Abstract
A genomic variant in the human ADTRP [androgen-dependent tissue factor (TF) pathway inhibitor (TFPI) regulating protein] gene increases the risk of coronary artery disease, the leading cause of death worldwide. TFPI is the TF pathway inhibitor that is involved in coagulation. Here, we report that adtrp and tfpi form a regulatory axis that specifies primitive myelopoiesis and definitive hematopoiesis, but not primitive erythropoiesis or vasculogenesis. In zebrafish, there are 2 paralogues for adtrp (i.e., adtrp1 and adtrp2). Knockdown of adtrp1 expression inhibits the specification of hemangioblasts, as shown by decreased expression of the hemangioblast markers, etsrp, fli1a, and scl; blocks primitive hematopoiesis, as shown by decreased expression of pu.1, mpo, and l-plastin; and disrupts the specification of hematopoietic stem cells (definitive hematopoiesis), as shown by decreased expression of runx1 and c-myb. However, adtrp1 knockdown does not affect erythropoiesis during primitive hematopoiesis (no effect on gata1 or h-bae1) or vasculogenesis (no effect on kdrl, ephb2a, notch3, dab2, or flt4). Knockdown of adtrp2 expression does not have apparent effects on all markers tested. Knockdown of adtrp1 reduced the expression of tfpi, and hematopoietic defects in adtrp1 morphants were rescued by tfpi overexpression. These data suggest that the regulation of tfpi expression is one potential mechanism by which adtrp1 regulates primitive myelopoiesis and definitive hematopoiesis.—Wang, L., Wang, X., Wang, L., Yousaf, M., Li, J., Zuo, M., Yang, Z., Gou, D., Bao, B., Li, L., Xiang, N., Jia, H., Xu, C., Chen, Q., Wang, Q. K. Identification of a new adtrp1-tfpi regulatory axis for the specification of primitive myelopoiesis and definitive hematopoiesis.
Keywords: hemangioblasts, vascular development, aggf1, coagulation
Coronary artery disease (CAD) and its major complication, myocardial infarction (MI), are the leading causes of death worldwide. Our first genome-wide association study for CAD in the Chinese Han population found that a genomic variant, rs6903956, in the C6orf105 gene was significantly associated with the risk of CAD and MI (1), and the finding was independently replicated in other independent studies (2–5). The C6orf105 gene is a putative gene without any specific biologic function identified at the time, but, later, Lupu et al. (6) found that C6orf105 regulated the expression and function of the tissue factor (TF) pathway inhibitor (TFPI) in response to androgen in endothelial cells. The C6orf105 gene was then named ADTRP (androgen-dependent TFPI regulating protein), which encodes the androgen-dependent TFPI regulating protein (6); however, the in vivo physiologic function of ADTRP is unknown.
Coagulation is linked to thrombosis and MI—the major complications of CAD. TFPI is the major inhibitor of the TF-initiated coagulation pathway (7). TFPI inhibits TF-FVIIa–dependent FXa generation (8). TFPI clearly plays an important role in the regulation of coagulation (9, 10), but little is known about its other physiologic roles.
In this study, we used zebrafish as a model system to investigate the physiologic role of the two paralogues of adtrp—adtrp1 and adtrp2. For the first time to our knowledge, we demonstrate an important role of adtrp1, but not adtrp2, during normal embryonic development, specifically, the specification of hemangioblasts, primitive myelopoiesis, and definitive hematopoiesis. We also show that adtrp1 regulates the expression of tfpi in vivo and that this regulation plays an important role in primitive myelopoiesis and definitive hematopoiesis. To date, hematopoiesis and coagulation are considered to be independent biologic processes, but this study mechanistically connects hematopoiesis and coagulation together via the adtrp1-tfpi regulatory axis.
MATERIALS AND METHODS
Zebrafish
Wild-type AB strain zebrafish, the Tg(kdrl:mCherry/c-myb:GFP) transgenic zebrafish, and the Tg(c-myb:GFP) transgenic zebrafish lines were used in this study. This study was approved by the ethics committee of Huazhong University of Science and Technology.
Identification and homology analysis of zebrafish adtrp1 and adtrp2
Full-length sequences of zebrafish adtrp1 and adtrp2 genes were identified by searching the National Center for Biotechnology Information database (Bethesda, MD, USA; http://www.ncbi.nlm.nih.gov/). The analysis of homology among human and zebrafish ADTRP protein sequences was carried out by using ClustalX software (University College Dublin, Dublin, Ireland; http://www.clustal.org/download/current/). Sequence data for adtrp2 differed between the latest 2015 version (ENSDART00000124898, 175 aa) and the 2014 version (ENSACAT00000008542, 242 aa). Our RT-PCR analysis revealed that the 2014 version is the correct adtrp2 (Supplemental Fig. 7A, B), although the 2015 version of adtrp2 could not be excluded as a result of its low expression level.
Morpholinos and microinjection
Morpholinos (MOs) were designed and synthesized by GeneTools (http://www.gene-tools.com/). Two MOs were designed for adtrp1, including adtrp1 MO1 (5′-CCAGTCTCGTGGAGGCAGCCATCAT-3′), which targets the translation initiation codon, AUG, to block the translation of the ADTRP1 protein, and adtrp1 MO2 (5′-AACAAACGAATGATCTCACCATTGC-3′), which spans the exon 3/intron 3 boundary that disrupts adtrp1 splicing. Standard MO (5′-CCTCTTACCTCAGTTACAATTTATA-3′) was used as negative control and does not pair with any zebrafish RNA sequences.
Because there are 2 potential, alternatively spliced isoforms of adtrp2 transcripts, we designed 4 MOs. Adtrp2 MO1 (5′-TCTGTTGCTGAAATACCAGTTTCAT-3′) targets the translation initiation codon, AUG, to block the translation of the ADTRP2 (2014 version) protein. Adtrp2 MO2 (5′-CGCCAGTCCAAAAAACACCATCTGT-3′) targets the translation initiation codon, AUG, to block the translation of the ADTRP2 (2015 version) protein. Adtrp2 MO3 (5′-AATACCCACACTCACCATTCCCACT-3′) spans the exon 2/intron 2 boundary, whereas adtrp2 MO4 (5′-GCGGAGCACAAATCATACTCACCAT-3′) spans the exon 4/intron 4 boundary. MO3 and MO4 were designed to disrupt adtrp2 splicing.
MOs were injected into the 1- to 2-cell stage zebrafish embryos by using a pneumatic picopump (World Precision Instruments, Sarasota, FL, USA) as we and others have described previously (11, 12). Doses used for microinjection of adtrp1 MO1, adtrp1 MO2, adtrp2 MO1, adtrp2 MO2, adtrp2 MO3, and adtrp2 MO4 were 4, 16, 4, 2, 2, and 2 ng, respectively. The effectiveness of each MO was verified (Supplemental Figs. 2 and 7).
Synthesis of mRNA and microinjection into zebrafish embryos
The coding region of zebrafish adtrp1 was PCR amplified from cDNA that was prepared from embryonic mRNA—with primers 5′-CCCAAGCTTATGATGGCAGCTTCAACTAGGCTGGGA-3′ and 5′-AGAGGATCCGTGGTGTCCTGCAGACATCTA-3′—and subcloned into the pSP64 vector (pSP64-zadtrp1). Plasmid pSP64-zadtrp1 was linearized and used to prepare capped zebrafish adtrp1 mRNA with SP6 RNA polymerase and the mMESSAGE mMACHINE system (Thermo Fisher Scientific, Waltham, MA, USA) as we have previously described (11, 13). For tfpi, mRNA samples were prepared by using primers 5′-CGGAATTCCACACACACTTCTCCATATTACT-3′ and 5′-GCTCTAGAAGTGGTTTAGGTTTTGGTTTCA-3′, and for adtrp2 by using primers 5′-CGGAATTCCACACACACTTCTCCATATTACT-3′ and 5′-GCTCTAGAAGTGGTTTAGGTTTTGGTTTCA-3′. We have previously described the construct for the preparation of a constitutively active form of human AKT1 mRNAs (hmyr-AKT) and aggf1 mRNA (13).
Concentrations of mRNA samples for microinjection were 200 ng/μl for adtrp1 mRNA, 100 ng/μl for tfpi mRNA, 75 ng/μl for aggf1 mRNA, 75 ng/μl for hmyr-AKT mRNA, and 50 ng/μl for adtrp2 mRNA.
Capped mRNA samples—adtrp1, tfpi, aggf1, GFP, hmyrAKT, and adtrp2—were prepared by using the mMESSAGE mMACHINE SP6 kit and injected into 1- to 2-cell stage zebrafish embryos at the yolk/cytoplasm boundary as previously described (11, 13).
Real-time quantitative RT-PCR analysis
Real-time quantitative RT-PCR analysis was carried out in a 10-μl reaction volume on a QuantStudio 12K Flex Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) as described previously (11–17). The actb gene that encodes β-actin was used as internal control. Primers used for quantifying tfpi were 5′-CTAAACAGGAAGCAGAGT-3′ and 5′-TAGCGAAAGACTTGACAT-3′.
Whole-mount in situ hybridization
Antisense RNA probes were prepared as we described previously (11–14). Sequences of primers for preparation of the adtrp1 probe were 5′-GATGGCTGCCTCCACGAG-3′ and 5′-GCTGTGCGACTGGGATATCTG-3′. Primers for adtrp2 were 5′-TTGCCACATAGCTGCTTTCA-3′ and 5′-CCCATCCTAAATAAGCGAGACC-3′. Whole-mount in situ hybridization was performed as we previously described (11–14).
