Abstract
Signaling via the transient receptor potential (TRP) ion channel C6 plays a pivotal role in hereditary and sporadic glomerular kidney disease. Several studies have identified gain-of-function mutations of TRPC6 and report induced expression and enhanced channel activity of TRPC6 in association with glomerular diseases. Interfering with TRPC6 activity may open novel therapeutic pathways. TRPC6 channel activity is controlled by protein expression and stability as well as intracellular trafficking. Identification of regulatory phosphorylation sites in TRPC6 and corresponding protein kinases is essential to understand the regulation of TRPC6 activity and may result in future therapeutic strategies. In this study, an unbiased phosphoproteomic screen of human TRPC6 identified several novel serine phosphorylation sites. The phosphorylation site at serine 14 of TRPC6 is embedded in a basophilic kinase motif that is highly conserved across species. We confirmed serine 14 as a target of MAPKs and proline-directed kinases like cyclin-dependent kinase 5 (Cdk5) in cell-based as well as in vitro kinase assays and quantitative phosphoproteomic analysis of TRPC6. Phosphorylation of TRPC6 at serine 14 enhances channel conductance by boosting membrane expression of TRPC6, whereas protein stability and multimerization of TRPC6 are not altered, making serine 14 phosphorylation a potential drug target to interfere with TRPC6 channel activity.—Hagmann, H., Mangold, N., Rinschen, M. M., Koenig, T., Kunzelmann, K., Schermer, B., Benzing, T., Brinkkoetter, P. T. Proline-dependent and basophilic kinases phosphorylate human TRPC6 at serine 14 to control channel activity through increased membrane expression.
Keywords: MAPK, cyclin-dependent kinase 5, proteinuria, podocyte, glomerular disease
Canonical transient receptor potential ion channels are widely expressed nonselective Ca2+ channels (1). Their activation leads to depolarization of the cell membrane, triggering activation of other plasma membrane or intracellular ion channels (2, 3). Consistently, transient receptor potential (TRP) ion channel C6 plays an essential role in regulating cytosolic free Ca2+ levels and in initiating or amplifying a multitude of secondary messengers (1). TRPC6 belongs to the family of channels that assemble to tetrameric structures of homo- or heteromers (4). Accordingly, TRPC6 coassembles with TRPC3 and TRPC7. Coassembling with TRPCs and multimerization requires 4 ankyrin repeats and 1 coiled-coil domain in the N terminus as well as 1 coiled-coil domain in the C terminus of TRPC6 (1, 4).
Signaling via TRPC6 has been associated with human diseases, such as cardiac hypertrophy, depression, and anxiety disorders, and it plays a pivotal role in the development of proteinuric kidney disease (1, 5–8). Hyperactivity of TRPC6 due to gain-of-function mutations that increase opening probability or membrane abundance of the channel protein causes functional impairment and loss of glomerular visceral epithelial cells, called podocytes. This process finally results in the loss of protein in the urine (proteinuria) and renal failure with the histologic picture of hereditary focal and segmental glomerulosclerosis, i.e., glomerular scarring (9, 10). In addition, TRPC6 is induced and hyperactive in sporadic glomerular diseases such as membranous nephropathy and diabetic nephropathy (11–13). Meanwhile, it is well accepted that interfering with TRPC6 activity may be a causal therapeutic strategy in glomerular disease (14–16). Nevertheless, no leverage point for treatment has yet been identified in TRPC6 (17, 18).
In recent years, phosphorylation-dependent regulation of TRPC6 channel activity has earned more attention (19–21). Phosphorylation at serine 448 and threonine 69 inhibits TRPC6 activity, making these sites less ideal targets for therapeutic interventions (19, 20). The activating phosphorylation at tyrosine 284 leads to enhanced trafficking to the plasma membrane. However, simultaneously, membrane abundance is reduced via phosphorylation-dependent interaction with regulatory proteins (21). Identification of additional phosphorylation-dependent molecular switches in TRPC6 and understanding their role in TRPC6 regulation will eventually open new gateways toward targeted therapy in TRPC6-associated diseases.
MATERIALS AND METHODS
Cell culture, transfection, and cell treatment
HEK 293T and HeLa cells were grown under standard conditions at 37°C in 5% CO2 in DMEM (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (Sigma-Aldrich, St. Louis, MO, USA). Transfection was performed with cells of 60 to 80% confluence by using plasmid DNA using the calcium phosphate method for HEK 293T cells, or GeneJuice (Novagen, North Ryde, NSW, Australia) for HeLa cells according to the manufacturer’s instructions. Cultured human podocytes were grown under permissive conditions at 33°C. Transient transfection was achieved with Lipofectamine 2000 (Thermo Fisher Scientific) according to the manufacturer’s instructions. Briefly, cells were grown to 50% confluence on collagen-coated glass coverslips; Lipofectamine 2000 reagent and plasmid DNA were mixed in Opti-MEM medium and added to the cells. Hypertonic stress was applied through prewarmed PBS containing 582 mOsm/L sucrose 30 min before fixation. For MAPK inhibition, SB 203580 was added to culture medium at 5 µM. For protein stability assays, HEK 293T cells were transiently transfected as indicated and treated with 25 µg/ml cycloheximide (Sigma-Aldrich) for the indicated durations 48 h after transfection.
Phosphoproteomic profiling of HEK 293T cells expressing TRPC6
HEK 293T cells were transfected with TRPC6 wild-type (WT) cDNA, and cell lysates were generated as previously described (22). Peptides were reduced, alkylated, and digested with trypsin. Then peptides were desalted and fractionated using offline strong cation exchange chromatography (22). Fractions were desalted, and phosphopeptides were enriched using IMAC- FeNTA columns (22), and peptides were cleaned up using stage tips (23). Phosphopeptides were analyzed on an Orbitrap XL machine (Thermo Fisher Scientific) coupled to a nano-LC device with a 90 min gradient with a setup as previously described (24). Data were searched using the proteome discoverer 1.4 platform (Thermo Fisher Scientific) using the Sequest search algorithm. Search settings were as follows: minimum precursor mass, 350 Da; maximum precursor mass, 5000 Da; S/N threshold (FT only), 1.5; maximum missed cleavage sites, 2; precursor mass tolerance, 10 ppm; fragment mass tolerance, 0.8 Da; and consider only b and y ions. Dynamic modifications were phospho/ +79.966 Da (on S, T, Y) and oxidation/ +15.995 Da (on M), with 4 maximal allowed modifications. Static modification was carbamidomethyl/ +57.021 Da (on C). All data reported had a target false discovery rate of <0.01. PhosphoRS (25) was enabled and scores filtered (>0.75). The mass spectrometry proteomics data have been deposited in the ProteomeXchange Consortium via the Proteomics Identifications (PRIDE) (26, 27) partner repository (http://www.ebi.ac.uk/pride). The data set “Phosphoproteomic profiling of HEK 293T cells expressing TRPC6” was deposited under project accession number PXD005482.
Quantitative phosphoproteomic analysis of TRPC6 phosphorylation
HEK 293T cells were transfected with human TRPC6 WT, mouse TRPC6 WT, cyclin-dependent kinase 5 (CDK5) WT, and dominant-negative CDK5 (Cdk5-dn) cDNA as indicated. Subsequently, cells were lysed in lysis buffer containing 8 M urea, 100 mM ammonium bicarbonate, and 1× protease/phosphatase inhibitor (Thermo Fisher Scientific). Proteins were digested with trypsin and samples were desalted. Phosphopeptides were isolated by Fe-NTA IMAC columns, cleaned up using stage tips, and run on a LTQ Orbitrap XL with a 90-min gradient as previously described (24) or a QExactive mass spectrometer with a 2.5-h gradient as previously described (28). An MS1-based label-free quantification approach was used. Quantification of QExactive data was performed by MaxQuant 1.5 (29, 30) and searched against a human reference proteome database (UniProt, with no isoforms; www.uniprot.org). The search settings were default. Exceptions were LFQ option enabled, variable modification was phosphorylation, and match between run options was enabled. Quantification of LTQ Orbitrap MS1 data was carried out by Sequest and QUIL software as previously described (31). All quantification data for the phosphopeptide containing S14 were normalized and expressed as a fraction of 1.
