Abstract
Bone atrophy and its related fragility fractures are frequent, late side effects of radiotherapy in cancer survivors and have a detrimental impact on their quality of life. In another study, we showed that parathyroid hormone 1-34 and anti-sclerostin antibody attenuates radiation-induced bone damage by accelerating DNA repair in osteoblasts. DNA damage responses are partially regulated by the ubiquitin proteasome pathway. In the current study, we examined whether proteasome inhibitors have similar bone-protective effects against radiation damage. MG132 treatment greatly reduced radiation-induced apoptosis in cultured osteoblastic cells. This survival effect was owing to accelerated DNA repair as revealed by γH2AX foci and comet assays and to the up-regulation of Ku70 and DNA-dependent protein kinase, catalytic subunit, essential DNA repair proteins in the nonhomologous end-joining pathway. Administration of bortezomib (Bzb) reversed the loss of trabecular bone structure and strength in mice at 4 wk after focal radiation. Histomorphometry revealed that Bzb significantly increased the number of osteoblasts and activity in the irradiated area and suppressed the number and activity of osteoclasts, regardless of irradiation. Two weeks of Bzb treatment accelerated DNA repair in bone-lining osteoblasts and thus promoted their survival. Meanwhile, it also inhibited bone marrow adiposity. Taken together, we demonstrate a novel role of proteasome inhibitors in treating radiation-induced osteoporosis.—Chandra, A., Wang, L., Young, T., Zhong, L., Tseng, W.-J., Levine, M. A., Cengel, K., Liu, X. S., Zhang, Y., Pignolo, R. J., Qin, L. Proteasome inhibitor bortezomib is a novel therapeutic agent for focal radiation-induced osteoporosis.
Keywords: osteoblast, DNA repair, bone, irradiation, apoptosis
As an effective treatment that damages tumor DNA and induces tumor cell death, radiotherapy has been successfully used in patients with cancer, alone or in conjunction with chemotherapy, for many decades (1). Clinical radiotherapy is undergoing constant refinement, and current technologies are increasingly focused on mitigating its harmful effect on the neighboring tissues (2). Nonetheless, the deleterious effect of ionizing radiation on bone adjacent to tumors is a well-recognized late side effect of radiotherapy that frequently leads to osteoradionecrosis and osteoporotic- or stress-induced fractures (3, 4). Antiresorptive drugs (bisphosphonates) are sometimes used to treat this side effect, but there is limited and ambiguous evidence of the clinical efficacy of this approach. Studies by our group (5–7) and others (8–11) have demonstrated that focal radiation does not always stimulate osteoclastogenesis. Depending on the dosage, treatment regimen, and animal model, radiation either has no effect or reduces the number of osteoclasts. Thus, searching for novel therapeutics to treat radiation-induced bone damage is of clinical importance.
Skeletal homeostasis relies on the balance of new bone formation by osteoblasts and removal of old bone by osteoclasts, a process often termed bone remodeling (12). Tipping this delicate balance toward less bone formation and more bone resorption inevitably leads to osteoporosis. Using a small-animal radiation research platform (SARRP), a focal irradiator that mimics patient focal radiotherapy in rodents, we demonstrated that drastically suppressed bone formation is the major cause of radiation damage on bone (5–7). Therefore, 2 anabolic bone-forming agents, parathyroid hormone (PTH) (rhPTH1-34, teriparatide; Forteo, Eli Lilly, New York, NY, USA) (5, 6), which received U.S. Food and Drug Administration (FDA) approval for postmenopausal osteoporosis in 2003, and a monoclonal antibody against sclerostin (Scl-Ab, romosozumab), which completed a phase 3 clinical trial in 2016 (7), were tested for their efficacy in our animal models of focal radiation. Although these therapies delivered surprisingly robust bone formation and alleviated structural and mechanical damage in irradiated bones, both PTH (13) and, to a theoretical extent, Scl-Ab have clinical concerns for being oncogenic in nature. Mechanistic studies reveal that they up-regulate Ku70 protein to promote DNA repair and thus cell survival in osteoblasts after radiation (7, 14). Osteoblasts are the most sensitive cells among mesenchymal lineage cells toward radiation. By preserving those cells, either PTH or Scl-Ab treatment maintains a sufficient level of bone-forming activity within the irradiated field to avoid bone structural deterioration.
Ku70 belongs to the nonhomologous end-joining (NHEJ) pathway that repairs DNA double-strand breaks (DSBs), the most lethal type of DNA lesions caused by radiation. It is critical in initiating and assembling DNA repair machinery at DSBs (15). Recruited by the heterodimer of Ku70/Ku80 to DSBs, DNA-PKc (DNA-dependent protein kinase, catalytic subunit) phosphorylates and alters the activity of many proteins, including itself, to mediate DNA repair through the NHEJ pathway (16). Like most other eukaryotic intracellular proteins, both Ku70 (17) and DNA-PKc (18) are degraded via the ubiquitin–proteasome system, in which proteins are tagged by ubiquitin and then degraded by the 26S proteasome complex. In pertinent studies, the proteasome inhibitor MG132 significantly prolonged the half-lives of those DNA repair proteins in cultured cells. In the clinic, bortezomib (Bzb) is the first proteasome inhibitor approved by the FDA for treating multiple myeloma, and it has potential for treating other hematologic malignancies, some solid tumors, and autoimmune and inflammatory diseases (19, 20).
Preclinical and clinical data also showed that these inhibitors have a beneficial effect on bone remodeling by stimulating bone formation and suppressing bone resorption (21). In this study, we demonstrated in culture and in an animal model that suppressing the 26S proteasome by proteasome inhibitors accelerates DNA repair in osteoblasts and protects them from radiation-induced apoptosis, thus protecting bones from radiation damage. Our data suggest that Bzb could be a novel therapeutic agent to treat radiation-induced osteoporosis.
MATERIALS AND METHODS
Irradiation of cultured cells and calvariae
Osteoblastic UMR106-01 cells were cultured in growth medium (minimum essential medium supplemented with 5% fetal bovine serum plus 100 IU/ml penicillin and 100 µg/ml streptomycin). Primary calvarial osteoblasts (POBs) digested from rat neonatal calvariae (22) were cultured in growth medium with 10% fetal bovine serum and 1% nonessential amino acids. MC3T3-E1 subclone 4 cells were maintained in 10% αMEM plus 100 IU/ml penicillin and 100 μg/ml streptomycin. Calvariae from neonatal mouse pups were harvested and cultured in 10% DMEM on a wired mesh (23, 24). For the radiation experiments, cells and calvariae were irradiated by X-Rad 320i (PxiPrecision X-Ray, North Branford, CT, USA) to a final dose of 8 Gy at a rate of 1.04 Gy/min, with or without 20 nM MG132 (Millipore-Sigma, St. Louis, MO, USA) added to the medium at 30 min before the radiation.
Cell culture staining
To detect cell death, cultured cells were stained with ethidium bromide/acridine orange (EB/AO; Millipore-Sigma) and cells with condensed chromatin were counted, as described elsewhere (24). For immunofluorescence staining, cells were fixed in 4% paraformaldehyde (PFA) and incubated with antibodies against γ-H2AX (9718S, RRID: AB_2118009) and cleaved caspase-3 (9661, RRID: AB_2341188; both from Cell Signaling Technology, Danvers, MA, USA) at room temperature for 1 h followed by Alexa Fluor–conjugated fluorescent secondary antibodies Alexa Fluor-594 (A11012, RRID: AB_10562717) and -488 (A-21206, RRID: AB_141708; both from Thermo Fisher Scientific, Waltham, MA, USA). Images were obtained with a fluorescence microscope (Eclipse 90i; Nikon, Melville, NY, USA) and quantified with ImageJ plugins (National Institutes of Health, Bethesda, MD, USA; https://imagej.nih.gov/ij/plugins/), either to quantify the nuclear intensity (cleaved caspase-3) or to measure the number of γ-H2AX foci per cell. At least 100 cells per sample were analyzed.