TUNEL assays
TUNEL assays were performed by using the In Situ Cell Death Detection Kit KGA7061 (KeyGEN BioTech, Jiangsu, China) with 3-d postfertilization (dpf) embryos. Embryos were fixed with 4% paraformaldehyde for 2 h, digested with proteinase K (20 μg/ml) at 30°C for 30 min, and treated with a breaking agent (Google Biotechnology, Mountain View, CA, USA) at 30°C for 90 min. Embryos were stained with the TUNEL Kit and blocked with block solution (PBS, 10 mg/ml bovine serum albumin, 10% fetal bovine serum, and 0.1% Tween-20) for 2 h at room temperature. Embryos were then incubated with rabbit anti-GFP serum (1:500 dilution; Thermo Fisher Scientific), followed by incubation with a goat FITC-conjugated anti-rabbit secondary Ab (1:200 dilution; Thermo Fisher Scientific).
O-Dianisdine staining
Staining of hemoglobin was carried out in zebrafish by o-dianisdine as previously described (11, 14, 18).
Image acquisition
Embryos were analyzed under an Olympus SZX16 stereo-microscope (Olympus, Tokyo, Japan). Images were obtained with an Olympus DP72 digital camera by using cellSens software (version 1.6; Olympus), and processed with Adobe Photoshop CS8 software and Adobe Illustrator CS6 (Adobe Systems, San Jose, CA, USA). Zebrafish embryos were also scanned by an Olympus Fluoview FV1000 confocal laser scanning microscope. Images were processed with FV10-ASW 3.0 Viewer and Adobe Photoshop CS8 software.
Statistical analysis
Each experiment was repeated at least 3 times. Statistical analysis was carried out by using the Student’s t test. Difference was considered to be significant with values of P ≤ 0.05.
RESULTS
Molecular cloning and expression of zebrafish adtrp1 and adtrp2
Zebrafish have 2 paralogous genes for mammalian ADTRP: adtrp1 and adtrp2 (Supplemental Fig. 1A). Phylogenetic analysis for these genes is shown in Supplemental Fig. 1B. The expression profile of adtrp1 and adtrp2 is shown in Supplemental Fig. 1C–E.
Adtrp1 regulates the specification of hemangioblasts
Etsrp and fli are hemangioblast markers that belong to the ETS family of transcriptional factors and act at the top of the transcriptional network for the specification of hemangioblasts (19, 20). Whole-mount in situ hybridization demonstrated that the expression of etsrp and fli was dramatically reduced in embryos that were injected with adtrp1 MO1 compared with control Std-MO (Fig. 1A–F and Supplemental Fig. 2). These data suggest that adtrp1 is involved in the differentiation of hemangioblasts from the mesoderm.
Figure 1.
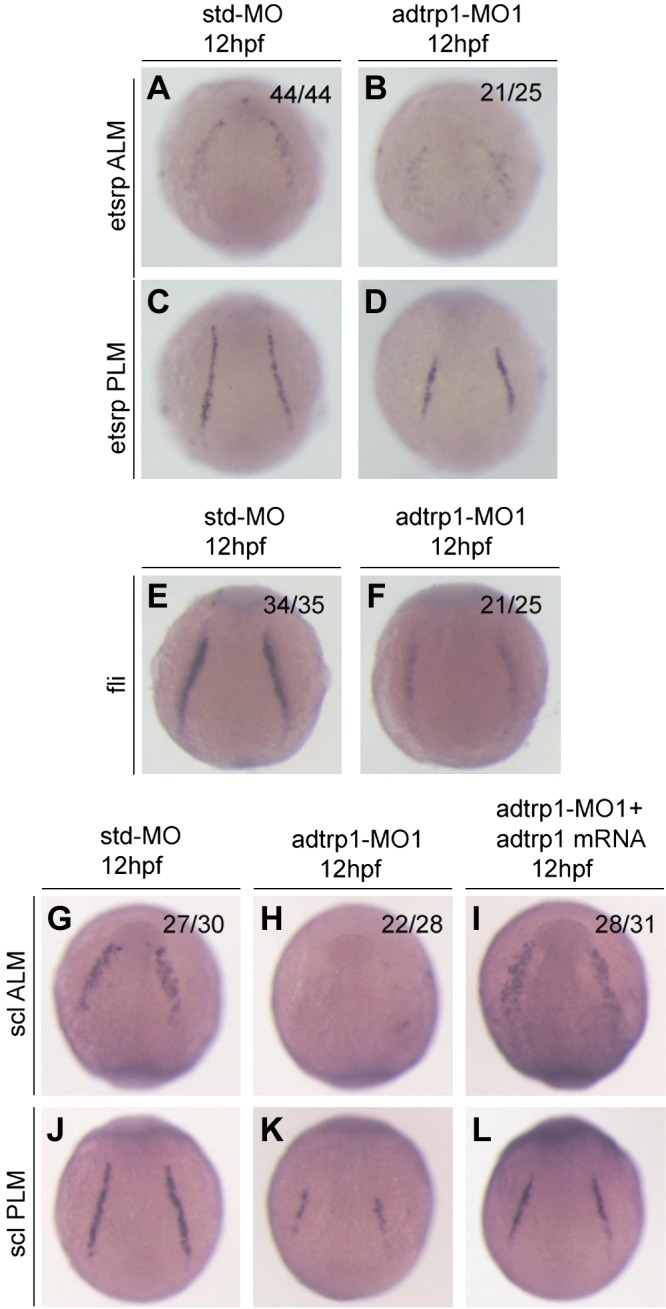
Knockdown of adtrp1 expression disrupts the development of hemangioblasts during embryogenesis. A–D) adtrp1 MO1 reduced the signal intensity of the hemagioblast marker, etsrp, in both anterior lateral mesoderm (ALM) (A, B) and posterior lateral mesoderm (PLM) (C, D). E, F) adtrp1 MO1 reduced the signal intensity of the hemagioblast marker, fli1a. G–L) adtrp1 MO1 reduced the signal intensity of the hemagioblast marker, scl, in both ALM (G–I) and PLM (J–L). Zebrafish embryos at the 1- to 2-cell stage were injected with 4 ng of control Std-MO (A, C, E, G, J), 4 ng adtrp1 MO1 (B, D, F, H, K), or 4 ng adtrp1 MO1 together with 50 pg adtrp1 mRNA (I, L), grown to appropriate embryonic stages (12 hpf), and used for whole-mount in situ hybridization with the antisense probe for each marker. Images of ALM are shown in an anterior view with the ventral side at the top, and PLM images are shown in a dorsal view with the anterior view to the top. The ratio in each image indicates the number of embryos with the image shown over the total number of embryos analyzed.
To confirm the role of adtrp1 in the differentiation of hemangioblasts, we characterized scl, which is another marker for the differentiation of hemangioblasts. Expression of scl was markedly reduced in adtrp1 MO1 morphants compared with control embryos that were injected with Std-MO at 12 h postfertilization (hpf; Fig. 1G, H, J, K). Coinjection of adtrp1 MO1 and zebrafish adtrp1 mRNA rescued the decreased expression level of scl that was caused by adtrp1 MO1 (Fig. 1G–L). These data indicate the specificity of adtrp1 MO1 on the specification of hemangioblasts. These findings with adtrp1 MO1 were independently confirmed with adtrp1 MO2 (Supplemental Figs. 2 and 3A–C′).
Adtrp1 is involved in myeloid development, but not erythroid development, during primitive hematopoiesis
Hematopoiesis is a complicated and hierarchical process that occurs in 2 successive waves that are referred to as primitive and definitive hematopoiesis (21–26). Gata1 and pu-1 encode 2 transcription factors that regulate primitive hematopoiesis (24, 27). To assess the role of adtrp1 in primitive hematopoiesis, we performed whole-mount in situ hybridization in adtrp1 morphants for gata1, a marker for erythroid progenitors. The expression level of gata1 did not differ between adtrp1 MO1 morphants and control Std-MO morphants (Fig. 2A–D). A similar observation was made for hbae1, another marker for the development of erythroid cells (Fig. 2E–H). These data suggest that adtrp1 is not involved in the development of erythroid cells during primitive hematopoiesis.
Figure 2.
