite challenge (24). House dust mite extract activates apical Cl− channels in primary nasal cells (7), Calu-3 bronchial adenocarcinoma cells (25, 26), and mouse primary tracheal epithelial cells (27), possibly via PAR-2.
Because PAR-2 is a GPCR tied to inositol trisphosphate production and intracellular Ca2+ signaling (15), we examined whether PAR-2 activates acute Ca2+ responses in primary sinonasal epithelial cells and the effect on motile cilia beating, which drives mucociliary clearance, the main physical defense of the airways (28). Ca2+ generally increases ciliary beat frequency (CBF) (29), although some Ca2+ agonists do not increase CBF, either because of insufficient magnitude or because of localization of the Ca2+ response. We previously observed that histamine elicits Ca2+ responses from primary sinonasal epithelial cells that are sufficient (at high histamine concentrations) to elicit Ca2+-activated fluid secretion but not increase CBF (30). Moreover, because PARs can activate PKC (26, 31–33), which can slow CBF (34), and β-arrestin–dependent pathways (32, 35), PARs could have a range of effects on CBF. To our knowledge, no one has yet investigated CBF responses to PAR-2 stimulation in primary differentiated human airway cells. Understanding how PAR-2 affects CBF will contribute to our understanding of the regulation of mucociliary clearance. This is of paramount importance to elucidating new therapeutics for diseases characterized by mucostasis, including cystic fibrosis (36), acute rhinosinusitis, and CRS (37).
MATERIALS AND METHODS
Reagents and solutions
Unless indicated, all reagents and solutions were as previously described (30, 38). Fura-2-acetoxymethyl ester (AM) and 4-amino-5-metholamino-2′,7′-difluorofluorescein (DAF-FM) diacetate, 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA)-AM, and l-NG-nitroarginine methyl ester (l-NAME) were from Thermo Fisher Scientific (Waltham, MA, USA). AY-NH2, 2-furoyl-LIGRLO-amide (2FLI), anti–PAR-2 (ab138479), and anti-GLUT1 (ab15309) rabbit pAbs, anti–PAR-2 mouse mAb (SAM11), and anti-Na+K+ATPase rabbit mAb (ab76020) were from Abcam (Cambridge, MA, USA). Anti-NKCC1 T9 was from Developmental Studies Hybridoma Bank (Iowa City, IA, USA). FSLLRY-NH2 and tcY-NH2 were from Bio-Techne (Minneapolis, MN, USA). BTP2/YM58483 was from Cayman Chemical (Ann Arbor, MI, USA). All other reagents were from MilliporeSigma (St. Louis, MO, USA).
Culture of human cell lines
16HBE14o− (16HBE) and CFBE41o− (CFBE) epithelial cells were from Dr. Dieter C. Gruenert (Institute for Human Genetics at University of California, San Francisco, San Francisco, CA, USA) (39). BEAS-2B and NCI-H292 cells were from American Type Culture Collection (Manassas, VA, USA). Cells were cultured in minimal essential medium with Earle’s salts (minimum essential medium Eagle, 16HBE, CFBE), Roswell Park Memorial Institute (RPMI)-1640 (H292), or F12K (BEAS-2B) plus 10% fetal bovine serum. Confluent cells were dissociated with trypsin and seeded on Greiner BioOne Thincerts coated with bovine serum albumin (BSA), type I bovine collagen, and human fibronectin. Cells were grown to 100% confluence (∼5 d), then washed apically and exposed to air for ≥14 d to allow full polarization. 16HBE and CFBE tight junction formation was confirmed by transepithelial electrical resistance.
Generation of primary human sinonasal air–liquid interface cultures from residual surgical material
All experimental protocols were carried out in accordance with University of Pennsylvania guidelines regarding use of residual clinical material. Patients undergoing sinonasal surgery were recruited from the Department of Otorhinolaryngology at the University of Pennsylvania with institutional review board approval (800614) and written, informed consent in accordance with the U.S. Department of Health and Human Services Code of Federal Regulations Title 45 (CFR 46.116). Inclusion criteria included patients ≥18 yr old undergoing sinonasal surgery for sinonasal disease (CRS) or other procedures (e.g., transnasal approaches to the skull base) in which tissue was classified as “control.” Exclusion criteria included a history of systemic inheritable disease (e.g., granulomatosis with polyangiitis, cystic fibrosis, or systemic immunodeficiencies) or use of antibiotics, oral corticosteroids, or antibiologics (e.g., Xolair, omalizumab; Genentech, South San Francisco, CA, USA) within 1 mo of surgery. Individuals ≤18 yr old, pregnant women, and cognitively impaired persons were not included; 21 patient samples (13 female, 8 male) were used for air–liquid interface (ALI) culture, including 3 control patients, 17 patients with CRS, and 1 patient with an aspergilloma fungal ball. Mean age was 56 ± 3.5 yr (median, 60.4 yr); 43% (9/21) had nasal polyps.
Human sinonasal epithelial cells were enzymatically dissociated and grown to confluence in proliferation medium (DMEM/Ham’s F-12 plus) for 7 d (38). Confluent cells were dissociated and seeded on Corning Life Sciences (Tewksbury, MA, USA) Transwells coated with BSA, type I bovine collagen, and fibronectin. When culture medium was removed from the upper compartment, the basolateral medium was changed to a differentiation medium (1:1 DMEM:Ham’s F-12 plus) containing human epidermal growth factor (0.5 ng/ ml), epinephrine (5 ng/ml), bovine pituitary extract (0.13 mg/ml), hydrocortisone (0.5 ng/ml), insulin (5 ng/ml), triiodothyronine (6.5 ng/ml), and transferrin (0.5 ng/ml), supplemented with 100 U/ml penicillin, 100 g/ml streptomycin, 0.1 nM retinoic acid, and NuSerum (BD Biosciences, Franklin Lakes, NJ, USA) as previously described (38).
Dissociated ciliated cells were removed from healthy nasal inferior turbinate samples from patients with inverted papilloma; turbinate was outside the tumor margins but was removed by necessity to access the tumor. Cells were dissociated via cytology brush and seeded onto coverslips previously coated with several layers of Cell-Tak (Corning Life Sciences). Cells were left to attach to the Cell-Tak for 60 min before either live cell imaging or paraformaldehyde fixation for immunofluorescence.
Generation of mouse nasal septum ALI cultures
Nasal septal tissue was obtained from C57BL/6J mice euthanized for other experimental purposes with Institutional Animal Care and Use Committee approval. No animals were sacrificed for the experiments in this study. PAR-2-knockout (B6.Cg-F2rl1tm1Mslb/J) and congenic wild-type mice were from The Jackson Laboratory (Bar Harbor, ME, USA). Mouse primary cells were dissociated from nasal septum and cultured at the ALI as previously described (40) and were used for experiments after 3 wk exposure to air for full differentiation. Cells were differentiated in DMEM/F12K medium containing 2% NuSerum.
Par-2 RT-PCR, Western blotting, and immunofluorescence
Human PAR-2 and PAR-4 samples were amplified by PCR using an iCycler (Bio-Rad, Hercules, CA, USA), RedTaq DNA Polymerase (MilliporeSigma), and the following primers: 5′-GCTGGTCACCATCCCTTTGT-3′ and 5′-TCTGCTTTACAGTGCGGACA-3′ (hPAR-2) and 5′-CCTGGTTTATCTCTACCGGCG-3′ and 5′-CTCTCGTCGTAGACGCTGGG-3′ (hPAR-4) from Integrated DNA Technologies (Coralville, IA, USA). cDNA was prepared from cultured cells using Trizol (Thermo Fisher Scientific) with rDNAse I (Ambion, Austin, TX, USA) and an iScript cDNA Synthesis Kit (Bio-Rad). The expected amplimer sizes of 500 bp (PAR-2) and 200 bp (PAR-4) were observed with a genomic DNA-positive control, and no amplification was noted when the template was omitted. For PAR-2 Western blot analysis, half of the protein isolated from a 0.33-cm2 primary sinonasal ALI was loaded into each lane of a NuPage 4–12% Bis–Tris gel. Separated proteins were transferred to nitrocellulose and blocked with 5% milk in 50 mM Tris, 150 mM NaCl, and 0.05% Tween-20. Anti-PAR-2 antibody was used at 1:1000 in 5% BSA in Tris–Tween for 1.5 h, followed by secondary goat anti-rabbit IgG-horseradish peroxidase at 1:10,000 for 1.5 h.