Comet assay
DNA damage was analyzed with a Comet Assay kit (Trevigen, Gaithersburg, MD, USA). Images were taken with a similar background. At least 50 cells per sample were examined with the Comet Assay plugin from ImageJ to measure the DNA content in the comet head and tail. DNA damage was quantified as a percentage of tail DNA content.
Small interfering RNA knockdown study
POBs at 50–60% confluence were transfected with 10 nM small interfering RNAs (siRNAs) Ku70 (RNC.RNAI.N139080.12.1 and RNC.RNAI.N139080.12.3, IDT, Newark, NJ, USA) or NC1-control duplex (TriFECTa siRNA kit; IDT) using Lipofectamine 2000 (Thermo Fisher Scientific) according to the manufacturer’s protocol. One day later, the cells were irradiated and treated with 20 nM MG132 for comet assay.
Immunoblot analysis
Cell lysis was performed in a modified RIPA buffer (1× RIPA buffer, 2 mM EDTA, 50 mM β-glycerophosphate, 1 mM Na3VO4, 1 mM PMSF, and protease inhibitor cocktail). Protein (15 μg) was electrophoresed by SDS-PAGE and then transferred to a PVDF membrane (Bio-Rad, Hercules, CA, USA). Antibodies against Ku70 (ab3114, RRID: AB_2219041; Abcam Inc., Cambridge, MA, USA), DNA-PKc (A300-519A, RRID: AB_451044; Bethyl Laboratories, Montgomery, TX, USA), and glyceraldehyde 3-phosphate dehydrogenase (60004-1-Ig, RRID: AB_2107436; Proteintech, Manchester, United Kingdom) were used to detect protein amounts using secondary antibodies conjugated with horseradish peroxidase followed by chemiluminescence (Clarity; Bio-Rad), and visualized with a Chemidoc-XRS system (Bio-Rad) with a supercooled camera.
Animal study design
All animal use in this study was reviewed and approved by the Institutional Animal Care and Use Committee at the University of Pennsylvania. Specific pathogen-free male 8-wk-old C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). Col2-Cre Rosa-Tomato (Col2/Tomato) mice were generated by breeding Col2-Cre mice and Rosa-Tomato mice from The Jackson Laboratory. In accordance with the standards for animal housing, mice were group housed at 23–25°C with a 12-h light/dark cycle and allowed free access to water and standard laboratory pellets. At 10 wk of age, the mice received a clinically relevant radiation dose of 8 Gy at the distal metaphyseal region of the right femur by SARRP (Xstrahl, Suwanee, GA, USA) twice on d 1 and 3. The radiation was delivered in a 5 × 5 mm square collimated field centered at the metaphysis about 1 mm from the growth plate at a rate of 1.65 Gy/min with the aid of built-in micro–computed tomography (µCT) and X-ray. Mice were then divided in 2 groups (9 per group) receiving either vehicle (1× PBS) or 1 mg/kg Bzb (LC Laboratories, Woburn, MA, USA), once every 4 d from d 1 for 4 wk. Based on our previous finding that focal radiation from SARRP on the right leg has no confounding effects on the contralateral leg (6), we used the left leg as the control. Serum was collected at euthanasia for osteocalcin (Mouse Osteocalcin Enzyme Immunoassay Kit; Alfa Aesar, Ward Hill, MA, USA) and C-telopeptide of type 1 collagen (CTX-I) (RatLaps EIA; Immunodiagnostic Systems Inc., Gaithersburg, MD, USA) ELISAs. Bzb injections did not affect the body weight (vehicle: 22.94 ± 0.31 g; Bzb: 23.74 ± 0.27 g) and the general morphology of vital organs in animals (Supplemental Fig. 1).
µCT analysis
Analysis by µCT (μCT 35; Scanco Medical AG, Brüttisellen, Switzerland) was performed on the distal metaphysis of bilateral femurs harvested 4 wk after radiation. A 0–4.1-mm region above the growth plate of the distal end of the femur was scanned at 6 µm isotropic voxel size to acquire a total of 686 µCT slices per scan. All images were first smoothed by a gaussian filter (σ = 1.2, support = 2.0) and then a threshold corresponding to 30% of the maximum available range of image gray scale values were applied. The secondary spongiosa region at 0.6–1.8 mm from the highest point of the growth plate was contoured for trabecular bone analysis. Three-dimensional standard microstructural analysis was performed to determine the geometric trabecular bone volume/total volume (BV/TV) fraction, trabecular thickness (Tb.Th), trabecular separation (Tb.Sp), trabecular number (Tb.N), and structure model index (SMI). Microstructural finite element analysis (FEA) of thresholded whole-bone images was generated by conversion of individual bone voxels to an 8-node brick element. Young’s modulus of 15 GPa and a Poisson ratio of 0.3 were set while using the bone tissue as an isotropic, linear elastic material. Bone stiffness was quantified by applying a uniaxial compression along the axial direction and was further analyzed by measuring the linear elasticity.
Static and dynamic bone histomorphometry
Subcutaneous injections of xylenol orange (90 mg/kg) and calcein (15 mg/kg) (Millipore-Sigma) were administered 9 and 2 d before harvest. After µCT imaging, femurs were processed for methyl methacrylate embedding and sectioning. Goldner’s trichrome-stained sections were used for static measurements and unstained sections for dynamic measurements. Images obtained via an Eclipse 90i microscope were quantified with Bioquant Osteo Software (Bioquant Image Analysis, Nashville, TN, USA). The main parameters assessed were trabecular bone perimeter [bone surface (BS)], osteoblast number (Ob.N), osteoclast number (Oc.N), single- and double-labeled surface, and interlabel width. Mineralizing surface (MS) and surface-referent bone formation rate (BFR/BS, µm3/µm2/d) were calculated as described earlier (25).
Immunohistochemistry
After treatments, cultured calvariae were fixed in 4% PFA overnight, decalcified in 10% EDTA for 2 d and then processed and paraffin embedded for sectioning. Sections (5 μm thick) were cut parallel to the plane of the sagittal suture, and equivalent areas were assessed. Target immunogens were detected using secondary antibodies conjugated with Alexa Fluor-594 (for cleaved-caspase-3 and γ-H2AX), 488 (for Ku70), and 647 (for DNA-PKc).
After euthanasia, mouse tissues, such as heart, lung, liver, and kidney, were collected for paraffin-embedded sections followed by hematoxylin and eosin staining to examine the toxicity of Bzb. Bilateral femurs were harvested, fixed with 4% PFA, and decalcified with 10% EDTA for 3 wk before processing for frozen sections. The sections were air dried and then rehydrated with 1× PBS briefly, and permeabilized with 1% Triton X-100. After rehydration with PBS, sections were subjected to fluorescent TUNEL staining with the Apoptag in situ peroxidase kit (Millipore-Sigma) or antibodies against perilipin (9349; RRID: AB_10829911; Cell Signaling Technology), and against endomucin (sc-65495; RRID: AB_2100037; Santa Cruz Biotechnology, Dallas, TX, USA), followed by secondary antibodies conjugated with Alexa Fluor-488 (Thermo Fisher Scientific). Images were taken at ×200 magnification with an Eclipse 90i microscope and quantified with ImageJ.
Statistics
Data are expressed as means ± sem and analyzed by paired Student’s t test, for comparison of irradiated and nonirradiated contralateral femurs, and by unpaired Student’s t test, for comparison of vehicle and Bzb-treated samples followed by Bonferroni adjustment for multiple comparisons using Prism 5 software (GraphPad Software, La Jolla, CA, USA). For cell culture experiments, observations were repeated independently at least 3 times with a similar conclusion. Only data from a representative experiment with triplicates per group (immunofluorescence staining) or duplicates per group (comet assay) are presented. Differences reaching P < 0.05 were significant.