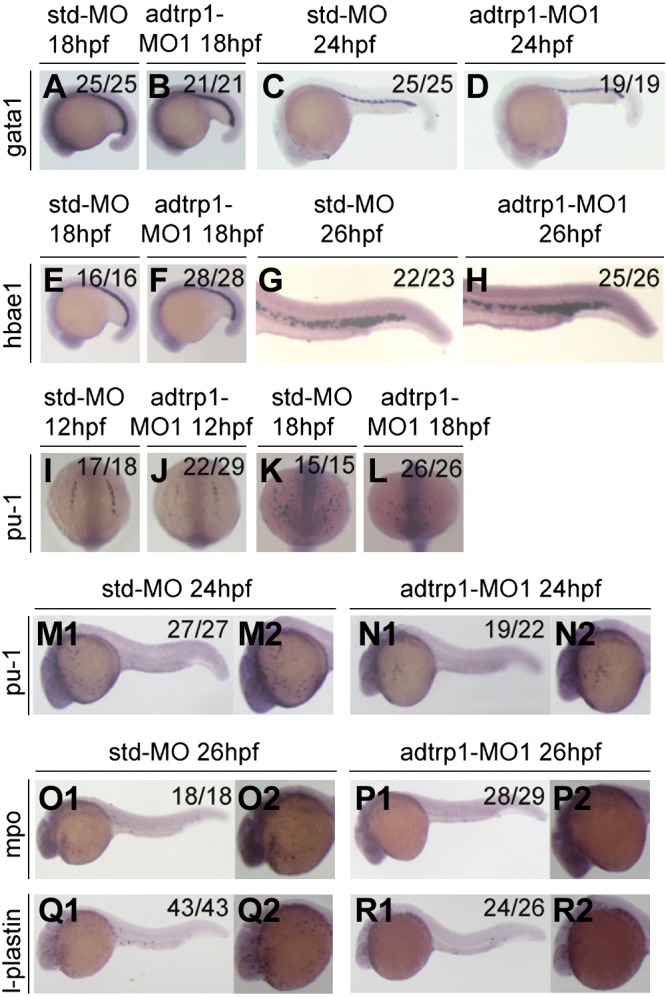
Knockdown of adtrp1 expression disrupts the specification of primitive myelopoiesis during embryogenesis. A–H) Knockdown of adtrp1 expression did not affect the expression levels of gata1 (18 hpf, A, B; 24 hpf, C, D) and hbae1 (18 hpf, E, F; 26 hpf, G, H)—markers for erythropoiesis during primitive hematopoiesis. I–N2) Knockdown of adtrp1 expression reduced the expression level of pu-1 (12 hpf, I, J; 18 hpf, K, L; 24 hpf, M1–N2), a myeloid progenitor marker. O1–R2) Knockdown of adtrp1 expression reduced the expression levels of mpo (26 hpf; O1–P2), a granulocyte marker, and l-plastin (26 hpf; Q1–R2), a monocyte/macrophage cell marker.
We then assessed the role of adtrp1 in the development of myeloid cells during primitive hematopoiesis. Whole-mount in situ hybridization was performed in adtrp1 morphants for pu-1, a marker for myeloid progenitors. The expression of pu-1 was markedly down-regulated by adtrp1 MO1 compared with Std-MO (Fig. 2I–N2). We also investigated the expression levels of markers for granulocytes and monocytes/macrophages. Mpo, a marker for granulocytes, displayed a markedly decreased expression level in adtrp1 MO1 morphants compared with Std-MO morphants (Fig. 2O1–P2). Similarly, l-plastin, a marker for monocytes/macrophages, also displayed a reduced expression level in adtrp1 MO1 morphants compared with Std-MO morphants (Fig. 2Q1–R2). Coinjection of in vitro synthesized adtrp1 mRNA rescued the decreased expression levels of pu-1 and mpo in adtrp1 morphants (data not shown). These data suggest that adtrp1 is involved in the development of myeloid cells during primitive hematopoiesis.
Findings with adtrp1 MO1 were independently confirmed with adtrp1 MO2 (Supplemental Fig. 3D–G).
Adtrp1 is involved in definitive hematopoiesis
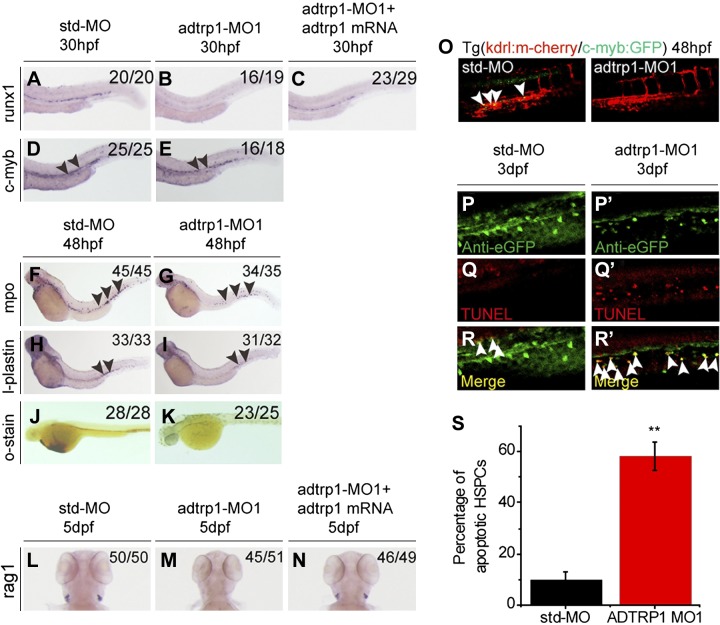
We also investigated whether adtrp1 affected definitive hematopoiesis; runx1 and c-myb are genes that regulate definitive hematopoiesis (11). Whole-mount in situ hybridization was carried out with markers for definitive hematopoiesis, including runx1 and c-myb—markers for the specification of hematopoietic stem cells (HSCs). The expression level of runx1 was dramatically reduced in adtrp1 MO1 morphants compared with control Std-MO morphants, and the effect of adtrp1 MO1 was rescued by adtrp1 mRNA (Fig. 3A–C). Findings with adtrp1 MO1 were independently confirmed with adtrp1 MO2 (Supplemental Fig. 3H, I).
Figure 3.
Adtrp1 is involved in definitive hematopoiesis. A–E) Knockdown of adtrp1 expression reduced the expression levels of runx1 (30 hpf; A–C) and c-myb (30 hpf; D, E), 2 independent HSC markers. Reduced runx1 signal by adtrp1 knockdown can be rescued by zebrafish adtrp1 mRNA (50 pg). Black arrowheads mark the differences between 2 groups. F–I) Knockdown of adtrp1 expression reduced the expression levels of mpo (48 hpf; F, G), a marker for granulocytes, and l-plastin (48 hpf; H, I), a marker for monocytes/macrophages. J–N) Knockdown of adtrp1 expression reduced the number of erythroid cells in the circulation at 48 hpf, as shown by o-dianisidine staining (J, K), and abolishes the signal of rag1 (L–N), a marker for lymphocytes, at 5 dpf. Reduced rag1 signal by adtrp1 knockdown can be rescued by zebrafish adtrp1 mRNA (50 pg). O) The number of cmyb+kdrl+ cells in Tg(cmyb:GFP/kdrl:mCherry) embryos was decreased in adtrp1 morphants compared with control Std-MO morphants. White arrowheads mark cmyb+kdrl+ cells in the ventral wall of the dorsal aorta region at 48 hpf. P–R′) Double immunostaining of c-myb:GFP and TUNEL at 3 dpf in adtrp1 morphants (P′, Q′, R′) and control embryos (P, Q, R). The signal for apoptotic HSPCs—TUNEL and GFP double-positive cells (white arrows)—was increased in adtrp1 morphants compared with Std-MO morphants. S) Quantitative analysis of images in panels P–R′. Error bars represent sem. **P < 0.01 (n > 10/group).
The effect of adtrp1 MO1 on c-myb expression was not as obvious as on runx1 expression using whole-mount in situ hybridization (Fig. 3D, E). Thus, we used a more sensitive approach to determine the effect of knockdown of adtrp1 on c-myb. We injected adtrp1 MO1 and Std-MO into 1- to 2-cell stage embryos from cmyb:GFP/kdrl:mCherry fish and examined the GFP signal for c-myb in the context of red mcherry endothelial cell signal for kdrl (cmyb:GFP and kdrl:mCherry double-positive yellow signal). The yellow signal with both c-myb and kdrl expression almost disappeared in adtrp1 MO1 morphants compared with Std-MO morphants (Fig. 3O). Findings with adtrp1 MO1 were independently confirmed with adtrp1 MO2 (Supplemental Fig. 3J, K). These data suggest that adtrp1 is involved in the specification of HSCs in the ventral wall of the dorsal aorta.
Previous studies have shown that the reduced signal for HSCs or hematopoietic stem progenitor cells (HSPCs) could be caused by apoptosis (28). TUNEL assays displayed a significantly increased number of apoptotic EGFP-positive HSPCs in the caudal hematopoietic tissue region of adtrp1 morphants compared with that of Std-MO morphants at 3 dpf (Fig. 3P–S).
We also assessed the role of adtrp1 in the development of myeloid cells during definitive hematopoiesis. Whole-mount in situ hybridization was performed in adtrp1 morphants for mpo (a marker for granulocytes) and l-plastin (a marker for monocytes/macrophages). The abundance of both markers was reduced in adtrp1 morphants compared with Std-MO morphants (Fig. 3F–I).
To determine whether adtrp1 affects the specification of erythrocytes during definitive hematopoiesis, we performed whole-mount in situ hybridization and O-dianisidine staining, and found that the hemoglobin level was abolished in adtrp1 morphants compared with control Std-MO morphants (Fig. 3J, K and Supplemental Fig. 3L, M).
To further determine the effect of adtrp1 on definitive hematopoiesis, we performed whole-mount in situ hybridization for 5-dpf embryos for rag1, a marker for lymphocytes. Expression of rag1 was abolished in adtrp1 morphants compared with control Std-MO morphants (Fig. 3L, M). Coinjection of in vitro synthesized adtrp1 mRNA rescued the decreased expression of rag1 in adtrp1 morphants (Fig. 3N).
The effect of adtrp1 knockdown on the specification of hemangioblasts and hematopoiesis was confirmed by quantitative real-time RT-PCR (Supplemental Fig. 4).
