Abstract
The tumor necrosis factor receptor–associated factor 2 (TRAF2) is a second messenger adaptor protein that plays an essential role in propagating TNF-α-mediated signaling pathways. Modulation of TRAF2 activity by ubiquitination is well studied; however, the deubiquitinating enzyme (DUB), which regulates TRAF2 stability, has not been identified. Here we reveal USP48 as the first identified DUB to deubiquitinate and stabilize TRAF2 in epithelial cells. Down-regulation of USP48 increases K48-linked polyubiquitination of TRAF2 and reduces TRAF2 protein levels. Interestingly, USP48 only targets the TRAF2 related to JNK pathway, not the TRAF2 related to NF-κB and p38 pathways. USP48 is serine phosphorylated in response to TNF-α. The phosphorylation is catalyzed by glycogen synthase kinase 3β (GSK3β), ultimately resulting in increases in USP48 DUB activity. Furthermore, we reveal a new biologic function of TRAF2 that contributes to epithelial barrier dysfunction, which is attenuated by knockdown of USP48. Inhibition of TRAF2/JNK pathway increases E (epithelial)-cadherin expression and enhances epithelial barrier integrity, while knockdown of USP48 attenuates TNF-α/JNK pathway and increases E-cadherin expression and cell–cell junction in epithelial cells. These data, taken together, indicate that USP48 stabilizes TRAF2, which is promoted by GSK3β-mediated phosphorylation. Further, down-regulation of USP48 increases E-cadherin expression and epithelial barrier integrity through reducing TRAF2 stability.—Li, S., Wang, D., Zhao, J., Weathington, N. M., Shang, D., Zhao, Y. The deubiquitinating enzyme USP48 stabilizes TRAF2 and reduces E-cadherin-mediated adherens junctions.
Keywords: deubiquitination, phosphorylation, signal pathway, epithelial barrier integrity
The epithelium forms a continuous physical barrier that protects against environmental challenge. It plays important roles in host defense and is regulated in large part by epithelial cell–cell junctions (1), composed of tight junctions, adherens junctions, and desmosomes. Cadherins constitute a large family of cell surface proteins, including E (epithelial)-, N (neural)-, and VE (vascular–endothelial)-cadherin (2), and they represent the major adhesion molecules in adherens junctions. E-cadherin mediates homotypic interaction between neighboring epithelial cells and is thus critical for the maintenance of epithelial barrier integrity (3). Loss of E-cadherin disrupts establishment of cell–cell junction and results in increases in epithelial permeability (4, 5) and motility (6). Mechanistic insights into E-cadherin regulation could greatly enhance our understanding of the pathogenesis and approach to treatment human disorders caused by epithelial barrier dysfunction.
In acute infectious diseases or a systemic inflammatory response, TNF-α is highly up-regulated and epithelial layers of organ tissues lose barrier integrity, causing fluid handling at the tissue level and impairing organ function. At the cellular level, TNF-α signaling results in loss of E-cadherin (7). TNF receptor–associated factors (TRAFs) are a family of adaptor proteins that bind directly to the cytoplasmic regions of TNF receptor (TNFR) superfamily. Among the 7 TRAF family members (TRAF1–TRAF7), all except TRAF1 possess a RING finger domain and E3 ubiquitin ligase activity critical for their signaling (8, 9). All TRAFs contain a zinc-finger motif, a coiled coil domain, and a C-terminal TRAF domain (10, 11). TRAF2 regulates multiple cellular responses. Upon TNF-α stimulation, TRAF2 is recruited to TNFR1 or TNFR2, leading to the activation of NF-κB and JNK signaling pathways (12–15). While JNK has been shown to play an important role in regulating E-cadherin junctions (16–18), the effect of TRAF2 on E-cadherin expression has not been studied.
Protein ubiquitination is a reversible covalent modification whereby ubiquitin E3 ligases conjugate the 8-kDa ubiquitin singly or in chains to target proteins’ acceptor lysine amino acid residues to regulate stability, activity, and localization of the target proteins. This process is integral to many biologic processes, such as cell cycle control, DNA damage repair, immune response, and signal transduction (19). A family of deubiquitinating enzymes (DUBs) cleave ubiquitin or ubiquitin-like proteins from the target proteins and determine target proteins’ fate with downstream impact on cellular processes (20). DUB activities are regulated through a number of different mechanisms, including substrate-induced conformational changes, adaptor protein binding, proteolytic cleavage, and posttranslational modifications (21, 22). For instance, IKKγ-mediated CYLD phosphorylation negatively affects the deubiquitinating activity of CYLD (23). Akt phosphorylates USP14 and increases its DUB activity (24). Thus, priming phosphorylation events seem to be common controllers of DUB catalytic activity.
Molecular regulation of TRAF2 stability and activity by the ubiquitin system is a topic of recent investigation. The ubiquitin editing protein A20 targets and ubiquitinates TRAF2 for its lysosomal degradation (25). E3 ubiquitin ligases, FBXL2, siah2, and cIAP-1 ubiquitinate TRAF2 to facilitate its proteasomal degradation (26–28). It has also been shown that DUBs, such as USP4, USP25, and CYLD, deubiquitinate TRAF2 and modulate its signal transduction (8, 29, 30). While these DUB enzymes affect TRAF2 activity, modulation of TRAF2 protein stability by a DUB enzyme has not been described. The USP48 DUB, also known as USP31, has been shown to associate with TRAF2 (31) without any description of USP48 action on TRAF2 activity or stability.
In the present study, we demonstrate that USP48 deubiquitinates and stabilizes TRAF2 in epithelial cells. Knockdown of USP48 increases abundance of K48-linked polyubiquitinated TRAF2. Glycogen synthase kinase 3β (GSK3β)-mediated USP48 phosphorylation enhances its DUB activity. Furthermore, knockdown of USP48 attenuates TNF-α-induced phosphorylation of JNK1 and c-Jun, but not p38 and I-κB. Knockdown of USP48 also increases E-cadherin mRNA and protein levels through destabilization of TRAF2 and inactivation of the TRAF2-TNIK-JNK pathway, with resultant enhancement of epithelial barrier integrity. This study reveals that GSK3β activates USP48, which in turn stabilizes TRAF2, permitting potent TNF-α-mediated activation of JNK and repression of E-cadherin-mediated epithelial barrier integrity.
MATERIALS AND METHODS
Cell culture and reagents
Human lung epithelial cells [Beas2B and human bronchial epithelial cells; American Type Culture Collection (ATCC), Manassas, VA, USA] and murine lung epithelial 12 (MLE12) cells (ATCC) were cultured with medium supplemented with hydrocortisone, insulin, transferrin, estrogen, selenium, 10% fetal bovine serum, and antibiotics at 37°C in 5% CO2 incubator. A549 cells were cultured with RPMI 1640 medium containing 10% fetal bovine serum and antibiotics. HEK293 cells were cultured in DMEM containing 10% fetal bovine serum and antibiotics. Human Usp11 small interfering RNA (siRNA), immobilized protein A/G beads, antibodies against TRAF1, TRAF3–6, and control IgG were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies against phospho-JNK1, JNK1, phospho-cJun, phospho-IKKα/β, IKKβ, phospho-P38, P38, P100/P52, I-κB, TRAF2, hemagglutinin (HA) tag, K48 ubiquitin, K63 ubiquitin, and ubiquitin were from Cell Signaling Technology (Danvers, MA, USA). Superfect transfection reagent was from Qiagen (Germantown, MD, USA). V5 antibody, E-cadherin, the mammalian expression plasmid pcDNA3.1/V5-His TOPO, Escherichia coli Top 10 competent cells, and Lipofectamine RNAiMax reagent were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Antibodies against to USP11 and USP48 were obtained from Abcam (Cambridge, MA, USA). Cycloheximide (CHX), leupeptin, and β-actin antibodies were from Sigma-Aldrich (St. Louis, MO, USA). Human Usp48 siRNA and control siRNA were purchased from Thermo Fisher Scientific. Mouse usp48 shRNA was purchased from GE Dharmacon (Lafayette, CO, USA). Phospho-serine (p-Serine) antibody, KY-05009, and MG132 were from EMD Millipore (Billerica, MA, USA). Horseradish peroxidase–conjugated goat anti-rabbit and anti-mouse secondary antibodies were obtained from Bio-Rad (Hercules, CA, USA). TWS119 was from Cayman Chemicals (Ann Arbor, MI, USA). All materials used in the experiments were the highest grades commercially available.
Construction of plasmids
Human Usp48 cDNA was inserted into pcDNA3.1D/His-V5 TOPO vector. Intracellular domain 886–890 deletion mutants of Usp48 were generated by PCR with specific primers designed to target the USP48 cDNA sequence. Site-directed mutagenesis was performed to generate Usp48 mutants according to the manufacturer’s instructions (Agilent Technologies, Santa Clara, CA, USA). Plasmid pEBB-3xMyc-TRAF2 (44104) was a gift from W. Hahn (Addgene, Cambridge, MA, USA).