Cell-based phosphorylation assay and coimmunoprecipitation
HEK 293T cells were transiently transfected with V5-tagged TRPC6 constructs and kinases as indicated. After incubation for 48 h, the cells were lysed in lysis buffer (10 mM Tris, pH 8.0, 150 mM NaCl, 50 mM NaF, 2% SDS in ddH2O) (all from Sigma-Aldrich) in the presence of protease inhibitors (Roche, Basel, Switzerland). Lysates were homogenized by sonication with a digital sonifier (Branson Ultrasonics, Danbury, CT, USA) and diluted (1:10) in dilution buffer containing 1% Triton X-100, 150 mM NaCl, 10 mM Tris, pH 8.0, and 2 mM EDTA before immunoprecipitation. TRPC6 protein was precipitated from the samples by using a specific antibody directed against the V5 or FLAG epitope tag on protein A–coated Sepharose beads. Samples were separated on SDS-PAGE, then protein blotted on PVDF membranes, followed by immunoblot analysis using specific antibodies detecting phosphorylated serine in a CDK motif (Cell Signaling Technology, Danvers, MA, USA), the V5 epitope tag (Bio-Rad, Hercules, CA, USA), the FLAG-epitope tag (Sigma-Aldrich), the hemagglutinin epitope tag (Covence, Princeton, NJ, USA), or the myc epitope tag (Cell Signaling Technology). Visualization of immunoblots was achieved with chemiluminescence.
Radioactive in vitro phosphorylation assay
V5-tagged TRPC6 constructs were expressed in HEK 293T cells. Cells were lysed and TRPC6 protein precipitated as previously described. Precipitates were transferred into 5× reaction buffer (40 mM MOPS, pH 7, 1 mM EDTA) with ATP (final concentration 0.1 mM) and 32P-ATP [ATP, (γ-32P)- 3000 Ci/mM, 5 mCi/ml; final concentration 0.2 mCi/ml] and recombinant Cdk5/p35 (EMD Millipore, Billerica, MA, USA) in a dilution of 1:25,000 was added. Samples were incubated for 30 min at 30°C. The reaction was stopped by adding SDS-PAGE loading buffer and boiling. Samples were separated on SDS-PAGE, gels were dried in a vacuum and heated gel dryer (Vacuubrand, Wertheim, Germany), and autoradiography detected on phosphor storage plates, which were imaged on a Typhoon imager (GE Healthcare Life Sciences, Little Chalfont, United Kingdom).
Whole-cell voltage clamp in Xenopus oocytes
Oocytes were collected from Xenopus laevis according to German regulations governing animal experiments. Oocytes were defolliculated for 1 h at 18°C with 1.5 mg/ml collagenase type V (Sigma-Aldrich) in OR2 solution (mM): 82.5 NaCl, 2 KCl, 1 MgCl2, and 5 HEPES pH 7.55. Oocytes were injected with 10 ng (47 nl double-distilled water) of cRNA encoding empty plasmid (mock vector), TRPC6, Cdk5, p53, and/or podocin. cRNA was transcribed in vitro after linearization using the appropriate promoter and RNA polymerase (T3, T7, or SP6). Oocytes were maintained in ND97 solution (mM): 96 NaCl, 2 KCl, 1.8 CaCl2, 1 MgCl2, 5 HEPES, 2.5 Na-pyruvate, 0.5 theophylline, and 0.01 mg/ml 1 gentamycin, pH 7.55 at 18°C. At 2 to 4 d after injection, membrane currents were measured by impaling 2 electrodes (Harvard Apparatus, Holliston, MA, USA) filled with 3 M KCl, which had a resistance of <1 MΩ. The bath was continuously perfused at a rate of 5 ml/min with ND96 solution (in mM): 96 NaCl, 2 KCl, 1.8 CaCl2, 1 MgCl2, 5 HEPES, and 2.5 Na-pyruvate, pH 7.55. The voltage drop across the serial resistance was optimized to 0 using 2 bath electrodes and a virtual ground head stage. Oocytes were continuously voltage clamped between −80 and +40 mV (oocyte clamp amplifier; Warner Instruments, Hamden, CT, USA) in steps of 10 mV, each for 1 s. All experiments were conducted at room temperature.
Velocity gradient centrifugation
A 2-ml discontinuous sucrose gradient (40–5%) was layered on top of a 60% sucrose cushion in an ultracentrifuge tube (Beckman Coulter, Brea, CA, USA). Cell lysates were generated as previously described and added on top of the gradient. Samples were subjected to centrifugation for 22 h at 215,000 g at 4°C in a Beckman Coulter SW-60Ti rotor. After centrifugation, 14 fractions (150 µl each) were collected and analyzed by SDS-PAGE. V5 epitope tag–specific polyclonal antibody (Bio-Rad) was used for immunoblotting.
Surface biotinylation assay
HEK 293T cells were grown in 6-well plates and transfected as indicated. The experiments were performed at 4°C. Cells were incubated with 1 mg/ml NHS-S-S-Biotin (Thermo Fisher Scientific), washed with buffer containing 100 mM glycine, and lysed in detergent buffer as previously described. Biotinylated protein was bound to streptavidin beads (Pierce, Rockford, IL, USA). After washing 3 times in detergent buffer, precipitates and lysates were analyzed by SDS-PAGE.
Immunohistochemistry and immunofluorescence
HeLa cells and human podocytes grown on collagen-coated coverslips were transfected with V5-tagged constructs as indicated using GeneJuice reagent or Lipofectamine 2000, respectively, and incubated for 24 h. Cells were then fixed in 4% paraformaldehyde, permeabilized using 0.1% Triton X-100 in PBS, and stained with V5 polyclonal antibody (Bio-Rad) diluted in 1% bovine serum albumin–PBS for 1 to 2 h at room temperature. Cells were washed repeatedly with PBS, incubated with fluorescence-labeled secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA, USA), and mounted in Prolong Gold antifade containing DAPI (Thermo Fisher Scientific). Images were acquired by an LSM 710/Axiobserver Z1 confocal microscope operated by ZEN 2009 software (Carl Zeiss, Jena, Germany).
RESULTS
Phosphoproteomics screen identifies novel basophilic and proline-directed phosphorylation sites in human TRPC6
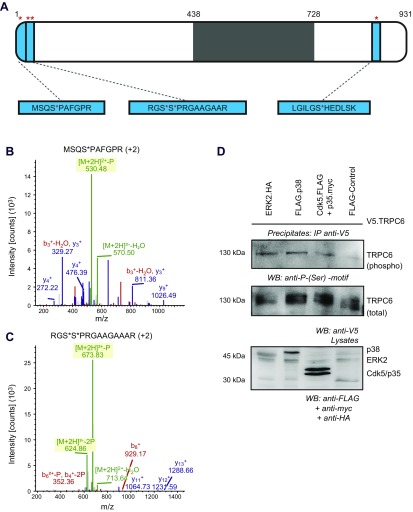
To identify novel phosphorylation sites in human TRPC6, we transiently transfected untreated HEK 293T cells with untagged human TRPC6 cDNA. After solubilization in urea buffer and purification of phosphopeptides from whole-cell lysates, analysis by mass spectrometry revealed 2241 phosphopeptides in total. Among these were a number of novel as well as previously published TRPC6 phosphorylation sites. Exemplarily, serine 814 was phosphorylated in our sample, as previously published (32). As novel phosphorylation sites, serine 4 and the double-phosphorylation site at serines 13 and 14 were identified (Fig. 1A–C and Supplemental Fig. 1). Serines 4 and 14 are followed by a proline residue. As surrounding residues of a phosphosite determine substrate specificity, the proline in +1 is suggestive of phosphorylation by proline-directed kinases. In addition, the motif around serines 13 and 14 is basophilic, pointing toward recognition of the motif by various basophilic protein kinases. Consistently, prediction algorithms estimate MAPK as well as cyclin-dependent kinases as crucial kinases for phosphorylation at serine 4 and 14 (Supplemental Fig. 2) (33). To analyze candidate kinases that could induce TRPC6 phosphorylation, HEK 293T cells were transiently transfected with V5-tagged human TRPC6 and either ERK kinase, p38-MAPK, Cdk5 together with its activator p35, or control protein. After purification with a V5-specific antibody, immunoblot of the precipitates was performed using a specific antibody recognizing the phosphorylated serine motif. Immunoreactivity at the size of TRPC6 in samples containing ERK kinase, p38-MAPK, or Cdk5/p35 represented TRPC6 phosphorylation (Fig. 1D), whereas only a weak signal was detected when TRPC6 was coexpressed with control protein.
Figure 1.
