Abstract
Leukocytes express formyl-peptide receptors (FPRs), which sense microbe-associated molecular pattern (MAMP) molecules, leading to leukocyte chemotaxis and activation. We recently demonstrated that phenol-soluble modulin (PSM) peptides from highly pathogenic Staphylococcus aureus are efficient ligands for the human FPR2. How PSM detection by FPR2 impacts on the course of S. aureus infections has remained unknown. We characterized the specificity of mouse FPR2 (mFpr2) using a receptor-transfected cell line, homeobox b8 (Hoxb8), and primary neutrophils isolated from wild-type (WT) or mFpr2−/− mice. The influx of leukocytes into the peritoneum of WT and mFpr2−/− mice was analyzed. We demonstrate that mFpr2 is specifically activated by PSMs in mice, and they represent the first secreted pathogen-derived ligands for the mFpr2. Intraperitoneal infection with S. aureus led to lower numbers of immigrated leukocytes in mFpr2−/− compared with WT mice at 3 h after infection, and this difference was not observed when mice were infected with an S. aureus PSM mutant. Our data support the hypothesis that the mFpr2 is the functional homolog of the human FPR2 and that a mouse infection model represents a suitable model for analyzing the role of PSMs during infection. PSM recognition by mFpr2 shapes leukocyte influx in local infections, the typical infections caused by S. aureus.—Weiss, E., Hanzelmann, D., Fehlhaber, B., Klos, A., von Loewenich, F. D., Liese, J., Peschel, A., Kretschmer, D. Formyl-peptide receptor 2 governs leukocyte influx in local Staphylococcus aureus infections.
Keywords: mFpr2, PSMs, innate immunity, neutrophil, Hoxb8
Staphylococcus aureus, particularly community-associated methicillin-resistant S. aureus, is an important human pathogen and responsible for local infections, such as superficial skin and wound infections. In addition, S. aureus often causes severe systemic infections afflicting particularly immunocompromised and elderly patients (1, 2). Polymorphonuclear neutrophils (PMNs) are of particular importance for the effective clearance of S. aureus infections, and they contribute to the massive inflammation typically accompanying S. aureus infections (3). How neutrophils and other leukocytes are recruited to local infection sites, in particular, in early phases, has remained incompletely understood.
Neutrophils migrate to the focus of infection in response to bacterial molecules, such as formylated peptides. The ability of neutrophils to detect and respond to such chemotactic MAMP molecules is crucial for host defense against invading bacteria (4). All bacteria release formylated peptides, for example, the N-terminal signal peptides released from secreted proteins (5) as a consequence of the use of formyl-methionine as the first amino acid in bacterial protein biosynthesis. In addition, PSMs, which represent the most abundant peptides secreted by S. aureus, are potent neutrophil chemoattractants (6). S. aureus produces several PSMs, including the short α-type PSMs (PSMα1–4 and δ-toxin) and the twice-as-long β-type PSMs (PSMβ1, PSMβ2) (7). At low concentrations, PSMs induce recruitment of human neutrophils (6). Furthermore, PSMαs induce the release of TLR2-activating lipoproteins from bacterial cells and at high concentrations, lysis of human cells (8, 9). It has been shown that formyl-peptide- or PSM-induced chemotaxis depends on activation of human FPR1 or -2. The 2 receptors appear to discriminate between ligands based on peptide length and folding state (10). Short, formylated peptides usually have no defined structure, whereas the longer PSMs adopt α-helical, amphipathic structures, which favor detection by FPR1 or FPR2, respectively (10).
Mouse neutrophils also respond to formylated peptides and PSMs, but their repertoire of FPR-related receptors differs from that of human neutrophils. They include 8 orthologs (in contrast to 3 in humans), 3 of which are expressed on mouse neutrophils (11). mFpr1 and mFpr2 (previously named mFpr-rs2) are expressed on naive mouse neutrophils. In contrast, mFpr3 (previously named mFpr-rs1) is expressed only in some mouse lineages and only upon stimulation (12, 13). The murine mFpr1 has been shown to respond with high efficiency to short formylated peptides but to prefer the sequence fMIFL over fMLF, the most potent formylated peptide for the human FPR1 (14). mFpr1 is 77% identical to the human FPR1 amino acid sequence, and studies with knockout mice have shown that it represents the functional counterpart to human FPR1 (15). The mouse mFpr2 is structurally most similar to human FPR2; it possesses 76% amino acid sequence identity (11, 16). In spite of the high similarity between these 2 receptors, mFpr2 responds only very weakly to several peptide agonists that strongly activate human FPR2 (17, 18). However, it has been shown that the FPR2-specific antagonist WRW4 inhibits the activation of the mFpr2 (19). This result suggests that mFpr2 is the functional counterpart of the human FPR2. Despite the sequence similarity between mouse and human FPRs, there are crucial species-specific differences. Inhibitory proteins for human FPR1 (chemotaxis inhibitory protein of S. aureus) or FPR2 are secreted by many S. aureus strains and have been shown to be specific for human FPRs but to be inactive against mFprs (20–22). The unclear relation between human FPR and mFPR homologs has impeded the analysis of the role of the PSM-sensing receptor in infection in mouse models (11).
To clarify the role of FPR2 in S. aureus infections, we compared the ligand specificities of human FPRs and mFPRs and found mFpr2 to respond strongly and specifically to all S. aureus PSMs but not to short formylated peptides. In contrast, mFpr1 was not activated by PSMs but by formyl-Met-Ile-Phe-Leu (fMIFL) (14). When mice were infected intraperitoneally with S. aureus, lack of mFpr2 led to strongly reduced numbers of immigrated leukocytes at early but not in later stages of infection, indicating that this receptor may play a crucial role in the clearance of superficial, early infections.
MATERIALS AND METHODS
Cells and bacteria
The plasmid pQCXIN-EF1α was used for transfection of rat basophilic leukemia (RBL; RBL-2H3, CRL 1593; American Type Culture Collection, Manassas, VA, USA; and subclone; gift from Peter Monk, The University of Sheffield, Sheffield, United Kingdom) cells. mFpr cDNAs (16) were cloned in plasmid pcDND3.1 (Thermo Fisher Scientific, Waltham, MA, USA). The cDNAs of mFpr1 and mFpr2 were amplified by PCR with primers containing restriction sites for NotI, BamHI, and PacI (Supplemental Table 1). cDNAs of Fprs were cloned into pQCXIN-EF1α via compatible restriction sites to yield translational fusions with an N-terminal FLAG-tag and maintained in Escherichia coli under ampicillin selection. pQCXIN-EF1α-mFpr1 and pQCXIN-EF1α-mFpr2 were isolated using an endotoxin-free isolation kit from Qiagen (Germantown, MD, USA) and further used for transfection of RBL-2H3 cells using nucleofector technology. Transfection efficiency was analyzed via an antibody against the FLAG-tag (mouse anti-FLAG, isotype control mouse IgG1, secondary antibody anti-mouse IgG-phycoerythrin; MilliporeSigma, Billerica, MA, USA), expressed as part of the mFpr receptors on the surface of the receptor-transfected RBL cells.