Plasmid and siRNA transfection
Cells were subcultured on 6-well plates, 35-mm plates, or 10-mm dishes to 70 to 90% confluence. Superfect transfection reagent was added to the mixture containing varying amounts of plasmid and 200 μl of Opti-medium, then incubated for 10 min to allow transfection reagent/DNA complexes to form. The mixture was then added directly to the cells with complete medium. MLE12 cells grown on 100-mm plates (70–90% confluence) were transfected with plasmids using Lonza electroporation transfection according to the manufacturer’s protocol (Lonza, Basel, Switzerland). siRNAs and Lipofectamine RNAiMax reagent were diluted separately in Opti-MEM medium, then incubated together for 5 min at room temperature. Transfection mix was replaced with complete cell culture medium after 3 h. Analysis of the transfected cells was performed 24 and 72 h later.
Immunoprecipitation and in vivo ubiquitin assay
Cells were washed with cold PBS and collected in cell lysis buffer. For immunoprecipitation, equal amounts of cell lysates (1 mg) were incubated with specific primary antibody overnight at 4°C, followed by the addition of 40 μl of protein A/G agarose beads and incubation for additional 2 h at 4°C. The immunoprecipitated complex was washed 3 times with PBS and analyzed by immunoblotting with the indicated antibodies. For the in vivo ubiquitin assay, we performed a modified protocol under denaturing conditions. After cells were treated as indicated in the presence of TNF-α + CHX and proteasome and lysosome inhibitors, cells were washed and collected with cold PBS. After centrifuging at 1000 rpm for 5 min, supernatant was removed, and 1 μl of ubiquitin aldehyde and 1 μl of N-ethylmaleimide were added into the cell pellet. SDS lysis buffer (2%; 50 to 80 μl) was added according to the cell amount. Cell lysates were then sonicated on ice for 12 s, followed by boiling at 100°C for 10 min. The denatured samples were diluted with Tris-buffered saline. The following steps were the same as normal immunoprecipitation.
RNA isolation, reverse transcription, and real-time quantitative PCR
Total RNA was isolated from cultured Beas2B cells using the NucleoSpin RNA extraction kit (Clontech Laboratories, Mountain View, CA, USA) according to the manufacturer’s instructions, and RNA was quantified by spectrophotometry. cDNA was prepared using the iScript cDNA synthesis kit (Bio-Rad). Real-time quantitative PCR (qPCR) was performed to assess expression of Usp48, Traf2, Traf6, and E-cadherin using primers designed based on human mRNA sequences. Usp48 primers were as follows: (forward) 5′-CAGTAAAGGGCAGCGATGGA-3′ and (reverse) 5′-TCTGCATCACCATCTTGCTCA-3′; Traf2 primers, (forward) 5′-TGCGACCGTTGGGGCT-3′ and (reverse) 5′-GAGAAGCCGGGCTGTAGCAA-3′; Traf6 primers, (forward) 5′-TTGGCTGAGCGGAGTCGT-3′ and (reverse) 5′-AAGAAAGCTGGGTCCCTTCAG-3′; and E-cadherin primers, (forward) 5′-GTCAGTTCAGACTCCAGCCC-3′ and (reverse) 5′-GCTGCGGCTCCAAGGG-3′. Real-time qPCR was performed using iQ SYBR Green Supermix and the iCycler Real-Time PCR Detection System (Bio-Rad).
In vitro deubiquitination assays
USP48-V5, USP48C98S-V5, and USP48 Δ886–890-V5 were translated using the Transcription/Translation System (Promega, Madison, WI, USA) as directed by the manufacturer. Recombinant USP48 and USP48 mutant proteins were incubated with specific primary antibodies and protein A/G agarose beads for 2 h. The immunoprecipitated complex was washed twice with PBS. Enzymes and substrates were prepared in ubiquitinated (Ub)-AMC buffer containing 50 mM Tris-HCl (pH 7.5), 1 mM EDTA, 1 mM ATP, 5 mM MgCl2, 1 mM DTT, and 1 mg/ml bovine serum albumin as previously described (24). The reaction mixtures were subjected to 10% SDS-PAGE, followed by blotting with an ubiquitin antibody.
Coimmunofluorescence analysis by confocal microscopy
Beas2B cells were cultured in glass-bottomed dishes. Cells were transfected with plasmids for 48 h; cells were then fixed with 3.7% formaldehyde. After blocking in 1% bovine serum albumin in Tris-buffered saline with 0.1% Tween 20 for 1 h, cells were incubated with 1:200 dilution of primary antibody for 1 h, followed by a 1:200 dilution of fluorescence-conjugated secondary antibodies sequentially for immunostaining. Images were captured by an Eclipse TE 300 inverted confocal microscope (Nikon, Tokyo, Japan). Tomographic and 3-dimensional images were analyzed by NIS-Elements software (Nikon).
Measurement of transepithelial electrical resistance by electric cell-substrate impedance sensing system
A549, Beas2B, or human bronchial epithelial cells were grown on gold microelectrodes till confluence. Transepithelial electrical resistance (TEER) across the epithelial monolayer was dynamically measured by the electric cell-substrate impedance sensing (ECIS) system (Applied BioPhysics, Foster City, CA, USA). Increases in TEER indicate enhancement of epithelial barrier integrity.
Quantification and statistical analysis
Phosphorylated USP48-V5, phosphorylated USP48, E-cadherin, ZO-1, claudin 1, mono-Ub, TRAF2, and Ub-TRAF2 levels and TRAF2 intensities were quantified by ImageJ software (Image Processing and Analysis in Java; National Institutes of Health, Bethesda, MD, USA; http://imagej.nih.gov/). All results were subjected to statistical analysis by 2-way ANOVA and, wherever appropriate, Student’s t test. Data are expressed as means ± sd of triplicate samples from at least 3 independent experiments, and P < 0.05 was considered statistically significant.
RESULTS
USP48 stabilizes TRAF2 protein, not other TRAFs
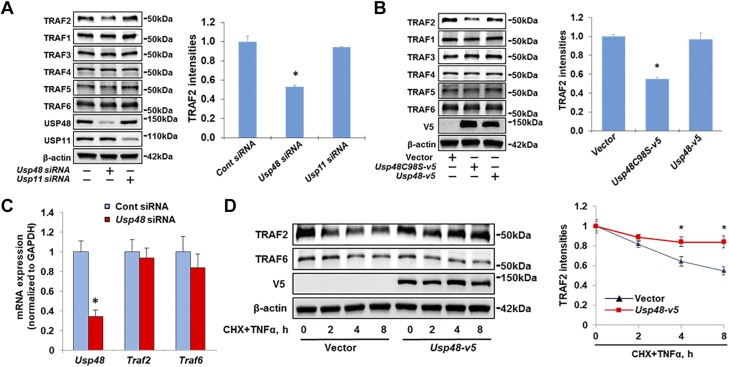
It has been shown that USP48 is an interacting partner of TRAF2 in a yeast 2-hybrid screen (31); however, the effects of USP48 on TRAF2 protein stability and activity have not been reported. To test the functional significance of this interaction, we examined whether USP48 affects TRAF2 protein levels. As shown in Fig. 1A, Usp48 siRNA transfection significantly reduced USP48 expression, as well as endogenous TRAF2 protein levels, without altering TRAF1 and TRAF3–6 abundance in Beas2B cells. TRAF2 levels were not changed by knockdown of another DUB USP11. Similarly, knockdown of USP48 reduced TRAF2 protein levels in both MLE12 and HEK293 cells, without altering other TRAF levels (Supplemental Fig. S1). Cysteine 98 is a key residue for USP48 deubiquitinating activity. In a similar fashion, overexpression of USP48C98S-V5, a catalytically inactive mutant that behaves as a dominant-negative enzyme (31, 32), decreased the endogenous TRAF2 protein levels (Fig. 1B). Knockdown of USP48 had no effect on Traf2 mRNA abundance (Fig. 1C) in Beas2B, implying that the effects of USP48 might be through modulating TRAF2 protein stability. Compared to cells transfected with empty vector, overexpression of USP48 protected TRAF2 degradation in the presence of TNF-α and CHX in Beas2B (Fig. 1D). Taken together, these data suggest that USP48 specifically stabilizes TRAF2.
Figure 1.
USP48 stabilizes TRAF2. A) Knockdown of USP48 decreases TRAF2 levels. Beas2B cells were transfected with control siRNA, Usp48 siRNA, or Usp11 siRNA for 3 d; then TRAF2, TRAF1, TRAF3, TRAF4, TRAF5, TRAF6, USP48, USP11, and β-actin levels were analyzed by Western blot analysis. *P < 0.01 compared to control siRNA-transfected cells. B) Inhibition of USP48 decreases TRAF2 levels. Beas2B cells were transfected with Usp48 wild-type or Usp48 cysteine mutant plasmid as indicated for 48 h; cell lysates were analyzed by immunoblotting with antibodies to TRAF2, TRAF1, TRAF3, TRAF4, TRAF5, TRAF6, V5, and β-actin. *P < 0.01 compared to vector-transfected cells. C) Knockdown of USP48 has no effect on TRAF2 mRNA levels. Beas2B cells were transfected with control siRNA or Usp48 siRNA and incubated for 3 d. Total RNAs were extracted and analyzed by real-time qPCR with Usp48, Traf2, and Traf6 primers. Relative expression of Usp48, Traf2, and Traf6 were normalized to Gapdh. *P < 0.01 compared to control siRNA. D) USP48 overexpression increases TRAF2 half-life. Beas2B cells were transfected with empty vector or V5-tagged Usp48 plasmid and incubated for 48 h. Cells were then treated with CHX (20 μg/ml) plus TNF-α (10 ng/ml) for indicated times. Cell lysates were examined by immunoblotting with V5, TRAF2, TRAF6, and β-actin antibodies. *P < 0.01 compared to empty vector-transfected cells. Shown are representative blots from at least 3 independent experiments. Data are presented as means ± sd.