Effect of adtrp1 overexpression on the specification of hemangioblasts and hematopoiesis
We studied the effects of overexpression of adtrp1 on the differentiation of hemangioblasts and hematopoiesis by injecting 200 pg adtrp1 mRNA into 1- to 2-cell zebrafish embryos. Whole-mount in situ hybridization demonstrated that, compared with injection of 200 pg control gfp mRNA, injection of 200 pg adtrp1 mRNA increased expression levels of scl (Fig. 4A, B) and runx1 (Fig. 4C, D) as well as o-dianisidine staining of hemoglobin (Fig. 4E, F). Moreover, the injection of adtrp1 mRNA dramatically increased the level of the cmyb:GFP and kdrl:mCherry double-positive signal for HSCs compared with injection of 100 pg control GFP mRNA in transgenic cmyb:GFP/kdrl:mCherry fish (Fig. 4G).
Figure 4.
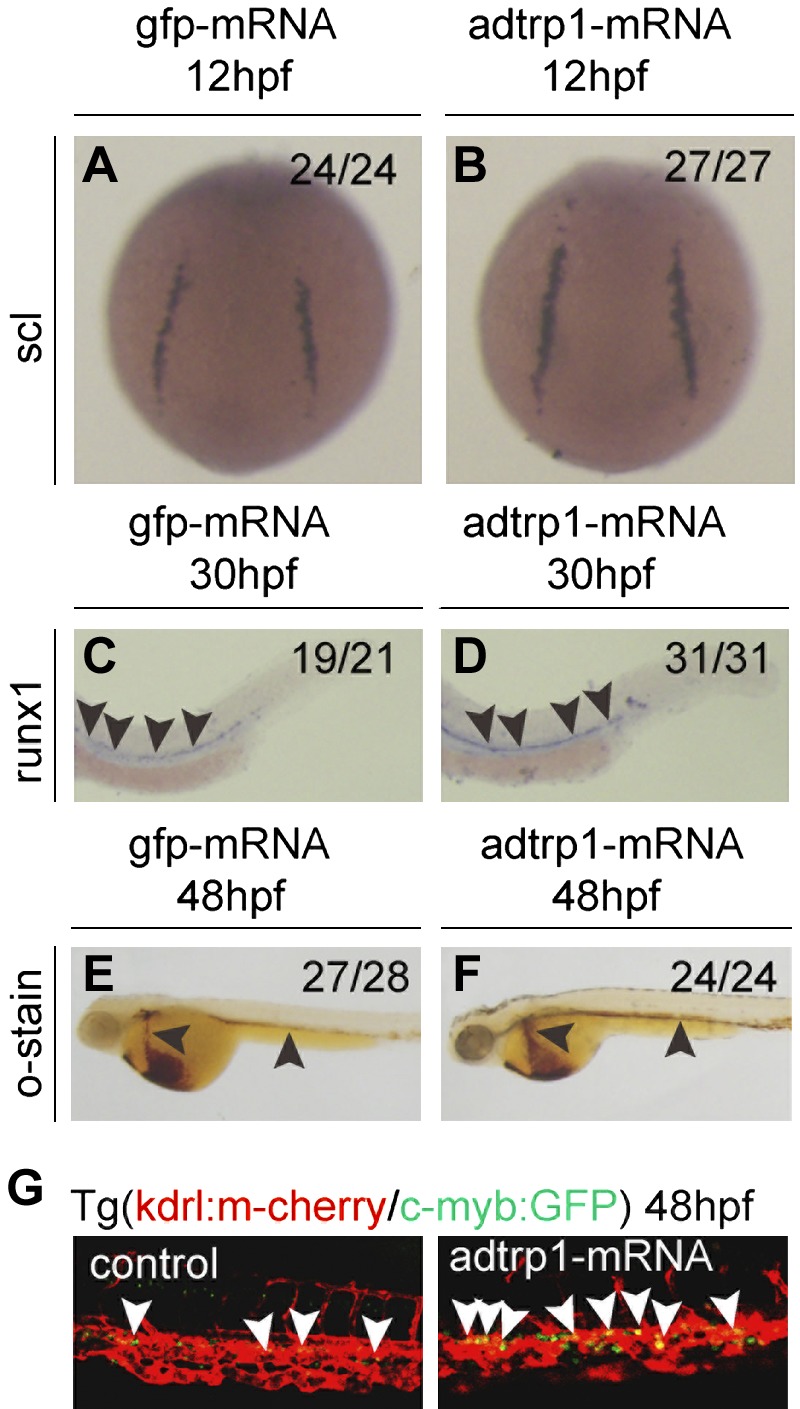
Overexpression of adtrp1 increases the expression levels of scl and runx1 and the number of HSCs. A, B) Overexpression of adtrp1 increased the expression level of scl at 12 hpf. C–F) Overexpression of adtrp1 increased the expression level of runx1 (C, D), a marker for HSCs at 30 hpf, and the number of erythrocytes with o-dianisidine staining (E, F) at 48 hpf. G) The number of cmyb+kdrl+ cells in Tg(cmyb:GFP/kdrl:mCherry) embryos was increased in embryos that were injected with adtrp1 mRNA compared with control GFP mRNA. White arrowheads mark cmyb+kdrl+ cells in the ventral wall of the dorsal aorta region at 48 hpf. Embryos were injected with 200 pg of control gfp mRNA group or 200 pg of adtrp1 mRNA, grown to appropriate embryonic stages, and used for whole-mount in situ hybridization with the antisense probe for each marker. Embryos at 12 hpf are shown in a dorsal view with the anterior to the top. Embryos before 48 hpf are shown in a lateral view with the anterior to the left. Black arrowheads mark the differences between 2 groups.
Effect of adtrp1 on the specificationof vascular endothelial cells and vascular development
As vascular endothelial cells (ECs) are differentiated from the same hemangioblasts as hematopoietic cells, we studied the effect of adtrp1 MO1 on the development of vascular systems. The adrenomedullin receptor encoded by admr, also known as kdrl, is expressed in all vascular ECs in early zebrafish embryos (29). Expression level of kdrl was slightly increased by adtrp1 MO1 (Supplemental Fig. 5A, B). We also examined notch3 and ephrinB2a, markers for arterial endothelial cell differentiation (30, 31), at 28 hpf. Expression levels of notch3 and ephrinB2a were also slightly increased by adtrp1 MO1 (Supplemental Fig. 5C–F). Similar observations were made for the venous markers, dab2 and flt4, markers for venous EC differentiation (Supplemental Fig. 5G–J) (32). Overall, the effect of adtrp1 knockdown on vascular EC differentiation was small.
Knockdown of adtrp1 does not affect the development of the endoderm, ectoderm, nervous system, notochord, somites, pronephros, or heart
We investigated whether adtrp1 regulated the development of other systems in addition to hematopoiesis derived from the mesoderm. Whole-mount in situ hybridization demonstrated that the expression of an endoderm-specific marker, sox17, and an ectoderm marker, krox20 (11), was not affected in adtrp1 morphants compared with control Std-MO morphants (Supplemental Fig. 6A–D). A similar analysis revealed no obvious difference for elavl3, a nervous system marker for the differentiation of the ectoderm (33), between adtrp1 morphants and control embryos that were injected with Std-MO (Supplemental Fig. 6E, F). Moreover, no differences were observed for the expression of other mesoderm markers not related to hematopoiesis between adtrp1 morphants and Std-MO morphants, including cdh17 (a marker for pronephric duct) (34), shha (a marker for notochord) (35), mylz (a marker for somites) (36), and cmlc2 (a marker for cardiac differentiation; Supplemental Fig. 6G–N) (12). These data suggest that adtrp1 specifically affects hematopoiesis in zebrafish.
Adtrp2 is not involved in the specificationof hemangioblasts, hematopoiesis, and vascular development
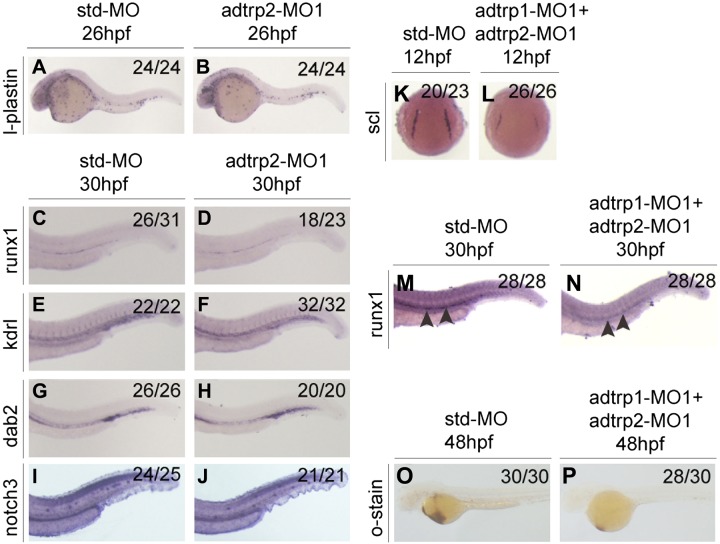
Whole-mount in situ hybridization with adtrp2 MO1 morphants and Std-MO morphants did not identify obvious differences for the expression of l-plastin at 26 hpf, which suggests that adtrp2 does not specify primitive hematopoiesis (Fig. 5A, B and Supplemental Fig. 7). Similar analysis showed that adtrp2 MO1 did not affect the expression of runx1 (Fig. 5C, D), which suggests that adtrp2 is not involved in the differentiation of HSCs during definitive hematopoiesis. We also analyzed the vascular endothelial marker, kdrl, venous marker, dab2, and arterial marker, notch3, by using whole-mount in situ hybridization, but did not detect obvious differences between adtrp2 MO1 morphants and Std-MO morphants (Fig. 5E–J). These data suggest that adtrp2 does not play a role in vascular development in zebrafish.