Strain USA300 (23) and the isogenic PSM deletion mutant (USA300∆αβδ) used for mouse stimulation and infection experiments have recently been described in detail (24). Bacterial culture supernatants were obtained by centrifugation of overnight cultures grown in tryptic soy broth and filtered through 0.2-μm-pore filters (MilliporeSigma).
Synthetic peptides
The PSM peptides were kindly provided by Stefan Stevanovic (University of Tübingen). fMIFL was synthesized by EMC Microcollections (Tübingen, Germany).
Isolation of primary neutrophils
Mouse leukocytes were obtained from the peritoneum of 12-wk-old female C57BL/6 mice (Envigo, Somerset, NJ, USA) or from mFpr2−/− mice with a genetic C57BL/6 background (bred in the animal facilities of the University Hospital Tübingen) (25) upon inducing an intraperitoneal inflammatory reaction by repeated casein injection, as previously described (26). All mice were held under specific pathogen-free conditions and provided food and water ad libitum. Animal experiments were performed in strict accordance with the European Health Law of the Federation of Laboratory Animal Science Associations. The protocol was approved by the Regierungspräsidium Tübingen (Anzeige 17.12.14)
Hoxb8 cell lines
Progenitors were derived from the bone marrow of the mouse strains mentioned above. The progenitors were retrovirally transduced with estrogen-regulated Hoxb8 and selected for 4 wk in the presence of stem cell factor (SCF) to generate neutrophil progenitor cell lines (27). Polyclonal progenitor cell lines were cultured in Opti-MEM + GlutaMax medium (Thermo Fisher Scientific), supplemented with 10% fetal calf serum, 30 µM 2-ME, 1 µM β-estradiol (MilliporeSigma), and 1% supernatant from SCF-producing Chinese hamster ovary cells. The SCF-producing cell line was kindly provided by Hans Häcker (St. Jude Children’s Research Hospital, Memphis, TN, USA). Differentiation was induced by β-estradiol removal.
Calcium flux assay
Calcium ion fluxes in primary mouse neutrophils, Hoxb8, and RBL cells were analyzed by stimulating cells loaded with Fluo-3-acetoxymethyl (Thermo Fisher Scientific) and monitoring fluorescence with a FACSCalibur flow cytometer or LSRFortessa (BD Biosciences, San Jose, CA, USA), as described recently (6). Synthetic chemoattractants were used at concentrations in the linear range of the dose-response curves. Measurements of 2000 events were performed, and calcium flux was expressed as relative fluorescence.
MIP2 production and chemotaxis
Macrophage inflammatory protein 2 (MIP2) was measured using an ELISA kit (R&D Systems, Minneapolis, MN, USA), according to the manufacturer’s instructions. Hoxb8 neutrophils were primed with 100 ng/ml of the synthetic diacylated lipopeptide S-[2,3-bis(palmitoyloxy)-(2RS)-propyl]-(R)-cysteine cysteine-serine-lysine 4 (Thermo Fisher Scientific) for 24 h. Hoxb8 neutrophils were incubated with indicated concentrations of the different peptides for 5 h at 37°C, 5% CO2. The cells were centrifuged for 10 min at 250 g and 4°C, and cell supernatants were stored at −20°C until use.
Chemotaxis of primary or Hoxb8 neutrophils toward staphylococcal supernatants or synthetic peptides was determined by using fluorescence-labeled neutrophils that migrated through a 3-µm-pore polycarbonate Transwell filter, as recently described (26). Synthetic chemoattractants were used at concentrations in the linear range of the dose-response curves. Neutrophil migration from the upper to the lower Transwell chamber was only observed when the peptides were added to the lower chamber. When an even PSM concentration was present in the 2 chambers, no such migration was observed, thereby confirming that PSMs induce chemotactic migration in neutrophils. The relative fluorescence measured was corrected for the buffer control.
Degranulation assay
RBL cells were preincubated with 10 µM cytochalasin B in HBSS + 0.1% bovine serum albumin + 20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid for 15 min on ice, followed by 15 min at 37°C. Subsequently, the cells were stimulated for 10 min with the indicated amounts of agonists at 37°C. Triton X-100 (0.1%) was used as a positive control. The reaction was terminated by addition of ice-cold PBS, and the supernatants were collected by centrifugation. The amount of released β-glucuronidase was quantified by incubating 20 µl supernatant with 20 µl, 10 mM 4-methylumbelliferyl β-d-glucuronide hydrate in 0.1 M sodium acetate (pH 4.0) and 0.1% Triton X-100 for 15 min at 37°C. The reaction was terminated by addition of 300 µl, 50 mM glycine and 5 mM EDTA in H2O (pH 10.4). Fluorescence was measured at 365 nm excitation and 460 nm emission wavelengths.
Cytotoxicity assay
RBL cells (105/ml) or Hoxb8 cells (106/ml) were incubated for 1 h in a humidified 37°C incubator with PSMα2, PSMα3, or δ-toxin at final concentrations of 0.5, 2, or 4 μM, respectively, in Roswell Park Memorial Institute (RPMI; 1640; Merck; Darmstadt, Germany) medium without phenol red, and release of cytoplasmic lactate dehydrogenase was determined in triplicate (Roche Applied Science, Mannheim, Germany). The percentage lactate dehydrogenase release was calculated relative to that observed in detergent-lysed cells (maximal lysis) as a measure for cell lysis as described in Kretchmer et al. (6).
Peptide-binding assay
Hoxb8 cells (105) were incubated with increasing concentrations of 5-carboxytetramethylrhodamine-labeled PSMα3 for 2 h at 37°C under agitation. Cells were washed with ice-cold PBS containing 0.1% human serum albumin, and cell-associated fluorescence was measured using a FACSCalibur flow cytometer, as described in Kretschmer et al. (6).
Mouse infection assay
In the mouse peritonitis model, 4 × 108 (for 3 h) or 5 × 107 (for 24 h) colony-forming units of live S. aureus USA300 wild type (WT) or USA300Δαβδ were injected into the peritoneum of 6- to 8-wk-old female C57BL/6 WT or mFpr2−/− mice. Three or 24 h after infection, the mice were euthanized with CO2. Subsequently, peritoneal exudates were collected, and leukocytes were stained and counted as previously described (9). FITC-conjugated α−mouse Ly6G antibody, a marker for neutrophils, and a corresponding isotype control were purchased from BD Biosciences. A phycoerythrin-labeled α−mouse F4/80 antibody, a marker for macrophages, and the corresponding isotype control were purchased from Bio-Rad Laboratories (Hercules, CA, USA) and Thermo Fisher Scientific, respectively. Samples were analyzed on a FACSCalibur flow cytometer (Supplemental Fig. 4). All mice were held under specific pathogen-free conditions and provided food and water ad libitum. Animal experiments were performed in strict accordance with the European Health Law of the Federation of Laboratory Animal Science Associations. The protocol was approved by the Regierungspräsidium Tübingen (H4/09)
Statistics
Statistical analysis was performed using Graph Pad Prism 5.0 (GraphPad Software, La Jolla, CA, USA). Unpaired 2-tailed Student’s t test was used to compare 2 data groups unless otherwise noted.