Identification of novel serine phosphosites in TRPC6-MAPK and Cdk5 phosphorylate TRPC6 at serine residues. A) Human TRPC6 was transiently expressed in HEK 293T cells. After purification of phosphopeptides from crude cell lysates, liquid chromatographic tandem mass spectrometry analysis identified novel phosphorylation sites. Serine residues in positions 4, 13/14, and 814 (red asterisks) were identified as phosphorylation targets. Schematic shows TRPC6 and phosphopeptides (blue). Transmembrane domains and intra- or extracellular loops are shown in gray. B, C) Mass-to-charge ratio spectra of serine 4 and 13/14 phosphorylation sites. Green, peaks with phosphorylation loss; red, b ions; blue, y ions. D) HEK 293T cells were transiently transfected as indicated. After immunoprecipitation of V5.TRPC6, immunoblot analysis was performed with phospho-serine-specific motif antibody, showing serine phosphorylation of TRPC6 in presence of ERK, p38, and Cdk5/p35. Restaining was performed with V5-specific antibody.
Atypical cyclin-dependent kinase 5 phosphorylates human TRPC6 at serine 14
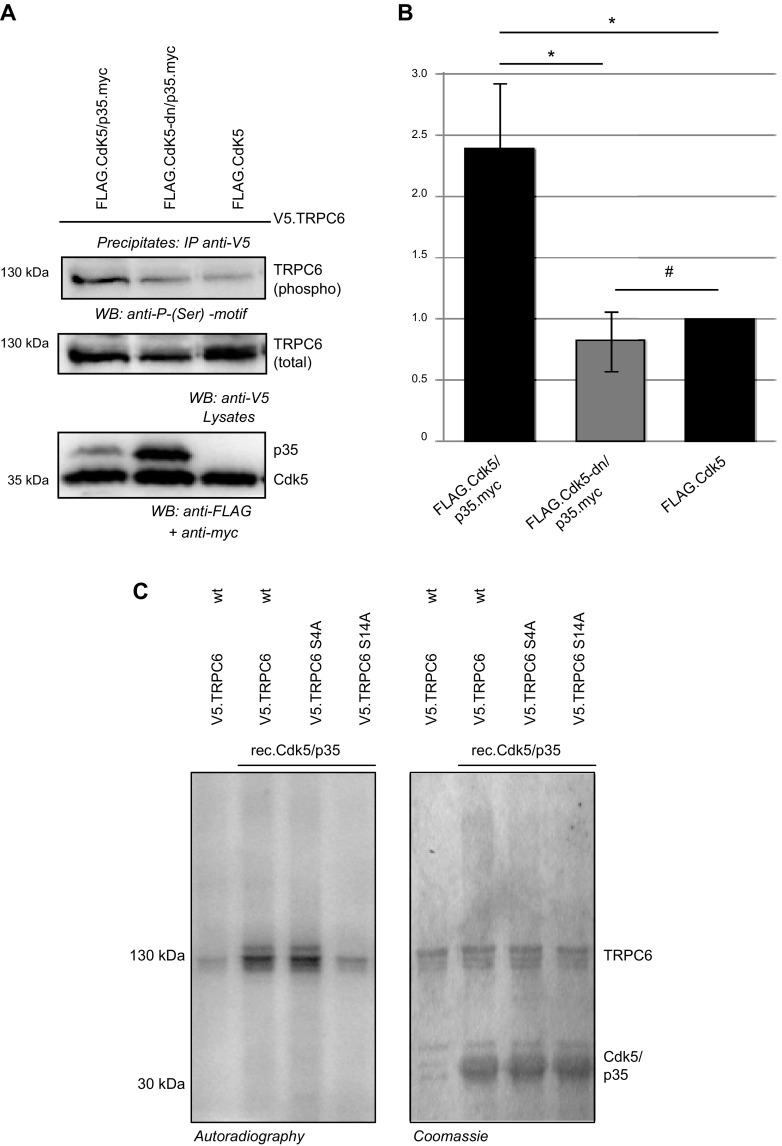
In contrast to MAPKs, Cdk5 with its specific activator p35 is not expressed in cultured cell lines such as HEK 293T cells. To ease experimental design, Cdk5-driven phosphorylation of TRPC6 was studied exemplarily in cell culture experiments and in vitro kinase assays. HEK 293T cells were transiently transfected with human TRPC6 and Cdk5/p35 or human TRPC6 and dominant-negative Cdk5/p35 (Cdk5-dn/p35). After purification of TRPC6 from cell lysates via immunoprecipitation, immunoblot analysis using a specific antibody recognizing phosphorylated CDK substrates revealed predominant immunoreactivity in precipitates of cells transfected with the WT form of Cdk5 together with its activator p35 (Fig. 2A, B).
Figure 2.
Cdk5/p35 phosphorylates TRPC6 at serine 14. A) HEK 293T cells were transiently transfected as indicated. After immunoprecipitation for V5.TRPC6, immunoblot analysis was performed with phospho-serine-specific motif antibody, showing TRPC6 serine phosphorylation only in presence of WT Cdk5/p35. B) Densitometry of phospho-serine motif immunoblot normalized to Cdk5 WT transfected cells detects significantly more phosphorylation in Cdk5/p35 transfected cells compared to Cdk5-dn/p35 and Cdk5 WT alone (n = 3; Student’s t test). #P > 0.05 (not significant); *P < 0.05. C) TRPC6 WT and phospho-ablating mutants of TRPC6 were analyzed in radioactive in vitro kinase assay. TRPC6 WT (WT), TRPC6 S4A, or TRPC6 S14A (all V5 tagged) were expressed in HEK 293T cells and precipitated from cell lysates. After in vitro addition of recombinant Cdk5/p35 and 32P-labeled ATP to immunoprecipitates of TRPC6, phosphorylation of TRPC6 was detected by autoradiography after SDS-PAGE. TRPC6 phosphorylation is abrogated when serine 14 is replaced by alanine. Coomassie blue–stained gel served as loading control.
To dissect the specific target residue of Cdk5-driven phosphorylation, TRPC6 phospho mutants were generated by replacing serine 4 or serine 14 by alanine via site-directed mutagenesis to abrogate phosphorylation. The respective mutant proteins were purified by immunoprecipitation after expression in HEK 293T cells. In vitro phosphorylation after addition of recombinant Cdk5/p35 and 32P-labeled ATP was abrogated in the TRPC6 S14A mutant, whereas TRPC6 WT and TRPC6 S4A showed stable phosphorylation as detected by autoradiography. Balanced input was verified by Coomassie blue staining of the SDS-PAGE (Fig. 2C).
Serine 14 of TRPC6 is highly conserved across species
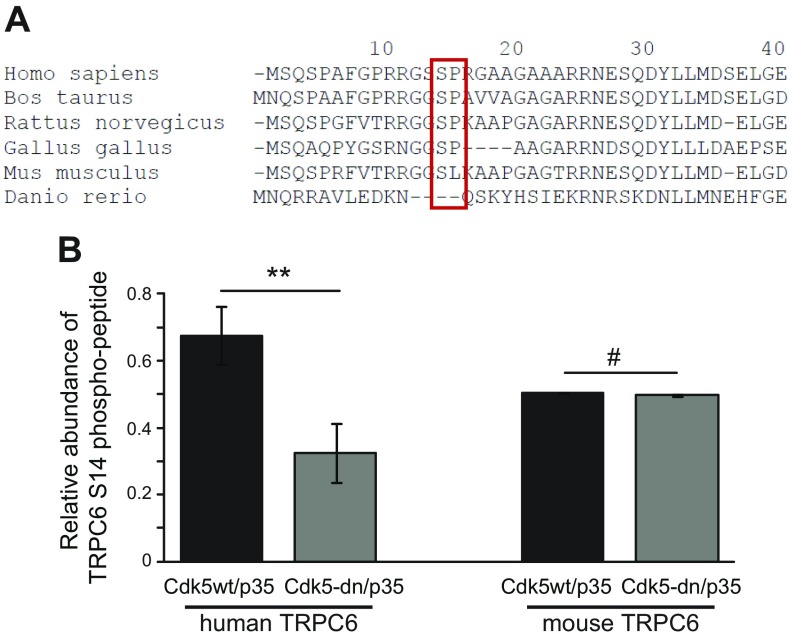
The serine at position 14 in the human amino acid sequence is highly conserved across mammalian species and is even found in birds (Fig. 3A). In addition, the kinase motif featuring a proline residue in position +1 is also well conserved in most species, with the exception of Mus musculus. Interestingly, in the mouse, the proline is replaced by leucine, thereby potentially shifting kinase preference at this site (34).