Figure 5.
Adtrp2 is not involved in the regulation of hematopoiesis and vasculogenesis. A–D) Knockdown of adtrp2 expression did not reduce the expression levels of l-plastin (A, B) and runx1 (C, D), markers for hematopoiesis. E–J) Knockdown of adtrp2 expression did not reduce the expression levels of kdrl (E, F), dab2 (G, H), and notch3 (G, H), markers for vasculogenesis. K–P) Coinjection of adtrp1 MO1 and adtrp2 MO1 reduced the expression levels of scl (K, L) and runx1 (M, N) using whole-mount in situ hybridization and the number of erythroid cells in the circulation by o-dianisidine staining (O, P) as in adtrp1 MO1 morphants. Black arrowheads mark the differences between 2 groups.
Knockdown of both adtrp1 and adtrp2 by coinjection of adtrp1 MO1 and adtrp2 MO1 yielded results that were identical to embryos that were injected with adtrp1 MO1 alone with whole-mount in situ hybridization data for scl, runx1, and o-dianisdine staining (Fig. 5K–P). Coinjection of in vitro synthesized adtrp2 mRNA did not rescue the decreased expression levels of l-plastin, mpo, rag1, and hemoglobin in adtrp1 MO1 morphants (Supplemental Fig. 8). These data suggest that the effect of adtrp1 on hematopoiesis is independent of adtrp2. Moreover, adtrp1 knockdown did not affect the expression levels of adtrp2 and aig1 (Supplemental Fig. 9).
Similar to adtrp2 MO1, no apparent effects were observed for adtrp2 MO3, which disrupted the splicing of the 2014 version of adtrp2 transcript and resulted in the deletion of exon 2 (Supplemental Figs. 7 and 10). We also studied the effects of the knockdown of the 2015 version of adtrp2 transcript, with adtrp2 MO2 inhibiting the translation and MO4 disrupting splicing (Supplemental Figs. 7, 11, and 12). No apparent effects were observed for adtrp2 MO2 (Supplemental Fig. 11). For MO4, no effects were observed on the levels of scl and etsrp (Supplemental Fig. 12). The levels of l-plastin, mpo, rags, and o-dianisidine were reduced by MO4, but the effects were not rescued by adtrp2 overexpression (Supplemental Fig. 12), which may suggest that the observed effects were not specific to adtrp2.
Adtrp1 acts upstream of tfpi to regulate hematopoiesis in zebrafish
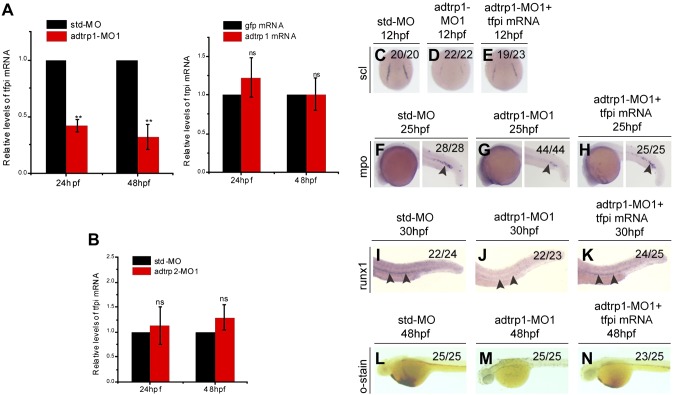
Human ADTRP protein was shown to be colocalized with TFPI in human ECs and to regulate the expression of TFPI in ECs (6); however, it remains to be determined whether ADTRP regulates TFPI expression in vivo. Real-time RT-PCR analysis demonstrated that the expression level of tfpi mRNA in the adtrp1 MO1 group was reduced by more than 2-fold at 24 hpf and by approximately 3-fold at 48 hpf compared with the Std-MO group (Fig. 6A); however, overexpression of zebrafish adtrp1 by injection of 200 pg mRNA did not affect the tfpi expression level (Fig. 6A). Knockdown of zebrafish adtrp2 did not affect the tfpi expression level (Fig. 6B).
Figure 6.
Adtrp1 regulates hematopoiesis by controlling the expression level of tfpi. A) Real-time RT-PCR analysis for tfpi in embryos that were injected with adtrp1 MO1 (left panel; red bars) or adtrp1 mRNA (right panel; red bars) and control embryos that were injected with Std-MO (left panel; black bars) or gfp mRNA (right panel; black bars). B) Real-time RT-PCR analysis for tfpi in embryos that were injected with adtrp2 MO1 (red bars) and control embryos that were injected with Std-MO (black bars). C–N) Tfpi overexpression rescued developmental defects of adtrp1 morphants. Zebrafish embryos (1- to 2-cell stage) were injected with Std-MO (4 ng; C, F, I, L), adtrp1 MO1 (4 ng; D, G, J, M), or adtrp1 MO1 (4 ng) together with tfpi mRNA (100 pg) (E, H, K, N). Embryos were used for whole-mount in situ hybridization with antisense probes for scl at 6 somites (C–E), mpo at 25 hpf (F–H), and runx1 at 30 hpf (I–K). Embryos were collected at 48 hpf for o-dianisidine staining (L–N). Coinjection of tfpi mRNA rescued abnormal hematopoietic phenotypes in adtrp1 morphants. Quantitative data are shown as means ± sem from 3 independent experiments (n = 3). Black arrowheads denote the differences between 2 groups. ns, not significant (P > 0.05).**P < 0.01.
We characterized the expression profile of tfpi during zebrafish embryogenesis using whole-mount in situ hybridization. From 6 to 12 hpf, tfpi expression was ubiquitous in these embryos (Supplemental Fig. 13A, B), which is similar to the expression pattern of adtrp1 (Supplemental Fig. 1C). At 24–48 hpf, the expression pattern of tfpi was different than that of adtrp1 (Supplemental Fig. 13C, D). The expression of tfpi was limited to the vicinity of the dorsal aorta (the region of hemogenic endothelium; Supplemental Fig. 13C), whereas the expression of adtrp1 was localized in the brain areas (Supplemental Fig. 1C). By 72 hpf, tfpi expression was reduced or had disappeared (Supplemental Fig. 13E).
Because adtrp1 regulates the expression level of tfpi, we hypothesized that tfpi can rescue the abnormal hematopoietic phenotypes that are caused by adtrp1 MO1. To test this hypothesis, we coinjected tfpi mRNA with adtrp1 MO1 in 1- to 2-cell stage embryos. Whole-mount in situ hybridization showed that the decreased expression levels of scl, mpo, and runx1 as well as the reduced o-dianisdine staining in adtrp1 morphants were partially rescued by expression of tfpi (Fig. 6C–N). These data suggest that adtrp1 acts upstream of tfpi to regulate the specification of hemangioblasts, primitive myelopoiesis, and definitive hematopoiesis.
Adtrp1 acts independently of aggf1
We have previously reported that the angiogenic factor gene, aggf1, acted upstream of scl, etsrp, and fli in the specification of hemangioblasts, hematopoiesis, and vasculogenesis (11). Similar to aggf1, adtrp1 acts upstream of scl, etsrp, and fli; therefore, we determined the relationship between adtrp1 and aggf1 during embryogenesis. First, we coinjected capped aggf1 mRNA with adtrp1 MO1 into 1- to 2-cell embryos, then examined the expression levels of scl, runx1, and o-dianisidine staining for hemoglobin. The rescue effect of the overexpression of aggf1 was moderate for the reduced expression levels of scl and runx1 in adtrp1 morphants and negative for o-dianisidine staining (Fig. 7). Overexpression of adtrp1 did not recue any phenotype of aggf1 morphants (Fig. 7). Together, these data suggest that adtrp1 and aggf1 act independently in the specification of hemangioblasts.
Figure 7.