RESULTS
PSMs induce calcium release and degranulation in mFpr2-expressing RBL cells
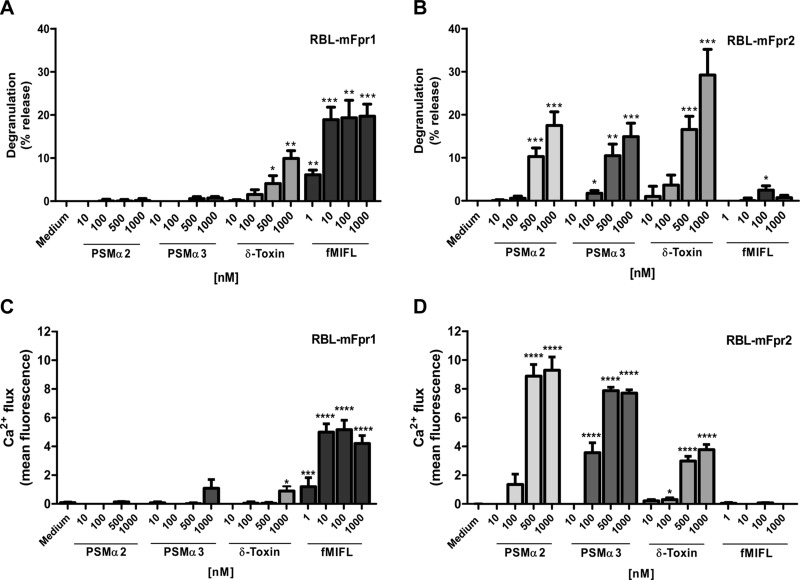
mFpr1 and mFpr2 are regarded as the murine orthologs of human FPR1 and FPR2, respectively, but a number of studies have suggested that mFprs exhibit less-defined ligand-binding properties than human FPRs (28). To analyze if mFprs differ in their capacities to discriminate between short formylated peptides and PSMs from human FPRs, RBL cells were stably transfected with mFpr1 or mFpr2 and found to express the receptors (Supplemental Fig. 1). mFpr1- and mFpr2-transfected but not untransfected RBL cells responded to fMIFL and PSMs, respectively, by mobilization of calcium from intracellular stores and by release of granule components (Fig. 1), thereby confirming that the 2 receptors were functionally expressed. When these cells were stimulated with PSMα2 or -α3 or δ-toxin, we observed degranulation (Fig. 1A, B) and strong calcium influx (Fig. 1C, D and Supplemental Fig. 2) in mFpr2-transfected RBL cells but hardly any response in mFpr1-transfected or in control cells. mFpr2-transfected cells did not respond to fMIFL, which confirms previous studies (14, 29) and indicates that mFpr1 and mFpr2 have similar capacities to discriminate between short formylated peptides and PSMs as the human homologs. At very high PSM concentrations (above 1 µM), some unspecific activation was observed (data not shown), which was most probably a result of the toxicity of the peptides (Supplemental Fig. 3).
Figure 1.
PSMs induce specific activation and degranulation of mFpr2-expressing RBL cells. PSMs specifically do not activate mFpr1–transfected RBL cells (A, C), but activate mFpr2 (B, D) at indicated concentrations. Data represent means ± sem of at least 3 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 vs. corresponding medium control (53), as calculated by Student’s t test.
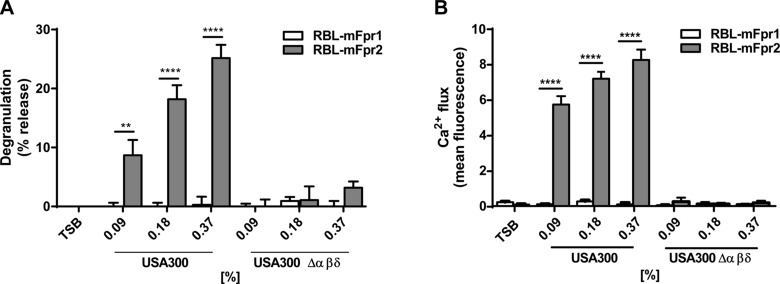
Human FPR2 has been reported to respond only to culture filtrates of PSM-secreting (WT) S. aureus but not of PSM-deficient mutants (6, 30). mFpr2 was found to respond in a similar way, because mFpr2-transfected RBL cells were only activated by culture filtrates of the S. aureus USA300 WT but not of the isogenic mutant lacking the PSMα and -β and δ-toxin genes (Δαβδ; Fig. 2). mFpr1-transfected RBL cells hardly responded to culture filtrates of S. aureus, which are known to contain formylated peptides at concentrations that did not cause toxicity (below 0.375%). Similar observations were previously made with human FPR1, which demonstrated that short formylated peptides are, by far, less concentrated in S. aureus culture filtrates than PSMs. Thus, PSMs have similar agonist properties for mouse and human FPRs.
Figure 2.
Culture filtrates from PSM-expressing S. aureus induce specific activation and degranulation of mFpr2-expressing RBL cells. Culture filtrates of PSM-expressing S. aureus USA300 WT, but not from an isogenic PSM deletion mutant, induce degranulation (A) and calcium influx (B) in mFpr2, but not mFpr1, transfected RBL cells at indicated concentrations. Data represent means ± sem of at least 3 independent experiments. TSB, tryptic soy broth. **P < 0.01, ****P < 0.0001 vs. corresponding medium control, as calculated by Student’s t test.
Mouse neutrophils are activated by PSMs via mFpr2
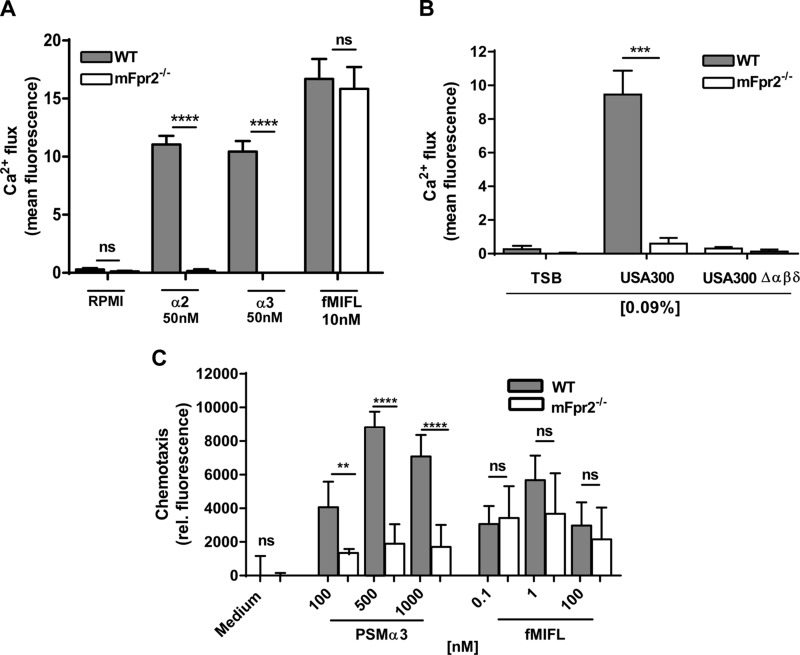
To confirm these findings with primary cells, neutrophils were isolated from WT and mFpr2−/− mice, and intracellular calcium mobilization was monitored after stimulation with mFpr1 and mFpr2 ligands. Indeed, WT neutrophils were activated by fMIFL, PSMα2, and PSMα3. In contrast, neutrophils from mFpr2−/− mice only responded to fMIFL but not to PSMs (Fig. 3A). Likewise, PSMα3 and fMIFL were chemoattractive for neutrophils of WT mice, whereas only fMIFL attracted neutrophils of mFpr2−/− mice (Fig. 3C). Thus, primary mouse neutrophils respond to Fpr agonists in a similar way as Fpr-transfected RBL cells. When neutrophils from WT mice were stimulated with S. aureus culture filtrates, only samples of the S. aureus parental strain but not the isogenic PSM mutant elicited calcium ion influx at concentrations below toxicity (below 0.375%). None of the S. aureus culture filtrates activated mFpr2−/− neutrophils (Fig. 3B). These findings confirm that only the mFpr2 detects PSM peptides and that insufficient amounts of mFpr1 ligands are present in S. aureus culture filtrates.