USP48 deubiquitinates TRAF2 in cytoplasm
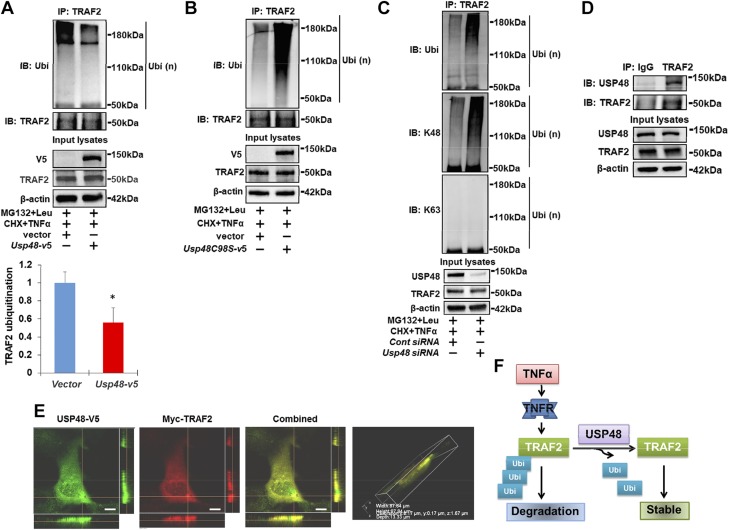
To investigate whether USP48 regulates TRAF2 stability by modulating the ubiquitination state of TRAF2, cellular ubiquitination assays were performed to examine the effect of USP48 on Ub-TRAF2 levels. Overexpression of USP48 reduced the Ub-TRAF2 levels (Fig. 2A), whereas the levels of Ub-TRAF2 were increased by overexpression of the inactive USP48C98S-V5 (Fig. 2B). TRAF2 has been known to be polyubiquitinated with K48- and K63-linked ubiquitin chains (33–35). Further, we examined which type of ubiquitination is regulated by USP48. As shown in Fig. 2C, K48-linked polyubiquitination is the dominant modification of TRAF2 in lung epithelial cells. Knockdown of USP48 increased the abundance of Ub-TRAF2, mostly K48-linked (and not K63-linked) polyubiquitinated TRAF2. Coimmunoprecipitation confirmed that TRAF2 was associated with USP48 (Fig. 2D). Further, we examined the cellular location of TRAF2 and USP48 by cotransfection of Myc-Traf2 and Usp48-v5 plasmids. As shown in Fig. 2E, both Myc-TRAF2 and USP48-V5 were colocalized in the cytoplasm of Beas2B cells. These data collectively suggest that USP48 associates with TRAF2 in the cytoplasm and that USP48 deubiquitinates and thus stabilizes TRAF2 (Fig. 2F).
Figure 2.
USP48 deubiquitinates TRAF2 in cytoplasm. A) Overexpression of USP48 reduces TRAF2 ubiquitination. Beas2B cells were cotransfected with HA-Ubi + vector or HA-Ubi + Usp48-v5 plasmids and incubated for 48 h. Denatured cell lysates were subjected to immunoprecipitation with TRAF2 antibody, followed by immunoblotting with ubiquitin or TRAF2 antibody. Input lysates were analyzed by immunoblotting with V5, TRAF2, and β-actin antibodies. B) Inhibition of USP48 activity elevates TRAF2 ubiquitination. Beas2B cells were cotransfected with HA-Ubi + vector or HA-Ubi + Usp48c98s-v5 plasmids and incubated for 48 h. Denatured cell lysates were subjected to immunoprecipitation with TRAF2 antibody, followed by immunoblotting with ubiquitin or TRAF2 antibody. Input lysates were analyzed by immunoblotting with V5, TRAF2, and β-actin antibodies. C) Knockdown of USP48 increases K48-linked polyubiquitination of TRAF2. Beas2B cells were transfected with control siRNA or Usp48 siRNA, then transfected with HA-Ubi plasmids and incubated for 3 d. Denatured cell lysates were subjected to immunoprecipitation with TRAF2 antibody, followed by immunoblotting with ubiquitin, K48 ubiquitin, and K63 ubiquitin antibodies. Input lysates were analyzed by immunoblotting with USP48, TRAF2, and β-actin antibodies. D) USP48 is associated with TRAF2. HEK293 cell lysates were subjected to immunoprecipitation with IgG or anti-TRAF2 antibody, then analyzed by immunoblotting with USP48 or TRAF2 antibody. Input cell lysates were immunoblotted with USP48, TRAF2, and β-actin antibodies. Shown are representative blots from at least 3 independent experiments. E) USP48 is colocalized with TRAF2. Beas2B cells were cotransfected with Myc-tagged Traf2 (Myc-TRAF2) and V5-tagged Usp48 (Usp48-v5) and incubated for 24 h. Localization of Myc-TRAF2 and USP48-V5 were detected by immunostaining with antibodies to Myc tag (red) and V5 tag (green). Scale bars, 5 µm. F) Scheme showing USP48 deubiquitinates and stabilizes TRAF2 (n = 3). *P < 0.01 compared to vector transfected cells.
TNF-α induces USP48 site-specific phosphorylation, which is mediated by GSK3β
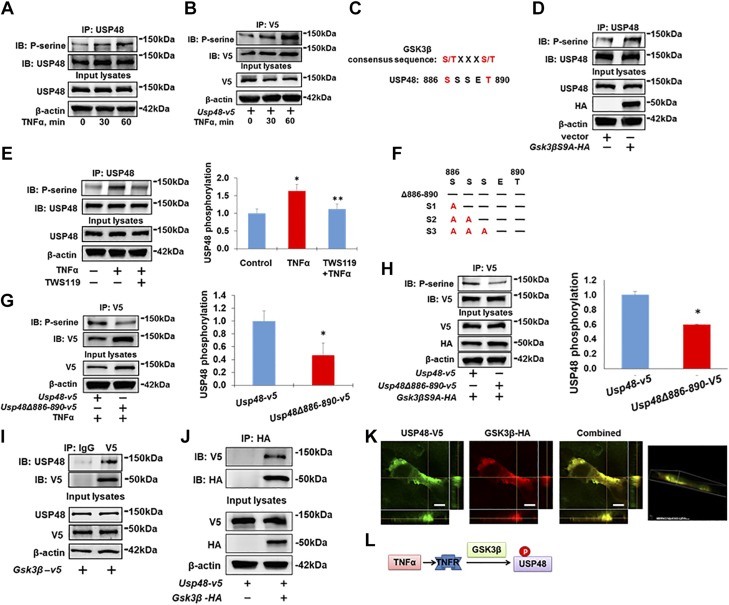
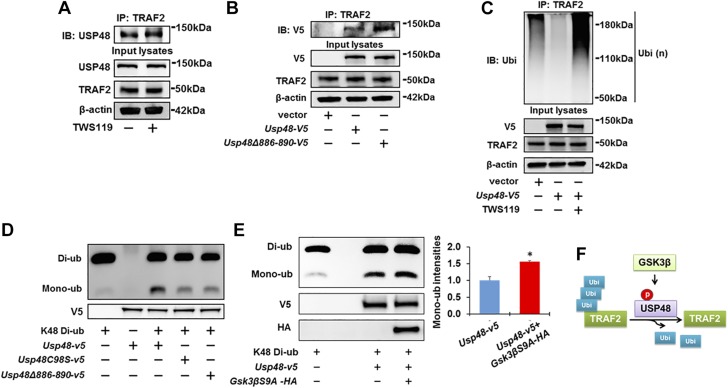
Recent studies have illuminated that the catalytic activity of DUBs can be regulated by their posttranslational modifications (20, 21), including phosphorylation (36). We found that TNF-α induced rapid serine phosphorylation of both endogenous USP48 (Fig. 3A) and overexpressed USP48-V5 (Fig. 3B) within 30 min. GSK3β, a downstream molecule of TNF-α (37–39), regulates the phosphorylation of multiple signaling proteins (40, 41), which share a common phosphorylation motif Ser/Thr-X-X-X-Ser/Thr (42). USP48 contains a consensus sequence motif (Ser886-S-S-E-Thr890) for phosphorylation by GSK3β (Fig. 3C). Thus, we next determined whether USP48 is a substrate of GSK3β. Overexpression of a constitutively active form of GSK3β (GSK3βS9A-HA) triggered phosphorylation of USP48 (Fig. 3D), whereas pretreatment with a GSK3β inhibitor, TWS119, attenuated the TNF-α-induced phosphorylation of USP48 (Fig. 3E), indicating that the TNF-α-induced phosphorylation of USP48 is mediated by GSK3β.
Figure 3.