Figure 3.
TRPC6 phosphorylation site at serine 14 is conserved across species. A) TRPC6 protein sequence was analyzed by CLUSTAL-O algorithm. Sequence analysis and alignment of TRPC6 reveals high conservation of domain around serine 14 across species. Proline at +1 position is not conserved in mice. B) HEK 293T cells expressing human or mouse TRPC6 and active Cdk5/p35 or Cdk5-dn/p35 were subjected to quantitative phosphoproteomics. Abundance of phospho-S14 TRPC6 is plotted for cells expressing active Cdk5/p35 compared to dominant-negative Cdk5/p35 (n = 3; Student’s t test). #P > 0.05 (not significant); **P < 0.01.
To analyze Cdk5-driven phosphorylation of human and mouse TRPC6 at serine 14, phosphoproteomic analysis was performed to quantify abundance of TRPC6 phospho-S14 peptides from lysates of HEK 293T cells expressing human or mouse TRPC6 and Cdk5/p35 or Cdk5-dn/p35. For human TRPC6, phosphorylated serine 14 was highly abundant in the lysates containing active Cdk5/p35. Significantly less phosphorylation of serine 14 of human TRPC6 was found in cells expressing Cdk5-dn/p35 (Fig. 3B). For mouse TRPC6, which is lacking the proline residue in +1, quantitative phosphoproteomic analysis using mouse TRPC6 coexpressed with WT Cdk5/p35 or Cdk5-dn/p35 showed no difference in the relative abundance of phospho-S14 peptides. This indicates that mouse TRPC6 is not a phosphorylation substrate of Cdk5/p35.
Phosphorylation of human TRPC6 at serine 14 regulates channel activity
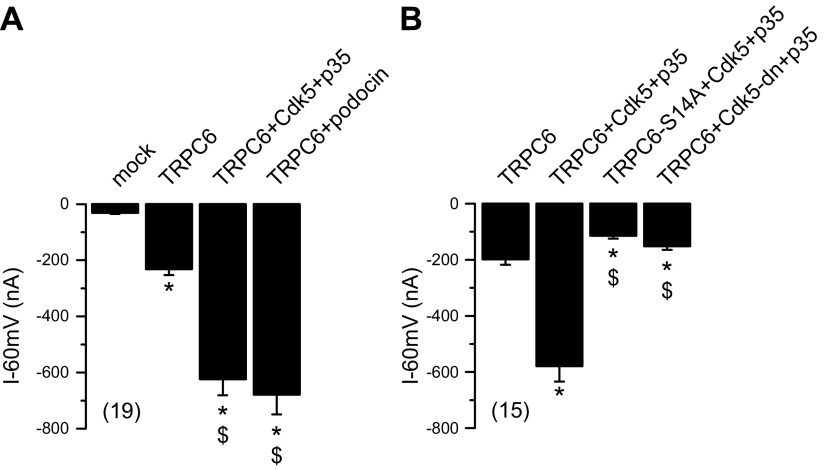
The mechanisms underlying activation and regulation of TRPC6 channels are still not fully understood (1). To investigate the channel activity of TRPC6, double-electrode voltage-clamp experiments were conducted in Xenopus oocytes. Expression of TRPC6 and exposure to 10 µM 1-oleoyl-2-acetyl-sn-glycerol (OAG) induced a negative (inward) membrane current (Fig. 4A) that was inhibited by removal of extracellular Na+ (data not shown). OAG had no effect on mock-injected oocytes. Coexpression of TRPC6 with WT Cdk5 and p35 enhanced whole-cell currents generated by TRPC6 (Fig. 4A). Whole-cell currents in the presence of Cdk5/p35 reached levels similar to those obtained by coexpression of TRPC6 with podocin, as previously reported (35). The enhanced activity of TRPC6 upon coexpression of Cdk5/p35 was shown to depend on phosphorylation at serine 14: activation of whole-cell currents by Cdk5/p35 was not seen in the presence of the phospho-deficient mutant TRPC6 S14A (Fig. 4B). Moreover, coexpression of Cdk5-dn/p35 inhibited augmentation of TRPC6 currents. No whole-cell currents were induced by Cdk5/p35 in the absence of TRPC6 (data not shown).
Figure 4.
Phosphorylation of TRPC6 at serine 14 modulates channel activity. TRPC6 channel activity was assessed in double-electrode voltage-clamp experiments in Xenopus oocytes after stimulation with 10 µM OAG. Coexpression of Cdk5 WT and p35 leads to enhanced conductance of TRPC6 channel. A) Summary of whole-cell currents measured at clamp voltage Vc = −60 mV. TRPC6 induced inward current that was augmented in presence of Cdk5/p35, similar to coexpression with podocin. OAG had no effect on mock-injected oocytes. B) Summaries of effects of coexpression with phospho-deficient mutant TRPC6-S14A or Cdk5-dn. Data are presented as means ± sem; values in parentheses indicate number of experiments. *P < 0.05 vs. mock vector (A) or TRPC6 (B) injection (ANOVA); $P < 0.05 vs. TRPC6 (A) or TRPC6 + Cdk5 + p35 (B) (ANOVA).
Phosphorylation at serine 14 enhances membrane expression of human TRPC6 without affecting stability and multimerization
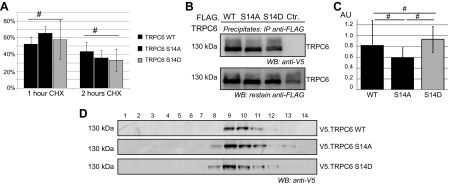
TRPC6 conductance is affected by intracellular trafficking, protein stability, and multimerization of TRPC subunits. To elucidate the mechanism underlying the enhanced TRPC6 conductance after serine 14 phosphorylation, protein stability, membrane abundance, and multimerization were analyzed in cell culture studies. In addition to the phospho-deficient mutant TRPC6 S14A, a phospho-mimicking mutant, TRPC6 S14D, was generated by site-directed mutagenesis. Then protein decay was studied in cells expressing TRPC6 WT, S14D, and S14A using cycloheximide assays (c = 25 µg/ml, 1 and 2 h). There was no significant difference in the intensity of the bands comparing the different time points normalized to baseline expression levels. This indicates equal protein turnover in the WT TRPC6 and the phospho mutants of TRPC6 (Fig. 5A). Because multimerization controls TRPC6 activity (4) and may be regulated by phosphorylation, we studied multimerization of TRPC6 subunits in response to phosphorylation at serine 14 by various experimental methods. First, homomeric protein interaction of TRPC6 was studied in cell culture in immunoprecipitation experiments. HEK 293T cells were transiently transfected with FLAG-tagged TRPC6 WT and cotransfected with V5.TRPC6 WT, V5.TRPC6 S14A, or V5.TRPC6 S14D. Pull-down for FLAG and immunoblot for V5 revealed equally strong reactivity in TRPC6 WT–, TRPC6 S14A– and TRPC6 S14D–containing samples (Fig. 5B, C), indicating equally affine interaction with TRPC6 WT. The experiment was also performed with V5 antibody for immunoprecipitation and yielded the same results (data not shown). In addition, complex size of native TRPC6 complexes containing V5-tagged TRPC6 WT, TRPC6 S14A, or TRPC6 S14D was studied by timed ultracentrifugation in a sucrose gradient. Immunoblot for V5 after velocity gradient ultracentrifugation detected TRPC6 immunoreactivity in fractions 8, 9, 10, and 11 in all samples, without any shift determined by the phospho-deficient or phospho-mimicking mutant (Fig. 5D).
Figure 5.
Phosphorylation at serine 14 does not alter protein stability or oligomerization characteristics of TRPC6. A) HEK 293T cells were transiently transfected with V5-tagged constructs as indicated and treated with 25 µg/ml cycloheximide for time indicated. Band density was quantified after immunoblot analysis of cell lysates with V5-specific antibody and normalized to untreated control. No significant difference in protein stability was detected for TRPC6 WT and phospho mutants (n = 3; Student’s t test). B) Multimerization of TRPC6 was assessed in coimmunoprecipitation experiments. FLAG- and V5-tagged TRPC6 cDNA constructs were transiently expressed in HEK 293T cells as indicated. After immunoprecipitation with FLAG-specific antibody, immunoblotting was performed with V5-specific antibody and restaining of precipitates with FLAG-specific antibody. C) Densitometry quantification of V5 immunoblot shows no significant difference in multimerization for TRPC6 WT and phospho mutants (AU, arbitrary units; n = 3; Student’s t test). D) To investigate complex size of native TRPC6 multimers, HEK 293T cells were transiently transfected with V5-tagged TRPC6 cDNA constructs as indicated, and cell lysates were subjected to velocity gradient centrifugation. Analysis of fractions on immunoblot using V5-specific antibody shows similar sedimentation pattern of TRPC6 WT and TRPC6 S14A and S14D (n = 3). #P > 0.05 (not significant).