RESULTS
Proteasome inhibition promotes cell survival in irradiated osteoblasts
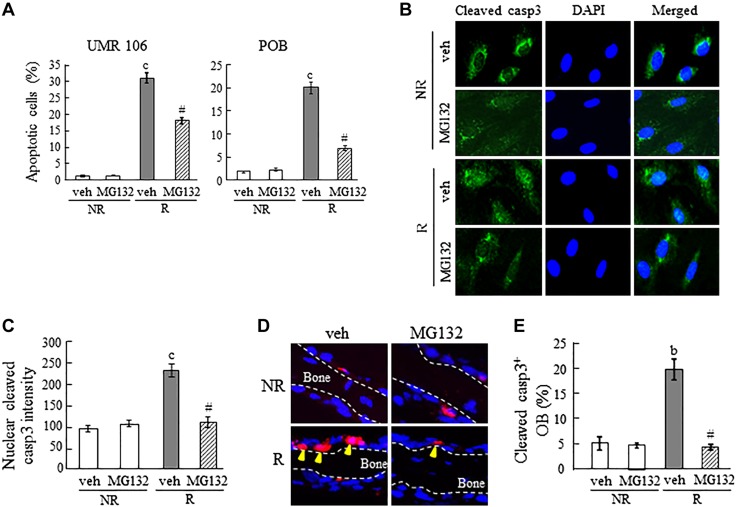
To investigate whether inhibiting proteasome activity can rescue osteoblastic cells after radiation, we treated UMR106-01 cells and POBs with MG132, a peptide aldehyde that specifically inhibits 20S proteasome activity, immediately before irradiating them. Similar to our previous studies, radiation caused a remarkable increase in the death of the cells quantified via markers of apoptosis. MG132 partially but significantly reduced the percentage of apoptotic cells in both cell types after radiation (Fig. 1A). Accumulation of cleaved caspase-3 in the nucleus indicates the initiation of apoptosis (26). Upon irradiation, nuclear intensity of cleaved caspase-3 was enhanced by 2.4-fold in MC3T3 cells, but this effect was alleviated by MG132 cotreatment (Fig. 1B, C). Furthermore, in ex vivo mouse calvarial organ culture, we observed that the percentage of cleaved caspase-3+ osteoblasts lining the BS was increased 4.3-fold at 6 h after radiation, and this increase was almost completely blocked by MG132 cotreatment (Fig. 1D, E). Note that in the above experiments, MG132 itself had no effects on cell death of nonirradiated osteoblastic cells. Thereby, these data provide the first evidence that inhibiting proteasome activity attenuates radiation-induced cell death in osteoblastic cells.
Figure 1.
Proteasome inhibitor MG132 blocks radiation-induced apoptosis in osteoblastic cells. A) An apoptotic assay was performed in UMR106 cells and rat POBs at 2 d after radiation, with or without 20 nM MG132 treatment. The percentages of apoptotic osteoblastic cells were quantified. B) Immunofluorescence staining of cleaved caspase-3 (casp3) in MC3T3 cells at 6 h after radiation, with and without MG132 treatment. C) Fluorescence intensity of nuclear staining for cleaved caspase-3. D) Immunohistochemistry of cleaved caspase-3 in cultured mouse calvariae at 6 h after radiation, with or without MG132 treatment (n = 3 calvariae/group). E) The percentages of cleaved caspase-3+ osteoblasts. NR, nonirradiated; R, irradiated; veh, vehicle. bP < 0.01, cP < 0.001 vs. NR; #P < 0.001 vs. veh R.
Proteasome inhibition accelerates DNA repair in irradiated osteoblasts
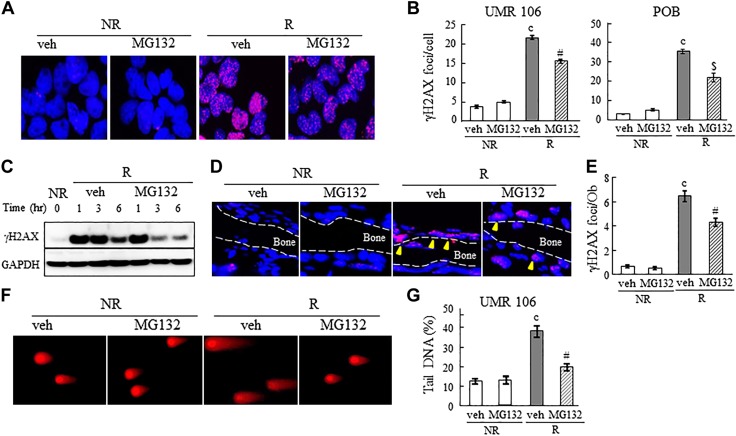
Radiation causes cell death mainly by its DNA-damaging actions. Among all DNA lesions created by radiation, DSB is the most lethal one and therefore must be repaired for cells to survive. To investigate whether inhibiting proteasome activity accelerates DNA repair, particularly resolving DSBs, in osteoblastic cells, we measured the amount of γH2AX, a marker for DSBs, and performed a Comet assay in the osteoblastic cells to quantify DNA damage and repair after radiation. Immunofluorescence revealed that radiation caused substantial increases in the number of γH2AX foci in both UMR106 cells (5.8-fold) and POBs (13.1-fold) at 4 h after radiation. Prior treatment of MG132 significantly reduced the γH2AX focus numbers (by 29 and 39% in these cells, respectively) (Fig. 2A, B). The marked increase in γH2AX protein was also detected as soon as 1 h after radiation by Western blot analysis and sustained until 6 h (Fig. 2C). Note that γH2AX protein was barely detectable in nonirradiated cells over a 6-h time course, regardless of vehicle or MG132 treatment (Supplemental Fig. 2). Although it did not change the initial induction of γH2AX, MG132 greatly reduced the amount of γH2AX starting from 3 h after irradiation. Robust elevation of the number of γH2AX foci in BS osteoblasts was similarly observed in the calvarial organ cultures after radiation (Fig. 2D, E). MG132 treatment significantly reduced the amount of this DSB marker at 6 h after irradiation. Damage to the chromatin upon ionizing radiation can also be detected by a Comet assay. When assessed by electrophoresis, the volume of a comet tail directly correlates to the extent of DNA fragmentation in a given cell (27). We observed a 3.0-fold increase in radiation-induced DNA fragmentation in UMR106-01 cells (Fig. 2F, G). MG132 significantly lowered the percentage of tail DNA and thus attenuated DNA damage from radiation.
Figure 2.
Inhibition of proteasome function promotes DNA repair in irradiated osteoblastic cells. A) MG132 (20 nM) reduced the number of γH2AX foci in UMR106 cells and POBs at 4 h after irradiation, as shown by immunofluorescence staining. Representative images of UMR cells are presented. B) Quantification of γH2AX foci per cell is shown. C) Time course analysis of γH2AX protein amount in UMR106 cells after radiation with or without MG132 treatment by immunoblotting. D) DNA damage was detected by γH2AX staining in mouse calvarial organ culture at 6 h after radiation with or without MG132 treatment (n = 3 calvariae/group). E) The number of γH2AX foci per osteoblast was quantified on the BS. F, G) A Comet assay was performed in UMR106 cells to detect DNA damage at 6 h after radiation, with or without MG132 treatment. F) Representative images of UMR106 cells are presented. G) The percentage of tail DNA was quantified. NR, nonirradiated; R, irradiated; veh, vehicle. cP < 0.001 vs. NR; $P < 0.01, #P < 0.001 vs. veh R.