GSK3β phosphorylates USP48. A, B) TNF-α induces USP48 phosphorylation. Beas2B cells (A) or USP48-V5-overexpressing Beas2B cells (B) were treated with TNF-α (10 ng/ml, 0, 30, and 60 min), assessed by immunoprecipitation with antibody to USP48 (A) or V5 (B), and by immunoblot analysis of precipitates with p-Serine and USP48 (A) or V5 (B) antibodies. Input lysates were analyzed with USP48 (A) or V5 (B), and β-actin antibodies. C) USP48 has GSK3β consensus phosphorylation motif (aa 886–890). D) GSK3β phosphorylates USP48. Beas2B cells were transfected with empty vector or HA-tagged GSK3β mutant with S9A (Gsk3βS9A-HA) and incubated for 48 h. Cell lysates were immunoprecipitated with USP48 antibody, and immunoblot analysis of precipitates was performed with p-Serine and USP48 antibodies. Input lysates were analyzed with USP48, HA, and β-actin antibodies. E) Inhibition of GSK3β attenuates TNF-α-induced phosphorylation of TRAF2. Beas2B cells were treated with or without TWS119 (10 μM) for 1 h; cells were then treated with or without TNF-α (10 ng/ml) for 1 h. Cell lysates were immunoprecipitated with USP48 antibody, and immunoblot analysis of precipitates was performed with p-Serine and USP48 antibodies. Input lysates were analyzed with USP48 and β-actin antibodies (n = 3). *P < 0.01 compared to control (untreated); **P < 0.01 compared to TNF-α-treated cells. F) USP48 mutants harboring various S/A substitutions. G) Identification of phosphorylation motif within USP48 in response to TNF-α treatment. Beas2B cells were transfected with Usp48-v5 or V5-tagged USP48 deletion mutant (Usp48Δ886–890-v5) plasmids and incubated for 48 h. Cells were then treated with TNF-α (10 ng/ml) for 1 h. Cell lysates were immunoprecipitated with V5 antibody, and immunoblot analysis of precipitates was performed with p-Serine and V5 antibodies. Input lysates were immunoblotted with V5 and β-actin antibodies (n = 3). *P < 0.01 compared to Usp48-v5 transfected cells. H) Identification of GSK3β phosphorylation motif within USP48. Beas2B cells were cotransfected with Usp48-v5 + Gsk3βS9A-HA or Usp48Δ886–890-v5 + Gsk3βS9A-HA plasmids and incubated for 48 h. Cell lysates were immunoprecipitated with V5 antibody, and immunoblot analysis of precipitates was performed with p-Serine and V5 antibodies. Input lysates were immunoblotted with V5, HA, and β-actin antibodies. n = 3, *P < 0.01 compared to Usp48-v5 transfected cells. I) Endogenous USP48 is associated with GSK3β-V5. Overexpressed GSK3β-V5 in HEK293 cells was immunoprecipitated with IgG or V5 antibody, then analyzed by immunoblotting with USP48 antibody. Input cell lysates were immunoblotted with USP48, V5, and β-actin antibodies. J) USP48-V5 is associated with GSK3β-HA. HEK293 cells were cotransfected with Usp48-v5 with or without Gsk3β-HA and incubated for 48 h. Cell lysates were subjected to immunoprecipitation with HA antibody, followed by immunoblotting with V5 antibody. Input lysates were analyzed by immunoblotting with V5, HA, and β-actin antibodies. Shown are representative blots from at least 3 independent experiments. K) USP48-V5 is colocalized with GSK3β-HA. Beas2B cells were cotransfected with Gsk3β-HA and Usp48-v5 and incubated for 24 h. Localization of GSK3β-HA and USP48-V5 were detected by immunostaining with antibodies to HA tag (red) and V5 tag (green). Scale bars, 5 µm. L) Scheme showing TNF-α induces USP48 phosphorylation through GSK3β.
To further confirm that Ser886-S-S-E-Thr890 within USP48 is a GSK3β phosphorylation site, several USP48 site mutants within the aa 886–890 region were generated and subjected to phosphorylation analysis (Fig. 3F). We observed that deletion of this putative GSK3β motif (USP48Δ886–890-V5) significantly reduced USP48 phosphorylation in the presence of TNF-α (Fig. 3G) or active GSK3βS9A-HA (Fig. 3H). Further, a USP48 triple-serine mutant (S886–888/A) also exhibited a reduction in USP48 phosphorylation that was not observed in single- or double-serine mutations, such as USP48S886A, USP48S887A, and USP48S886/887A (Supplemental Fig. S2).
Coimmunoprecipitation studies revealed that GSK3β was associated with endogenous USP48 (Fig. 3I) and overexpressed USP48 (Fig. 3J). Further, colocalization of GSK3β-HA and USP48-V5 in the cytoplasm was confirmed by coimmunofluorescence staining by confocal microscopy (Fig. 3K). Taken together, these results suggest that USP48 is a substrate of GSK3β (Fig. 3L).
GSK3β-mediated phosphorylation of USP48 facilitates TRAF2 stabilization
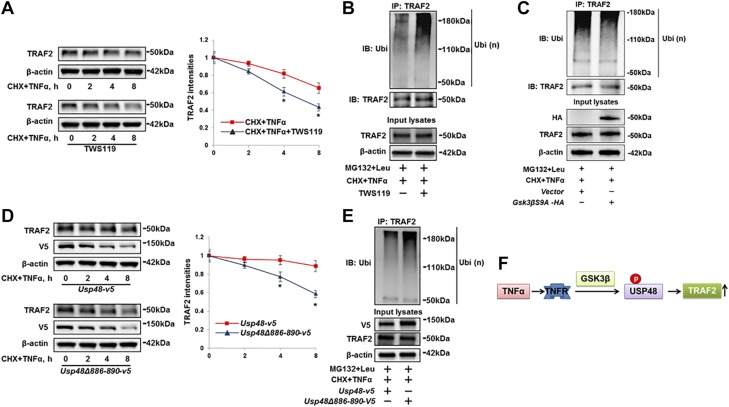
To investigate whether GSK3β-mediated phosphorylation of USP48 influences TRAF2 stabilization, cells were incubated with TWS119 before CHX and TNF-α combination treatment. TWS119 augmented TRAF2 degradation (Fig. 4A) and ubiquitination (Fig. 4B), while overexpression of active GSK3βS9A-HA reduced Ub-TRAF2 levels (Fig. 4C). Consistent with the pharmacologic studies, genetic manipulation of USP48 phosphorylation by overexpression of USP48Δ886–890 promoted TRAF2 degradation (Fig. 4D) and increased TRAF2 ubiquitination (Fig. 4E) compared to USP48 wild type. These data suggest that USP48 phosphorylation by GSK3β serves as a critical mechanism for regulating TRAF2 ubiquitination and degradation (Fig. 4F).
Figure 4.
USP48 phosphorylation is required for TRAF2 ubiquitination and stability. A) Inhibition of GSK3β promotes TRAF2 degradation. Beas2B cells were treated with or without TWS119 (10 μM) for 1 h; then cells were treated with CHX (20 μg/ml) plus TNF-α (10 ng/ml) for 0 to 8 h. Cell lysates were examined by immunoblotting with TRAF2 and β-actin antibodies. Data are presented as means ± sd (n = 3). *P < 0.01 compared to CHX + TNF-α-treated cells. B) Inhibition of GSK3β promotes TRAF2 ubiquitination. Beas2B cells were treated with TWS119 (10 μM) for 1 h. Denatured cell lysates were subjected to immunoprecipitation with TRAF2 antibody, followed by immunoblotting with ubiquitin antibody. Input lysates were immunoblotted with TRAF2 and β-actin antibodies. C) Overexpression of GSK3βS9A reduces TRAF2 ubiquitination. Beas2B cells were transfected with empty vector or Gsk3β-HA plasmids and incubated for 48 h. Denatured cell lysates were subjected to immunoprecipitation with TRAF2 antibody, followed by immunoblotting with ubiquitin or TRAF2 antibody. Input lysates were immunoblotted with HA, TRAF2, and β-actin antibodies. D) Phosphorylation motif mutant of USP48 loses effect of USP48 on stabilization of TRAF2. Beas2B cells were transfected with Usp48-v5 or Usp48Δ886–890-v5 and incubated for 48 h. Cells were then treated with CHX (20 μg/ml) plus TNF-α (10 ng/ml) for 0 to 8 h. Cell lysates were examined by immunoblotting with TRAF2, V5, and β-actin antibodies (n = 3). *P < 0.01 compared to Usp48-v5-transfected cells. E) Overexpression of GSK3β phosphorylation motif mutant of USP48 increases TRAF2 ubiquitination. Beas2B cells were cotransfected with Usp48-v5 or Usp48Δ886–890-v5 plasmids for 48 h. Denatured cell lysates were subjected to immunoprecipitation with TRAF2 antibody, followed by immunoblotting with ubiquitin antibody. Input lysates were analyzed by immunoblotting with V5, TRAF2, and β-actin antibodies. Shown are representative blots from at least 3 independent experiments. F) Scheme showing GSK3β-mediated USP48 phosphorylation increases TRAF2 stability. Data are presented as means ± sd.