Figure 3.
Primary neutrophils of mice are specifically activated by PSMs via mFpr2. PSMs induce calcium influx in primary neutrophils of mice. A, B) Calcium influx is induced by PSMα2, PSMα3, and fMIFL (A) or culture filtrates of USA300 WT or its isogenic PSM mutant (0.09%; culture filtrates diluted in RPMI 1640) (B) in neutrophils isolated from WT or mFpr2−/− mice. C) Migration is induced by PSMα3 or fMIFL in neutrophils of WT or mFpr2−/− mice. Data represent means ± sem of at least 3 independent experiments. **P < 0.01, ***P < 0.001, ****P < 0.0001; ns, not significantly different vs. mFpr2−/− neutrophils, as calculated by Student’s t test.
Hoxb8 neutrophils expressing mFpr2 behave like primary neutrophils
In contrast to human neutrophils, mouse neutrophils cannot be isolated in sufficient numbers from blood (31) but need to be collected from the mouse peritoneum upon intraperitoneal injection of casein, which leads to priming of the cells and may alter their responses to inflammatory stimuli (32). To overcome these problems, neutrophil precursor cells from the bone marrow of WT or mFpr2−/− mice were immortalized by stable transfection with estrogen-regulated transcription factor Hoxb8, according to an established protocol (27). The resulting Hoxb8 cells remain immortal as long as estrogen is added to growth medium, but they differentiate into mature neutrophils within 4 d in the absence of estrogen. Hoxb8 neutrophils have previously been shown to behave like primary neutrophils in terms of activation, chemotaxis, opsonophagocytosis, and killing (33).
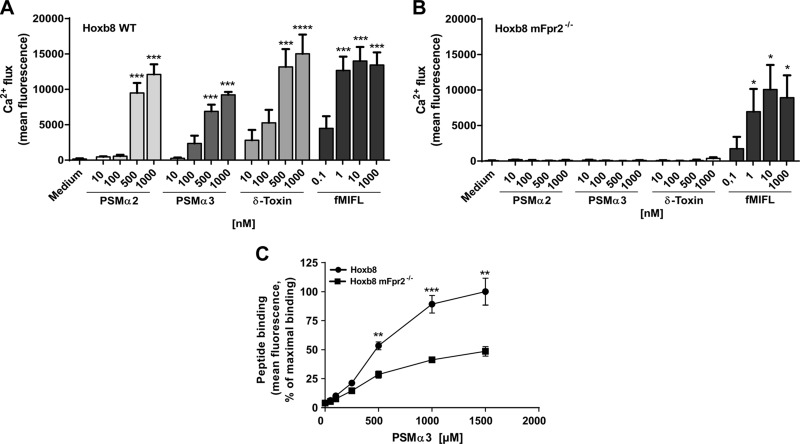
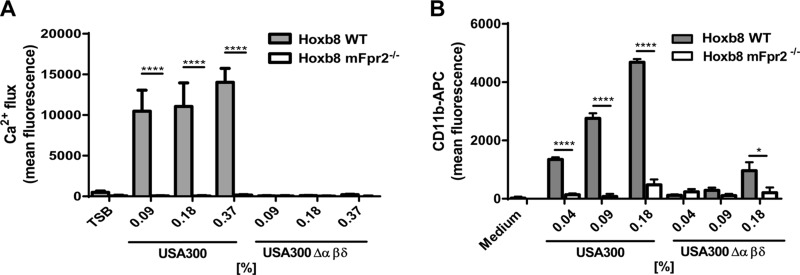
Stimulation of Hoxb8 neutrophils with various concentrations of fMIFL, PSMα2, PSMα3, or δ-toxin induced calcium influx in WT Hoxb8 cells, whereas only fMIFL was able to stimulate calcium influx in mFpr2−/− Hoxb8 neutrophils (Fig. 4A, B). Furthermore, fluorescent-labeled PSMα3 showed significant, stronger binding to WT Hoxb8 neutrophils than to mFpr2−/− Hoxb8 cells (Fig. 4C). Moreover, culture filtrates from the S. aureus parental strain activated only WT Hoxb8, whereas those from the PSM mutant activated neither WT nor mFpr2−/− Hoxb8 (Fig. 6A) at concentrations below the toxicity threshold. These findings support the results with primary neutrophils isolated from the peritoneum of mice and demonstrate that the Hoxb8 cells behave like primary mouse neutrophils.
Figure 4.
Hoxb8 neutrophils expressing mFpr2 are activated by PSMs. A, B) Calcium influx is induced by PSMα2, PSMα3, δ-toxin, or fMIFL in WT Hoxb8 (A) or mFpr2−/− Hoxb8 (B). C) 5-Carboxytetramethylrhodamine-labeled PSMα3 binds specifically to mFpr2-expressing Hoxb8 cells. Data represent means ± sem of at least 3 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 vs. corresponding medium control (A, B) or vs. mFpr2−/− Hoxb8 cells (C), as calculated by Student’s t test.
Figure 6.
Hoxb8 neutrophils expressing mFpr2 are activated through culture filtrates of PSM-expressing USA300. Culture filtrates of PSM-expressing S. aureus USA300, but not from an isogenic PSM deletion mutant, induce calcium influx (A) and CD11b up-regulation (B) in WT but not in Hoxb8 mFpr2−/− cells at indicated concentrations. Data represent means ± sem of at least 3 independent experiments. *P < 0.05; ****P < 0.0001 vs. mFpr2−/− neutrophils, as calculated by Student’s t test.