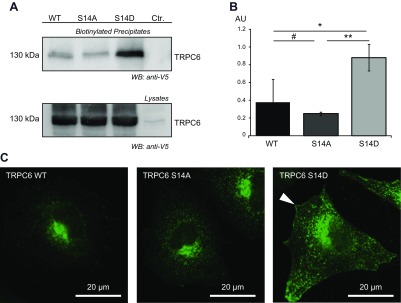
To explain the enhanced conductance of TRPC6 in response to phosphorylation at serine 14 seen in the voltage-clamp experiments, we next investigated the membrane abundance of TRPC6 in surface biotinylation assays. V5-tagged TRPC6 constructs were transiently expressed, and cells were subjected to surface biotinylation before lysis. Biotinylated proteins were precipitated by streptavidin-mediated pull-down. After separation on SDS-PAGE and immunoblot for V5, significantly more immunoreactivity was found in the precipitate fraction of phospho-mimicking TRPC6 S14D, indicating the presence of more TRPC6 S14D in the cell membrane (Fig. 6A, B). In addition, HeLa cells were transiently transfected with TRPC6 WT, TRPC6 S14A, or TRPC6 S14D, all V5 tagged. Immunofluorescence staining using V5-specific antibody resulted in a strong membrane staining in V5.TRPC6 S14D-transfected cells, whereas cells expressing V5.TRPC6 WT or V5.TRPC6 S14A showed accumulation of immunoreactivity in intracellular organelles, especially the endoplasmic reticulum (Fig. 6C).
Figure 6.
Phosphorylation of TRPC6 at serine 14 affects surface expression of TRPC6. A) HEK 293T cells were transiently transfected as indicated. After surface biotinylation, cell lysis and streptavidin precipitation immunoblot analysis were performed using V5-specific antibody. B) Densitometry of V5 immunoblot of biotinylated precipitates detects significantly more TRPC6 protein for phospho-mimicking mutant S14D (AU, arbitrary units; n = 3; t test). #P > 0.05 (not significant); *P < 0.05, **P < 0.01. C) HeLa cells transiently expressing V5-tagged TRPC6 cDNA constructs as indicated were stained with V5-specific antibody. At equal exposure times, more prominent immunoreactivity for V5 was found in plasma membrane of cells expressing TRPC6 S14D (white arrowhead).
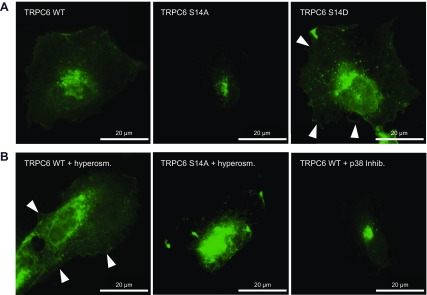
In cultured human podocytes, transient expression of V5-tagged TRPC6 constructs and immunofluorescence staining with a V5-specific antibody revealed the same subcellular distribution of TRPC6 S14A and S14D as seen in HeLa cells. V5.TRPC6 S14D was localizing to the plasma membrane, and accumulation of V5.TRPC6 S14A was identified in the endoplasmic reticulum (Fig. 7A). Activation of endogenous MAPK/p38 in human podocytes can be achieved by hyperosmotic stress (Supplemental Fig. 3). Human podocytes transiently expressing V5.TRPC6 WT or V5.TRPC6 S14A were incubated in hyperosmolar solution for 30 min before fixation and staining with V5-specific antibody. The staining pattern in cells expressing WT TRPC6 recapitulated the plasma membrane distribution of the phospho-mimicking TRPC6 S14D mutant, whereas activation of MAPK/p38 did not significantly alter the subcellular distribution of TRPC6 S14A (Fig. 7B). Interestingly, V5.TRPC6 WT appeared more dispersed in human podocytes compared to the staining pattern seen in HeLa cells (Fig. 7A). After inhibition of MAPK activation with SB 203580 after transfection of V5.TRPC6 WT, immunoreactivity was again found restricted to the endoplasmic reticulum (Fig. 7B). The dispersed staining pattern of V5.TRPC6 WT in cultured human podocytes likely reflects activation of endogenous p38 in response to transfection reagents.
Figure 7.
TRPC6 phosphorylation at serine 14 affects plasma membrane abundance in cultured human podocytes. A) Human podocytes transiently expressing V5-tagged TRPC6 cDNA constructs as indicated were stained with V5-specific antibody. Strong immunoreactivity for V5 is found in plasma membrane of cells expressing TRPC6 S14D (white arrowhead), whereas for TRPC6 S14A perinuclear accumulation is detected at equal exposure times. B) After hypertonic stress, V5.TRPC6 WT prominently localizes in plasma membrane of human podocytes. In contrast, V5.TRPC6 S14A reactivity is mostly restricted to endoplasmic reticulum. Treatment of V5.TRPC6 WT expressing human podocytes with MAPK/p38 inhibitor SB 203580 abrogates plasma membrane localization. Lysates separated in SDS-PAGE were analyzed by immunoblot with phospho-p38 and total-p38 specific antibody.
DISCUSSION
TRPC6 activity plays a pivotal role in the development of glomerular disease. Several gain-of-function mutations of TRPC6 have been identified to cause late-onset focal segmental glomerulosclerosis (9, 10, 36–38). This suggests that a chronic calcium load is detrimental to podocytes, causing podocyte detachment from the glomerular basement membrane, podocyte loss in the urine, or apoptosis (39–41). Meanwhile, compelling data exist for a role of enhanced TRPC6 activity or induced expression of TRPC6 in sporadic disease, such as membranous nephropathy, minimal change glomerulopathy, and diabetic nephropathy (12, 42, 43). In the rat model of streptozotocin-induced diabetic nephropathy and in the mouse model of doxorubicin-induced nephropathy, TRPC6 expression levels and basal intracellular calcium concentrations were higher in treated animals compared to control (12, 44). Furthermore, the TRPC6 channel open probability was increased more than 5-fold in patch-clamp experiments of glomerular podocytes in response to TRPC6 activation by angiotensin II (45). Complementary TRPC6-knockout mice were protected from angiotensin II–induced proteinuria (46). In addition, overexpression of WT or disease causing TRPC6 variants in mice was sufficient to cause low-grade proteinuria (47). Early on, a therapeutic benefit of direct inhibition of TRPC6 was assumed (48). However, development of agents blocking the channel function of TRPC6 was not sufficiently fruitful as a result of a lack of specificity and bioavailability of inhibitors affecting the channel pore (49–51). A better understanding of activation and regulation mechanisms of TRPC6 is warranted to tailor specific pharmacologic interventions. Interfering with phosphorylation events that regulate TRPC6 protein targeting and metabolism may be an aspect to future therapy.
The activation of MAPK and ERK1/2 in glomerular disease in podocytes is well established (52–56). Data from human kidney biopsy samples suggest a role for ERK and p38 activation in diabetic nephropathy in podocytes (57). It has been speculated that the activation of ERK may induce and activate TRPC6 and contribute to podocyte damage (58). However, an interaction of ERK or MAPK with TRPC6 has never been described. In addition to the widely expressed MAPKs, kinases specifically expressed in podocytes (and neurons) such as Cdk5 direct cell differentiation and protect from apoptosis in states of glomerular disease and may play a role in the modulation of TRPC6 (59–61).
In an unbiased phosphoproteomic screen, we identified a novel serine phosphorylation site in TRPC6 that is controlled by endogenous kinases such as MAPKs p38, ERK1/2, and proline-directed Cdk5. We confirmed phosphorylation of serine 14 of TRPC6 by MAPK and Cdk5 in cell-based and in vitro kinase assays, and in quantitative phosphoproteomics. The phosphorylation site at serine 14 is highly conserved across species.