Phosphorylation of USP48 by GSK3β enhances USP48 DUB activity
To understand the mechanisms by which USP48 phosphorylation regulates TRAF2 stability, we first examined whether USP48 phosphorylation by GSK3β influences the association between TRAF2 and USP48. TWS119 (Fig. 5A) or overexpression of Δ886–890 deletion mutant (Fig. 5B) had no effect on the association between USP48 and TRAF2, suggesting that phosphorylation of USP48 does not prevent its binding to substrate. Interestingly, we found that TWS119 treatment of cells attenuated USP48-mediated deubiquitination of TRAF2 (Fig. 5C). Thus, we hypothesized that the phosphorylation may influence the catalytic activity of USP48. USP48 has been known to preferentially depolymerize K48-linked polyubiquitin chains (32). Consistent with this finding, our data demonstrate a USP48 deubiquitinase activity on K48-linked Ub-TRAF2 (Fig. 2C). We performed an in vitro DUB assay to assess the impact of USP48 phosphorylation on hydrolysis of K48-diubiquitin. USP48 wild-type protein, USP48C98S mutant protein, and USP48Δ886–890 mutant protein were synthesized in a reticulocyte lysate transcription and translation system. Cleavage of K48-diubiquitin was decreased in the presence of USP48C98S or USP48Δ886–890 mutant proteins compared to wild-type USP48 (Fig. 5D). Furthermore, USP48 showed a greatly enhanced enzymatic activity after incubation with active GSK3βS9A-HA (Fig. 5E). Taken together, these results demonstrate that USP48 phosphorylation by GSK3β enhances its DUB catalytic activity (Fig. 5F).
Figure 5.
Phosphorylation of USP48 by GSK3β increases USP48 activity. A) Inhibition of GSK3β has no effect on association between USP48 and TRAF2. Beas2B cells were treated with or without TWS119 (10 μM) for 1 h. Cell lysates were then immunoprecipitated with TRAF2 antibody, followed by immunoblotting with USP48 antibody. Input cell lysates were immunoblotted with USP48, TRAF2, and β-actin antibodies. B) Deletion of phosphorylation motif of USP48 has no effect on USP48 interaction with TRAF2. Beas2B cells were transfected with empty vector, Usp48-v5, or Usp48Δ886–890-v5 as indicated and incubated for 48 h. Cell lysates were then immunoprecipitated with TRAF2 antibody, followed by immunoblotting with V5 antibody. Input cell lysates were immunoblotted with V5, TRAF2, and β-actin antibodies. C) Inhibition of GSK3β attenuates USP48 effect on deubiquitination of TRAF2. Beas2B cells were transfected with empty vector or Usp48-v5 and incubated for 48 h. Cells were then treated with or without TWS119 (10 μM) for 1 h. Denatured cell lysates were subjected to immunoprecipitation with TRAF2 antibody, followed by immunoblotting with ubiquitin antibody. Input lysates were immunoblotted with V5, TRAF2, and β-actin antibodies. D) Phosphorylation motif deletion mutant of USP48 loses DUB activity. Recombinant USP48 wild-type protein, USP48C98S mutant protein, and USP48Δ886–890 mutant protein cleavage of Lys48 Ub chain linkages were analyzed by immunoblotting with ubiquitin antibody. V5 antibody was used for input lysate immunoblotting. E) GSK3βS9A increases USP48 DUB activity. Dimeric Ub cleavage analysis was performed of immunoprecipitates derived from recombinant USP48 incubated with or without GSK3βS9A. Reaction mixtures were analyzed with ubiquitin antibody. V5 and HA antibodies were used for input lysate immunoblotting (n = 3). *P < 0.01 compared to Usp48-v5. Shown are representative blots from at least 3 independent experiments. F) Scheme showing GSK3β-mediated USP48 phosphorylation increases USP48 DUB activity.
Knockdown of USP48 attenuates TRAF2-dependent activation of JNK1 and increases E-cadherin expression
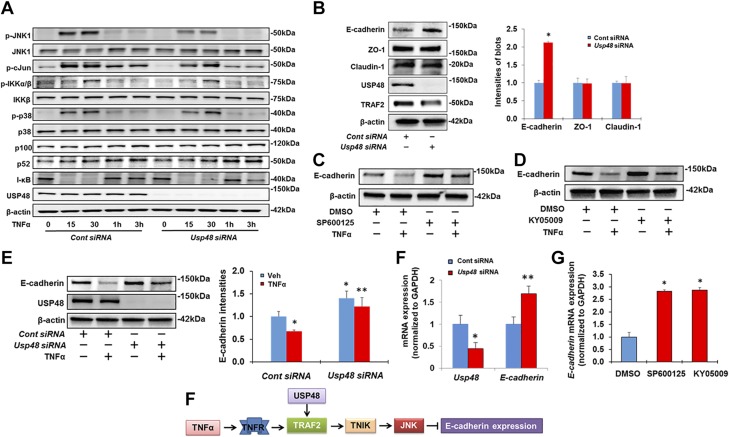
Next, we evaluated whether the USP48 regulation of TRAF2 protein stability affects its downstream biologic signaling. As shown in Fig. 6A, knockdown of USP48 attenuated TNF-α-induced phosphorylation of JNK1 and c-Jun, but not of p38 and I-κB. This is consistent with a study showing that TRAF2 mediates JNK1 and c-Jun phosphorylation in response to TNF-α (43). However, down-regulation of USP48 had no effect on TNF-α-induced cytokine release (Supplemental Fig. S3).
Figure 6.
Knockdown of USP48 attenuates TRAF2-dependent activation of JNK1, therefore increasing E-cadherin expression. A) Knockdown of USP48 attenuates TNF-α-induced phosphorylation of JNK1 and c-Jun. Beas2B cells were transfected with control siRNA or Usp48 siRNA, and incubated for 3 d. Cells were then treated with TNF-α (10 ng/ml) for 0 to 3 h. Cell lysates were examined by immunoblotting with indicated antibodies. B) Knockdown of USP48 increases E-cadherin expression. Beas2B cells were transfected with control siRNA or Usp48 siRNA for 3 d. Then E-cadherin, ZO-1, claudin 1, USP48, TRAF2, and β-actin levels were analyzed by Western blot analysis (n = 3). *P < 0.01 compared to control siRNA transfected cells. C) Inhibition of JNK attenuates TNF-α-induced reduction of E-cadherin. Beas2B cells were pretreated with SP600125 for 1 h. Cells were then added with TNF-α (10 ng/ml) for 48 h. E-cadherin and β-actin levels were analyzed by Western blot analysis. D) Inhibition of TNIK attenuates TNF-α-induced reduction of E-cadherin. Beas2B cells were pretreated with KY-05009 for 1 h. Cells were then added with TNF-α (10 ng/ml) for 48 h. E-cadherin and β-actin levels were analyzed by Western blot analysis. E) Knockdown of USP48 attenuates TNF-α-induced reduction of E-cadherin. Beas2B cells were transfected with control siRNA or Usp48 siRNA and incubated for 72 h. Cells were then treated with TNF-α (10 ng/ml) for 48 h. E-cadherin, USP48, and β-actin levels were analyzed by Western blot analysis (n = 3). *P < 0.01 compared to vehicle + control siRNA; **P < 0.01 compared to TNF-α + control siRNA. Shown are representative blots from at least 3 independent experiments. F) Knockdown of USP48 increases E-cadherin mRNA levels. Beas2B cells were transfected with control siRNA or Usp48 siRNA and incubated for 3 d. RNA was extracted and analyzed by real-time qPCR with Usp48 and E-cadherin primers. Relative expression of Usp48 and E-cadherin was normalized to Gapdh (n = 3). *P < 0.01 compared to control siRNA; **P < 0.01 compared to control siRNA. G) Inhibition of JNK or TNIK increases E-cadherin mRNA levels. Beas2B cells were treated with SP600125 (10 μM) or KY-05009 (10 μM) for 24 h. RNA was extracted and analyzed by real-time qPCR with E-cadherin primers. Relative expression of E-cadherin was normalized to Gapdh. *P < 0.01 compared to DMSO. H) Scheme showing down-regulation of USP48 or inhibition of TNIK and JNK increases E-cadherin expression. Data are presented as means ± sd.
Because JNK plays an important role in E-cadherin-mediated cell adhesion in epithelial cells (16, 17), we tested whether USP48 modulates E-cadherin expression. USP48 siRNA transfection significantly increased E-cadherin levels without altering tight junction proteins ZO-1 and claudin 1 (Fig. 6B). Similar results were obtained with A549 lung epithelial cells (Supplemental Fig. S4A). To investigate whether USP48 regulates E-cadherin expression through inhibition of JNK, we treated Beas2B cells with a JNK inhibitor, SP600125, and found that SP600125 increased E-cadherin expression in dose- and time-dependent manners (Supplemental Fig. S4B, C), as well as attenuated TNF-α-induced reduction of E-cadherin expression (Fig. 6C). The TRAF2 and Nck interacting kinase (TNIK) is a signaling kinase downstream of TRAF2, and its overexpression activates the JNK pathway (44, 45). An inhibitor of TNIK, KY-05009, attenuated TNF-α-induced phosphorylation of JNK1, but not p38, I-κB, or IL-8 release in epithelial cells (Supplemental Fig. S5A, B). Consistent with the above results from JNK inhibition, KY-05009 treatment increased E-cadherin expression in dose- and time-dependent manners (Supplemental Fig. S5C, D) and abrogated E-cadherin reduction by TNF-α (Fig. 6D). Knockdown of USP48 partially attenuated the TNF-α-mediated reduction of E-cadherin expression (Fig. 6E) without influencing TGF-β1-induced down-regulation of E-cadherin (Supplemental Fig. S6). We next wanted to test whether USP48 regulates E-cadherin gene transcription. Real-time qPCR studies confirmed that down-regulation of USP48 (Fig. 6F) or inhibition of JNK or TNIK (Fig. 6G) increased E-cadherin mRNA levels. These data suggest that USP48 regulates E-cadherin expression through the TRAF2-TNIK-JNK pathway (Fig. 6H).