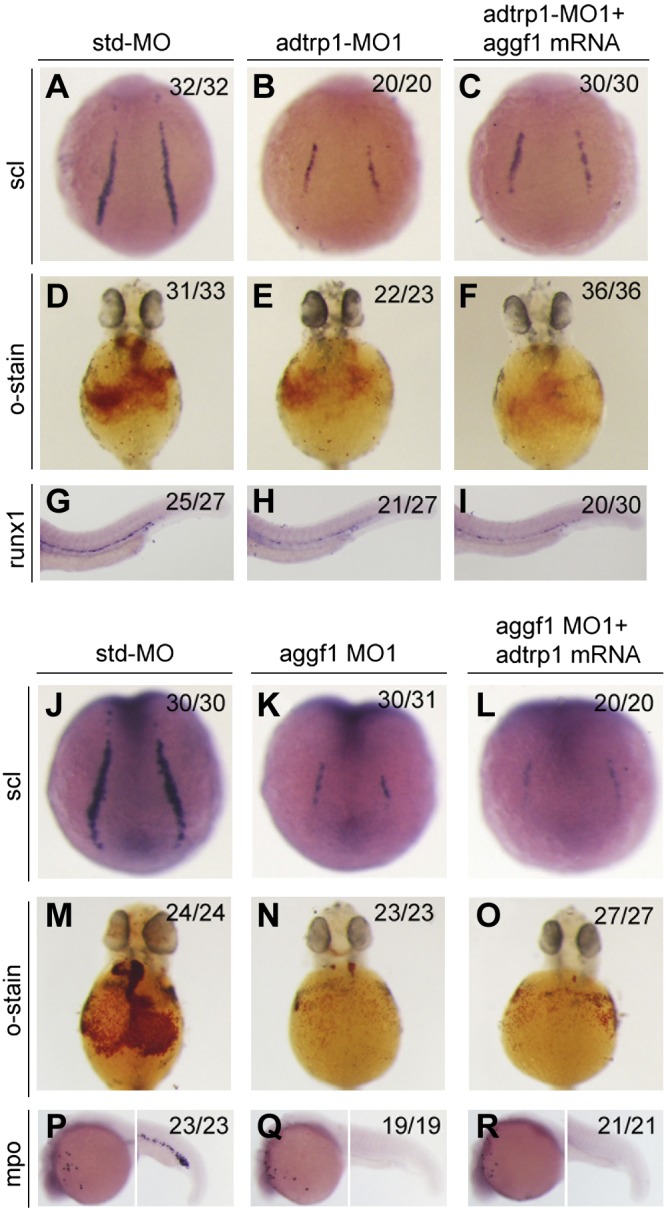
A–I) Aggf1 overexpression did not rescue the defects in adtrp1 morphants. J–R) Overexpression of adtrp1 did not rescue the developmental defects of aggf1 morphants.
Overexpression of a constitutively active form of AKT did not rescue the phenotypes of adtrp1 morphants
We recently found that the overexpression of ADTRP activated AKT in HepG2 and Eahy926 ECs, which promotes cell proliferation and migration (37, 38); therefore, we performed a rescue study with constitutively activated AKT to determine whether it can rescue defects in adtrp1 morphants. We coinjected adtrp1 MO1 with mRNA for a constitutively active form of human AKT (myristoylated AKT or myrAKT) into 1- to 2-cell embryos and examined the expression levels of scl, runx1, and o-dianisidine staining for hemoglobin, but no rescue was observed (Supplement Fig. 14). These data suggest that adtrp1 does not act via the AKT pathway in the specification of hemangioblasts and hematopoiesis.
DISCUSSION
In this study, we used zebrafish as a model organism to characterize the in vivo physiologic functions of adtrp1 and adtrp2. Knockdown of adtrp1 expression by two different MOs inhibited the specification of hemangioblasts, primitive myelopoiesis, and definitive hematopoiesis (Figs. 1–3). Overexpression of adtrp1 by injection of adtrp1 mRNA into zebrafish embryos increased hemangioblasts (increased scl expression) and definitive hematopoiesis (increased runx1 expression and increased number of kdrl/cmyb double-positive cells; Fig. 4). These results suggest that adtrp1 plays an important role in hemangioblast specification and hematopoiesis in zebrafish embryogenesis. It will be of interest to further characterize the phenotypes on hemangioblast specification and hematopoiesis in zebrafish that are deficient in adtrp1 in the future.
We then uncovered a molecular mechanism by which adtrp1 regulates the specification of hemangioblasts, primitive myelopoiesis, and definitive hematopoiesis—the adtrp1-tfpi regulatory axis. Our data suggest that tfpi is involved in adtrp1-mediated hematopoiesis. We found that knockdown of adtrp1 significantly reduced the expression levels of the tfpi gene (Fig. 6A, B) in zebrafish embryos and that overexpression of tfpi rescued the phenotypes that are observed in adtrp1 morphants (Fig. 6C–N). These data extend the previous finding in vitro that human ADTRP regulates the expression of TFPI in ECs (6) to that in vivo. Of most interest, these data suggest that adtrp1 regulates the specification of hemangioblasts and hematopoiesis by acting upstream of tfpi. Whole-mount in situ hybridization demonstrated that at 6 and 12 hpf during zebrafish embryogenesis, both adtrp1 and tfpi were expressed all over the animal pole; however, at 24 and 48 hpf, tfpi expression was observed mainly in the vicinity of the dorsal aorta (Supplemental Fig. 13), whereas adtrp1 expression was localized in the brain area (Supplemental Fig. 1). Because HSCs emerge at the ventral wall of the dorsal aorta, strong expression of tfpi at the dorsal aorta at 24 and 48 hpf is consistent with its role in rescuing the hematopoietic defects that are associated with adtrp1 knockdown. The role of TFPI was previously thought to be limited to the inhibition of coagulation, but our data suggest that TFPI also plays an important role in hematopoiesis, which links hematopoiesis and coagulation. TFPI was observed to interact with heparin sulfate proteoglycan GPC3, which inhibits the activity of serine protease CD26, increasing the proliferation and migration of HSPCs and decreasing HSPC retention in bone marrow (39). Broxmeyer et al. (40) showed that CD26, also known as dipeptidylpeptidase, can cleave the N terminus of G-CSF and decrease its activity. Stachura et al. (41) showed that G-CSF is involved in primitive and definitive hematopoiesis as well as the specification and proliferation of HSCs in zebrafish. Altogether, we propose a putative signaling pathway for adtrp1-mediated hematopoiesis. ADTRP1 up-regulates the expression of TFPI, which binds to GPC3, inhibits the activity of CD26, and blocks the cleavage of G-CSF, leading to increased G-CSF activity and increased primitive myelopoiesis and specification and proliferation of HSCs (definitive hematopoiesis). However, the overexpression of zebrafish tfpi only partially rescued the abnormal phenotypes in adtrp1 morphants (Fig. 6C–N); therefore, adtrp1 may also regulate primitive myelopoiesis and definitive hematopoiesis via other unidentified signaling pathways.
Human ADTRP has been identified as a susceptibility gene for CAD and MI (1); however, the underlying molecular mechanism remains to be identified. Hasegawa et al. (42) reported the interesting result that G-CSF treatment inhibited the progression of atherosclerosis and neointimal formation in MI and balloon injury rabbits. As discussed in the putative signaling pathway for adtrp1-mediated hematopoiesis section, ADTRP may increase the activity of G-CSF, thereby conferring a protective role for the risk of atherosclerosis and CAD, whereas the ADTRP variant reduces the expression of ADTRP, which leads to increased cleavage of G-CSF and an increased risk of atherosclerosis and CAD; however, there is controversy regarding the role of either protection for or risk of G-CSF in atherosclerosis. Takai et al. (43) did not identify any adverse effect of G-CSF on atherosclerotic lesions in high cholesterol–fed miniature swine. Haghighat et al. (44) reported that G-CSF exacerbated atherosclerosis in ApoE−/− knockout mice. Hu et al. (45) demonstrated that G-CSF exacerbated atherosclerosis in rabbits that were fed a high-fat diet. The different results may be a result of different animal models and different doses used in different reports. Nevertheless, it is of interest to further explore the role of G-CSF in the ADTRP-TFPI regulatory pathway and the pathogenesis of atherosclerosis and CAD in the future. Moreover, Chinetti-Gbaguidi (46) reported a high expression level of ADTRP in human macrophages and atherosclerotic lesions, where its expression is regulated by peroxisome proliferator-activated receptor-γ. Together with our finding that adtrp1 is required for the differentiation of monocytes and macrophages in zebrafish (Fig. 2), the role of adtrp in atherosclerosis and CAD may also be related to its function in monocytes and macrophages.
Human ADTRP was shown to be expressed in ECs (6). We demonstrated that human ADTRP regulates the expression of many downstream genes and promotes EC proliferation, migration, and capillary-tube formation as well as the adhesion of monocytes to ECs and the transmigration of monocytes across a layer of endothelial cells—the two initiating events in atherosclerosis. Our data in this study, however, do not identify any obvious roles for adtrp1 or adtrp2 in vascular development and the specification of arterial and venous ECs in zebrafish. Our study focused on the embryonic developmental stage of zebrafish and it is likely that adtrp1 or adtrp2 may play an important role in angiogenesis and the maintenance of vasculature integrity in adult zebrafish by regulating the functions of ECs. This issue may be addressed by future studies with knockout zebrafish that are deficient in adtrp1, adtrp2, or both genes, or knockout mice that are deficient in Adtrp.
It is common that zebrafish have multiple paralogs for a mammalian gene. Similarly, zebrafish have two ADTRP paralogous genes—adtrp1 and adtrp2—although humans and other mammals have only one copy of the ADTRP gene. Paralogues in zebrafish usually play a similar physiologic role; however, adtrp1 and adtrp2 display different expression patterns during embryogenesis and play different physiologic roles. Although adtrp1 is involved in the specification of hemangioblasts, primitive myelopoiesis, and definitive hematopoiesis, adtrp2 is not involved in these processes. The physiologic roles of adtrp2, therefore, remain to be identified.
Our data suggest that adtrp1 regulates the differentiation of hemangioblasts from the mesoderm because knockdown of adtrp1 expression reduced the expression of etsrp, fli1, and scl—markers for hemangioblasts (Fig. 1). Hemangioblasts act as common progenitors that are then differentiated into many different ECs and hematopoietic cells. We have previously shown that the angiogenic factor, AGGF1, acts as the upstream regulator that mediates the differentiation of hemangioblasts, thereby specifying the differentiation of ECs and all blood cells from both primitive and definitive hematopoiesis (11). Of interest, although adtrp1 also regulates the differentiation of hemangioblasts, it is not required for the specification of ECs or vasculogenesis and primitive erythropoiesis. These findings suggest the heterogeneity of hemangioblasts. There may be multiple types of hemangioblasts—for example, aggf1-type hemangioblasts that are involved in the differentiation of ECs and all hematopoietic cells and adtrp1-type hemangioblasts with a more limited role in primitive myelopoiesis and definitive hematopoiesis (no role in vasculogenesis and primitive erythropoiesis). This is consistent with our finding that aggf1 and adtrp1 act independently of each other (Fig. 7).