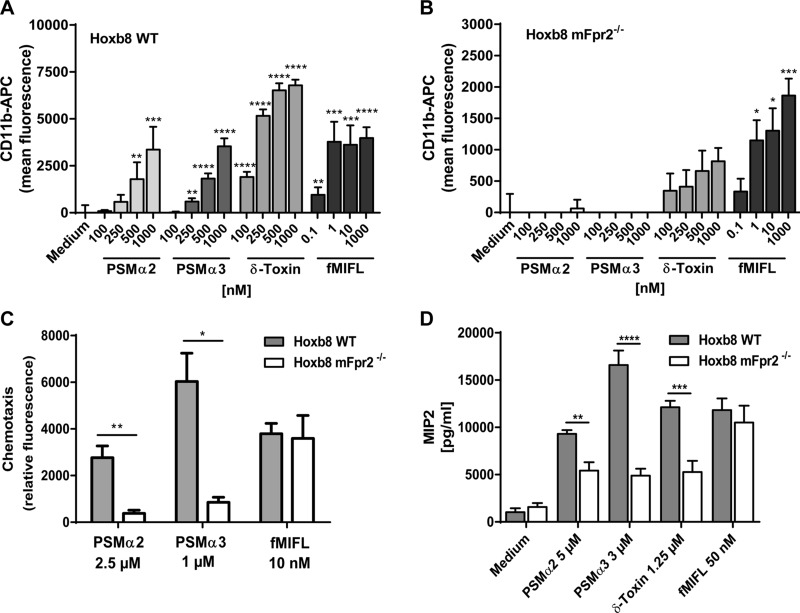
CD11b is important for effective phagocytosis of pathogens, and its expression can be used as a marker for neutrophil activation. Hoxb8 neutrophils were then used to study up-regulation of CD11b expression, chemotaxis, and chemokine release. For chemotaxis, we observed the typical bell-shaped curves for all tested peptides (data not shown) and show the optimal concentrations in Fig. 5. Stimulation of WT and mFpr2−/− Hoxb8 with fMIFL induced CD11b up-regulation, chemotaxis, and cytokine release in both cell types (Fig. 5). In contrast, PSMs induced robust activation of WT but no or only very weak activation of mFpr2−/− Hoxb8 in all 3 assays (Fig. 5). Furthermore, only WT Hoxb8 but not mFpr2−/− Hoxb8 responded to S. aureus WT culture filtrates. Supernatants from the S. aureus PSM mutant had no activity for any of the Hoxb8 neutrophils (Fig. 6B).
Figure 5.
Hoxb8 neutrophils expressing mFpr2 up-regulate CD11b and migrate toward and release MIP2 upon PSM stimulation. A, B) PSMs (α2, α3, δ-toxin) and fMIFL induce CD11b up-regulation in WT Hoxb8 cells (A) or mFpr2−/− Hoxb8 cells (B). C) Migration is induced by 2.5 µM PSMα2, 1 µM PSMα3, or 10 nM fMIFL in WT Hoxb8 or mFpr2−/− Hoxb8 cells. D) MIP2 secretion is induced by PSMs and fMIFL in WT and mFpr2−/− Hoxb8 cells. APC, allophycocyanin. Data represent means ± sem of at least 3 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 vs. corresponding medium control (A, B) or vs. mFpr2−/− neutrophils (C, D), as calculated by Student’s t test.
Early leukocyte recruitment in peritoneal S. aureus infection is mediated via mFpr2
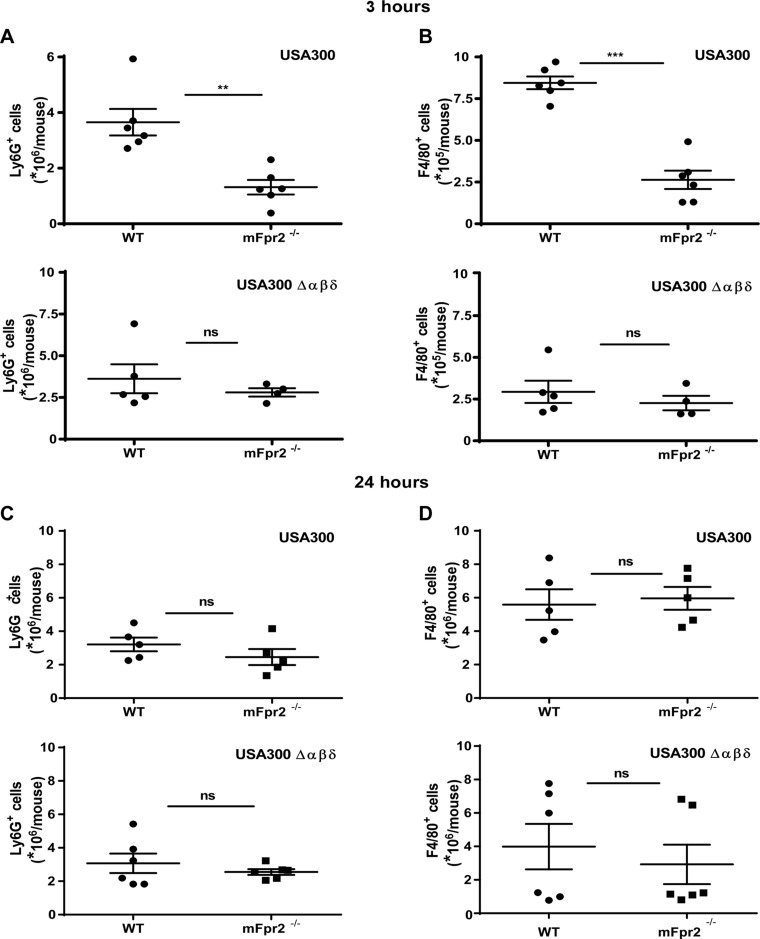
The mFpr2 receptor behaved like the human FPR2 in all tested parameters so far in response to S. aureus PSMs, indicating that mFpr2−/− mice could be useful for studying the role of mFpr2 in S. aureus infection. To analyze if local leukocyte recruitment after S. aureus infection is influenced by mFpr2, WT and mFpr2−/− mice were infected intraperitoneally for 3 h with USA300 or the isogenic PSM mutant. Subsequently, the numbers of infiltrated granulocytes and macrophages were determined. Notably, >2-fold lower numbers of granulocytes and macrophages were detected in the peritoneum of mFpr2−/− mice compared with WT mice after infection with USA300. In contrast, the isogenic PSM mutant caused lower numbers of macrophages compared with infection with the parental strain, and there were no differences in leukocyte counts between WT and mFpr2−/− mice (Fig. 7). Twenty-four hours after infection, no difference between WT and mFpr2−/− mice was observed in the recruitment of neutrophils and macrophages, neither with the PSM-producing USA300 nor with the isogenic PSM mutant (Fig. 7). Thus, mFpr2 plays an important role in recruiting leukocytes in early phases of local S. aureus infections.
Figure 7.
Leukocyte infiltration into the peritoneum of WT or mFpr2−/− mice, induced by S. aureus USA300. A, B) Infiltration of granulocytes (A) or macrophages (B) into the peritoneum of WT or mFpr2−/− mice, 3 h after intraperitoneal infection with USA300 WT or USA300Δαβδ. C, D) Infiltration of granulocytes (C) or macrophages (D) into the peritoneum of WT or mFpr2−/− mice, 24 h after intraperitoneal infection with USA300 WT or USA300Δαβδ. Data represent mean values of 4–6 mice. **P < 0.01, ***P < 0.001; ns, not significantly different (WT vs. mFpr2−/− mice), as calculated by Student’s t test.