USP48 and JNK activity modulate epithelial barrier function
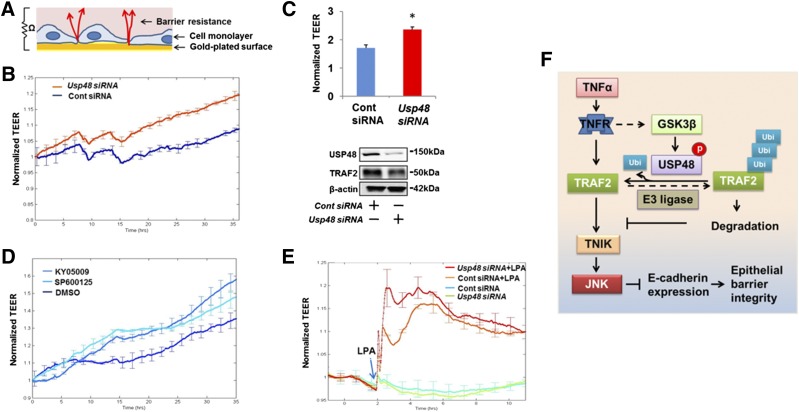
To evaluate the role of the USP48-TRAF2-TNIK-JNK-E-cadherin pathway on epithelial barrier integrity, we used an ECIS system to measure TEER (Fig. 7A). Increases in TEER reflect enhancement of epithelial barrier integrity. Depletion of cellular USP48 by Usp48 siRNA increased TEER over time with reduced USP48 and TRAF2 abundance (Fig. 7B, C), while down-regulation of USP48 had no effect on cell proliferation (Supplemental Fig. S7). Inhibition of JNK activity, either directly with SP600125 or by TNIK inhibition with KY-05009, also enhanced TEER over a 36 h time course in Beas2B (Fig. 7D), indicating that down-regulation of USP48 or inhibition of TNIK and JNK promotes epithelial barrier function. Because the immune effector molecule lysophosphatidic acid (LPA) has been shown to rapidly increase barrier integrity in epithelial cell culture (4), we added LPA to cells depleted of USP48 by siRNA and measured TEER. Figure 7E shows that USP48 depletion facilitated an augmented epithelial barrier in response to LPA. These functional studies indicate that attenuation of the USP48-TRAF2-TNIK-JNK pathway promotes epithelial barrier integrity (Fig. 7F).
Figure 7.
Knockdown of USP48 or inhibition of TNIK and JNK promotes epithelial barrier integrity. A) Scheme showing principle of ECIS system. B) Knockdown of USP48 increases TEER in A549 cells. A549 cells cultured on ECIS gold electrodes were transfected with control siRNA or Usp48 siRNA. After transfection, changes in TEER at 0 to 36 h were measured with ECIS. Data are presented as means ± sd (n = 4). C) Knockdown of USP48 increases TEER in Beas2B cells. Beas2B cells cultured on ECIS gold electrodes were transfected with control siRNA or Usp48 siRNA. After transfection, changes in TEER at 48 h were measured with ECIS. Data are presented as means ± sd (n = 4). P < 0.01 compared to control siRNA–transfected cells. The rest of the cells were analyzed by USP48, TRAF2, and β-actin immunoblotting. D) Inhibition of TNIK or JNK increases TEER. Beas2B cells cultured on ECIS gold electrodes were treated with DMSO (10 μM), KY-05009 (10 μM), or SP600125 (10 μM), and changes in TEER at 0 to 36 h were measured with ECIS. Data are presented as means ± sd, n = 4. E) Knockdown of USP48 promotes LPA-increased TEER. Human bronchial epithelial cells cultured on ECIS gold electrodes were transfected with control siRNA or Usp48 siRNA and incubated for 3 d. Cells were then challenged with LPA (0.5 μM), and changes in TEER were measured with ECIS. F) Scheme showing GSK3β phosphorylates and activates USP48, thus stabilizing TRAF2 and influencing TNIK/JNK-mediated E-cadherin expression and epithelial barrier integrity.
DISCUSSION
DUBs play key roles in the regulation of protein stability and signal transduction. USP48 is widely expressed in all normal tissues, suggesting that it participates in multiple essential deubiquitinating reactions (31, 46). However, the physiologic function of USP48 remains largely unknown. One recent study demonstrates that USP48 deubiquitinates the Na+-H+ exchanger (NHE3) and plays a role in blood pressure regulation (47). Tzimas et al. (31) have shown that USP48 is involved in the inhibition of NF-κB activation, a theme also presented in the study by Schweitzer and Naumann (32) in which the COP9 signalosome (CSN) and USP48 cooperatively control the nuclear turnover of RelA. These data suggest that USP48 regulates NF-κB signaling pathway through interaction with RelA in the nucleus. In the current study, we demonstrate that USP48 targets and deubiquitinates TRAF2, thereby stabilizing TRAF2 and regulating TNF-α-induced activation of JNK1, but not NF-κB and p38, therefore reducing E-cadherin expression. In epithelial cells, USP48 is mainly localized in the cytoplasm, not in nuclei, a difference from prior studies that may be due to cell types. This study reveals a new biologic function of USP48 in regard to regulation of TRAF2 stability and E-cadherin expression in epithelial cells.
To our knowledge, there is no report regarding the role of DUB enzymes in the regulation of TRAF2 stability. TRAF2 undergoes K48- and K63-linked polyubiquitination after ligation of the TNFR1 (33–35). E3 ubiquitin ligases, such as siah2, cIAP-1, and A20, promote TRAF2 ubiquitination and degradation (26, 27, 48). These studies indicate that ubiquitination plays an important role in remaining TRAF2 levels. Recent studies have been focused on investigating the effect of DUBs on TRAF2-mediated signaling pathways. Deubiquitination of TRAF2 by the DUBs CYLD or USP4 significantly inhibits TNF-α-induced NF-κB activation (8, 30). USP25 deubiquitinates TRAF2 to inhibit RIG-I-like receptor–mediated IFN signaling (29). Collectively, these DUBs negatively regulate TRAF2 activation without modulating TRAF2 protein stability. We demonstrate here that knockdown of USP48 increases the mass of K48-linked Ub-TRAF2 and reduces total TRAF2 protein levels in epithelial cells. Conversely, the overexpression of USP48 reduces TRAF2 K48-linked ubiquitination with a corresponding increase in total TRAF2 in the cytoplasm. This study identifies that USP48 stabilizes TRAF2 and regulates TRAF2-mediated signaling pathway. TRAF2 is a central molecule in the regulation of TNF-α-induced cellular responses. Our data suggest that targeting USP48 may modulate the TNF-α/TRAF2 pathway.
We noticed that knockdown of USP48 does not completely eliminate TRAF2 protein. Our data show that USP48 affects part of TRAF2-mediated signaling pathways, suggesting that USP48 specifically targets TNIK/JNK pathway–related TRAF2 without altering NF-κB and p38 pathway–related TRAF2 in epithelial cells. Though TRAF2 is known to regulate both NF-κB and JNK pathways in a variety of cell types, Tada et al. (43) reported that TRAF2 deficiency impaired JNK activation without affecting NF-κB pathway in mouse embryo fibroblast. The mechanisms by which USP48/TRAF2 specifically targets the JNK pathway have not been revealed. It is possible that TRAF2 stability is regulated by multi-DUBs; among them, USP48-associated TRAF2 contributes to JNK pathway, and other DUB-associated TRAF2 regulates NF-κB and p38. Our study provides unique evidence that targeting USP48-associated TRAF2 may precisely modulate the TNF-α-induced JNK pathway in lung epithelial cells. This is import for development of a strategy for treating human disorders caused by TNF-α-induced JNK activation in future precision medicine.
It is well known that disruption of E-cadherin link between neighboring cells impairs establishment of tight junctions (5). Loss of E-cadherin in the cell–cell junctions significantly loosens the cell–cell connection and increases cell motility (49, 50). Down-regulation of E-cadherin expression has been well studied, but few reports have focused on up-regulation of E-cadherin. We have shown that Src kinase up-regulates E-cadherin translation in A549 cells (51). Here, we present evidence that USP48 depletion increases E-cadherin mRNA levels in epithelial cells. Recent studies have implied that inhibition of JNK enhances E-cadherin redistribution to plasma membrane and promotes adherens junctions formation (16, 17). Niu et al. (18) show that JNK regulates E-cadherin degradation through the E3 ubiquitin ligase MDM2. In addition to evidence that JNK regulates E-cadherin redistribution and stability, our data reveal JNK suppression of E-cadherin expression. We discover a new molecular mechanism by which attenuation of the USP48-TRAF2-TNIK-JNK pathway up-regulates E-cadherin expression. The specific transcription factor involved in E-cadherin RNA induction is not studied here. However, Snail is a repressor of E-cadherin gene expression in epithelial tumor cells (52), and JNK is known to regulate Snail expression at the transcriptional level via the AP-1 transcription factor complex (53). It is tempting to speculate that the effects we observe for USP48 and E-cadherin expression may be exerted by JNK modulation of Snail expression. Investigations on whether USP48 influences Snail transcription or stability may be an informative area of future study.