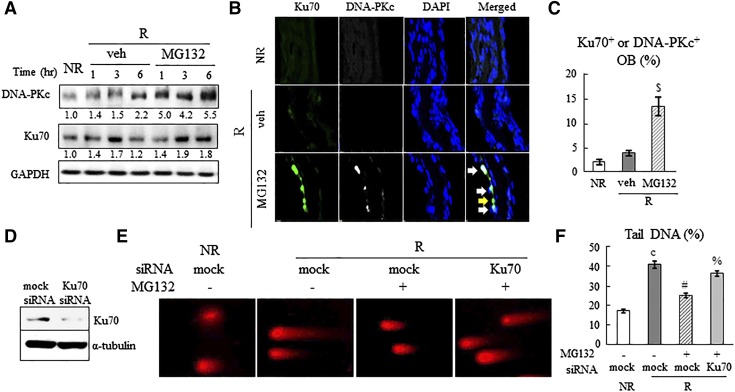
DSBs are repaired predominantly by the NHEJ pathway, which involves the recruitment of the NHEJ pathway proteins Ku70 and DNA-PKc (28). We previously demonstrated that DNA repair and cell survival in irradiated osteoblasts after PTH and Scl-Ab treatments require Ku70 protein (14). Western blots revealed that Ku70 and DNA-PKc amounts are quickly elevated after radiation (Fig. 3A). Blocking proteasome activity by MG132 further increased the amount of Ku70 at 6 h and of DNA-PKc at 1, 3, and 6 h after radiation. MG132 did not affect the levels of those proteins in nonirradiated cells (Supplemental Fig. 3). The increases in DNA repair proteins by MG132 were further confirmed in the calvarial organ culture. Radiation alone did not cause detectable staining of DNA-PKc and Ku70 in calvarial osteoblasts (Fig. 3B). However, in the presence of MG132, the percentage of osteoblasts with either Ku70 or DNA-PKc staining increased significantly (Fig. 3B, C). Most NHEJ+ osteoblasts stained for both markers, although some had only Ku70, but not DNA-PKc staining (Fig. 3B). This finding is consistent with the general notion that Ku70 binding to the DSB site precedes the recruitment of DNA-PKc. To investigate whether the NHEJ pathway is the dominant pathway mediating the rescue effect of MG132, we knocked down the expression of Ku70 in POBs via a siRNA approach (Fig. 3D). The Comet assay clearly showed that a reduction in Ku70 blocks DNA repair in the presence of MG132 (Fig. 3E, F). In summary, our data demonstrate that inhibiting proteasome activity accelerates DNA repair in osteoblastic cells and, hence, blocks radiation-induced death of these cells.
Figure 3.
Proteasome inhibition upregulates NHEJ pathway proteins to stimulate DNA repair. A) MG132 (20 nM) increases Ku70 and DNA-PKc amounts in UMR106 cells after radiation, as shown by immunoblot analysis. The band intensities listed below the blot were quantified by densitometric analysis and normalized against GAPDH. B) Immunofluorescence staining of Ku70 (green) and DNA-PKc (white) in irradiated mouse calvarial organ culture at 6 h in the presence or absence of MG132. Merged images are shown with white arrows indicating cells having both proteins and a yellow arrow indicating a cell having only Ku70 (n = 3 calvariae/group). C) The percentage of Ku70+ or DNA-PKc+ osteoblasts was quantified. $P < 0.01 vs. veh R. D) Western blot shows that Ku70 siRNA decreases Ku70 protein amount in POBs at 16 h after transfection. E, F) Comet assay shows that knocking down Ku70 abolishes MG132-induced DNA repair in osteoblastic cells. POBs were pretransfected with 10 nM mock or Ku70 siRNA, irradiated, cultured for another 6 h in the presence or absence of MG132, and subjected to Comet assay. E) Representative images. F) Quantification of the percentage of tail DNA. NR, nonirradiated; R, irradiated; veh, vehicle. cP < 0.001 vs. NR. #P < 0.001 vs. R+veh+mock siRNA; %P < 0.01 vs. R+MG132+mock siRNA.
Bzb protects trabecular bone architecture and strength from radiation damage
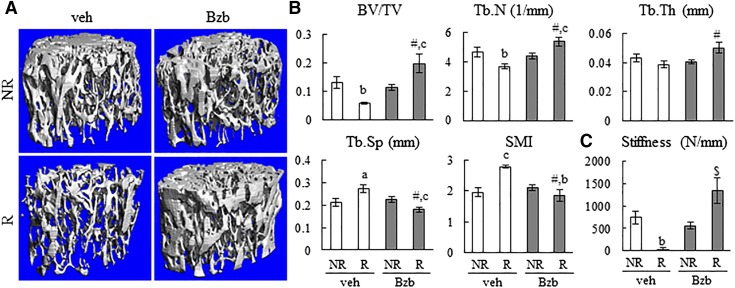
Derived from MG132, Bzb is a therapeutic version of proteasome inhibitor for treating multiple myeloma and other cancers. To explore the potential of this drug for alleviating radiation-induced osteoporosis, we studied the effect of Bzb on trabecular bone in our mouse focal radiation model. Consistent with our previous reports (6, 7), we observed marked trabecular bone architectural damage in the distal femoral area 4 wk after irradiation with significant decreases in BV/TV (54%) and Tb.N (21%), and a significant increase in Tb.Sp (28%) and SMI (43%) (Fig. 4A, B). Furthermore, μ-FEA revealed a 95% decrease in overall trabecular stiffness in the irradiated bones compared to that of nonirradiated controls (Fig. 4C). By contrast, 4 wk of Bzb injections robustly preserved trabecular bone architecture and strength after focal radiation to the same or even better level than in vehicle-treated, nonirradiated bones, resulting in ∼3.2-, 1.5-, 1.3-, and 36.7-fold increases in BV/TV, Tb.N, Tb.Th, and stiffness, respectively, with a 34% decrease in both Th.Sp and SMI, compared to vehicle-treated irradiated bones. Note that under the nonirradiation condition, Bzb did not affect overall trabecular bone structure (vehicle-treated nonirradiated vs. Bzb-treated nonirradiated bones). These data indicate that Bzb and radiation have synergistic effects on increasing trabecular bone mass.
Figure 4.
Bzb alleviates trabecular bone damage in mice caused by SARRP radiation. A) 3D reconstructed μCT images of trabecular bone from WT mice treated with vehicle or Bzb injections for 4 wk after focal radiation. NR, nonirradiated left femur; R, irradiated right femur, veh vehicle. B) μCT measurement of trabecular bone structural parameters (n = 9 mice/group). C) Trabecular bone stiffness was measured by μCT and FEA. NR, nonirradiated; R, irradiated; veh, vehicle. aP < 0.05, bP < 0.01, cP < 0.001 R vs. NR; $P < 0.01, #P < 0.001 Bzb vs. veh.