Immunofluorescence of submerged cells was carried out with the PAR-2 mouse mAb SAM11 (1:50 in Dulbecco PBS + 1% BSA, 2% normal donkey serum, and 0.2% saponin) or rabbit pAb using previously described methods (41) on BEAS-2B cells with AlexaFluor-conjugated donkey secondary antibodies (Thermo Fisher Scientific). Cells were visualized with an IX-83 spinning-disk confocal microscope (Olympus, Tokyo, Japan) at ×60 [1.4 numerical aperture (NA), oil]. Dissociated primary cells were fixed with 4% formaldehyde followed by 3 min permeabilization with −20°C methanol and blocking with 1% BSA and 2% normal donkey serum in the presence of 0.25% saponin for 1 h. Colocalization was analyzed with the Mander’s coefficients plugin for ImageJ (National Institutes of Health, Bethesda, MD, USA; https://imagej.nih.gov/ij/) as previously described (42). Because 2 different mouse antibodies were used in primary-cell experiments (either tubulin and NKCC1 or tubulin and PAR-2 SAM11), mouse antibodies were directly labeled for these experiments (Zenon Labeling Kit; Thermo Fisher Scientific), as previously described (42). Complete lack of overlap between labeled antibodies demonstrates the specificity of the labeling protocol.
Imaging of Ca2+, reactive nitrogen species, Cl− permeability, and CBF
Ca2+ and reactive nitrogen species were imaged in ALI using Fura-2 and DAF-FM, respectively, as previously described (38). Cultures were loaded with Fura-2-AM (10 µM apically) for 2 h. Cultures were similarly loaded with 10 µM DAF-FM diacetate for 90 min. Imaging was performed with an Olympus IX-83 microscope equipped with a ×10 (0.4-NA UPlanApo) objective, xenon lamp (Sutter Instruments, Novato, CA, USA), computer-controlled filter wheels (Sutter Instruments), and scientific CMOS camera (Hamamatsu Photonics, Hamamatsu City, Japan). Images were analyzed using MetaMorph software (Molecular Devices, Sunnyvale, CA, USA). Background was estimated by imaging unloaded ALI at identical settings. Fura-2 images used 340/380 excitation (79002; Chroma Technology, Bellows Falls, VT, USA). DAF-FM measurements used a standard FITC filter set (49002; Chroma Technology) to measure raw fluorescence values to compare experiments performed under identical conditions and settings, as previously described (38). Dissociated cells were loaded with fura-2 (2 µM) for 20 min. Experiments used HBSS, buffered with 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, at pH 7.4, with 1.5 mM Ca2+ and either 5.5 mM glucose (basolateral side) or 0 glucose (apical side). Experiments performed in the absence of Ca2+ (0-Ca2+) used cultures loaded with BAPTA-AM (10 µM; 15 min preincubation) and extracellular solutions containing no added Ca2+ and 1 mM EGTA (3, 38–40). Peak decay time constants (τ) were calculated by fitting the exponential decays of individual experiments in Prism (GraphPad Software, La Jolla, CA, USA). Because of potential errors introduced with calibration of ratios to intracellular free Ca2+ concentration ([Ca2+]i) and because comparisons made here did not require absolute measure of [Ca2+]i, we report data as 340/380 ratios.
Rhod2 was loaded as previously described (43) by incubation in 5-µM rhod2-AM in HBSS at 4°C for 18 h, followed by 1 h in 5 µM rhod2-AM in serum-free F12K at 37°C. Rhod2 was visualized on a spinning-disk confocal microscope (Olympus digital scanning unit, IX-83; ×30, 1.05-NA silicone oil objective). 6-Methoxy-N-(3-sulfopropyl)quinolinium (SPQ) was loaded for 6 h incubation with apical PBS containing 20 mM SPQ at 37°C and imaged with a DAPI filter set (49000; Chroma Technology). Fluorescence was normalized to time 0 (F/F0), as previously described (30, 44). NO3− substitution experiments were performed with control HBSS (containing 138 mM NaCl, 5.3 mM KCl, 0.34 mM Na2HPO4, 0.44 mM KH2PO4, 0.41 mM MgSO4, 0.5 mM MgCl2, and 1.3 mM CaCl2), for a total [Cl−] of 147 mM. Low-Cl− solution substituted 138 mM NaNO3 and 5.3 mM KNO3 for NaCl and KCl, respectively, for a total apical [Cl−] of 4 mM (∼37-fold reduction). CBF was measured by Sisson-Ammons video analysis (45) or ciliaFA (46) as previously described (30, 38, 47) at ∼28–30°C. Cultures were imaged on either a Leica microscope [×20/0.8-NA objective; Leica Microsystems, Mannheim, Germany; Basler (Ahrensburg, Germany) A602f camera] or an Olympus microscope [×20/0.8-NA objective and Hammamatsu Flash 4.0 camera] at 60–100 frames/s.
Data analysis and statistics
Images were acquired and analyzed in MetaMorph, MetaFluor (Molecular Devices), and/or ImageJ/Fiji. Multiple comparisons were made with 1-way ANOVA, with Bonferroni (preselected pairwise comparisons), Tukey-Kramer (comparing all values), or Dunnett’s (comparing to control value) posttest. A value of P < 0.05 was considered statistically significant. All data are means ± sem.
RESULTS
Airway epithelial cells express PAR-2
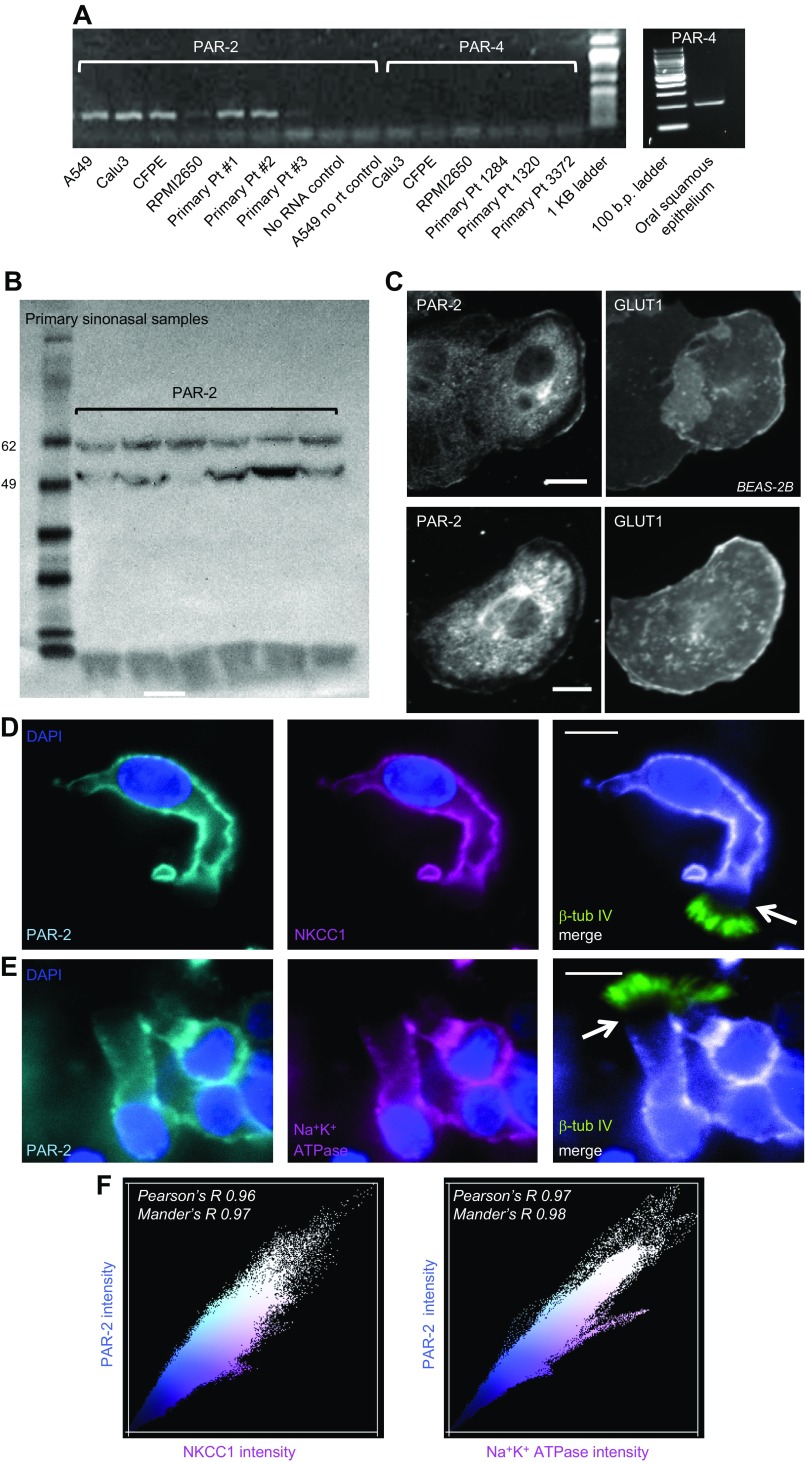
Reverse transcription-PCR confirmed previous observations of PAR-2 expression in cultured airway cell lines and primary sinonasal epithelial cells grown from residual surgical specimens and differentiated at the ALI (30, 38, 48). ALI culture of primary airway epithelial cells is a well-accepted in vitro method of recapitulating the airway epithelium. Primary airway cell ALIs differentiate into both ciliated and goblet cells, mimicking many aspects of in vivo physiology (49). We detected PAR-2 expression in all cells examined, including primary cells from 3 individual patients with CRS (Fig. 1A). PAR-4 was not detected by reverse transcription-PCR in airway cells (Fig. 1A). Par-2 expression in primary sinonasal ALIs was confirmed by Western blot (Fig. 1B), and membrane localization was observed via immunofluorescence of submerged BEAS-2B cells (Fig. 1C). Colocalization was observed with Glut-1, which polarizes in differentiated airway cells but is a general plasma membrane maker in submerged airway cells (Fig. 1C). The intracellular staining also observed likely reflected trafficking PAR-2 and/or nonspecific staining with that primary antibody because it was not observed with secondary antibodies only.