DUBs are considered to be promising drug targets for treatment of infectious diseases and cancer. However, little is known about how the DUBs are functionally regulated. The catalytic activity of DUBs is regulated in multiple mechanisms, including protein–protein interaction and posttranslational modification (21). In the present study, we demonstrate that GSK3β-mediated USP48 phosphorylation enhances its DUB activity. GSK3β has been implicated in various essential cellular functions, such as glycogen metabolism, cell cycle control, cell differentiation, cell adhesion, and inflammation (54–57). This transient USP48 phosphorylation occurs on a GSK3β consensus motif in the C-terminal region of USP48. Deletion of the phosphorylation motif from USP48 negatively affects the deubiquitinating activity of USP48 on TRAF2. These data are consistent with another report that demonstrated that CK2-mediated phosphorylation of USP48 enhances its ubiquitin chain trimming activity (32). However, Schweitzer and Naumann (32) show that USP48 phosphorylation by CK2 occurs in the nucleus, while our study reveals that GSK3β mediates cytoplasmic USP48 phosphorylation and activation. Further, our data suggest that USP48 phosphorylation has no effect on its association with TRAF2. Collectively, USP48 activation is regulated by phosphorylation. Different protein kinases catalyze its phosphorylation depending on its localization within the cells.
In summary, we provide a new framework for studying TRAF2 protein stabilization. In our cellular model system, GSK3β phosphorylates USP48, resulting in increased activation of USP48, which deubiquitinates TRAF2 to enhance TRAF2 stability and TNF-α-mediated JNK signaling. USP48 deficiency attenuates TNF-α-induced JNK activation, thereby increasing E-cadherin expression and epithelial barrier integrity. This GSK3β–USP48→TRAF2→TNIK→JNK→E-cadherin cascade may play an important role in cellular responses and could represent a new pharmacologic target that partially suppresses TNF-α signaling through TRAF2/JNK pathway and enhances epithelial barrier integrity in the setting of acute illness.
ACKNOWLEDGMENTS
This work was supported, in part, by U.S. National Institutes of Health (NIH) National Heart, Lung, and Blood Institute Grants R01 HL112791 and HL131665 (to Y.Z.), NIH National Institute of General Medical Sciences Grant R01GM115389 (to J.Z.), American Lung Association Biomedical Research Grant RG350146 (to J.Z.), and American Heart Association Grant 16GRNT30660001 (to Y.Z.). The authors declare no conflicts of interest.
Glossary
- CHX
cycloheximide
- DUB
deubiquitinating enzyme
- E-cadherin
epithelial cadherin
- ECIS
electric cell-substrate impedance sensing
- GSK3β
glycogen synthase kinase 3β
- HA
hemagglutinin
- LPA
lysophosphatidic acid
- MLE12
murine lung epithelial 12
- p-Serine
phospho-serine
- qPCR
quantitative PCR
- siRNA
small interfering RNA
- TEER
transepithelial electrical resistance
- TNFR
TNF receptor
- TNIK
TRAF2 and Nck interacting kinase
- TRAF
TNF receptor–associated factor
- TRAF1–7
TNF receptor–associated factor 1–7
- Ub
ubiquitinated
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
S. Li, D. Wang, J. Zhao, and N. M. Weathington performed experiments and analyzed the data; N. M. Weathington, D. Shang, and Y. Zhao wrote and revised the article and supported the study; and D. Shang and Y. Zhao jointly designed, oversaw, and directed the study.
REFERENCES
- 1.Nawijn M. C., Hackett T. L., Postma D. S., van Oosterhout A. J., Heijink I. H. (2011) E-cadherin: gatekeeper of airway mucosa and allergic sensitization. Trends Immunol. 32, 248–255 [DOI] [PubMed] [Google Scholar]
- 2.Hulpiau P., van Roy F. (2009) Molecular evolution of the cadherin superfamily. Int. J. Biochem. Cell Biol. 41, 349–369 [DOI] [PubMed] [Google Scholar]
- 3.Van den Bossche J., Malissen B., Mantovani A., De Baetselier P., Van Ginderachter J. A. (2012) Regulation and function of the E-cadherin/catenin complex in cells of the monocyte–macrophage lineage and DCs. Blood 119, 1623–1633 [DOI] [PubMed] [Google Scholar]
- 4.He D., Su Y., Usatyuk P. V., Spannhake E. W., Kogut P., Solway J., Natarajan V., Zhao Y. (2009) Lysophosphatidic acid enhances pulmonary epithelial barrier integrity and protects endotoxin-induced epithelial barrier disruption and lung injury. J. Biol. Chem. 284, 24123–24132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Capaldo C. T., Macara I. G. (2007) Depletion of E-cadherin disrupts establishment but not maintenance of cell junctions in Madin-Darby canine kidney epithelial cells. Mol. Biol. Cell 18, 189–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamouille S., Xu J., Derynck R. (2014) Molecular mechanisms of epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 15, 178–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carayol N., Campbell A., Vachier I., Mainprice B., Bousquet J., Godard P., Chanez P. (2002) Modulation of cadherin and catenins expression by tumor necrosis factor-alpha and dexamethasone in human bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 26, 341–347 [DOI] [PubMed] [Google Scholar]
- 8.Xiao N., Li H., Luo J., Wang R., Chen H., Chen J., Wang P. (2012) Ubiquitin-specific protease 4 (USP4) targets TRAF2 and TRAF6 for deubiquitination and inhibits TNFα-induced cancer cell migration. Biochem. J. 441, 979–986 [DOI] [PubMed] [Google Scholar]
- 9.Alvarez S. E., Harikumar K. B., Hait N. C., Allegood J., Strub G. M., Kim E. Y., Maceyka M., Jiang H., Luo C., Kordula T., Milstien S., Spiegel S. (2010) Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature 465, 1084–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie P. (2013) TRAF molecules in cell signaling and in human diseases. J. Mol. Signal. 8, 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aggarwal B. B. (2003) Signalling pathways of the TNF superfamily: a double-edged sword. Nat. Rev. Immunol. 3, 745–756 [DOI] [PubMed] [Google Scholar]
- 12.Micheau O., Tschopp J. (2003) Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 114, 181–190 [DOI] [PubMed] [Google Scholar]
- 13.Borghi A., Verstrepen L., Beyaert R. (2016) TRAF2 multitasking in TNF receptor–induced signaling to NF-κB, MAP kinases and cell death. Biochem. Pharmacol. 116, 1–10 [DOI] [PubMed] [Google Scholar]
- 14.Jin J., Xiao Y., Hu H., Zou Q., Li Y., Gao Y., Ge W., Cheng X., Sun S. C. (2015) Proinflammatory TLR signalling is regulated by a TRAF2-dependent proteolysis mechanism in macrophages. Nat. Commun. 6, 5930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J., Yan J., Jiang S., Wen J., Chen L., Zhao Y., Lin A. (2012) Site-specific ubiquitination is required for relieving the transcription factor Miz1-mediated suppression on TNF-α-induced JNK activation and inflammation. Proc. Natl. Acad. Sci. USA 109, 191–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee M. H., Koria P., Qu J., Andreadis S. T. (2009) JNK phosphorylates beta-catenin and regulates adherens junctions. FASEB J. 23, 3874–3883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee M. H., Padmashali R., Koria P., Andreadis S. T. (2011) JNK regulates binding of alpha-catenin to adherens junctions and cell-cell adhesion. FASEB J. 25, 613–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niu W., Wang Y., Wang Z., Xin Q., Wang Y., Feng L., Zhao L., Wen J., Zhang H., Wang C., Xia G. (2016) JNK signaling regulates E-cadherin junctions in germline cysts and determines primordial follicle formation in mice. Development 143, 1778–1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhoj V. G., Chen Z. J. (2009) Ubiquitylation in innate and adaptive immunity. Nature 458, 430–437 [DOI] [PubMed] [Google Scholar]
- 20.Reyes-Turcu F. E., Ventii K. H., Wilkinson K. D. (2009) Regulation and cellular roles of ubiquitin-specific deubiquitinating enzymes. Annu. Rev. Biochem. 78, 363–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kessler B. M., Edelmann M. J. (2011) PTMs in conversation: activity and function of deubiquitinating enzymes regulated via post-translational modifications. Cell Biochem. Biophys. 60, 21–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fraile J. M., Quesada V., Rodríguez D., Freije J. M., López-Otín C. (2012) Deubiquitinases in cancer: new functions and therapeutic options. Oncogene 31, 2373–2388 [DOI] [PubMed] [Google Scholar]