Bzb preserves bone formation in irradiated bones
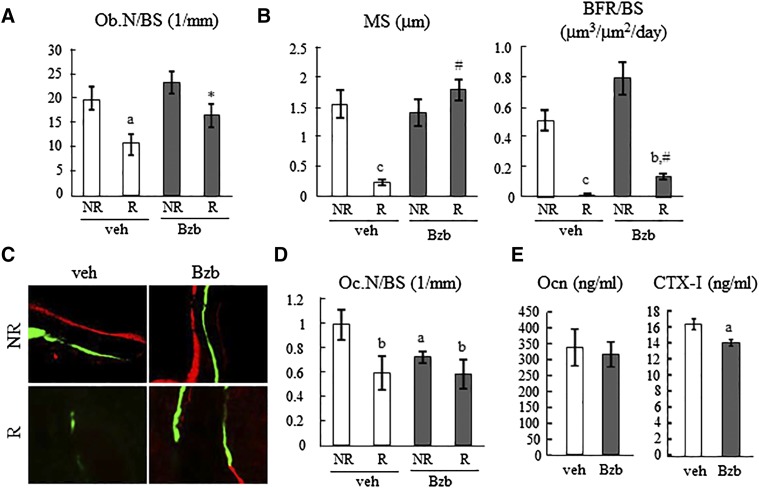
To investigate the underlying cellular mechanism, we performed bone histomorphometric assessment on bilateral femurs from vehicle- and Bzb-treated mice at 4 wk after focal radiation. In vehicle-treated animals, bone formation parameters, including Ob.N/BS, MS, and BFR, were remarkably suppressed in the irradiated area (Fig. 5A–C). Bzb injections did not alter bone formation in nonirradiated bones. However, in irradiated bones, Bzb injections substantially increased Ob.N/BS and MS by 1.5- and 7.5-fold, respectively, compared to vehicle injections and raised BFR to a measurable level compared to a nondetectable level in vehicle samples. Nevertheless, except MS, Ob.N/BS, and BFR in Bzb-treated irradiated samples were not restored to the same level as vehicle-treated nonirradiated samples. Both Bzb and radiation significantly reduced bone resorption (Fig. 5D). Radiation alone, Bzb alone, and Bzb plus radiation decreased Oc.N/BS by 40, 27, and 41% compared to vehicle and nonirradiated groups. No synergistic effect was detected between radiation and Bzb treatments. The subsequent serum chemistry analysis revealed that Bzb injections did not change the level of overall bone formation marker (osteocalcin) but significantly (by 20%) reduced the level of CTX-I (Fig. 5E).
Figure 5.
Bzb preserves trabecular Ob.N and bone-forming activity after SARRP radiation. A) Ob.N/BS was measured in the distal femur trabecular region from WT mice treated with either vehicle or Bzb injections for 4 wk after focal radiation based on static histomorphometry (n = 5 mice/group). B) MS and BFR were quantified based on dynamic histomorphometry (n = 5 mice/group). aP < 0.05, bP < 0.01, cP < 0.001 R vs. NR; *P < 0.05, #P < 0.001 Bzb vs. veh. C) Representative images show double labels, xylenol orange (red) and calcein (green), in trabecular bone after radiation and Bzb treatment. D) Bzb reduces the number of osteoclasts regardless of radiation (n = 5 mice/group). aP < 0.05, bP < 0.01 vs. veh NR. E) Serum osteocalcin (Ocn) and CTX-I levels in mice with or without Bzb treatment for 4 wk post irradiation were quantified (n = 9 mice/group). NR, nonirradiated; R, irradiated; veh, vehicle. aP < 0.05 vs. veh.
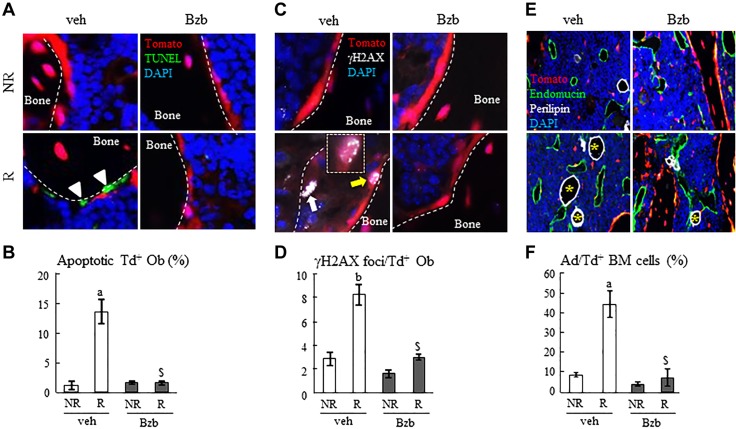
We had established a fluorescent reporter mouse model, Col2/Tomato, to visualize mesenchymal lineage cells within bone marrow (7). In these mice, all bone marrow mesenchymal progenitors, adipocytes, osteoblasts, and osteocytes, but not hematopoietic lineage cells and endothelial cells, are labeled with Tomato signal. We used this unique model to delineate how Bzb affects osteoblasts and bone marrow adipocytes after radiation. To detect early cellular changes, we harvested femurs at 2 wk after radiation. At this time point, trabecular bone structure and strength have not been significantly altered by either radiation or Bzb injections (Supplemental Fig. 4). However, TUNEL staining had already detected a great increase in Td+ osteoblasts that were undergoing apoptosis (Fig. 6A, B). Bzb injections remarkably decreased this percentage from 13.6 to 1.5% after radiation, blocking the radiation-induced apoptosis in osteoblasts. Cell death is preceded by the DNA damage and repair events as shown by the increase in γH2AX focus levels. γH2AX staining revealed that Bzb treatment indeed reduces the average number of γH2AX foci per osteoblast and thus accelerates DNA repair in osteoblasts in irradiated bones (Fig. 6C, D). In line with our previous report (7), osteocytes were also sensitive toward radiation. Bzb was also able to attenuate radiation-induced apoptosis in osteocytes (Supplemental Fig. 5). Radiation profoundly increased marrow adiposity at this time point (Fig. 6E, F and Supplemental Fig. 6) because it quickly shifted the cell fate of mesenchymal progenitors toward adipocytes (7). Bzb reduced the adiposity and the size of these bone marrow adipocytes after radiation, thus reversing the adipogenic cell fate of mesenchymal progenitors. In conclusion, these results demonstrated that Bzb treatment protects bone-forming osteoprogenitors and osteoblasts from radiation-induced damage and thus maintains the integrity of trabecular bone after radiation.
Figure 6.
Bzb injections promote trabecular osteoblast survival and DNA repair and suppress marrow adiposity in mice after SARRP radiation. A) TUNEL staining revealed that 2 wk of Bzb injections reversed the radiation-induced osteoblast apoptosis (green, indicated by white arrowheads) in the trabecular bone of Col2/Tomato mice. Dashed lines: trabecular bone perimeters. B) Quantification of the percentage of TUNEL+ cells among Tomato+ osteoblasts within the metaphysis. C) Immunofluorescent staining of γH2AX foci (white, indicated by arrows) in trabecular osteoblasts at 2 wk after radiation and Bzb injections. Inset: a magnified image of an osteoblast with γH2AX foci (indicated by a yellow arrow in lower magnification image). D) Quantification of the number of γ-H2AX foci per Tomato+ osteoblast on the BS. E) Immunofluorescent staining of perilipin (white outline) to detect adipocytes (yellow asterisks) in bone marrow at 2 wk after radiation and Bzb injections. Endomucin staining shows bone marrow blood vessels (green outline). F) Quantification of the percentage of perilipin+ adipocytes among Tomato+ bone marrow (BM) mesenchymal cells (n = 3 femurs/group). NR, nonirradiated; R, irradiated; veh, vehicle. aP < 0.05, bP < 0.01 R vs. NR; $P < 0.01 Bzb vs. veh.
DISCUSSION
Clinical radiotherapy, although effective in killing cancer cells, exerts collateral damage on adjacent bones, rendering a plethora of insults to bone cells and matrix. Our past studies using a unique focal irradiator, SARRP, that mimics clinical radiotherapy in rodents revealed that suppressing bone formation, rather than activating bone resorption, is the major mechanism mediating the radiation-induced bone deterioration. Among the bone-forming cells, the osteoblast is the most radiosensitive one; it rapidly undergoes cell death owing to the insufficient repair of DNA damage induced by radiation. In this study, we demonstrated that proteasome inhibitors accelerate DNA repair in osteoblastic cells and thus promote cell survival by raising the amounts of 2 key NHEJ pathway proteins: Ku70 and DNA-PKc. Furthermore, we provided the first proof of principle evidence that Bzb, the first proteasome inhibitor approved for clinical treatment, is capable of preserving the number and activity of BS osteoblasts in mice after focal radiation via a similar DNA repair mechanism. This bone anabolic effect, together with its action in suppressing bone resorption, leads to the protection of integrity and mechanical strength of the trabecular bone after radiation.