Figure 1.
Expression of PAR-2 in airway epithelial cells. A) PAR-2 expression in primary airway epithelial cell lines and primary cells from 3 patients was assayed by reverse transcription-PCR. Oral squamous epithelium cDNA was used as a control for PAR-4. b.p., base pair; Pt, patient; rt, reverse transcription. B) PAR-2 expression was confirmed by Western blot in primary sinonasal ALI culture samples (6 separate patients, unrelated to patients in A. C) Immunofluorescence using a PAR-2 antibody in submerged BEAS-2B cells revealed plasma membrane–localized staining. GLUT1 was a control for membrane localization. D, E) Immunofluorescence of PAR-2 in primary dissociated sinonasal ciliated cells showing colocalization with NKCC1 (D) and Na+K+ ATPase (E). A distinct gap was noted (arrows) between the base of the cilia (labeled with β-tubulin IV), corresponding to the apical cell body membrane and the start of basolateral NKCC1/Na+K+ ATPase immunofluorescence. PAR-2 rabbit pAb was used with mouse mAb anti-NKCC1 (top). PAR-2 mouse mAb was used with rabbit mAb anti-Na+K+ATPase (bottom). Scale bars, 10 µm. F) Representative scatter plots of PAR-2 intensity and either NKCC or Na+K+ ATPase. For all dissociated cells imaged (n = 15 with NKCC1 and n = 17 with Na+K+ ATPase), Pearson’s correlation coefficient (Pearson’s R) and Mander’s overlap coefficient (Mander’s R) for basolateral marker and PAR-2 were both ≥0.95.
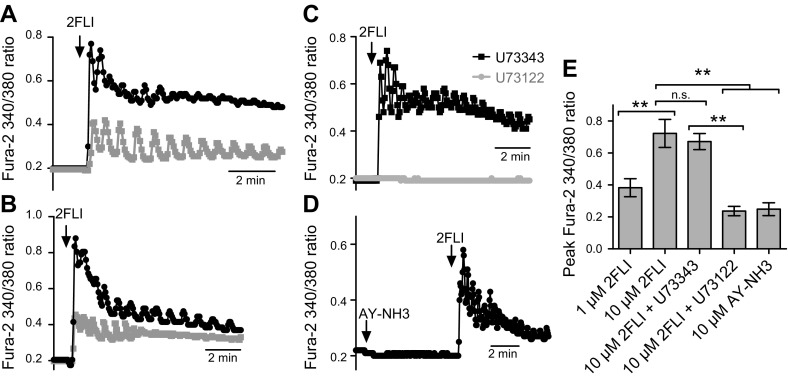
Dissociated primary sinonasal ciliated cells from turbinate exhibited PAR-2 immunofluorescence that colocalized with the well-established basolateral Na+K+2Cl− cotransporter [NKCC1 (44) and Na+/K+ ATPase (36); Fig. 1D–F]. Living dissociated ciliated cells were loaded with the Ca2+-sensitive dye fura-2, and single cells were identified by DRAQ5 staining of live-cell nuclei. Single ciliated cells exhibited robust increases in [Ca2+]i in response to a peptide PAR-2 agonist, 2FLI (Fig. 2A, B) that was inhibited by a phospholipase C inhibitor (U73122) but not by the inactive control (U73343; Fig. 2C). As a PAR-4–activating peptide, AY-NH2 did not elevate [Ca2+]i (Fig. 2D). Results are summarized in Fig. 2E.
Figure 2.
Ca2+ signaling in acutely dissociated nasal turbinate ciliated cells in response to PAR-2 stimulation. A, B) PAR-2 agonist 2FLI [10 µM (black) or 1 µM (gray)]–activated [Ca2+]i elevation in ciliated cells. Traces in representative cells from 2 separate patients. A total of 14 cells from 3 separate patients were imaged at each concentration. C) 2FLI responses were blocked by preincubation with PLC inhibitor U73122 but not by inactive analog U73343 (n = 6 cells from 2 patients imaged for each condition). D) PAR-4 agonist AY-NH2 did not elevate [Ca2+]i (n = 6 cells from 2 patients). E) Bar graph showing peak results from experiments as in A–D. N.s., not significant. **P < 0.01.
Basolateral PAR-2 activates an increase in [Ca2+]i in ALI cultures of bronchial epithelial cell lines and primary sinonasal cells
To begin to investigate the mechanisms of PAR2 signaling in the airway, we tested the acute effects of trypsin, a PAR-2–activating protease, on [Ca2+]i in 16HBE ALIs. Basolaterally applied trypsin dose-dependently elevated Ca2+ in a classic GPCR-induced, PLC/inositol trisphosphate–mediated kinetic pattern (Supplemental Fig. 1A). Trypsin-induced Ca2+ responses were abolished upon heat inactivation (Supplemental Fig. 1B, C) and were blocked by U73122 (Supplemental Fig. 1D). The responses to trypsin were closely mimicked by 2FLI (Supplemental Fig. 1E, F). AY-NH2 had no effect on [Ca2+]i (Supplemental Fig. 1G). 2FLI-induced [Ca2+]i increases were blocked by a PAR-2 antagonist (FSLLRY-NH2) but not by a PAR-4 antagonist (tcY-NH2) (Supplemental Fig. 1H). 2FLI-induced Ca2+ responses were also observed in CFBE41o− (39), BEAS-2B, and NCI-H292 ALIs (Supplemental Fig. 1I–K).
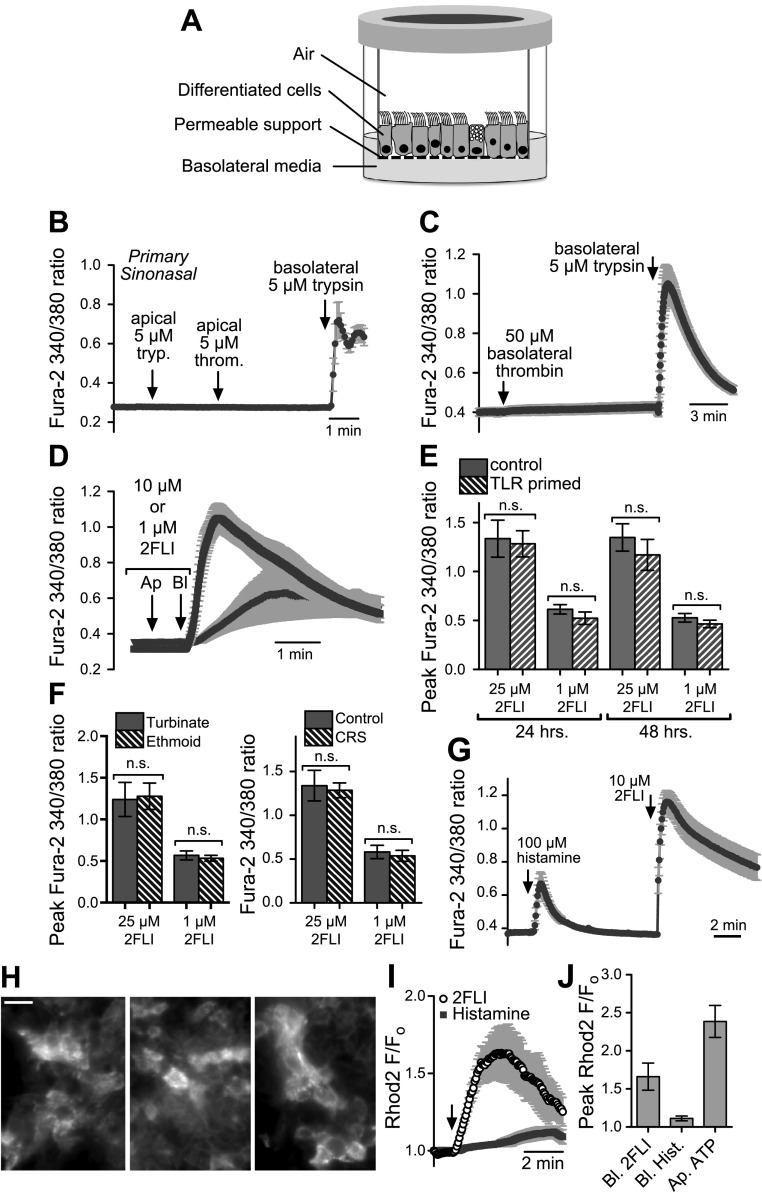
Primary sinonasal epithelial ALIs (Fig. 3A) likewise exhibited [Ca2+]i responses when cells were treated with basolateral trypsin (Fig. 3B–C) or 2FLI (Fig. 3D). Apical trypsin or apical or basolateral thrombin (PAR-4–activating protease) were without effect (Fig. 3B, C). Because PAR-2 has been implicated in inflammation, we examined 2FLI-induced peak [Ca2+]i responses in control cells treated for 24 or 48 h [reflects the time over which an increase in cytokine release is detected in primary, sinonasal ALIs (38, 48)] with a TLR agonist cocktail (LPS, lipoteichoic acid, FSL-1, and polyI:C) applied apically to mimic pathogen exposure but found no significant differences (Fig. 3E). ALIs used in Fig. 3A–E were primarily derived from the ethmoid sinuses of patients with CRS. In separate experiments, we tested ALIs derived from 2 distinct anatomic regions: inferior turbinate (n = 3 patients, 3 ALIs each) and ethmoid sinus (n = 3 patients, 3 ALIs each). No differences were observed (Fig. 3F). Likewise, no differences were observed between ALIs derived from patients with CRS (n = 3 patients; 3 ALIs each) or control individuals (n = 3 patients, 3 ALIs each; Fig. 3F).