Multiple studies have suggested that hemangioblasts generate HSCs (definitive hematopoiesis) via a hemogenic endothelium stage, a process that is referred to as endothelium transition to hematopoietic cells (47–50). Our data indeed show that the knockdown of adtrp1 reduced the number of kdrl+cmyb+ double-positive cells (Fig. 3), whereas the overexpression of adtrp1 increased the number of kdrl+cmyb+ cells (Fig. 4); however, our data suggest that adtrp1 is not involved in vascuologenesis or the differentiation of ECs (Fig. 5). On the contrary, expression levels of EC markers kdrl, efnb2a, notch3, dab2, and flt4 were slightly increased in adtrp1 morphants (Supplemental Fig. 5). These data argue that there are specialized aortic vascular ECs that are differentiated into hematopoietic cells by endothelium transition to hematopoietic cells, but the number of these cells is small (kdrl+cmyb+ cells; Figs. 3 and 4). The differentiation of specialized aortic vascular ECs may be affected by adtrp1, but could not be detected because of the limitation of current technology. Alternatively, some specialized hemangioblasts differentiate directly into hematopoietic cells without first differentiating into ECs. Future studies are needed to carefully distinguish these two possibilities.
In summary, our studies unveiled the important in vivo function of adtrp1 in the specification of hemangioblasts, primitive myelopoiesis, and definitive hematopoiesis in zebrafish. Adtrp1 acts upstream of tfpi involved in coagulation in the regulation of hematopoiesis. This study provides an understanding of the differentiation of hemangioblasts and hematopoietic cells, which may have general implications for regeneration medicine for hematologic diseases.
ACKNOWLEDGMENTS
This work was supported by the China National Natural Science Foundation (Grants 31430047, 81630003, and 91439129), Chinese National Basic Research (973 Programs 2013CB531101 and 2012CB517801), Hubei Province’s Outstanding Medical Academic Leader Program, Hubei Province Natural Science Programs (2016CFB224 and 2014CFA074), and the U.S. National Institutes of Health (NIH), National Heart, Lung, and Blood Institute (Grants R01-HL121358 and R01-HL126729). Other related research projects on ADTRP in the Q.K.W. Laboratory received funding from Bayer Health.
Glossary
- ADTRP
androgen-dependent tissue factor pathway inhibitor regulating protein
- CAD
coronary artery disease
- dpf
days postfertilization
- EC
endothelial cell
- hpf
hours postfertilization
- HSC
hematopoietic stem cell
- HSPC
hematopoietic stem progenitor cell
- MI
myocardial infarction
- MO
morpholino
- TF
tissue factor
- TFPI
tissue factor pathway inhibitor
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
Li. Wang designed and performed experiments, analyzed data, and drafted the manuscript; X. Wang performed some experiments and analyzed data; Lo. Wang, M. Yousaf, J. Li, M. Zuo, Z. Yang, D. Gou, L. Li, and N. Xiang constructed some plasmids and performed some experiments; C. Xu and Q. Chen provided valuable assistance and critically revised the manuscript; B. Bao and H. Jia provided Tg(kdrl:mCherry/c-myb:GFP) transgenic zebrafish; and Q. K. Wang designed and supervised the project, assisted with data analysis and interpretation, and critically revised the manuscript.
REFERENCES
- 1.Wang F., Xu C.-Q., He Q., Cai J.-P., Li X.-C., Wang D., Xiong X., Liao Y.-H., Zeng Q.-T., Yang Y.-Z., Cheng X., Li C., Yang R., Wang C.-C., Wu G., Lu Q.-L., Bai Y., Huang Y.-F., Yin D., Yang Q., Wang X.-J., Dai D.-P., Zhang R.-F., Wan J., Ren J.-H., Li S.-S., Zhao Y.-Y., Fu F.-F., Huang Y., Li Q.-X., Shi S.-W., Lin N., Pan Z.-W., Li Y., Yu B., Wu Y.-X., Ke Y.-H., Lei J., Wang N., Luo C.-Y., Ji L.-Y., Gao L.-J., Li L., Liu H., Huang E.-W., Cui J., Jia N., Ren X., Li H., Ke T., Zhang X.-Q., Liu J.-Y., Liu M.-G., Xia H., Yang B., Shi L.-S., Xia Y.-L., Tu X., Wang Q. K. (2011) Genome-wide association identifies a susceptibility locus for coronary artery disease in the Chinese Han population. Nat. Genet. 43, 345–349 [DOI] [PubMed] [Google Scholar]
- 2.Guo C. Y., Gu Y., Li L., Jia E. Z., Li C. J., Wang L. S., Yang Z. J., Cao K. J., Ma W. Z. (2012) Association of SNP rs6903956 on chromosome 6p24.1 with angiographical characteristics of coronary atherosclerosis in a Chinese population. PLoS One 7, e43732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tayebi N., Ke T., Foo J. N., Friedlander Y., Liu J., Heng C. K. (2013) Association of single nucleotide polymorphism rs6903956 on chromosome 6p24.1 with coronary artery disease and lipid levels in different ethnic groups of the Singaporean population. Clin. Biochem. 46, 755–759 [DOI] [PubMed] [Google Scholar]
- 4.Dechamethakun S., Ikeda S., Arai T., Sato N., Sawabe M., Muramatsu M. (2014) Associations between the CDKN2A/B, ADTRP and PDGFD polymorphisms and the development of coronary atherosclerosis in Japanese patients. J. Atheroscler. Thromb. 21, 680–690 [DOI] [PubMed] [Google Scholar]
- 5.Huang E. W., Peng L. Y., Zheng J. X., Wang D., Tan X. H., Yang Z. Y., Li X. M., Wu Q. P., Tang S. B., Luo B., Quan L., Liu S. P., Liu X. S., Li Z. H., Shi H., Lv G. L., Zhao J., Liu C., Cheng J. D. (2016) Investigation of associations between ten polymorphisms and the risk of coronary artery disease in Southern Han Chinese. J. Hum. Genet. 61, 389–393 [DOI] [PubMed] [Google Scholar]
- 6.Lupu C., Zhu H., Popescu N. I., Wren J. D., Lupu F. (2011) Novel protein ADTRP regulates TFPI expression and function in human endothelial cells in normal conditions and in response to androgen. Blood 118, 4463–4471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomassen M. C., Heinzmann A. C., Herfs L., Hartmann R., Dockal M., Scheiflinger F., Hackeng T. M., Rosing J. (2015) Tissue factor-independent inhibition of thrombin generation by tissue factor pathway inhibitor-α. J. Thromb. Haemost. 13, 92–100 [DOI] [PubMed] [Google Scholar]
- 8.Peraramelli S., Thomassen S., Heinzmann A., Rosing J., Hackeng T. M., Hartmann R., Scheiflinger F., Dockal M. (2014) Inhibition of tissue factor:factor VIIa-catalyzed factor IX and factor X activation by TFPI and TFPI constructs. J. Thromb. Haemost. 12, 1826–1837 [DOI] [PubMed] [Google Scholar]
- 9.Maroney S. A., Mast A. E. (2015) New insights into the biology of tissue factor pathway inhibitor. J. Thromb. Haemost. 13(Suppl 1), S200–S207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wood J. P., Ellery P. E., Maroney S. A., Mast A. E. (2014) Biology of tissue factor pathway inhibitor. Blood 123, 2934–2943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li L., Chen D., Li J., Wang X., Wang N., Xu C., Wang Q. K. (2014) Aggf1 acts at the top of the genetic regulatory hierarchy in specification of hemangioblasts in zebrafish. Blood 123, 501–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou J., Wang L., Zuo M., Wang X., Ahmed A. S., Chen Q., Wang Q. K. (2016) Cardiac sodium channel regulator MOG1 regulates cardiac morphogenesis and rhythm. Sci. Rep. 6, 21538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen D., Li L., Tu X., Yin Z., Wang Q. (2013) Functional characterization of Klippel-Trenaunay syndrome gene AGGF1 identifies a novel angiogenic signaling pathway for specification of vein differentiation and angiogenesis during embryogenesis. Hum. Mol. Genet. 22, 963–976 [DOI] [PubMed] [Google Scholar]
- 14.Su Z., Si W., Li L., Zhou B., Li X., Xu Y., Xu C., Jia H., Wang Q. K. (2014) MiR-144 regulates hematopoiesis and vascular development by targeting meis1 during zebrafish development. Int. J. Biochem. Cell Biol. 49, 53–63 [DOI] [PubMed] [Google Scholar]
- 15.Lu Q., Yao Y., Hu Z., Hu C., Song Q., Ye J., Xu C., Wang A. Z., Chen Q. (2016) Angiogenic factor AGGF1 activates autophagy with an essential role in therapeutic angiogenesis for heart disease. PLoS Biol. 14, e1002529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ye J., Yao Y., Song Q., Li S., Hu Z., Yu Y., Hu C., Da X., Li H., Chen Q., Wang Q. K. (2016) Up-regulation of miR-95-3p in hepatocellular carcinoma promotes tumorigenesis by targeting p21 expression. Sci. Rep. 6, 34034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang T., Yao Y., Wang J., Li Y., He P., Pasupuleti V., Hu Z., Jia X., Song Q., Tian X. L., Hu C., Chen Q., Wang Q. K. (2016) Haploinsufficiency of Klippel-Trenaunay syndrome gene Aggf1 inhibits developmental and pathological angiogenesis by inactivating PI3K and AKT and disrupts vascular integrity by activating VE-cadherin. Hum. Mol. Genet. 25, 5094–5110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paffett-Lugassy N. N., Zon L. I. (2005) Analysis of hematopoietic development in the zebrafish. Methods Mol. Med. 105, 171–198 [DOI] [PubMed] [Google Scholar]