DISCUSSION
S. aureus infections belong to the most frequent infectious diseases (2). Most of them remain superficial, for example, as minor skin or wound infections, and are often hardly recognized, because they are quickly and efficiently cleared by the innate immune system (34). Which mechanisms contribute to the detection and eradication of such infections has remained insufficiently explored. Innate immunity also contributes to severe infections, such as septicemia and pneumonia, which are always associated with massive inflammation, and it instructs the adaptive immune system if infections cannot easily be cured (34). Staphylococcal lipoproteins recognized by the TLR2 receptor are regarded as major MAMPs in S. aureus infections (35). In addition, other bacterial molecules, including peptidoglycan (36), DNA, RNA (37, 38), formylated peptides (26), and PSMs (6, 9), are thought to shape the course of S. aureus infections, but in which instances and to which extent they may instruct innate immunity remain elusive. Our study demonstrates that FPR2 and its staphylococcal ligands are crucial players in early S. aureus infections.
In contrast to other pattern recognition receptors, FPRs differ largely between mammalian species with different numbers of orthologs and specificities (12, 39, 40), probably because FPR peptide ligands can more easily be altered by pathogens than nonprotein MAMPs, which may provoke fast receptor coevolution. The FPR gene cluster has probably expanded and diversified to optimize the recognition of a wide range of peptides. A recent study supported this hypothesis and suggested that FPR genes have been subject to positive selection in the ongoing contact with pathogens (41). The mouse mFpr1 receptor has been clearly identified as the homolog of human FPR1, and mFpr1 knockout mice have been used to demonstrate that it contributes to the course of systemic Listeria monocytogenes infections (42, 43). Whether FPR1 also contributes to clearance of superficial infections remains to be analyzed. The association of FPR1 polymorphisms with more severe cases of periodontitis suggests that it contributes to the clearance of local infections (44).
The identity of the functional mFPR2 homolog has been more difficult to elucidate, as mice have several close FPR2 homologs, as outlined before (16). Nevertheless, recent studies supported the notion that mFpr2 has similar ligand specificities as human FPR2 (29). Interestingly, mFpr3, which has been proposed as a third FPR on the surface of mouse neutrophils, is functionally expressed only in some mouse strains (13). Our used mouse strain belongs to the mice, which express functional mFpr3, but our data demonstrate that only mFpr2 senses S. aureus PSMs. Moreover, mFpr2 responds to S. aureus culture filtrates much stronger than mFpr1, which reflects similar findings with human FPR1 and FPR2 (45). mFpr2 has been found to contribute to the severity of systemic L. monocytogenes infections, although ligands from Listeria that activate FPR2 or mFpr2 have not been reported yet (42). Additionally, it has been shown that lipoxin A4, an endogenous anti-inflammatory ligand of mFpr2, inhibits leukocyte recruitment in a pneumosepsis model in early sepsis, and the blocking of mFpr2 has exhibited beneficial effects to the host, increasing leukocyte migration, reducing bacterial load, and culminating with a survival increase (46). In contrast, we recently found that mFpr2 does not contribute to systemic S. aureus infection, probably as neutrophil chemotaxis is more relevant in local than in systemic infection (8). Our current study demonstrates that mFpr2 has a profound impact on early stages of local S. aureus infections governing the numbers of immigrated neutrophils and monocytes. mFpr2 did not seem to play a major role at later stages of infection, suggesting that other pattern recognition receptors may have more pronounced roles if a local S. aureus infection cannot be cleared rapidly. Interestingly, it has been shown that polymicrobial sepsis of Fpr2/3−/− mice that lack these receptors leads to an exacerbation of sepsis (47). It has been observed that the loss of Fpr2/3 correlates with increased plasma cytokine and chemokine levels, increased PMN amount, and reduced killing of E. coli by PMNs. It could be speculated that reduced activity of PMNs in these knockout mice is compensated by recruitment of more PMNs. As the authors used only double-knockout mice, the observed effects could, at least partially, be mediated by mFpr3 (47). Human FPR2 polymorphisms have been reported, but it is not clear yet if these may be associated with increased frequency or severity of superficial S. aureus infections. Nevertheless, such polymorphisms have been found to affect allergic diseases, such as chronic urticaria (48) and asthma (49), which are also associated with increased rates of S. aureus colonization (50). Thus, FPR2 function may affect S. aureus skin and airway colonization and infection.
It remains unclear which role FPR2 may play in infections caused by other pathogens. Culture filtrates of Enterococcus faecalis, Enterococcus faecium (45), and L. monocytogenes (51) contain FPR2 agonists whose identity has not been revealed yet, suggesting that FPR2 may be important for a wider range of bacterial pathogens and providing a potential explanation for the presence of an FPR2 homolog in mice, which seem to be less frequently infected by S. aureus than humans. Superficial, difficult-to-treat skin infections caused by community-associated methicillin-resistant S. aureus strains, such as USA300, have strongly increased in recent years (52). The targeting of FPR2 by immunomodulatory drugs may become a new strategy for treating such infections.
ACKNOWLEDGMENTS
The authors thank Cordula Gekeler (University of Tübingen) for technical support, Philip Murphy for providing vectors for mFpr1 and mFpr2 cloning, and Ji Ming Wang (both from the U.S. National Institutes of Health) for providing C57BL/6 mFpr2−/− mice. This work was supported by grants from the German Research Council (SFB685, SFB766, TRR156, and PE805/5-1 to A.P.; TRR34 to A.P. and D.K.; and KL 603/10-1 to A.K.) and the University of Tübingen Medical Faculty Fortüne Program (to D.K.). The authors declare no conflicts of interest.
Glossary
- fMIFL
formyl-Met-Ile-Phe-Leu
- FPR
formyl-peptide receptor
- Hoxb8
homeobox b8
- MAMP
microbe-associated molecular pattern
- mFpr2
mouse formyl-peptide receptor 2
- MIP2
macrophage inflammatory protein 2
- PMN
polymorphonuclear neutrophil
- PSM
phenol-soluble modulin
- RBL
rat basophilic leukemia
- SCF
stem cell factor
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
E. Weiss, A. Peschel, and D. Kretschmer designed research and wrote the paper; F. D. von Loewenich and J. Liese prepared Hoxb8 cells; D. Hanzelmann, B. Fehlhaber, and A. Klos prepared transfected RBL cells; and E. Weiss and D. Kretschmer conducted experiments.