Figure 3.
Basolateral PAR-2 stimulation activates Ca2+ signaling in primary sinonasal ALI cultures. A) Schematic of primary ALI culture. B) Apical trypsin (tryp.) or thrombin (throm.) had no effect on Ca2+ signaling, whereas basolateral trypsin activated a Ca2+ increase (n = 4 cultures from 4 individual patients) C) Basolateral thrombin had no effect, whereas basolateral trypsin activated a Ca2+ increase (n = 4 cultures from 4 individual patients). D) 2FLI activated Ca2+ responses in sinonasal ALIs. E) TLR priming [24–48 h stimulation with 2 µg/ml LPS, lipoteichoic acid (LTA), FSL-1, and 5 µg/ml polyI:C] had no effect on 2FLI-induced Ca2+ responses. F) Peak [Ca2+]i responses were not different in ALIs derived from turbinate or ethmoid sinus (left) or control vs. patients with CRS (right; ALIs from 3 individual samples for each type compared). N.s., not significant. G) Responses observed with 2FLI were greater than those observed with saturating (100 µM) histamine. H) Primary ALI cultures loaded with rhod-2 exhibited punctate mitochondrial fluorescence pattern. I, J) Basolateral 20 µM 2FLI, but not 100 µM histamine, caused an increase in rhod2 fluorescence, suggesting apical mitochondrial Ca2+ uptake.
Notably, a previously determined saturating concentration of basolateral histamine [100 µM (30)] elicited [Ca2+]i responses that were smaller in magnitude than those observed with 2FLI (Fig. 3G). Apical and basolateral Ca2+ signaling domains in airway epithelial cells have been postulated to be spatially separated by mitochondria (43). Mitochondrial Ca2+ uptake may limit the spread of signals from one domain to the other unless the signals are large enough. We loaded primary, sinonasal epithelial cells with the mitochondrial Ca2+ sensor rhod2 (43) and imaged the mitochondria at the apical plane of the ALIs with confocal microscopy (Fig. 3H). 2FLI (25 µM), but not histamine (100 µM), induced an increase in rhod2 fluorescence (Fig. 3I, J), suggesting that the larger 2FLI-induced Ca2+ signal more likely propagates to the apical domain of the cells.
Ca2+ influx is required for sustained PAR-2 [Ca2+]i elevation
Store-operated Ca2+ entry via Stim1/Orai1 Ca2+-release–activated channels was shown to be required for normal PAR-2–mediated Ca2+ signaling and cytokine secretion in primary bronchial cells and cell lines, such as BEAS-2B, although those studies were primarily performed in submerged cultures and not in cells grown at the ALI (50, 51). When 16HBE ALIs were stimulated with basolateral 2FLI in the absence of extracellular Ca2+ (0-Ca2+o; no Ca2+ added +1 mM EGTA on both apical and basolateral sides), the 2FLI-induced response (Supplemental Fig. 2A, B), like the apical purinergic ATP-stimulated response (Supplemental Fig. 2C, D), had a lower peak and faster decay than it did in the presence of extracellular Ca2+ (Supplemental Fig. 2E, F), suggesting that Ca2+ influx also has an important role in PAR-2 signaling in polarized cells. Interestingly, an 8-min treatment with 2FLI in 0-Ca2+o conditions resulted in a substantial, but not full, depletion of ATP-sensitive Ca2+ stores (Supplemental Fig. 2A), and vice versa (Supplemental Fig. 2C), suggesting that these distinct apical and basolateral agonists primarily activate Ca2+ release from shared intracellular stores but also elicit a small release from distinct stores. Global store depletion with the SR/ER-Ca2+–ATPase pump inhibitor thapsigargin for the same amount of time resulted in greater depletion of both ATP-sensitive and 2FLI-sensitive Ca2+ stores (Supplemental Fig. 2G).
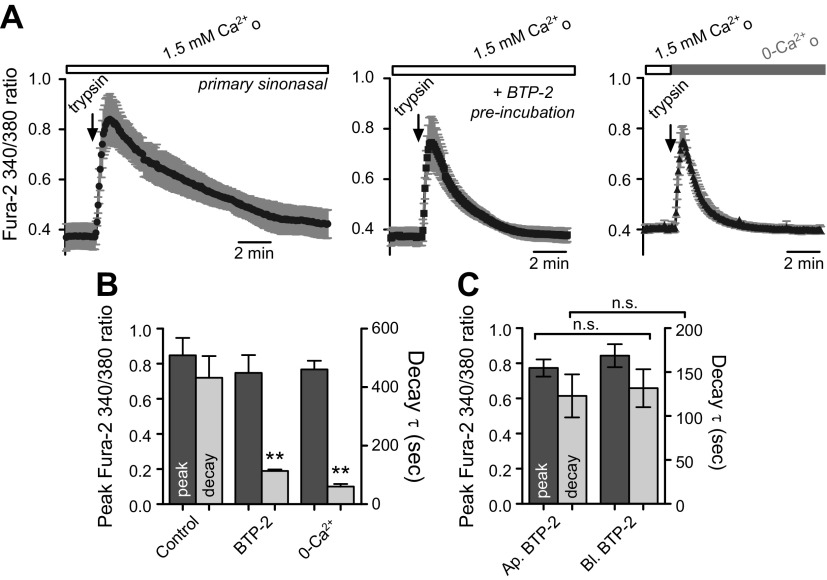
To confirm the involvement of Ca2+ influx in primary polarized sinonasal cells, ALIs were preincubated for 90 min in the presence of 10 nM BTP-2 (both apical and basolateral), a Ca2+-release–activated channel inhibitor. The decay of the trypsin-induced [Ca2+]i transient was markedly reduced (Fig. 4A, B), confirming a role for Ca2+ influx in sustained PAR-2 Ca2+ signaling. No differences were observed when cells were preincubated with BTP-2 on the apical vs. basolateral side (Fig. 4C), but preincubation time combined with the permeant nature of BTP-2 makes interpretation of these results difficult.
Figure 4.
Sustained PAR-2–activated Ca2+ signaling in sinonasal ALIs requires Ca2+-release–activated channel (CRAC)-mediated Ca2+ influx. A) Traces of basolateral trypsin-induced [Ca2+]i responses in the absence (top) or presence (middle) of the CRAC-inhibitor BTP-2 or in the absence of extracellular Ca2+ (0-Ca2+o + 1 mM EGTA). B) Quantification of peak fura-2 340/380 ratios and decay time constant (τ) under conditions above (n = 3–4 experiments each). **P < 0.01 vs. corresponding peak. C) Peak and decay constants were not different when cells were preincubated with apical or basolateral BTP-2. N.s., not significant.