- 19.Liu F., Walmsley M., Rodaway A., Patient R. (2008) Fli1 acts at the top of the transcriptional network driving blood and endothelial development. Curr. Biol. 18, 1234–1240 [DOI] [PubMed] [Google Scholar]
- 20.Ren X., Gomez G. A., Zhang B., Lin S. (2010) Scl isoforms act downstream of etsrp to specify angioblasts and definitive hematopoietic stem cells. Blood 115, 5338–5346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Notta F., Zandi S., Takayama N., Dobson S., Gan O. I., Wilson G., Kaufmann K. B., McLeod J., Laurenti E., Dunant C. F., McPherson J. D., Stein L. D., Dror Y., Dick J. E. (2016) Distinct routes of lineage development reshape the human blood hierarchy across ontogeny. Science 351, aab2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jagannathan-Bogdan M., Zon L. I. (2013) Hematopoiesis. Development 140, 2463–2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clements W. K., Traver D. (2013) Signalling pathways that control vertebrate haematopoietic stem cell specification. Nat. Rev. Immunol. 13, 336–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paik E. J., Zon L. I. (2010) Hematopoietic development in the zebrafish. Int. J. Dev. Biol. 54, 1127–1137 [DOI] [PubMed] [Google Scholar]
- 25.Warga R. M., Kane D. A., Ho R. K. (2009) Fate mapping embryonic blood in zebrafish: multi- and unipotential lineages are segregated at gastrulation. Dev. Cell 16, 744–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burns C. E., Galloway J. L., Smith A. C., Keefe M. D., Cashman T. J., Paik E. J., Mayhall E. A., Amsterdam A. H., Zon L. I. (2009) A genetic screen in zebrafish defines a hierarchical network of pathways required for hematopoietic stem cell emergence. Blood 113, 5776–5782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bukrinsky A., Griffin K. J., Zhao Y., Lin S., Banerjee U. (2009) Essential role of spi-1-like (spi-1l) in zebrafish myeloid cell differentiation. Blood 113, 2038–2046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao L., Li D., Ma K., Zhang W., Xu T., Fu C., Jing C., Jia X., Wu S., Sun X., Dong M., Deng M., Chen Y., Zhu W., Peng J., Wan F., Zhou Y., Zon L. I., Pan W. (2015) TopBP1 governs hematopoietic stem/progenitor cells survival in zebrafish definitive hematopoiesis. PLoS Genet. 11, e1005346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larson J. D., Wadman S. A., Chen E., Kerley L., Clark K. J., Eide M., Lippert S., Nasevicius A., Ekker S. C., Hackett P. B., Essner J. J. (2004) Expression of VE-cadherin in zebrafish embryos: a new tool to evaluate vascular development. Dev. Dyn. 231, 204–213 [DOI] [PubMed] [Google Scholar]
- 30.Gore A. V., Monzo K., Cha Y. R., Pan W., Weinstein B. M. (2012) Vascular Development in the Zebrafish, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, USA: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang H. U., Chen Z. F., Anderson D. J. (1998) Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell 93, 741–753 [DOI] [PubMed] [Google Scholar]
- 32.Aitsebaomo J., Portbury A. L., Schisler J. C., Patterson C. (2008) Brothers and sisters: molecular insights into arterial-venous heterogeneity. Circ. Res. 103, 929–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsu C. H., Lin J. S., Po Lai K., Li J. W., Chan T. F., You M. S., Tse W. K., Jiang Y. J. (2015) A new mib allele with a chromosomal deletion covering foxc1a exhibits anterior somite specification defect. Sci. Rep. 5, 10673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Butko E., Distel M., Pouget C., Weijts B., Kobayashi I., Ng K., Mosimann C., Poulain F. E., McPherson A., Ni C. W., Stachura D. L., Del Cid N., Espín-Palazón R., Lawson N. D., Dorsky R., Clements W. K., Traver D. (2015) Gata2b is a restricted early regulator of hemogenic endothelium in the zebrafish embryo. Development 142, 1050–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uribe R. A., Bronner M. E. (2015) Meis3 is required for neural crest invasion of the gut during zebrafish enteric nervous system development. Mol. Biol. Cell 26, 3728–3740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Groves J. A., Hammond C. L., Hughes S. M. (2005) Fgf8 drives myogenic progression of a novel lateral fast muscle fibre population in zebrafish. Development 132, 4211–4222 [DOI] [PubMed] [Google Scholar]
- 37.Juntilla M. M., Patil V. D., Calamito M., Joshi R. P., Birnbaum M. J., Koretzky G. A. (2010) AKT1 and AKT2 maintain hematopoietic stem cell function by regulating reactive oxygen species. Blood 115, 4030–4038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cornejo M. G., Mabialah V., Sykes S. M., Khandan T., Lo Celso C., Lopez C. K., Rivera-Muñoz P., Rameau P., Tothova Z., Aster J. C., DePinho R. A., Scadden D. T., Gilliland D. G., Mercher T. (2011) Crosstalk between NOTCH and AKT signaling during murine megakaryocyte lineage specification. Blood 118, 1264–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khurana S., Margamuljana L., Joseph C., Schouteden S., Buckley S. M., Verfaillie C. M. (2013) Glypican-3-mediated inhibition of CD26 by TFPI: a novel mechanism in hematopoietic stem cell homing and maintenance. Blood 121, 2587–2595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Broxmeyer H. E., Hoggatt J., O’Leary H. A., Mantel C., Chitteti B. R., Cooper S., Messina-Graham S., Hangoc G., Farag S., Rohrabaugh S. L., Ou X., Speth J., Pelus L. M., Srour E. F., Campbell T. B. (2012) Dipeptidylpeptidase 4 negatively regulates colony-stimulating factor activity and stress hematopoiesis. Nat. Med. 18, 1786–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stachura D. L., Svoboda O., Campbell C. A., Espín-Palazón R., Lau R. P., Zon L. I., Bartunek P., Traver D. (2013) The zebrafish granulocyte colony-stimulating factors (Gcsfs): 2 paralogous cytokines and their roles in hematopoietic development and maintenance. Blood 122, 3918–3928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hasegawa H., Takano H., Ohtsuka M., Ueda K., Niitsuma Y., Qin Y., Tadokoro H., Shiomi M., Komuro I. (2006) G-CSF prevents the progression of atherosclerosis and neointimal formation in rabbits. Biochem. Biophys. Res. Commun. 344, 370–376 [DOI] [PubMed] [Google Scholar]
- 43.Takai H., Miyoshi A., Yamazaki M., Adachi K., Katagiri K., Arakawa H., Katsuyama K., Ito T., Fujii E., Hayashi S., Kato A., Suzuki M. (2008) Granulocyte colony-stimulating factor has no adverse effects on atherosclerotic lesions in high cholesterol-fed miniature Swine. J. Vet. Med. Sci. 70, 943–950 [DOI] [PubMed] [Google Scholar]
- 44.Haghighat A., Weiss D., Whalin M. K., Cowan D. P., Taylor W. R. (2007) Granulocyte colony-stimulating factor and granulocyte macrophage colony-stimulating factor exacerbate atherosclerosis in apolipoprotein E-deficient mice. Circulation 115, 2049–2054 [DOI] [PubMed] [Google Scholar]
- 45.Hu Z., Zhang J., Guan A., Gong H., Yang M., Zhang G., Jia J., Ma H., Yang C., Ge J., Zou Y. (2013) Granulocyte colony-stimulating factor promotes atherosclerosis in high-fat diet rabbits. Int. J. Mol. Sci. 14, 4805–4816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chinetti-Gbaguidi G., Copin C., Derudas B., Vanhoutte J., Zawadzki C., Jude B., Haulon S., Pattou F., Marx N., Staels B. (2015) The coronary artery disease-associated gene C6ORF105 is expressed in human macrophages under the transcriptional control of PPARγ. FEBS Lett. 589, 461–466 [DOI] [PubMed] [Google Scholar]
- 47.Lancrin C., Sroczynska P., Stephenson C., Allen T., Kouskoff V., Lacaud G. (2009) The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature 457, 892–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eilken H. M., Nishikawa S., Schroeder T. (2009) Continuous single-cell imaging of blood generation from haemogenic endothelium. Nature 457, 896–900 [DOI] [PubMed] [Google Scholar]
- 49.Chen M. J., Yokomizo T., Zeigler B. M., Dzierzak E., Speck N. A. (2009) Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature 457, 887–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bertrand J. Y., Chi N. C., Santoso B., Teng S., Stainier D. Y., Traver D. (2010) Haematopoietic stem cells derive directly from aortic endothelium during development. Nature 464, 108–111 [DOI] [PMC free article] [PubMed] [Google Scholar]