In the past few years, the significance of phosphorylation at threonine, tyrosine, and serine residues in the regulation of TRPC6 channel activity has come into focus. Most identified phosphorylation sites inhibit TRPC6 channel activity: PKC isoforms were found to phosphorylate TRPC6 at serine 448, reducing channel conductance, possibly as a negative feedback mechanism (19, 62, 63). In addition, protein kinase G inhibits channel activity of TRPC6 by phosphorylation at threonine 69, which is protective in cardiac hypertrophy (20). In contrast, TRPC6 conductance is augmented by tyrosine phosphorylation at tyrosine 284 by Src kinases such as Fyn (64). Phosphorylation at tyrosine 284 of TRPC6 permits interaction with PLC γ1, which leads to recruitment of TRPC6 to the plasma membrane. At the same time, however, Y284-phosphorylated TRPC6 competitively binds to nephrin and other slit-diaphragm components, inhibiting surface expression of TRPC6 and reducing channel conductance (21).
Our data show that phosphorylation at serine 14 activates the TRPC6 channel in vitro and enhances channel conductance to an extent comparable to the augmentative effect of podocin on TRPC6 channel activity (35). The N-terminal domains of TRPC subunits play a role in the ability of the protein to oligomerize and affect cell surface expression (65, 66). We excluded an effect of serine 14 phosphorylation on multimerization and protein stability of TRPC6, and confirmed facilitated plasma membrane expression of the channel as the underlying mechanism of enhanced channel conductance after phosphorylation at serine 14. This mechanism was accurately recapitulated in cultured human podocytes after transient transfection of TRPC6 cDNA constructs.
While the serine 14 is conserved across species and also in mice, the proline in the +1 position is not. Hence, one might speculate whether this contributes to the observed difference in severity of some TRPC6-associated disease phenotypes between mice and humans. Exemplarily, transgenic animals expressing a disease-causingTRPC6 point mutation specifically in podocytes only present with mild proteinuria and minimal glomerular sclerosis while patients with a mutation in the homolog residue develop nephritic range proteinuria, focal and segmental glomerulosclerosis, and renal failure (9, 10, 47).
Our data suggest that phosphorylation at serine 14 in TRPC6 via MAPKs or Cdk5/p35 is a central pathway to the regulation of TRPC6 channel activity. It is tempting to speculate that this mechanism is relevant in human glomerular disease. However, because MAPKs operate a large number of pathways, and because Cdk5/p35 also refers protective signals in glomerular disease, blocking the superordinate kinases to serine 14 phosphorylation of TRPC6 will not be expedient. Instead, development of small molecules specifically blocking phosphorylation of TRPC6 serine 14 might be the key to exploiting this phosphorylation site as a therapeutic target.
ACKNOWLEDGMENTS
The authors thank A. Köser for technical support. This study was funded by the Köln Fortune Program of the University of Cologne to H.H. K.K. was supported by the Deutsche Forschungsgemeinschaft (SFB699A7). B.S. obtained funding from the Deutsche Forschungsgemeinschaft (SCHE1562/2 and 1562/6). T.B. received funding from the Deutsche Forschungsgemeinschaft (SFB635 and SFB829). P.T.B. received funding from the Deutsche Forschungsgemeinschaft (BR2955/4-1) and the Köln Fortune Program, University of Cologne. The authors declare no conflicts of interest.
Glossary
- Cdk5
cyclin-dependent kinase 5
- Cdk5-dn
dominant negative cyclin-dependent kinase 5
- OAG
1-oleoyl-2-acetyl-sn-glycerol
- TRP
transient receptor potential
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
H. Hagmann, M. M. Rinschen, and P. T. Brinkkoetter designed research; H. Hagmann, N. Mangold, M. M. Rinschen, T. Koenig, and K. Kunzelmann performed experiments; H. Hagmann, M. M. Rinschen, T. Koenig, K. Kunzelmann, B. Schermer, and P. T. Brinkkoetter analyzed data; and H. Hagmann, T. Benzing, and P. T. Brinkkoetter wrote the article.
REFERENCES
- 1.Dryer S. E., Reiser J. (2010) TRPC6 channels and their binding partners in podocytes: role in glomerular filtration and pathophysiology. Am. J. Physiol. Renal Physiol. 299, F689–F701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abramowitz J., Birnbaumer L. (2009) Physiology and pathophysiology of canonical transient receptor potential channels. FASEB J. 23, 297–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nilius B., Owsianik G., Voets T., Peters J. A. (2007) Transient receptor potential cation channels in disease. Physiol. Rev. 87, 165–217 [DOI] [PubMed] [Google Scholar]
- 4.Lepage P. K., Lussier M. P., Barajas-Martinez H., Bousquet S. M., Blanchard A. P., Francoeur N., Dumaine R., Boulay G. (2006) Identification of two domains involved in the assembly of transient receptor potential canonical channels. J. Biol. Chem. 281, 30356–30364 [DOI] [PubMed] [Google Scholar]
- 5.Kuwahara K., Wang Y., McAnally J., Richardson J. A., Bassel-Duby R., Hill J. A., Olson E. N. (2006) TRPC6 fulfills a calcineurin signaling circuit during pathologic cardiac remodeling. J. Clin. Invest. 116, 3114–3126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Onohara N., Nishida M., Inoue R., Kobayashi H., Sumimoto H., Sato Y., Mori Y., Nagao T., Kurose H. (2006) TRPC3 and TRPC6 are essential for angiotensin II–induced cardiac hypertrophy. EMBO J. 25, 5305–5316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leuner K., Kazanski V., Müller M., Essin K., Henke B., Gollasch M., Harteneck C., Müller W. E. (2007) Hyperforin—a key constituent of St. John’s wort specifically activates TRPC6 channels. FASEB J. 21, 4101–4111 [DOI] [PubMed] [Google Scholar]
- 8.Liu Y., Liu C., Qin X., Zhu M., Yang Z. (2015) The change of spatial cognition ability in depression rat model and the possible association with down-regulated protein expression of TRPC6. Behav. Brain Res. 294, 186–193 [DOI] [PubMed] [Google Scholar]
- 9.Winn M. P., Conlon P. J., Lynn K. L., Farrington M. K., Creazzo T., Hawkins A. F., Daskalakis N., Kwan S. Y., Ebersviller S., Burchette J. L., Pericak-Vance M. A., Howell D. N., Vance J. M., Rosenberg P. B. (2005) A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science 308, 1801–1804 [DOI] [PubMed] [Google Scholar]
- 10.Reiser J., Polu K. R., Möller C. C., Kenlan P., Altintas M. M., Wei C., Faul C., Herbert S., Villegas I., Avila-Casado C., McGee M., Sugimoto H., Brown D., Kalluri R., Mundel P., Smith P. L., Clapham D. E., Pollak M. R. (2005) TRPC6 is a glomerular slit diaphragm–associated channel required for normal renal function. Nat. Genet. 37, 739–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Möller C. C., Wei C., Altintas M. M., Li J., Greka A., Ohse T., Pippin J. W., Rastaldi M. P., Wawersik S., Schiavi S., Henger A., Kretzler M., Shankland S. J., Reiser J. (2007) Induction of TRPC6 channel in acquired forms of proteinuric kidney disease. J. Am. Soc. Nephrol. 18, 29–36 [DOI] [PubMed] [Google Scholar]