The 26S proteasome complex, a multisubunit complex composed of the 20S catalytic core and 19S regulatory units, is responsible for degrading most intracellular proteins. It not only degrades abnormal and damaged protein, but also controls the homeostasis of short-lived proteins involved in cell cycle, transcription, DNA repair process, apoptosis and many other cellular processes (29, 30). The entire process of protein degradation follows a sequence of well-orchestrated events including activation and conjugation of the ubiquitin molecules, recognition of the ubiquitinated substrates by the shuttle factors for the delivery to the 26S proteasome, and an ATP-dependent process of catalytic degradation of ubiquitin proteins. Dysregulation of this process plays an essential role in the cell growth, development, and survival of human cancer. Compared to normal cells, increased proteasome activity is observed in many malignant diseases and these tumorigenic cells are more dependent on proteasomal activity for their proliferation and survival (20). Proteasome inhibition results in growth suppression and apoptosis in tumor cells and one of first mechanisms discovered is the inhibition of NF-κB activity by preventing the degradation of NF-κB inhibitor I-κB (31, 32). It is important to note that normal cells are much more tolerant to proteasome inhibitors because of several reasons. First, cells possess several deubiquitinases to disassemble ubiquitin conjugates and recycle the proteins. Second, Bzb primarily blocks the chymotrypsin-like site in 20S subunit, leaving the other active sites in 20S and 19S subunits functional. Indeed, Bzb probably only inhibits protein degradation by 20–30% (33). Because osteoblasts are normal cells and generally nonproliferative in vivo, it is not surprising that proteasome inhibitors have the opposite effects on these cells to promote their survival instead of apoptosis. This differential effect of Bzb on cancer cells and normal bone-forming cells also warrants the safety of applying this drug to cancer patients receiving radiotherapy to prevent osteoporosis within tumor-neighboring bones.
It has been shown that during oxidative stress after ionizing radiation, the proteasome complex fails to assemble its 19S regulatory subunit with its 20S catalytic core (34). The 20S catalytic core now can degrade protein substrates irrespective of their ubiquitination status (35, 36). Proteasome inhibitors have been shown to be effective blockers of such unwanted proteasomal degradation (35). Both Ku70 and DNA-PKc are ubiquitinated and targeted to the proteasome (17, 18). With the inhibition of proteasomal catalytic core, both the ubiquitinated (targeted to the intact 26S proteasome) and the nascent (targeted to the stress-induced 20S) NHEJ pathway proteins may escape the degradation cycle. Furthermore, the deubiquitinases could act on NHEJ pathway proteins to make them available for DSB repair. During these early events that follow stress, the cellular machinery produces nascent NHEJ proteins as a preliminary transcriptional response to these stresses. Our data showed that MG132 treatment also reduced the cleavage of caspase-3 and prevented its nuclear localization. DNA-PKc is one of the substrates of cleaved caspase-3 (37–39), and we indeed observed that the reduction in cleaved caspase-3 nuclear localization coincided with the rise in DNA-PKc protein amounts.
Bzb is a dipeptidyl boronic acid that binds reversibly to the active site of threonine hydroxyl group in the β5-subunit of the 20S proteasome and inhibits its chymotrypsin-like activity (40). A major hallmark of multiple myeloma is bone disease characterized by lytic lesions that are associated with bone pain, fractures, and hypercalcemia. It has been well documented that Bzb treatment increases bone-forming markers, such as alkaline phosphatase, and suppresses bone resorption markers, such as CTX and TRAP-5b, in patients with multiple myeloma (41). Preclinical studies showed that Bzb primarily stimulates bone formation by promoting osteoblast differentiation using multifaceted mechanisms, such as up-regulation of Runx2 activity, active β-catenin level, osterix, and vitamin D signaling, stabilization of Runx2 protein, and down-regulation of DKK1, a Wnt pathway inhibitor (21). Our study uncovered a new mechanism for the anabolic bone action of Bzb, i.e., stimulation of DNA repair to preserve osteoblasts after radiation insult. In contrast to 2 previous reports that Bzb treatment increases trabecular bone mass in normal adult mice (42, 43), we did not observe significant changes in trabecular bone structure, Ob.N, and osteoblast activity in nonirradiated mouse femurs after Bzb treatment. This discrepancy could be related to the difference in mouse strains and treatment regimen (drug dosage, treatment interval, and/or treatment duration). Nevertheless, upon focal radiation, Bzb treatment remarkably increased bone formation by preserving Ob.N and osteoblast activity, suggesting that in our hands, the anabolic action of Bzb was more prominent under pathologic conditions. In line with previous reports, we found that Bzb suppressed osteoclast formation and resorptive activity, regardless of radiation treatment. This effect was most likely mediated by its modulation of p38, AP-1, and NF-κB pathways (44). However, this inhibition of bone resorption was apparently not strong enough to cause a significant increase in bone mass in nonirradiated bones after 2 and 4 wk of Bzb treatment. Longer treatment duration, such as 6 or 8 wk, is probably required to detect the increased bone mass phenotype in mice without radiation. One limitation of our study is that we used young adult mice in our experiments. However, we focused our analyses on the trabecular bone in the secondary spongiosa and thus minimized the confounding effects of skeletal growth at this stage.
We have previously demonstrated that PTH1-34 exerts its radioprotective action on bone mainly through activating the β-catenin pathway to accelerate DNA repair and block osteoblast apoptosis and so is the agent that directly stimulates the canonical Wnt pathway, such as Scl-Ab (14). More than a decade of clinical usage of teriparatide has revealed no carcinogenic effect (45), but prior preclinical studies in rats led to a black box warning from the FDA to prevent its usage in patients with prior external beam or implant radiation therapy involving the skeleton. Romosozumab activates the Wnt pathway by blocking sclerostin binding to Lrp5/6. Although a recent study in rats revealed no carcinogenicity risk after almost 2 yr of continuous romosozumab treatment (46), a theoretical concern of applying this drug to cancer patients still exists owing to the strong linkage between the Wnt pathway and cancer formation. Our finding that Bzb, a currently FDA-approved drug for cancer treatment, has similar bone anabolic actions as teriparatide and romosozumab after radiation, points to a new direction for treating radiotherapy-induced osteoporosis. However, as a cancer therapeutic, Bzb also comes with side effects including neurotoxicity, fatigue, gastrointestinal effects, and modest cytopenia. Peripheral sensory neuropathy, sometimes painful, is the most problematic side effect for many patients. Fortunately, it is often reversible after treatment interruption (47). In the future, we plan to test the effect of second-generation proteasome inhibitors, such as carfilzomib, which are more specific and have fewer side effects than Bzb. Because the proteasome inhibitor class of drugs is already known to be antineoplastic, we believe that these agents have all the ingredients for treating radiotherapy-induced osteoporosis, while giving physicians an added advantage of sensitizing the tumor microenvironment toward increasing doses of radiation.
ACKNOWLEDGMENTS
The authors thank Martin F. Heyworth (Corporal Michael J. Crescenz Veterans Affairs Medical Center), for critically editing the manuscript. This study was supported by U.S. National Institutes of Health (NIH) National Institute of Arthritis and Musculoskeletal and Skin Diseases Grants R01AR066098, P30AR050950 (Penn Center for Musculoskeletal Disorders), and K01AR066743 (to X.S.L.); NIH National Institute of Diabetes and Digestive and Kidney Diseases Grant R01DK095803 (to L.Q.); and NIH Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant 1K08HD049598 (to Y.Z.). The authors declare no conflicts of interest.