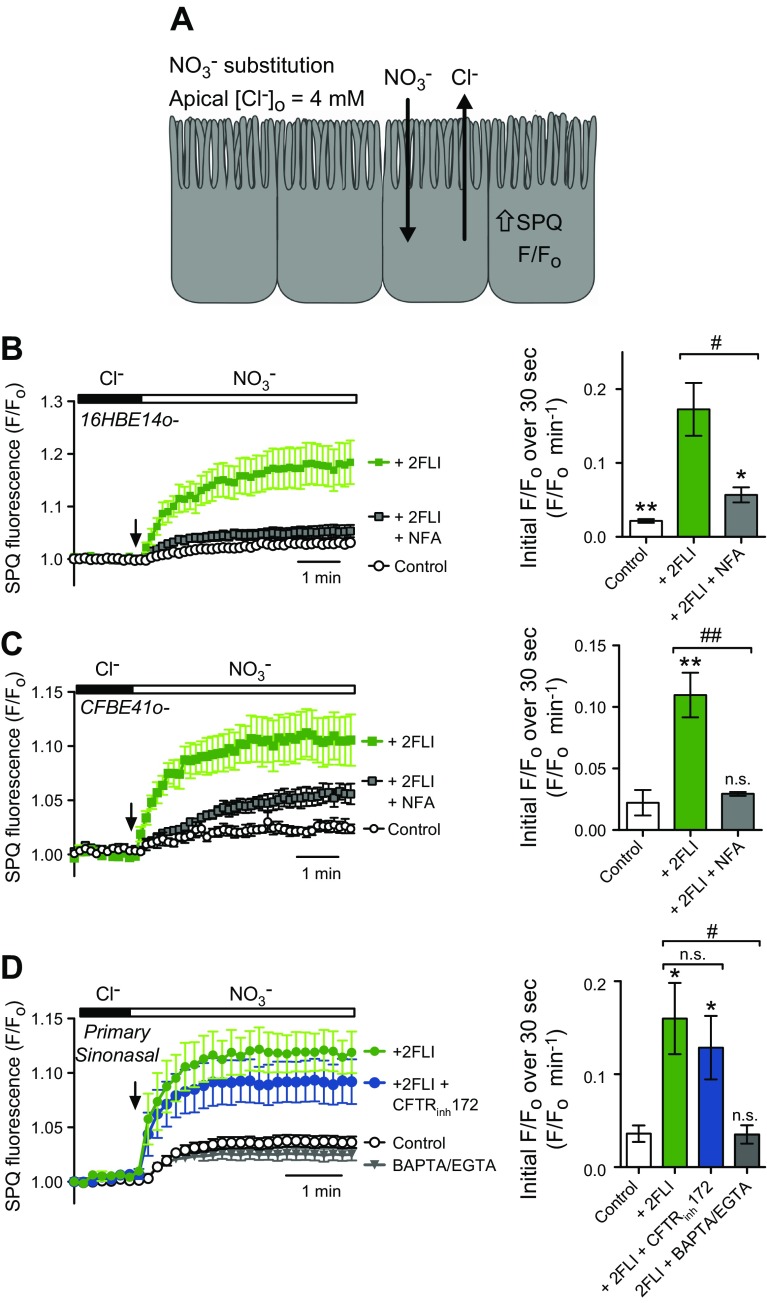
PAR-2 activates an apical membrane Ca2+-dependent Cl− permeability
Because PAR-2 has been extensively tied to epithelial ion transport, as previously described, we tested whether 2FLI induced activation of apical membrane Cl− permeability using SPQ, as previously described (30, 41, 44). SPQ is quenched by Cl− but not by NO3−. Because both Ca2+-activated TMEM16A and cAMP/PKA-activated cystic fibrosis transmembrane conductance regulator (CFTR) Cl− channels have a nearly equal permeability to Cl− and NO3−, apical substitution of NO3− for Cl− results in electroneutral efflux of Cl− and influx of NO3− along diffusional gradients, resulting in an increase in intracellular SPQ fluorescence (Fig. 5A). Simultaneous basolateral addition of 2FLI and apical NO3− substitution resulted in an increase in the rate of SPQ fluorescence change (roughly equal to the relative apical anion permeability) in both 16HBE (Fig. 5B) and CFBE41o− (Fig. 5C) ALIs. This increase was blocked in both cell lines by niflumic acid (NFA), a Ca2+-activated, Cl− channel blocker (41, 44, 52). Similar results were observed in primary, sinonasal ALIs (Fig. 5D). No inhibition of the 2FLI-induced increase in SPQ fluorescence change was observed in primary ALIs treated with the CFTR inhibitor CFTRinh172 (Fig. 5D), but the effects of 2FLI were completely abolished in 0-Ca2+o conditions [cells preloaded with BAPTA-AM, as previously described (41, 44, 52)] (Fig. 5D).
Figure 5.
Activation of PAR-2 increased apical membrane Cl− permeability. A) Schematic of NO3− substitution experiment. B) Increase in SPQ ΔF/F0 with 20 µM 2FLI stimulation and a block with 100 µM NFA in 16HBE ALIs (4–5 experiments each). C) Increase in SPQ ΔF/F0 with 20 µM 2FLI stimulation and a block with 100 µM NFA in CFBE41o− ALIs (3–6 experiments each). D) Increase in SPQ ΔF/F0 with 20 µM 2FLI stimulation and a block with BAPTA/EGTA, but not CFTRinh172 in primary sinonasal ALIs (5–6 experiments each). N.s., not significant. *P < 0.05, **P < 0.01 vs. control; #P < 0.05, ##P < 0.01.
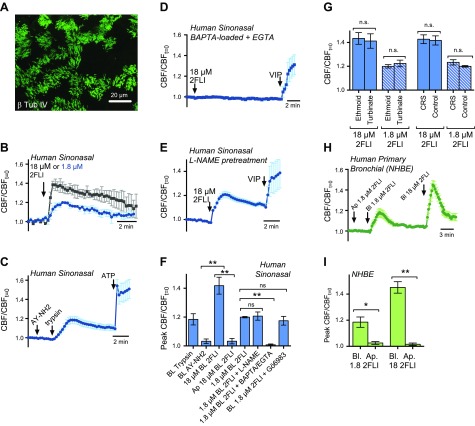
PAR-2 activates a Ca2+-dependent increase in CBF
To determine whether PAR-2 Ca2+ signals had an effect on CBF, we imaged CBF in differentiated, primary, sinonasal ALIs, which form functional motile cilia (Fig. 6A), as previously described (30, 34, 38). 2FLI induced a transient increase in CBF (Fig. 6B), which was similar to the response observed with trypsin but not the PAR-4 agonist AY-NH2 (Fig. 6C). Abolishment of Ca2+ signaling (0-Ca2+o and BAPTA preloading) blocked the 2FLI response (Fig. 6D). Abolishment of signaling with l-NAME had no discernable effect (Fig. 6E), despite low-level NO responses observed during 2FLI stimulation (Supplemental Fig. 2H, I). These results are summarized in Fig. 6F. The ALIs used in Fig. 6A–E were primarily derived from the ethmoid sinuses of patients with CRS. In separate experiments, CBF increases were compared, based on the anatomic site from which the ALIs were derived, and showed no difference between inferior turbinate (n = 3 patients, 3 ALIs each) and ethmoid sinus (n = 3 patients, 3 ALIs each) ALIs (Fig. 6G). Likewise, no differences were observed between ALIs derived from control individuals (n = 3 patients; 3 ALIs each, 9 ALIs total) or patients with CRS (n = 3 patients; 3 ALIs each; 9 ALIs total; Fig. 6G).
Figure 6.
Basolateral PAR-2 stimulation increases CBF in primary human sinonasal ALIs. A) β-tubulin IV staining of cilia in primary human ALI cultures. B) 2FLI (18 or 1.8 μM) increased CBF (normalized to initial CBF at time 0 (CFBt = 0). C) Trypsin, but not AY-NH2, increased CBF. Trace shows averages of 3 experiments each from 3 individuals (patients with CRS, ethmoid sinus-derived ALIs). D, E) 2FLI-activated increases in CBF were blocked by Ca2+ chelation (BAPTA-preloading and extracellular EGTA; D) but not by abolishment of NO signaling (l-NAME pretreatment. E) Traces show average of 3 experiments each from 3 individuals (patients with CRS, ethmoid sinus-derived ALIs). F) Peak CBF/CBFt = 0 values (n = 4–5 experiments from 4 to 5 patients each; 1 ALI per patient). G) Graphs show peak CBF values in response to 2FLI from ALIs from 3 separate turbinate samples compared with 3 separate ethmoid samples and 3 separate control patients compared with 3 separate patients with CRS. H) 2FLI (1.8 and 18 µM) increased CBF in primary bronchial ALI cultures. Trace shown is the average of 4 ALI cultures (2 donors; 2 ALIs each). NHBE, human bronchial epithelial cells. I) Peak CBF increases to apical and basolateral 2FLI. N.s., not signficant. *P < 0.05, **P < 0.01.