- 12.Ilatovskaya D. V., Levchenko V., Lowing A., Shuyskiy L. S., Palygin O., Staruschenko A. (2015) Podocyte injury in diabetic nephropathy: implications of angiotensin II–dependent activation of TRPC channels. Sci. Rep. 5, 17637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu B.-C., Song X., Lu X.-Y., Li D. T., Eaton D. C., Shen B.-Z., Li X.-Q., Ma H.-P. (2013) High glucose induces podocyte apoptosis by stimulating TRPC6 via elevation of reactive oxygen species. Biochim. Biophys. Acta 1833, 1434–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ilatovskaya D. V., Staruschenko A. (2015) TRPC6 channel as an emerging determinant of the podocyte injury susceptibility in kidney diseases. Am. J. Physiol. Renal Physiol. 309, F393–F397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang H., You Y., Lin X., Tang C., Gu X., Huang M., Qin Y., Tan J., Huang F. (2017) Inhibition of TRPC6 signal pathway alleviates podocyte injury induced by TGF-β1. Cell. Physiol. Biochem. 41, 163–172 [DOI] [PubMed] [Google Scholar]
- 16.Wang L., Jirka G., Rosenberg P. B., Buckley A. F., Gomez J. A., Fields T. A., Winn M. P., Spurney R. F. (2015) Gq signaling causes glomerular injury by activating TRPC6. J. Clin. Invest. 125, 1913–1926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pavenstädt H., Kriz W., Kretzler M. (2003) Cell biology of the glomerular podocyte. Physiol. Rev. 83, 253–307 [DOI] [PubMed] [Google Scholar]
- 18. Elger M., Kriz W. (1998) Podocytes and the development of segmental glomerulosclerosis. Nephrol. Dial. Transplant. 13, 1368–1373 [DOI] [PubMed] [Google Scholar]
- 19.Bousquet S. M., Monet M., Boulay G. (2010) Protein kinase C–dependent phosphorylation of transient receptor potential canonical 6 (TRPC6) on serine 448 causes channel inhibition. J. Biol. Chem. 285, 40534–40543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishida M., Watanabe K., Sato Y., Nakaya M., Kitajima N., Ide T., Inoue R., Kurose H. (2010) Phosphorylation of TRPC6 channels at Thr69 is required for anti-hypertrophic effects of phosphodiesterase 5 inhibition. J. Biol. Chem. 285, 13244–13253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanda S., Harita Y., Shibagaki Y., Sekine T., Igarashi T., Inoue T., Hattori S. (2011) Tyrosine phosphorylation–dependent activation of TRPC6 regulated by PLC-γ1 and nephrin: effect of mutations associated with focal segmental glomerulosclerosis. Mol. Biol. Cell 22, 1824–1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rinschen M. M., Pahmeyer C., Pisitkun T., Schnell N., Wu X., Maaß M., Bartram M. P., Lamkemeyer T., Schermer B., Benzing T., Brinkkoetter P. T. (2015) Comparative phosphoproteomic analysis of mammalian glomeruli reveals conserved podocin C-terminal phosphorylation as a determinant of slit diaphragm complex architecture. Proteomics 15, 1326–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rappsilber J., Mann M., Ishihama Y. (2007) Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat. Protoc. 2, 1896–1906 [DOI] [PubMed] [Google Scholar]
- 24.Kohli P., Bartram M. P., Habbig S., Pahmeyer C., Lamkemeyer T., Benzing T., Schermer B., Rinschen M. M. (2014) Label-free quantitative proteomic analysis of the YAP/TAZ interactome. Am. J. Physiol. Cell Physiol. 306, C805–C818 [DOI] [PubMed] [Google Scholar]
- 25.Taus T., Köcher T., Pichler P., Paschke C., Schmidt A., Henrich C., Mechtler K. (2011) Universal and confident phosphorylation site localization using phosphoRS. J. Proteome Res. 10, 5354–5362 [DOI] [PubMed] [Google Scholar]
- 26.Vizcaíno J. A., Deutsch E. W., Wang R., Csordas A., Reisinger F., Ríos D., Dianes J. A., Sun Z., Farrah T., Bandeira N., Binz P.-A., Xenarios I., Eisenacher M., Mayer G., Gatto L., Campos A., Chalkley R. J., Kraus H.-J., Albar J. P., Martinez-Bartolomé S., Apweiler R., Omenn G. S., Martens L., Jones A. R., Hermjakob H. (2014) ProteomeXchange provides globally coordinated proteomics data submission and dissemination. Nat. Biotechnol. 32, 223–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vizcaíno J. A., Csordas A., Del-Toro N., Dianes J. A., Griss J., Lavidas I., Mayer G., Perez-Riverol Y., Reisinger F., Ternent T., Xu Q. W., Wang R., Hermjakob H. (2016) 2016 update of the PRIDE database and its related tools. Nucleic Acids Res. 44, D447–D456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rinschen M. M., Bharill P., Wu X., Kohli P., Reinert M. J., Kretz O., Saez I., Schermer B., Höhne M., Bartram M. P., Aravamudhan S., Brooks B. R., Vilchez D., Huber T. B., Müller R.-U., Krüger M., Benzing T. (2016) The ubiquitin ligase Ubr4 controls stability of podocin/MEC-2 supercomplexes. Hum. Mol. Genet. 25, 1328–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cox J., Mann M. (2008) MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 26, 1367–1372 [DOI] [PubMed] [Google Scholar]
- 30.Cox J., Hein M. Y., Luber C. A., Paron I., Nagaraj N., Mann M. (2014) Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Mol. Cell. Proteomics 13, 2513–2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rinschen M. M., Wu X., König T., Pisitkun T., Hagmann H., Pahmeyer C., Lamkemeyer T., Kohli P., Schnell N., Schermer B., Dryer S., Brooks B. R., Beltrao P., Krueger M., Brinkkoetter P. T., Benzing T. (2014) Phosphoproteomic analysis reveals regulatory mechanisms at the kidney filtration barrier. J. Am. Soc. Nephrol. 25, 1509–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bousquet S. M., Monet M., Boulay G. (2011) The serine 814 of TRPC6 is phosphorylated under unstimulated conditions. PLoS One 6, e18121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xue Y., Ren J., Gao X., Jin C., Wen L., Yao X. (2008) GPS 2.0, a tool to predict kinase-specific phosphorylation sites in hierarchy. Mol. Cell. Proteomics 7, 1598–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller M. L., Jensen L. J., Diella F., Jørgensen C., Tinti M., Li L., Hsiung M., Parker S. A., Bordeaux J., Sicheritz-Ponten T., Olhovsky M., Pasculescu A., Alexander J., Knapp S., Blom N., Bork P., Li S., Cesareni G., Pawson T., Turk B. E., Yaffe M. B., Brunak S., Linding R. (2008) Linear motif atlas for phosphorylation-dependent signaling. Sci. Signal. 1, ra2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huber T. B., Schermer B., Müller R. U., Höhne M., Bartram M., Calixto A., Hagmann H., Reinhardt C., Koos F., Kunzelmann K., Shirokova E., Krautwurst D., Harteneck C., Simons M., Pavenstädt H., Kerjaschki D., Thiele C., Walz G., Chalfie M., Benzing T. (2006) Podocin and MEC-2 bind cholesterol to regulate the activity of associated ion channels. Proc. Natl. Acad. Sci. USA 103, 17079–17086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heeringa S. F., Möller C. C., Du J., Yue L., Hinkes B., Chernin G., Vlangos C. N., Hoyer P. F., Reiser J., Hildebrandt F. (2009) A novel TRPC6 mutation that causes childhood FSGS. PLoS One 4, e7771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Du J., Heeringa S. F., Möller C. C., Reiser J., Hildebrandt F., Yue L. (2009) Characterization of a novel TRPC6 mutant identified in FSGS patients. Biophys. J. 96, 264a [Google Scholar]
- 38.Mottl A. K., Lu M., Fine C. A., Weck K. E. (2013) A novel TRPC6 mutation in a family with podocytopathy and clinical variability. BMC Nephrol. 14, 104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kriz W., Shirato I., Nagata M., LeHir M., Lemley K. V. (2013) The podocyte’s response to stress: the enigma of foot process effacement. Am. J. Physiol. Renal Physiol. 304, F333–F347 [DOI] [PubMed] [Google Scholar]
- 40.Shankland S. J. (2006) The podocyte’s response to injury: role in proteinuria and glomerulosclerosis. Kidney Int. 69, 2131–2147 [DOI] [PubMed] [Google Scholar]
- 41.Brinkkoetter P. T., Ising C., Benzing T. (2013) The role of the podocyte in albumin filtration. Nat. Rev. Nephrol. 9, 328–336 [DOI] [PubMed] [Google Scholar]
- 42.Sonneveld R., van der Vlag J., Baltissen M. P. A., Verkaart S. A. J., Wetzels J. F. M., Berden J. H. M., Hoenderop J. G. J., Nijenhuis T. (2014) Glucose specifically regulates TRPC6 expression in the podocyte in an AngII-dependent manner. Am. J. Pathol. 184, 1715–1726 [DOI] [PubMed] [Google Scholar]