Glossary
- μCT
micro–computed tomography
- BFR
bone formation rate
- BS
bone surface
- BV/TV
bone volume/total volume
- Bzb
bortezomib
- CTX-I
C-telopeptide of type 1 collagen
- DNA-PKc
DNA-dependent protein kinase, catalytic subunit
- DSB
double-strand break
- FDA
U.S. Food and Drug Administration
- FEA
finite element analysis
- MS
mineralizing surface
- NHEJ
nonhomologous end joining
- Ob.N
osteoblast number
- Oc.N
osteoclast number
- PFA
paraformaldehyde
- POB
primary calvarial osteoblast
- PTH
parathyroid hormone
- SARRP
small-animal radiation research platform
- Scl-Ab
anti-Sclerostin antibody
- siRNA
small interfering RNA
- SMI
structure model index
- Tb.N
trabecular number
- Tb.Sp
trabecular separation
- Tb.Th
trabecular thickness
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
A. Chandra, M. A. Levine, K. Cengel, X. S. Liu, and L. Qin designed the study; A. Chandra, L. Wang, and T. Young conducted the study; A. Chandra and L. Wang collected the data; A. Chandra, W.-J. Tseng, L. Zhong, and L. Qin analyzed the data; A. Chandra, Y. Zhang, M. A. Levine, K. Cengel, X. S. Liu, R. J. Pignolo, and L. Qin interpreted the data; A. Chandra and L. Qin drafted the manuscript; A. Chandra, M. A. Levine, X. S. Liu, Y. Zhang, and L. Qin revised the manuscript content; A. Chandra, L. Wang, T. Young, L. Zhong, W.-J. Tseng, M. A. Levine, K. Cengel, X. S. Liu, Y. Zhang, R. J. Pignolo, and L. Qin approved the final version of manuscript; and L. Qin was responsible for the integrity of the data analysis.
REFERENCES
- 1.Ross G. M. (1999) Induction of cell death by radiotherapy. Endocr. Relat. Cancer 6, 41–44 [DOI] [PubMed] [Google Scholar]
- 2.Chargari C., Goodman K. A., Diallo I., Guy J. B., Rancoule C., Cosset J. M., Deutsch E., Magne N. (2016) Risk of second cancers in the era of modern radiation therapy: does the risk/benefit analysis overcome theoretical models? Cancer Metastasis Rev. 35, 277–288 [DOI] [PubMed] [Google Scholar]
- 3.Holt G. E., Griffin A. M., Pintilie M., Wunder J. S., Catton C., O’Sullivan B., Bell R. S. (2005) Fractures following radiotherapy and limb-salvage surgery for lower extremity soft-tissue sarcomas. A comparison of high-dose and low-dose radiotherapy. J. Bone Joint Surg. Am. 87, 315–319 [DOI] [PubMed] [Google Scholar]
- 4.Oh D., Huh S. J. (2014) Insufficiency fracture after radiation therapy. Radiat. Oncol. J. 32, 213–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chandra A., Lan S., Zhu J., Lin T., Zhang X., Siclari V. A., Altman A. R., Cengel K. A., Liu X. S., Qin L. (2013) PTH prevents the adverse effects of focal radiation on bone architecture in young rats. Bone 55, 449–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chandra A., Lin T., Tribble M. B., Zhu J., Altman A. R., Tseng W. J., Zhang Y., Akintoye S. O., Cengel K., Liu X. S., Qin L. (2014) PTH1-34 alleviates radiotherapy-induced local bone loss by improving osteoblast and osteocyte survival. Bone 67, 33–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chandra A., Lin T., Young T., Tong W., Ma X., Tseng W. J., Kramer I., Kneissel M., Levine M. A., Zhang Y., Cengel K., Liu X. S., Qin L. (2016) Suppression of sclerostin alleviates radiation-induced bone loss by protecting bone-forming cells and their progenitors through distinct mechanisms. J. Bone Miner. Res. 32, 360–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao X., Wu X., Frassica D., Yu B., Pang L., Xian L., Wan M., Lei W., Armour M., Tryggestad E., Wong J., Wen C. Y., Lu W. W., Frassica F. J. (2011) Irradiation induces bone injury by damaging bone marrow microenvironment for stem cells. Proc. Natl. Acad. Sci. USA 108, 1609–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ergün H., Howland W. J. (1980) Postradiation atrophy of mature bone. CRC Crit. Rev. Diagn. Imaging 12, 225–243 [PubMed] [Google Scholar]
- 10.Güngör T., Hedlund T., Hulth A., Johnell O. (1982) The effect of irradiation on osteoclasts with or without transplantation of hematopoietic cells. Acta Orthop. Scand. 53, 333–337 [DOI] [PubMed] [Google Scholar]
- 11.Sawajiri M., Mizoe J., Tanimoto K. (2003) Changes in osteoclasts after irradiation with carbon ion particles. Radiat. Environ. Biophys. 42, 219–223 [DOI] [PubMed] [Google Scholar]
- 12.Crockett J. C., Rogers M. J., Coxon F. P., Hocking L. J., Helfrich M. H. (2011) Bone remodelling at a glance. J. Cell Sci. 124, 991–998 [DOI] [PubMed] [Google Scholar]
- 13.Vahle J. L., Sato M., Long G. G., Young J. K., Francis P. C., Engelhardt J. A., Westmore M. S., Linda Y., Nold J. B. (2002) Skeletal changes in rats given daily subcutaneous injections of recombinant human parathyroid hormone (1-34) for 2 years and relevance to human safety. Toxicol. Pathol. 30, 312–321 [DOI] [PubMed] [Google Scholar]
- 14.Chandra A., Lin T., Zhu J., Tong W., Huo Y., Jia H., Zhang Y., Liu X. S., Cengel K., Xia B., Qin L. (2015) PTH1-34 blocks radiation-induced osteoblast apoptosis by enhancing DNA repair through canonical Wnt pathway. J. Biol. Chem. 290, 157–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grundy G. J., Moulding H. A., Caldecott K. W., Rulten S. L. (2014) One ring to bring them all--the role of Ku in mammalian non-homologous end joining. DNA Repair (Amst.) 17, 30–38 [DOI] [PubMed] [Google Scholar]
- 16.Goodwin J. F., Knudsen K. E. (2014) Beyond DNA repair: DNA-PK function in cancer. Cancer Discov. 4, 1126–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gama V., Yoshida T., Gomez J. A., Basile D. P., Mayo L. D., Haas A. L., Matsuyama S. (2006) Involvement of the ubiquitin pathway in decreasing Ku70 levels in response to drug-induced apoptosis. Exp. Cell Res. 312, 488–499 [DOI] [PubMed] [Google Scholar]
- 18.Jiang N., Shen Y., Fei X., Sheng K., Sun P., Qiu Y., Larner J., Cao L., Kong X., Mi J. (2013) Valosin-containing protein regulates the proteasome-mediated degradation of DNA-PKcs in glioma cells. Cell Death Dis. 4, e647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chitra S., Nalini G., Rajasekhar G. (2012) The ubiquitin proteasome system and efficacy of proteasome inhibitors in diseases. Int. J. Rheum. Dis. 15, 249–260 [DOI] [PubMed] [Google Scholar]
- 20.Dou Q. P., Zonder J. A. (2014) Overview of proteasome inhibitor-based anti-cancer therapies: perspective on bortezomib and second generation proteasome inhibitors versus future generation inhibitors of ubiquitin-proteasome system. Curr. Cancer Drug Targets 14, 517–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zangari M., Suva L. J. (2016) The effects of proteasome inhibitors on bone remodeling in multiple myeloma. Bone 86, 131–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shalhoub V., Conlon D., Tassinari M., Quinn C., Partridge N., Stein G. S., Lian J. B. (1992) Glucocorticoids promote development of the osteoblast phenotype by selectively modulating expression of cell growth and differentiation associated genes. J. Cell. Biochem. 50, 425–440 [DOI] [PubMed] [Google Scholar]