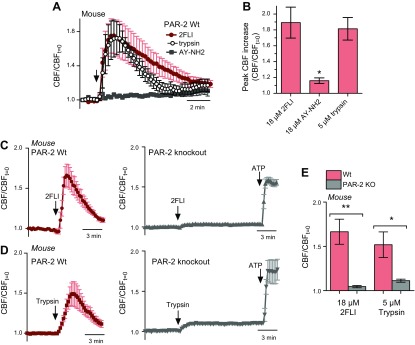
In normal human bronchial epithelial cells, CBF increased with basolateral, but not apical, 2FLI (Fig. 6H, I). The increase was not likely a consequence of secreted factors released during stimulation (Supplemental Fig. 2J, K) or intracellular alkalinization (Supplemental Fig. 2J). Trypsin and 2FLI induced CBF increases in mouse ALIs (Fig. 7A, B) grown from nasal septum. AY-NH2 had no effect (Fig. 7A, B). In ALIs from PAR-2-knockout mice, the CBF increase in response to trypsin or 2FLI was abolished (Fig. 7C–E), suggesting an essential role for PAR-2 in acute, trypsin-like, protease-induced airway CBF responses.
Figure 7.
Basolateral PAR-2 stimulation increases CBF in primary mouse septal ALIs. A, B) 2FLI and trypsin, but not AY-NH2, stimulated CBF in wild-type primary mouse nasal septal ALI cultures. Peak CBF increases were 1.89 ± 0.20 (2FLI), 1.16 ± 0.04 (AY-NH2), 1.81 ± 0.14 (trypsin). C, D) Basolateral 2FLI (C) and trypsin (D) stimulated CBF increases in wild-type (left red traces), but not PAR-2-knockout (PAR-2 KO), ALIs. E) Bar graph summarizing results from C and D. N.s. not significant. *P < 0.05, **P < 0.01.
DISCUSSION
In this study, we examined primary sinonasal epithelial cells grown at air-liquid interface (ALI) and confirmed several previous observations of PAR-2 expression and function from various primary and cultured cell models while further elucidating mechanisms of PAR-2 Ca2+ signaling. We also made the novel observation that PAR-2-activated Ca2+ signals increase CBF. This observation is important, as it suggests a novel role for PAR-2 in airway physiology, in addition to previous observations of PAR-2 activation of ion transport and cytokine secretion. This work demonstrates that PAR-2 is thus a previously overlooked mechanism that regulates acute Ca2+ and CBF responses. Importantly, in well-differentiated primary airway epithelial ALIs and dissociated cells, PAR-2 is basolaterally expressed in ciliated cells. Other studies have suggested both apical and basolateral expression of PAR-2 in mouse and rat tracheal ALIs (27, 53, 54). We found no evidence of apical PAR-2. This may reflect differences in cell culture conditions favoring enhanced polarization or species/tissue-specific differences. It is important to note that PAR-2 potentially expressed in basal cells may also play a role in amplifying the Ca2+ response in surface epithelial cells via gap junction communication or other secreted factors, though we found no evidence for dependence of PAR-2 CBF increases on secreted factors in this study. Nonetheless, our data demonstrate that activation of PAR-2 from the basolateral epithelial side activates a Ca2+ response while stimulation from the apical side elicited no response.
No differences were observed between ALIs derived from 2 separate sinonasal anatomic regions or between control or CRS ALIs. A caveat is that the sample size for this study (21 patient samples from which ALIs were grown) was reasonable for a basic airway biology study but is small for a clinical study. However, we and others (38, 49) have found that primary human airway cells normalize out during the 4–6-wk culture and differentiation process, with phenotype based overwhelmingly on genetics rather than patient history. For example, primary cystic fibrosis airway epithelium cells exhibit greater Ca2+ responses from inflammation, but that was only observed for ∼6–11 d of culture and was lost in older cultures (55). Although that limits studies of disease-related effects, baseline (nonpathologic) effects of PAR-2 can be studied without confounding from the secondary disease effects. Future studies are needed to clarify how PAR-2 contributes to disease progression in vivo. Despite well-studied links between PAR-2 and inflammation activation (4, 16, 18), acute TLR stimulation did not up-regulate PAR-2 Ca2+ signaling. Effects may still occur with other inflammatory mediators or longer TLR stimulation. A known polymorphism in PAR-2 that affects function exists (F240S) but only at a low frequency (e.g., ∼1:100 in white populations) (56). Future clinical studies are needed to correlate PAR-2 genetics with disease progression or severity, although that will likely require a large population of patients to study.
Our data here, combined with our previous study (30), demonstrate that various basolateral Ca2+-elevating receptors have differential effects on CBF, at least in vitro. We previously reported that basolateral histamine activates Ca2+ signals in primary sinonasal ALIs, but although those Ca2+ elevations caused activation of apical-membrane Cl− conductances at high levels of histamine, they did not affect CBF (30). Notably, maximum basolateral histamine-induced global Ca2+ signals are small (∼50%) in comparison with those evoked by apical ATP (30) or basolateral PAR-2 (this study). The greater Ca2+ response can likely overcome intracellular buffering barriers (e.g., by mitochondria), which may separate apical and basolateral cellular compartments, as previously suggested to exist in airway cells (43).
However, it is not yet fully clear how [Ca2+]i during strong (100 µM) histamine stimulation reaches a level that can activate fluid secretion (30), because apical Ca2+-activated Cl− channels would be expected to be above the apical mitochondrial buffer (43) without detectably elevating the CBF. Further diffusional barriers into the cilia may have an important role (57), or other messengers activated by histamine (e.g., PKC) may blunt the effects of Ca2+ on the CBF. Further studies of airway cell Ca2+ signal localization, including better methods to study localized [Ca2+]i directly within motile airway cell cilia, would explain those observations. Nonetheless, our data suggest that Ca2+ signals from distinct basolateral GPCRs differentially regulate apical components of sinonasal epithelial physiology. In Calu-3 cells, PAR-2–activated fluid secretion requires prostaglandin release and activation of CFTR. Our data suggest that in surface epithelial cells, PAR-2 Ca2+ signaling is sufficient to activate calcium-activated Cl− channel (CaCC)–mediated secretion that does not require CFTR.
Despite low-level 2FLI-stimulated NO production, which has been demonstrated to activate CFTR in some cells (58), we observed no CFTR activation or NO-dependent CBF increase. Apical stimulation of T2R bitter-taste receptors, which are expressed in sinonasal cilia (38, 42), elicits ∼3-fold higher NO levels, which we previously showed are associated with cGMP-dependent protein kinase G–dependent activation of CBF (38, 42). We hypothesize that the NO produced during PAR-2 stimulation is either too low to activate CFTR or is improperly localized to activate guanylyl cyclase. It is also possible that the NO-activated CFTR conductance is small relative to CaCC and thus difficult to detect with the optical methods used here. Future Ussing chamber studies are necessary to fully test this theory.
Exploitation of the basolateral serosal localization of PAR-2 may be useful with therapeutic agonists or antagonists that could be delivered systemically, which would require further in vivo validation of PAR-2 function in human airways. However, our demonstration of a novel role for PAR-2–activated Ca2+ signals in increasing CBF suggests that potential strategies to target PAR-2 for reducing inflammation should take into account a concomitant reduction in mucociliary clearance. Likewise, potential targeting of PAR-2 to increase CBF and fluid secretion must also account for potential effects of PAR-2–activated cytokine release. Future studies of this receptor and its signal transduction pathway in polarized, differentiated primary airway cells, as well as in in vivo models, will shed more light on whether and how this receptor can be pharmacologically targeted for respiratory diseases. Further in vivo work is also needed to understand how abnormalities of PAR-2 signaling may contribute to respiratory disease progression.
ACKNOWLEDGMENTS
The authors thank M. Victoria (Perelman School of Medicine) for excellent technical assistance with PCR experiments. This study was supported by grants from U.S. National Institutes of Health, National Institute on Deafness and Other Communication Disorders (Grant R03DC01386), the Cystic Fibrosis Foundation (Grant LEER16G0), and funding from the Department of Otorhinolaryngology at the University of Pennsylvania (to R.J.L). The authors declare no conflicts of interest.