REFERENCES
- 1.Van Belkum A., Melles D. C., Nouwen J., van Leeuwen W. B., van Wamel W., Vos M. C., Wertheim H. F., Verbrugh H. A. (2009) Co-evolutionary aspects of human colonisation and infection by Staphylococcus aureus. Infect. Genet. Evol. 9, 32–47 [DOI] [PubMed] [Google Scholar]
- 2.Thammavongsa V., Kim H. K., Missiakas D., Schneewind O. (2015) Staphylococcal manipulation of host immune responses. Nat. Rev. Microbiol. 13, 529–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foster T. J. (2004) The Staphylococcus aureus “superbug”. J. Clin. Invest. 114, 1693–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bloes D. A., Kretschmer D., Peschel A. (2015) Enemy attraction: bacterial agonists for leukocyte chemotaxis receptors. Nat. Rev. Microbiol. 13, 95–104 [DOI] [PubMed] [Google Scholar]
- 5.Bufe B., Schumann T., Kappl R., Bogeski I., Kummerow C., Podgórska M., Smola S., Hoth M., Zufall F. (2015) Recognition of bacterial signal peptides by mammalian formyl peptide receptors: a new mechanism for sensing pathogens. J. Biol. Chem. 290, 7369–7387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kretschmer D., Gleske A. K., Rautenberg M., Wang R., Köberle M., Bohn E., Schöneberg T., Rabiet M. J., Boulay F., Klebanoff S. J., van Kessel K. A., van Strijp J. A., Otto M., Peschel A. (2010) Human formyl peptide receptor 2 senses highly pathogenic Staphylococcus aureus. Cell Host Microbe 7, 463–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rautenberg M., Joo H. S., Otto M., Peschel A. (2011) Neutrophil responses to staphylococcal pathogens and commensals via the formyl peptide receptor 2 relates to phenol-soluble modulin release and virulence. FASEB J. 25, 1254–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanzelmann D., Joo H. S., Franz-Wachtel M., Hertlein T., Stevanovic S., Macek B., Wolz C., Götz F., Otto M., Kretschmer D., Peschel A. (2016) Toll-like receptor 2 activation depends on lipopeptide shedding by bacterial surfactants. Nat. Commun. 7, 12304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang R., Braughton K. R., Kretschmer D., Bach T. H., Queck S. Y., Li M., Kennedy A. D., Dorward D. W., Klebanoff S. J., Peschel A., DeLeo F. R., Otto M. (2007) Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat. Med. 13, 1510–1514 [DOI] [PubMed] [Google Scholar]
- 10.Kretschmer D., Rautenberg M., Linke D., Peschel A. (2015) Peptide length and folding state govern the capacity of staphylococcal β-type phenol-soluble modulins to activate human formyl-peptide receptors 1 or 2. J. Leukoc. Biol. 97, 689–697 [DOI] [PubMed] [Google Scholar]
- 11.Ye R. D., Boulay F., Wang J. M., Dahlgren C., Gerard C., Parmentier M., Serhan C. N., Murphy P. M. (2009) International Union of Basic and Clinical Pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol. Rev. 61, 119–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao J. L., Chen H., Filie J. D., Kozak C. A., Murphy P. M. (1998) Differential expansion of the N-formylpeptide receptor gene cluster in human and mouse. Genomics 51, 270–276 [DOI] [PubMed] [Google Scholar]
- 13.Stempel H., Jung M., Pérez-Gómez A., Leinders-Zufall T., Zufall F., Bufe B. (2016) Strain-specific loss of formyl peptide receptor 3 in the murine vomeronasal and immune systems. J. Biol. Chem. 291, 9762–9775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Southgate E. L., He R. L., Gao J. L., Murphy P. M., Nanamori M., Ye R. D. (2008) Identification of formyl peptides from Listeria monocytogenes and Staphylococcus aureus as potent chemoattractants for mouse neutrophils. J. Immunol. 181, 1429–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao J. L., Murphy P. M. (1993) Species and subtype variants of the N-formyl peptide chemotactic receptor reveal multiple important functional domains. J. Biol. Chem. 268, 25395–25401 [PubMed] [Google Scholar]
- 16.Hartt J. K., Barish G., Murphy P. M., Gao J. L. (1999) N-Formylpeptides induce two distinct concentration optima for mouse neutrophil chemotaxis by differential interaction with two N-formylpeptide receptor (FPR) subtypes. Molecular characterization of FPR2, a second mouse neutrophil FPR. J. Exp. Med. 190, 741–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang T. S., Wang J. M., Murphy P. M., Gao J. L. (2000) Serum amyloid A is a chemotactic agonist at FPR2, a low-affinity N-formylpeptide receptor on mouse neutrophils. Biochem. Biophys. Res. Commun. 270, 331–335 [DOI] [PubMed] [Google Scholar]
- 18.Tiffany H. L., Lavigne M. C., Cui Y. H., Wang J. M., Leto T. L., Gao J. L., Murphy P. M. (2001) Amyloid-beta induces chemotaxis and oxidant stress by acting at formylpeptide receptor 2, a G protein-coupled receptor expressed in phagocytes and brain. J. Biol. Chem. 276, 23645–23652 [DOI] [PubMed] [Google Scholar]
- 19.Onnheim K., Bylund J., Boulay F., Dahlgren C., Forsman H. (2008) Tumour necrosis factor (TNF)-alpha primes murine neutrophils when triggered via formyl peptide receptor-related sequence 2, the murine orthologue of human formyl peptide receptor-like 1, through a process involving the type I TNF receptor and subcellular granule mobilization. Immunology 125, 591–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Haas C. J., Veldkamp K. E., Peschel A., Weerkamp F., Van Wamel W. J., Heezius E. C., Poppelier M. J., Van Kessel K. P., van Strijp J. A. (2004) Chemotaxis inhibitory protein of Staphylococcus aureus, a bacterial antiinflammatory agent. J. Exp. Med. 199, 687–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prat C., Bestebroer J., de Haas C. J., van Strijp J. A., van Kessel K. P. (2006) A new staphylococcal anti-inflammatory protein that antagonizes the formyl peptide receptor-like 1. J. Immunol. 177, 8017–8026 [DOI] [PubMed] [Google Scholar]
- 22.Prat C., Haas P. J., Bestebroer J., de Haas C. J., van Strijp J. A., van Kessel K. P. (2009) A homolog of formyl peptide receptor-like 1 (FPRL1) inhibitor from Staphylococcus aureus (FPRL1 inhibitory protein) that inhibits FPRL1 and FPR. J. Immunol. 183, 6569–6578 [DOI] [PubMed] [Google Scholar]
- 23.Diep B. A., Otto M. (2008) The role of virulence determinants in community-associated MRSA pathogenesis. Trends Microbiol. 16, 361–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joo H. S., Cheung G. Y., Otto M. (2011) Antimicrobial activity of community-associated methicillin-resistant Staphylococcus aureus is caused by phenol-soluble modulin derivatives. J. Biol. Chem. 286, 8933–8940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen K., Le Y., Liu Y., Gong W., Ying G., Huang J., Yoshimura T., Tessarollo L., Wang J. M. (2010) A critical role for the g protein-coupled receptor mFPR2 in airway inflammation and immune responses. J. Immunol. 184, 3331–3335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dürr M. C., Kristian S. A., Otto M., Matteoli G., Margolis P. S., Trias J., van Kessel K. P., van Strijp J. A., Bohn E., Landmann R., Peschel A. (2006) Neutrophil chemotaxis by pathogen-associated molecular patterns--formylated peptides are crucial but not the sole neutrophil attractants produced by Staphylococcus aureus. Cell. Microbiol. 8, 207–217 [DOI] [PubMed] [Google Scholar]