- 23.Reiley W., Zhang M., Wu X., Granger E., Sun S. C. (2005) Regulation of the deubiquitinating enzyme CYLD by IkappaB kinase gamma–dependent phosphorylation. Mol. Cell. Biol. 25, 3886–3895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu D., Shan B., Lee B. H., Zhu K., Zhang T., Sun H., Liu M., Shi L., Liang W., Qian L., Xiao J., Wang L., Pan L., Finley D., Yuan J. (2015) Phosphorylation and activation of ubiquitin-specific protease-14 by Akt regulates the ubiquitin–proteasome system. eLife 4, e10510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li L., Soetandyo N., Wang Q., Ye Y. (2009) The zinc finger protein A20 targets TRAF2 to the lysosomes for degradation. Biochim. Biophys. Acta 1793, 346–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Habelhah H., Frew I. J., Laine A., Janes P. W., Relaix F., Sassoon D., Bowtell D. D., Ronai Z. (2002) Stress-induced decrease in TRAF2 stability is mediated by Siah2. EMBO J. 21, 5756–5765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X., Yang Y., Ashwell J. D. (2002) TNF-RII and c-IAP1 mediate ubiquitination and degradation of TRAF2. Nature 416, 345–347 [DOI] [PubMed] [Google Scholar]
- 28.Chen B. B., Coon T. A., Glasser J. R., McVerry B. J., Zhao J., Zhao Y., Zou C., Ellis B., Sciurba F. C., Zhang Y., Mallampalli R. K. (2013) A combinatorial F box protein directed pathway controls TRAF adaptor stability to regulate inflammation. Nat. Immunol. 14, 470–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhong H., Wang D., Fang L., Zhang H., Luo R., Shang M., Ouyang C., Ouyang H., Chen H., Xiao S. (2013) Ubiquitin-specific proteases 25 negatively regulates virus-induced type I interferon signaling. PLoS One 8, e80976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trompouki E., Hatzivassiliou E., Tsichritzis T., Farmer H., Ashworth A., Mosialos G. (2003) CYLD is a deubiquitinating enzyme that negatively regulates NF-kappaB activation by TNFR family members. Nature 424, 793–796 [DOI] [PubMed] [Google Scholar]
- 31.Tzimas C., Michailidou G., Arsenakis M., Kieff E., Mosialos G., Hatzivassiliou E. G. (2006) Human ubiquitin specific protease 31 is a deubiquitinating enzyme implicated in activation of nuclear factor-kappaB. Cell. Signal. 18, 83–92 [DOI] [PubMed] [Google Scholar]
- 32.Schweitzer K., Naumann M. (2015) CSN-associated USP48 confers stability to nuclear NF-κB/RelA by trimming K48-linked Ub-chains. Biochim. Biophys. Acta 1853, 453–469 [DOI] [PubMed] [Google Scholar]
- 33.Habelhah H., Takahashi S., Cho S. G., Kadoya T., Watanabe T., Ronai Z. (2004) Ubiquitination and translocation of TRAF2 is required for activation of JNK but not of p38 or NF-kappaB. EMBO J. 23, 322–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi C. S., Kehrl J. H. (2003) Tumor necrosis factor (TNF)-induced germinal center kinase-related (GCKR) and stress-activated protein kinase (SAPK) activation depends upon the E2/E3 complex Ubc13-Uev1A/TNF receptor–associated factor 2 (TRAF2). J. Biol. Chem. 278, 15429–15434 [DOI] [PubMed] [Google Scholar]
- 35.Wu C. J., Conze D. B., Li X., Ying S. X., Hanover J. A., Ashwell J. D. (2005) TNF-alpha induced c-IAP1/TRAF2 complex translocation to a Ubc6-containing compartment and TRAF2 ubiquitination. EMBO J. 24, 1886–1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ubersax J. A., Ferrell J. E. Jr (2007) Mechanisms of specificity in protein phosphorylation. Nat. Rev. Mol. Cell Biol. 8, 530–541 Erratum in: Nat. Rev. Mol Cell Biol. 2007;8:665. [DOI] [PubMed] [Google Scholar]
- 37.Amasheh M., Fromm A., Krug S. M., Amasheh S., Andres S., Zeitz M., Fromm M., Schulzke J. D. (2010) TNFalpha-induced and berberine-antagonized tight junction barrier impairment via tyrosine kinase, Akt and NFkappaB signaling. J. Cell Sci. 123, 4145–4155 [DOI] [PubMed] [Google Scholar]
- 38.Ramirez S. H., Fan S., Zhang M., Papugani A., Reichenbach N., Dykstra H., Mercer A. J., Tuma R. F., Persidsky Y. (2010) Inhibition of glycogen synthase kinase 3beta (GSK3beta) decreases inflammatory responses in brain endothelial cells. Am. J. Pathol. 176, 881–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwabe R. F., Brenner D. A. (2002) Role of glycogen synthase kinase-3 in TNF-alpha-induced NF-kappaB activation and apoptosis in hepatocytes. Am. J. Physiol. Gastrointest. Liver Physiol. 283, G204–G211 [DOI] [PubMed] [Google Scholar]
- 40.Leng S., Zhang W., Zheng Y., Liberman Z., Rhodes C. J., Eldar-Finkelman H., Sun X. J. (2010) Glycogen synthase kinase 3 beta mediates high glucose–induced ubiquitination and proteasome degradation of insulin receptor substrate 1. J. Endocrinol. 206, 171–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao J., Wei J., Mialki R. K., Mallampalli D. F., Chen B. B., Coon T., Zou C., Mallampalli R. K., Zhao Y. (2012) F-box protein FBXL19-mediated ubiquitination and degradation of the receptor for IL-33 limits pulmonary inflammation. Nat. Immunol. 13, 651–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.ter Haar E., Coll J. T., Austen D. A., Hsiao H. M., Swenson L., Jain J. (2001) Structure of GSK3beta reveals a primed phosphorylation mechanism. Nat. Struct. Biol. 8, 593–596 [DOI] [PubMed] [Google Scholar]
- 43.Tada K., Okazaki T., Sakon S., Kobarai T., Kurosawa K., Yamaoka S., Hashimoto H., Mak T. W., Yagita H., Okumura K., Yeh W. C., Nakano H. (2001) Critical roles of TRAF2 and TRAF5 in tumor necrosis factor–induced NF-kappa B activation and protection from cell death. J. Biol. Chem. 276, 36530–36534 [DOI] [PubMed] [Google Scholar]
- 44.Shkoda A., Town J. A., Griese J., Romio M., Sarioglu H., Knöfel T., Giehler F., Kieser A. (2012) The germinal center kinase TNIK is required for canonical NF-κB and JNK signaling in B-cells by the EBV oncoprotein LMP1 and the CD40 receptor. PLoS Biol. 10, e1001376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schürch C., Riether C., Matter M. S., Tzankov A., Ochsenbein A. F. (2012) CD27 signaling on chronic myelogenous leukemia stem cells activates Wnt target genes and promotes disease progression. J. Clin. Invest. 122, 624–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lockhart P. J., Hulihan M., Lincoln S., Hussey J., Skipper L., Bisceglio G., Wilkes K., Farrer M. J. (2004) Identification of the human ubiquitin specific protease 31 (USP31) gene: structure, sequence and expression analysis. DNA Seq. 15, 9–14 [DOI] [PubMed] [Google Scholar]
- 47.Armando I., Villar V. A., Jones J. E., Lee H., Wang X., Asico L. D., Yu P., Yang J., Escano C. S. Jr., Pascua-Crusan A. M., Felder R. A., Jose P. A. (2014) Dopamine D3 receptor inhibits the ubiquitin-specific peptidase 48 to promote NHE3 degradation. FASEB J. 28, 1422–1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dupoux A., Cartier J., Cathelin S., Filomenko R., Solary E., Dubrez-Daloz L. (2009) cIAP1-dependent TRAF2 degradation regulates the differentiation of monocytes into macrophages and their response to CD40 ligand. Blood 113, 175–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baum B., Georgiou M. (2011) Dynamics of adherens junctions in epithelial establishment, maintenance, and remodeling. J. Cell Biol. 192, 907–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prieto-García E., Díaz-García C. V., García-Ruiz I., Agulló-Ortuño M. T. (2017) Epithelial-to-mesenchymal transition in tumor progression. Med. Oncol. 34, 122 [DOI] [PubMed] [Google Scholar]
- 51.Dong S., Khoo A., Wei J., Bowser R. K., Weathington N. M., Xiao S., Zhang L., Ma H., Zhao Y., Zhao J. (2014) Serum starvation regulates E-cadherin upregulation via activation of c-Src in non-small-cell lung cancer A549 cells. Am. J. Physiol. Cell Physiol. 307, C893–C899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cano A., Pérez-Moreno M. A., Rodrigo I., Locascio A., Blanco M. J., del Barrio M. G., Portillo F., Nieto M. A. (2000) The transcription factor Snail controls epithelial–mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 2, 76–83 [DOI] [PubMed] [Google Scholar]
- 53.Li Y., Liu Y., Xu Y., Voorhees J. J., Fisher G. J. (2010) UV irradiation induces Snail expression by AP-1 dependent mechanism in human skin keratinocytes. J. Dermatol. Sci. 60, 105–113 [DOI] [PubMed] [Google Scholar]
- 54.Doble B. W., Woodgett J. R. (2003) GSK-3: tricks of the trade for a multi-tasking kinase. J. Cell Sci. 116, 1175–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang H., Brown J., Martin M. (2011) Glycogen synthase kinase 3: a point of convergence for the host inflammatory response. Cytokine 53, 130–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jope R. S., Johnson G. V. (2004) The glamour and gloom of glycogen synthase kinase-3. Trends Biochem. Sci. 29, 95–102 [DOI] [PubMed] [Google Scholar]
- 57.Cortés-Vieyra R., Bravo-Patiño A., Valdez-Alarcón J. J., Juárez M. C., Finlay B. B., Baizabal-Aguirre V. M. (2012) Role of glycogen synthase kinase-3 beta in the inflammatory response caused by bacterial pathogens. J. Inflamm. (Lond.) 9, 23 [DOI] [PMC free article] [PubMed] [Google Scholar]