- 43.Kim E. Y., Anderson M., Dryer S. E. (2012) Insulin increases surface expression of TRPC6 channels in podocytes: role of NADPH oxidases and reactive oxygen species. Am. J. Physiol. Renal Physiol. 302, F298–F307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nijenhuis T., Sloan A. J., Hoenderop J. G. J., Flesche J., van Goor H., Kistler A. D., Bakker M., Bindels R. J. M., de Boer R. A., Möller C. C., Hamming I., Navis G., Wetzels J. F. M., Berden J. H. M., Reiser J., Faul C., van der Vlag J. (2011) Angiotensin II contributes to podocyte injury by increasing TRPC6 expression via an NFAT-mediated positive feedback signaling pathway. Am. J. Pathol. 179, 1719–1732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ilatovskaya D., Palygin O., Lowing A., Levchenko V., Staruschenko A. (2015) Angiotensin II dependent regulation of TRPC6 calcium channels in the podocytes of the STZ-induced type 1 diabetic Dahl SS rats. FASEB J. 29 [Google Scholar]
- 46.Eckel J., Lavin P. J., Finch E. A., Mukerji N., Burch J., Gbadegesin R., Wu G., Bowling B., Byrd A., Hall G., Sparks M., Zhang Z. S., Homstad A., Barisoni L., Birbaumer L., Rosenberg P., Winn M. P. (2011) TRPC6 enhances angiotensin II–induced albuminuria. J. Am. Soc. Nephrol. 22, 526–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krall P., Canales C. P., Kairath P., Carmona-Mora P., Molina J., Carpio J. D., Ruiz P., Mezzano S. A., Li J., Wei C., Reiser J., Young J. I., Walz K. (2010) Podocyte-specific overexpression of wild type or mutant trpc6 in mice is sufficient to cause glomerular disease. PLoS One 5, e12859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Winn M. P., Daskalakis N., Spurney R. F., Middleton J. P. (2006) Unexpected role of TRPC6 channel in familial nephrotic syndrome: does it have clinical implications? J. Am. Soc. Nephrol. 17, 378–387 [DOI] [PubMed] [Google Scholar]
- 49.Seo K., Rainer P. P., Shalkey Hahn V., Lee D. I., Jo S.-H., Andersen A., Liu T., Xu X., Willette R. N., Lepore J. J., Marino J. P. Jr., Birnbaumer L., Schnackenberg C. G., Kass D. A. (2014) Combined TRPC3 and TRPC6 blockade by selective small-molecule or genetic deletion inhibits pathological cardiac hypertrophy. Proc. Natl. Acad. Sci. USA 111, 1551–1556; erratum, 111, 6115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maier T., Follmann M., Hessler G., Kleemann H.-W., Hachtel S., Fuchs B., Weissmann N., Linz W., Schmidt T., Löhn M., Schroeter K., Wang L., Rütten H., Strübing C. (2015) Discovery and pharmacological characterization of a novel potent inhibitor of diacylglycerol-sensitive TRPC cation channels. Br. J. Pharmacol. 172, 3650–3660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Washburn D. G., Holt D. A., Dodson J., McAtee J. J., Terrell L. R., Barton L., Manns S., Waszkiewicz A., Pritchard C., Gillie D. J., Morrow D. M., Davenport E. A., Lozinskaya I. M., Guss J., Basilla J. B., Negron L. K., Klein M., Willette R. N., Fries R. E., Jensen T. C., Xu X., Schnackenberg C. G., Marino J. P. Jr (2013) The discovery of potent blockers of the canonical transient receptor channels, TRPC3 and TRPC6, based on an anilino-thiazole pharmacophore. Bioorg. Med. Chem. Lett. 23, 4979–4984 [DOI] [PubMed] [Google Scholar]
- 52.Stambe C., Atkins R. C., Hill P. A., Nikolic-Paterson D. J. (2003) Activation and cellular localization of the p38 and JNK MAPK pathways in rat crescentic glomerulonephritis. Kidney Int. 64, 2121–2132 [DOI] [PubMed] [Google Scholar]
- 53.Koshikawa M., Mukoyama M., Mori K., Suganami T., Sawai K., Yoshioka T., Nagae T., Yokoi H., Kawachi H., Shimizu F., Sugawara A., Nakao K. (2005) Role of p38 mitogen-activated protein kinase activation in podocyte injury and proteinuria in experimental nephrotic syndrome. J. Am. Soc. Nephrol. 16, 2690–2701 [DOI] [PubMed] [Google Scholar]
- 54.Chen Z., Wan X., Hou Q., Shi S., Wang L., Chen P., Zhu X., Zeng C., Qin W., Zhou W., Liu Z. (2016) GADD45B mediates podocyte injury in zebrafish by activating the ROS-GADD45B-p38 pathway. Cell Death Dis. 7, e2068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu S., Ding J., Fan Q., Zhang H. (2010) The activation of extracellular signal–regulated kinase is responsible for podocyte injury. Mol. Biol. Rep. 37, 2477–2484 [DOI] [PubMed] [Google Scholar]
- 56.Bokemeyer D., Guglielmi K. E., McGinty A., Sorokin A., Lianos E. A., Dunn M. J. (1998) Different activation of mitogen-activated protein kinases in experimental proliferative glomerulonephritis. Kidney Int. Suppl. 67, S189–S191 [DOI] [PubMed] [Google Scholar]
- 57.Sakai N., Wada T., Furuichi K., Iwata Y., Yoshimoto K., Kitagawa K., Kokubo S., Kobayashi M., Hara A., Yamahana J., Okumura T., Takasawa K., Takeda S., Yoshimura M., Kida H., Yokoyama H. (2005) Involvement of extracellular signal–regulated kinase and p38 in human diabetic nephropathy. Am. J. Kidney Dis. 45, 54–65 [DOI] [PubMed] [Google Scholar]
- 58.Zhang H., Ding J., Fan Q., Liu S. (2009) TRPC6 up-regulation in Ang II–induced podocyte apoptosis might result from ERK activation and NF-kappaB translocation. Exp. Biol. Med. (Maywood) 234, 1029–1036 [DOI] [PubMed] [Google Scholar]
- 59.Brinkkoetter P. T., Olivier P., Wu J. S., Henderson S., Krofft R. D., Pippin J. W., Hockenbery D., Roberts J. M., Shankland S. J. (2009) Cyclin I activates Cdk5 and regulates expression of Bcl-2 and Bcl-XL in postmitotic mouse cells. J. Clin. Invest. 119, 3089–3101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brinkkoetter P. T., Wu J. S., Ohse T., Krofft R. D., Schermer B., Benzing T., Pippin J. W., Shankland S. J. (2010) p35, the non-cyclin activator of Cdk5, protects podocytes against apoptosis in vitro and in vivo. Kidney Int. 77, 690–699 [DOI] [PubMed] [Google Scholar]
- 61.Taniguchi Y., Pippin J. W., Hagmann H., Krofft R. D., Chang A. M., Zhang J., Terada Y., Brinkkoetter P., Shankland S. J. (2012) Both cyclin I and p35 are required for maximal survival benefit of cyclin-dependent kinase 5 in kidney podocytes. Am. J. Physiol. Renal Physiol. 302, F1161–F1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shi J., Ju M., Saleh S. N., Albert A. P., Large W. A. (2010) TRPC6 channels stimulated by angiotensin II are inhibited by TRPC1/C5 channel activity through a Ca2+- and PKC-dependent mechanism in native vascular myocytes. J. Physiol. 588, 3671–3682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ambrus L., Oláh A., Oláh T., Balla G., Saleem M. A., Orosz P., Zsuga J., Bíró K., Csernoch L., Bíró T., Szabó T. (2015) Inhibition of TRPC6 by protein kinase C isoforms in cultured human podocytes. J. Cell. Mol. Med. 19, 2771–2779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hisatsune C., Kuroda Y., Nakamura K., Inoue T., Nakamura T., Michikawa T., Mizutani A., Mikoshiba K. (2004) Regulation of TRPC6 channel activity by tyrosine phosphorylation. J. Biol. Chem. 279, 18887–18894 [DOI] [PubMed] [Google Scholar]
- 65.Schindl R., Frischauf I., Kahr H., Fritsch R., Krenn M., Derndl A., Vales E., Muik M., Derler I., Groschner K., Romanin C. (2008) The first ankyrin-like repeat is the minimum indispensable key structure for functional assembly of homo- and heteromeric TRPC4/TRPC5 channels. Cell Calcium 43, 260–269 [DOI] [PubMed] [Google Scholar]
- 66. Wedel B. J., Vazquez G., McKay R. R., St J Bird G., Putney J. W. Jr (2003) A calmodulin/inositol 1,4,5-trisphosphate (IP3) receptor-binding region targets TRPC3 to the plasma membrane in a calmodulin/IP3 receptor-independent process. J. Biol. Chem. 278, 25758–25765 [DOI] [PubMed] [Google Scholar]