Glossary
- 2FLI
2-furoyl-LIGRLO-amide
- 16HBE
16HBE14o−
- ALI
air–liquid interface
- AM
acetoxymethyl ester
- BAPTA
1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid
- BSA
bovine serum albumin
- [Ca2+]i
intracellular free Ca2+ concentration
- CaCC
calcium-activated Cl− channel
- CBF
ciliary beat frequency
- CFBE
CFBE41o−
- CFTR
cystic fibrosis transmembrane conductance regulator
- CRS
chronic rhinosinusitis
- DAF-FM
4-amino-5-metholamino-2′,7′-difluorofluorescein
- l-NAME
l-NG-nitroarginine methyl ester
- NA
numerical aperture
- NFA
niflumic acid
- NKCC1
Na+K+2Cl− cotransporter
- PAR
protease-activated receptor
- SPQ
6-methoxy-N-(3-sulfopropyl)quinolinium
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
D. B. McMahon and R. J. Lee developed the concept and wrote the paper; D. B. McMahon, A. D. Workman, M. A. Kohanski, R. M. Carey, J. R. Freund, B. M. Hariri, B. Chen, and R. J. Lee performed and designed experiments and analyzed data; A. D. Workman, M. A. Kohanski, B. Chen, L. J. Doghramji, N. D. Adappa, J. N. Palmer, and D. W. Kennedy recruited patients, maintained clinical databases and records, assisted with primary epithelial tissue procurement and cell culture, and contributed to data interpretation; and all authors contributed intellectually to the study and approved the final version of the manuscript.
REFERENCES
- 1.Peters T., Henry P. J. (2009) Protease-activated receptors and prostaglandins in inflammatory lung disease. Br. J. Pharmacol. 158, 1017–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cocks T. M., Moffatt J. D. (2001) Protease-activated receptor-2 (PAR2) in the airways. Pulm. Pharmacol. Ther. 14, 183–191 [DOI] [PubMed] [Google Scholar]
- 3.Lan R. S., Stewart G. A., Henry P. J. (2002) Role of protease-activated receptors in airway function: a target for therapeutic intervention? Pharmacol. Ther. 95, 239–257 [DOI] [PubMed] [Google Scholar]
- 4.Rothmeier A. S., Ruf W. (2012) Protease-activated receptor 2 signaling in inflammation. Semin. Immunopathol. 34, 133–149 [DOI] [PubMed] [Google Scholar]
- 5.Reed C. E., Kita H. (2004) The role of protease activation of inflammation in allergic respiratory diseases. J. Allergy Clin. Immunol. 114, 997–1008, quiz 1009 [DOI] [PubMed] [Google Scholar]
- 6.Dulon S., Candé C., Bunnett N. W., Hollenberg M. D., Chignard M., Pidard D. (2003) Proteinase-activated receptor-2 and human lung epithelial cells: disarming by neutrophil serine proteinases. Am. J. Respir. Cell Mol. Biol. 28, 339–346 [DOI] [PubMed] [Google Scholar]
- 7.Cho H. J., Choi J. Y., Yang Y. M., Hong J. H., Kim C. H., Gee H. Y., Lee H. J., Shin D. M., Yoon J. H. (2010) House dust mite extract activates apical Cl− channels through protease-activated receptor 2 in human airway epithelia. J. Cell. Biochem. 109, 1254–1263 [DOI] [PubMed] [Google Scholar]
- 8.Lee H. M., Kim H. Y., Kang H. J., Woo J. S., Chae S. W., Lee S. H., Hwang S. J. (2007) Up-regulation of protease-activated receptor 2 in allergic rhinitis. Ann. Otol. Rhinol. Laryngol. 116, 554–558 [DOI] [PubMed] [Google Scholar]
- 9.Ebert C. S. Jr., McKinney K. A., Urrutia G., Wu M., Rose A. S., Fleischman G. M., Thorp B., Senior B. A., Zanation A. M. (2014) Expression of protease-activated receptors in allergic fungal rhinosinusitis. Int. Forum Allergy Rhinol. 4, 266–271 [DOI] [PubMed] [Google Scholar]
- 10.Lee H. J., Yang Y. M., Kim K., Shin D. M., Yoon J. H., Cho H. J., Choi J. Y. (2012) Protease-activated receptor 2 mediates mucus secretion in the airway submucosal gland. PLoS One 7, e43188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho H. J., Lee H. J., Kim S. C., Kim K., Kim Y. S., Kim C. H., Lee J. G., Yoon J. H., Choi J. Y. (2012) Protease-activated receptor 2-dependent fluid secretion from airway submucosal glands by house dust mite extract. J. Allergy Clin. Immunol. 129, 529–535, 535 e1-5 [DOI] [PubMed] [Google Scholar]
- 12.Chokki M., Yamamura S., Eguchi H., Masegi T., Horiuchi H., Tanabe H., Kamimura T., Yasuoka S. (2004) Human airway trypsin-like protease increases mucin gene expression in airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 30, 470–478 [DOI] [PubMed] [Google Scholar]
- 13.Chokki M., Eguchi H., Hamamura I., Mitsuhashi H., Kamimura T. (2005) Human airway trypsin-like protease induces amphiregulin release through a mechanism involving protease-activated receptor-2-mediated ERK activation and TNF-α–converting enzyme activity in airway epithelial cells. FEBS J. 272, 6387–6399 [DOI] [PubMed] [Google Scholar]
- 14.Liu C., Li Q., Zhou X., Kolosov V. P., Perelman J. M. (2013) Human airway trypsin-like protease induces mucin5AC hypersecretion via a protease-activated receptor 2-mediated pathway in human airway epithelial cells. Arch. Biochem. Biophys. 535, 234–240 [DOI] [PubMed] [Google Scholar]
- 15.Bunnett N. W. (2006) Protease-activated receptors: how proteases signal to cells to cause inflammation and pain. Semin. Thromb. Hemost. 32(Suppl 1), 39–48 [DOI] [PubMed] [Google Scholar]
- 16.Zhou J., Perelman J. M., Kolosov V. P., Zhou X. (2013) Neutrophil elastase induces MUC5AC secretion via protease-activated receptor 2. Mol. Cell. Biochem. 377, 75–85 [DOI] [PubMed] [Google Scholar]
- 17.Ossovskaya V. S., Bunnett N. W. (2004) Protease-activated receptors: contribution to physiology and disease. Physiol. Rev. 84, 579–621 [DOI] [PubMed] [Google Scholar]
- 18.Homma T., Kato A., Bhushan B., Norton J. E., Suh L. A., Carter R. G., Gupta D. S., Schleimer R. P. (2016) Role of Aspergillus fumigatus in triggering protease-activated receptor-2 in airway epithelial cells and skewing the cells toward a T-helper 2 bias. Am. J. Respir. Cell Mol. Biol. 54, 60–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boitano S., Flynn A. N., Sherwood C. L., Schulz S. M., Hoffman J., Gruzinova I., Daines M. O. (2011) Alternaria alternata serine proteases induce lung inflammation and airway epithelial cell activation via PAR2. Am. J. Physiol. Lung Cell. Mol. Physiol. 300, L605–L614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsuwaki Y., Wada K., White T., Moriyama H., Kita H. (2012) Alternaria fungus induces the production of GM-CSF, interleukin-6 and interleukin-8 and calcium signaling in human airway epithelium through protease-activated receptor 2. Int. Arch. Allergy Immunol. 158(Suppl 1), 19–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Grady S. M., Patil N., Melkamu T., Maniak P. J., Lancto C., Kita H. (2013) ATP release and Ca2+ signalling by human bronchial epithelial cells following Alternaria aeroallergen exposure. J. Physiol. 591, 4595–4609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park M. K., Cho M. K., Kang S. A., Park H. K., Kim D. H., Yu H. S. (2014) Acanthamoeba protease activity promotes allergic airway inflammation via protease-activated receptor 2. PLoS One 9, e92726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cottrell G. S., Amadesi S., Schmidlin F., Bunnett N. (2003) Protease-activated receptor 2: activation, signalling and function. Biochem. Soc. Trans. 31, 1191–1197 [DOI] [PubMed] [Google Scholar]
- 24.De Boer J. D., Van’t Veer C., Stroo I., van der Meer A. J., de Vos A. F., van der Zee J. S., Roelofs J. J., van der Poll T. (2014) Protease-activated receptor-2 deficient mice have reduced house dust mite-evoked allergic lung inflammation. Innate Immun. 20, 618–625 [DOI] [PubMed] [Google Scholar]
- 25.Palmer M. L., Lee S. Y., Maniak P. J., Carlson D., Fahrenkrug S. C., O’Grady S. M. (2006) Protease-activated receptor regulation of Cl− secretion in Calu-3 cells requires prostaglandin release and CFTR activation. Am. J. Physiol. Cell Physiol. 290, C1189–C1198 [DOI] [PubMed] [Google Scholar]