- 23.Mohammad K. S., Chirgwin J. M., Guise T. A. (2008) Assessing new bone formation in neonatal calvarial organ cultures. Methods Mol. Biol. 455, 37–50 [DOI] [PubMed] [Google Scholar]
- 24.Chandra A., Lan S., Zhu J., Siclari V. A., Qin L. (2013) Epidermal growth factor receptor (EGFR) signaling promotes proliferation and survival in osteoprogenitors by increasing early growth response 2 (EGR2) expression. J. Biol. Chem. 288, 20488–20498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dempster D. W., Compston J. E., Drezner M. K., Glorieux F. H., Kanis J. A., Malluche H., Meunier P. J., Ott S. M., Recker R. R., Parfitt A. M. (2013) Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 28, 2–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Porter A. G., Jänicke R. U. (1999) Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 6, 99–104 [DOI] [PubMed] [Google Scholar]
- 27.Olive P. L., Banáth J. P. (2006) The comet assay: a method to measure DNA damage in individual cells. Nat. Protoc. 1, 23–29 [DOI] [PubMed] [Google Scholar]
- 28.Vignard J., Mirey G., Salles B. (2013) Ionizing-radiation induced DNA double-strand breaks: a direct and indirect lighting up. Radiother. Oncol. 108, 362–369 [DOI] [PubMed] [Google Scholar]
- 29.Elliot P. J., Adams J. (1999) Recent advances in understanding proteasome function. Curr. Opin. Drug Discov. Devel. 2, 484–490 [PubMed] [Google Scholar]
- 30.Konstantinova I. M., Tsimokha A. S., Mittenberg A. G. (2008) Role of proteasomes in cellular regulation. Int. Rev. Cell Mol. Biol. 267, 59–124 [DOI] [PubMed] [Google Scholar]
- 31.Hideshima T., Chauhan D., Richardson P., Mitsiades C., Mitsiades N., Hayashi T., Munshi N., Dang L., Castro A., Palombella V., Adams J., Anderson K. C. (2002) NF-kappa B as a therapeutic target in multiple myeloma. J. Biol. Chem. 277, 16639–16647 [DOI] [PubMed] [Google Scholar]
- 32.Palombella V. J., Rando O. J., Goldberg A. L., Maniatis T. (1994) The ubiquitin-proteasome pathway is required for processing the NF-kappa B1 precursor protein and the activation of NF-kappa B. Cell 78, 773–785 [DOI] [PubMed] [Google Scholar]
- 33.Kisselev A. F., Callard A., Goldberg A. L. (2006) Importance of the different proteolytic sites of the proteasome and the efficacy of inhibitors varies with the protein substrate. J. Biol. Chem. 281, 8582–8590 [DOI] [PubMed] [Google Scholar]
- 34.Wang X., Yen J., Kaiser P., Huang L. (2010) Regulation of the 26S proteasome complex during oxidative stress. Sci. Signal. 3, ra88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baugh J. M., Viktorova E. G., Pilipenko E. V. (2009) Proteasomes can degrade a significant proportion of cellular proteins independent of ubiquitination. J. Mol. Biol. 386, 814–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoyt M. A., Coffino P. (2004) Ubiquitin-free routes into the proteasome. Cell. Mol. Life Sci. 61, 1596–1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Casciola-Rosen L., Nicholson D. W., Chong T., Rowan K. R., Thornberry N. A., Miller D. K., Rosen A. (1996) Apopain/CPP32 cleaves proteins that are essential for cellular repair: a fundamental principle of apoptotic death. J. Exp. Med. 183, 1957–1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song Q., Lees-Miller S. P., Kumar S., Zhang Z., Chan D. W., Smith G. C., Jackson S. P., Alnemri E. S., Litwack G., Khanna K. K., Lavin M. F. (1996) DNA-dependent protein kinase catalytic subunit: a target for an ICE-like protease in apoptosis. EMBO J. 15, 3238–3246 [PMC free article] [PubMed] [Google Scholar]
- 39.Han Z., Malik N., Carter T., Reeves W. H., Wyche J. H., Hendrickson E. A. (1996) DNA-dependent protein kinase is a target for a CPP32-like apoptotic protease. J. Biol. Chem. 271, 25035–25040 [DOI] [PubMed] [Google Scholar]
- 40.Groll M., Berkers C. R., Ploegh H. L., Ovaa H. (2006) Crystal structure of the boronic acid-based proteasome inhibitor bortezomib in complex with the yeast 20S proteasome. Structure 14, 451–456 [DOI] [PubMed] [Google Scholar]
- 41.Zangari M., Terpos E., Zhan F., Tricot G. (2012) Impact of bortezomib on bone health in myeloma: a review of current evidence. Cancer Treat. Rev. 38, 968–980 [DOI] [PubMed] [Google Scholar]
- 42.Hurchla M. A., Garcia-Gomez A., Hornick M. C., Ocio E. M., Li A., Blanco J. F., Collins L., Kirk C. J., Piwnica-Worms D., Vij R., Tomasson M. H., Pandiella A., San Miguel J. F., Garayoa M., Weilbaecher K. N. (2013) The epoxyketone-based proteasome inhibitors carfilzomib and orally bioavailable oprozomib have anti-resorptive and bone-anabolic activity in addition to anti-myeloma effects. Leukemia 27, 430–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mukherjee S., Raje N., Schoonmaker J. A., Liu J. C., Hideshima T., Wein M. N., Jones D. C., Vallet S., Bouxsein M. L., Pozzi S., Chhetri S., Seo Y. D., Aronson J. P., Patel C., Fulciniti M., Purton L. E., Glimcher L. H., Lian J. B., Stein G., Anderson K. C., Scadden D. T. (2008) Pharmacologic targeting of a stem/progenitor population in vivo is associated with enhanced bone regeneration in mice. J. Clin. Invest. 118, 491–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Von Metzler I., Krebbel H., Hecht M., Manz R. A., Fleissner C., Mieth M., Kaiser M., Jakob C., Sterz J., Kleeberg L., Heider U., Sezer O. (2007) Bortezomib inhibits human osteoclastogenesis. Leukemia 21, 2025–2034 [DOI] [PubMed] [Google Scholar]
- 45.Cipriani C., Irani D., Bilezikian J. P. (2012) Safety of osteoanabolic therapy: a decade of experience. J. Bone Miner. Res. 27, 2419–2428 [DOI] [PubMed] [Google Scholar]
- 46.Chouinard L., Felx M., Mellal N., Varela A., Mann P., Jolette J., Samadfam R., Smith S. Y., Locher K., Buntich S., Ominsky M. S., Pyrah I., Boyce R. W. (2016) Carcinogenicity risk assessment of romosozumab: a review of scientific weight-of-evidence and findings in a rat lifetime pharmacology study. Regul. Toxicol. Pharmacol. 81, 212–222 [DOI] [PubMed] [Google Scholar]
- 47.Richardson P. G., Sonneveld P., Schuster M. W., Stadtmauer E. A., Facon T., Harousseau J. L., Ben-Yehuda D., Lonial S., Goldschmidt H., Reece D., Bladé J., Boccadoro M., Cavenagh J. D., Boral A. L., Esseltine D. L., Wen P. Y., Amato A. A., Anderson K. C., San Miguel J. (2009) Reversibility of symptomatic peripheral neuropathy with bortezomib in the phase III APEX trial in relapsed multiple myeloma: impact of a dose-modification guideline. Br. J. Haematol. 144, 895–903 [DOI] [PubMed] [Google Scholar]