- 27.Wang G. G., Calvo K. R., Pasillas M. P., Sykes D. B., Häcker H., Kamps M. P. (2006) Quantitative production of macrophages or neutrophils ex vivo using conditional Hoxb8. Nat. Methods 3, 287–293 [DOI] [PubMed] [Google Scholar]
- 28.Dahlgren C., Gabl M., Holdfeldt A., Winther M., Forsman H. (2016) Basic characteristics of the neutrophil receptors that recognize formylated peptides, a danger-associated molecular pattern generated by bacteria and mitochondria. Biochem. Pharmacol. 114, 22–39 [DOI] [PubMed] [Google Scholar]
- 29.Skovbakke S. L., Winther M., Gabl M., Holdfeldt A., Linden S., Wang J. M., Dahlgren C., Franzyk H., Forsman H. (2016) The peptidomimetic Lau-(Lys-βNSpe)6-NH2 antagonizes formyl peptide receptor 2 expressed in mouse neutrophils. Biochem. Pharmacol. 119, 56–65 [DOI] [PubMed] [Google Scholar]
- 30.Kretschmer D., Nikola N., Dürr M., Otto M., Peschel A. (2012) The virulence regulator Agr controls the staphylococcal capacity to activate human neutrophils via the formyl peptide receptor 2. J. Innate Immun. 4, 201–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swamydas M., Luo Y., Dorf M. E., Lionakis M. S. (2015) Isolation of mouse neutrophils. Curr. Protoc. Immunol. 110, 3.20.1–3.20.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rzodkiewicz P., Wojtecka-Lukasik E., Szukiewicz D., Schunack W., Maslinski S. (2010) Antihistaminic drugs modify casein-induced inflammation in the rat. Inflamm. Res. 59 (Suppl 2), S187–S188 [DOI] [PubMed] [Google Scholar]
- 33.McDonald J. U., Cortini A., Rosas M., Fossati-Jimack L., Ling G. S., Lewis K. J., Dewitt S., Liddiard K., Brown G. D., Jones S. A., Hallett M. B., Botto M., Taylor P. R. (2011) In vivo functional analysis and genetic modification of in vitro-derived mouse neutrophils. FASEB J. 25, 1972–1982 [DOI] [PubMed] [Google Scholar]
- 34.Belkaid Y., Tamoutounour S. (2016) The influence of skin microorganisms on cutaneous immunity. Nat. Rev. Immunol. 16, 353–366 [DOI] [PubMed] [Google Scholar]
- 35.Kawai T., Akira S. (2010) The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 11, 373–384 [DOI] [PubMed] [Google Scholar]
- 36.Xie X., Wang L., Gong F., Xia C., Chen J., Song Y., Shen A., Song J. (2012) Intracellular Staphylococcus aureus-induced NF-κB activation and proinflammatory responses of P815 cells are mediated by NOD2. J. Huazhong Univ. Sci. Technolog. Med. Sci. 32, 317–323 [DOI] [PubMed] [Google Scholar]
- 37.Bergstrøm B., Aune M. H., Awuh J. A., Kojen J. F., Blix K. J., Ryan L., Flo T. H., Mollnes T. E., Espevik T., Stenvik J. (2015) TLR8 senses Staphylococcus aureus RNA in human primary monocytes and macrophages and induces IFN-β production via a TAK1-IKKβ-IRF5 signaling pathway. J. Immunol. 195, 1100–1111 [DOI] [PubMed] [Google Scholar]
- 38.Krüger A., Oldenburg M., Chebrolu C., Beisser D., Kolter J., Sigmund A. M., Steinmann J., Schäfer S., Hochrein H., Rahmann S., Wagner H., Henneke P., Hornung V., Buer J., Kirschning C. J. (2015) Human TLR8 senses UR/URR motifs in bacterial and mitochondrial RNA. EMBO Rep. 16, 1656–1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Snyderman R., Pike M. C. (1980) N-Formylmethionyl peptide receptors on equine leukocytes initiate secretion but not chemotaxis. Science 209, 493–495 [DOI] [PubMed] [Google Scholar]
- 40.Oseas R. S., Boxer L. A., Butterick C., Baehner R. L. (1980) Differences in polymorphonuclear leukocyte aggregating responses among several species in response to chemotactic stimulation. J. Lab. Clin. Med. 96, 213–221 [PubMed] [Google Scholar]
- 41.Muto Y., Guindon S., Umemura T., Kőhidai L., Ueda H. (2015) Adaptive evolution of formyl peptide receptors in mammals. J. Mol. Evol. 80, 130–141 [DOI] [PubMed] [Google Scholar]
- 42.Liu M., Chen K., Yoshimura T., Liu Y., Gong W., Wang A., Gao J. L., Murphy P. M., Wang J. M. (2012) Formylpeptide receptors are critical for rapid neutrophil mobilization in host defense against Listeria monocytogenes. Sci. Rep. 2, 786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao J. L., Lee E. J., Murphy P. M. (1999) Impaired antibacterial host defense in mice lacking the N-formylpeptide receptor. J. Exp. Med. 189, 657–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y., Syed R., Uygar C., Pallos D., Gorry M. C., Firatli E., Cortelli J. R., VanDyke T. E., Hart P. S., Feingold E., Hart T. C. (2003) Evaluation of human leukocyte N-formylpeptide receptor (FPR1) SNPs in aggressive periodontitis patients. Genes Immun. 4, 22–29 [DOI] [PubMed] [Google Scholar]
- 45.Bloes D. A., Otto M., Peschel A., Kretschmer D. (2012) Enterococcus faecium stimulates human neutrophils via the formyl-peptide receptor 2. PLoS One 7, e39910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sordi R., Menezes-de-Lima O. Jr., Horewicz V., Scheschowitsch K., Santos L. F., Assreuy J. (2013) Dual role of lipoxin A4 in pneumosepsis pathogenesis. Int. Immunopharmacol. 17, 283–292 [DOI] [PubMed] [Google Scholar]
- 47.Gobbetti T., Coldewey S. M., Chen J., McArthur S., le Faouder P., Cenac N., Flower R. J., Thiemermann C., Perretti M. (2014) Nonredundant protective properties of FPR2/ALX in polymicrobial murine sepsis. Proc. Natl. Acad. Sci. USA 111, 18685–18690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang E. M., Kim S. H., Kim N. H., Park H. S. (2010) The genetic association of the FPRL1 promoter polymorphism with chronic urticaria in a Korean population. Ann. Allergy Asthma Immunol. 105, 96–97 [DOI] [PubMed] [Google Scholar]
- 49.Kim H. J., Cho S. H., Park J. S., Lee T. H., Lee E. J., Kim Y. H., Uh S. T., Chung I. Y., Kim M. K., Choi I. S., Park B. L., Shin H. D., Park C. S. (2012) Association analysis of formyl peptide receptor 2 (FPR2) polymorphisms and aspirin exacerbated respiratory diseases. J. Hum. Genet. 57, 247–253 [DOI] [PubMed] [Google Scholar]
- 50.Sharma A. D. (2012) Role of nasal carriage of Staphylococcus aureus in chronic urticaria. Indian J. Dermatol. 57, 233–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rabiet M. J., Huet E., Boulay F. (2005) Human mitochondria-derived N-formylated peptides are novel agonists equally active on FPR and FPRL1, while Listeria monocytogenes-derived peptides preferentially activate FPR. Eur. J. Immunol. 35, 2486–2495 [DOI] [PubMed] [Google Scholar]
- 52.Otto M. (2013) Community-associated MRSA: what makes them special? Int. J. Med. Microbiol. 303, 324–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Correia-de-Sá P., Noronha-Matos J. B., Timóteo M. A., Ferreirinha F., Marques P., Soares A. M., Carvalho C., Cavalcante W. L., Gallacci M. (2013) Bothropstoxin-I reduces evoked acetylcholine release from rat motor nerve terminals: radiochemical and real-time video-microscopy studies. Toxicon 61, 16–25 [DOI] [PubMed] [Google Scholar]