- 26.Sato S., Ito Y., Kondo M., Ohashi T., Ito S., Nakayama S., Shimokata K., Kume H. (2005) Ion transport regulated by protease-activated receptor 2 in human airway Calu-3 epithelia. Br. J. Pharmacol. 146, 397–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sherwood C. L., Daines M. O., Price T. J., Vagner J., Boitano S. (2014) A highly potent agonist to protease-activated receptor-2 reveals apical activation of the airway epithelium resulting in Ca2+-regulated ion conductance. Am. J. Physiol. Cell Physiol. 307, C718–C726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hariri B. M., Cohen N. A. (2016) New insights into upper airway innate immunity. Am. J. Rhinol. Allergy 30, 319–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salathe M. (2007) Regulation of mammalian ciliary beating. Annu. Rev. Physiol. 69, 401–422 [DOI] [PubMed] [Google Scholar]
- 30.Lee R. J., Chen B., Doghramji L., Adappa N. D., Palmer J. N., Kennedy D. W., Cohen N. A. (2013) Vasoactive intestinal peptide regulates sinonasal mucociliary clearance and synergizes with histamine in stimulating sinonasal fluid secretion. FASEB J. 27, 5094–5103 [DOI] [PubMed] [Google Scholar]
- 31.Lidington E. A., Steinberg R., Kinderlerer A. R., Landis R. C., Ohba M., Samarel A., Haskard D. O., Mason J. C. (2005) A role for proteinase-activated receptor 2 and PKC-epsilon in thrombin-mediated induction of decay-accelerating factor on human endothelial cells. Am. J. Physiol. Cell Physiol. 289, C1437–C1447 [DOI] [PubMed] [Google Scholar]
- 32.Jung S. R., Seo J. B., Deng Y., Asbury C. L., Hille B., Koh D. S. (2016) Contributions of protein kinases and β-arrestin to termination of protease-activated receptor 2 signaling. J. Gen. Physiol. 147, 255–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suzuki A., Ohno S. (2006) The PAR-aPKC system: lessons in polarity. J. Cell Sci. 119, 979–987 [DOI] [PubMed] [Google Scholar]
- 34.Lee R. J., Workman A. D., Carey R. M., Chen B., Rosen P. L., Doghramji L., Adappa N. D., Palmer J. N., Kennedy D. W., Cohen N. A. (2016) Fungal aflatoxins reduce respiratory mucosal ciliary function. Sci. Rep. 6, 33221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grimsey N., Lin H., Trejo J. (2014) Endosomal signaling by protease-activated receptors. Methods Enzymol. 535, 389–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee R. J., Foskett J. K. (2014) Ca2+ signaling and fluid secretion by secretory cells of the airway epithelium. Cell Calcium 55, 325–336 [DOI] [PubMed] [Google Scholar]
- 37.Stevens W. W., Lee R. J., Schleimer R. P., Cohen N. A. (2015) Chronic rhinosinusitis pathogenesis. J. Allergy Clin. Immunol. 136, 1442–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee R. J., Xiong G., Kofonow J. M., Chen B., Lysenko A., Jiang P., Abraham V., Doghramji L., Adappa N. D., Palmer J. N., Kennedy D. W., Beauchamp G. K., Doulias P.-T., Ischiropoulos H., Kreindler J. L., Reed D. R., Cohen N. A. (2012) T2R38 taste receptor polymorphisms underlie susceptibility to upper respiratory infection. J. Clin. Invest. 122, 4145–4159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gruenert D. C., Finkbeiner W. E., Widdicombe J. H. (1995) Culture and transformation of human airway epithelial cells. Am. J. Physiol. 268, L347–L360 [DOI] [PubMed] [Google Scholar]
- 40.Lee R. J., Chen B., Redding K. M., Margolskee R. F., Cohen N. A. (2014) Mouse nasal epithelial innate immune responses to Pseudomonas aeruginosa quorum-sensing molecules require taste signaling components. Innate Immun. 20, 606–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee R. J., Foskett J. K. (2010) Mechanisms of Ca2+-stimulated fluid secretion by porcine bronchial submucosal gland serous acinar cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 298, L210–L231 [DOI] [PubMed] [Google Scholar]
- 42.Hariri B. M., McMahon D. B., Chen B., Freund J. R., Mansfield C. J., Doghramji L. J., Adappa N. D., Palmer J. N., Kennedy D. W., Reed D. R., Jiang P., Lee R. J. (2017) Flavones modulate respiratory epithelial innate immunity: anti-inflammatory effects and activation of the T2R14 receptor. J. Biol. Chem. 292, 8484–8497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ribeiro C. M., Paradiso A. M., Livraghi A., Boucher R. C. (2003) The mitochondrial barriers segregate agonist-induced calcium-dependent functions in human airway epithelia. J. Gen. Physiol. 122, 377–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee R. J., Limberis M. P., Hennessy M. F., Wilson J. M., Foskett J. K. (2007) Optical imaging of Ca2+-evoked fluid secretion by murine nasal submucosal gland serous acinar cells. J. Physiol. 582, 1099–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sisson J. H., Stoner J. A., Ammons B. A., Wyatt T. A. (2003) All-digital image capture and whole-field analysis of ciliary beat frequency. J. Microsc. 211, 103–111 [DOI] [PubMed] [Google Scholar]
- 46.Smith C. M., Djakow J., Free R. C., Djakow P., Lonnen R., Williams G., Pohunek P., Hirst R. A., Easton A. J., Andrew P. W., O’Callaghan C. (2012) ciliaFA: a research tool for automated, high-throughput measurement of ciliary beat frequency using freely available software. Cilia 1, 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hariri B. M., Payne S. J., Chen B., Mansfield C., Doghramji L. J., Adappa N. D., Palmer J. N., Kennedy D. W., Niv M. Y., Lee R. J. (2016) In vitro effects of anthocyanidins on sinonasal epithelial nitric oxide production and bacterial physiology. Am. J. Rhinol. Allergy 30, 261–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee R. J., Kofonow J. M., Rosen P. L., Siebert A. P., Chen B., Doghramji L., Xiong G., Adappa N. D., Palmer J. N., Kennedy D. W., Kreindler J. L., Margolskee R. F., Cohen N. A. (2014) Bitter and sweet taste receptors regulate human upper respiratory innate immunity. J. Clin. Invest. 124, 1393–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fulcher M. L., Randell S. H. (2013) Human nasal and tracheo-bronchial respiratory epithelial cell culture. Methods Mol. Biol. 945, 109–121 [DOI] [PubMed] [Google Scholar]
- 50.Jairaman A., Yamashita M., Schleimer R. P., Prakriya M. (2015) Store-operated Ca2+ release-activated Ca2+ channels regulate PAR2-activated Ca2+ signaling and cytokine production in airway epithelial cells. J. Immunol. 195, 2122–2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jairaman A., Maguire C. H., Schleimer R. P., Prakriya M. (2016) Allergens stimulate store-operated calcium entry and cytokine production in airway epithelial cells. Sci. Rep. 6, 32311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee R. J., Foskett J. K. (2010) cAMP-activated Ca2+ signaling is required for CFTR-mediated serous cell fluid secretion in porcine and human airways. J. Clin. Invest. 120, 3137–3148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chow J. M., Moffatt J. D., Cocks T. M. (2000) Effect of protease-activated receptor (PAR)-1, -2 and -4-activating peptides, thrombin and trypsin in rat isolated airways. Br. J. Pharmacol. 131, 1584–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kunzelmann K., Sun J., Markovich D., König J., Mürle B., Mall M., Schreiber R. (2005) Control of ion transport in mammalian airways by protease activated receptors type 2 (PAR-2). FASEB J. 19, 969–970 [DOI] [PubMed] [Google Scholar]
- 55.Ribeiro C. M., Paradiso A. M., Schwab U., Perez-Vilar J., Jones L., O’neal W., Boucher R. C. (2005) Chronic airway infection/inflammation induces a Ca2+i-dependent hyperinflammatory response in human cystic fibrosis airway epithelia. J. Biol. Chem. 280, 17798–17806 [DOI] [PubMed] [Google Scholar]
- 56.Compton S. J., Cairns J. A., Palmer K. J., Al-Ani B., Hollenberg M. D., Walls A. F. (2000) A polymorphic protease-activated receptor 2 (PAR2) displaying reduced sensitivity to trypsin and differential responses to PAR agonists. J. Biol. Chem. 275, 39207–39212 [DOI] [PubMed] [Google Scholar]
- 57.Pablo J. L., DeCaen P. G., Clapham D. E. (2017) Progress in ciliary ion channel physiology. J. Gen. Physiol. 149, 37–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dong Y. J., Chao A. C., Kouyama K., Hsu Y. P., Bocian R. C., Moss R. B., Gardner P. (1995) Activation of CFTR chloride current by nitric oxide in human T lymphocytes. EMBO J. 14, 2700–2707 [DOI] [PMC free article] [PubMed] [Google Scholar]