Abstract
Phagocytosis after myocardial infarction (MI) is a prerequisite to cardiac repair. Recruited monocytes clear necrotic cardiomyocytes and differentiate into cardiac macrophages. Some studies have linked apoptotic cell receptors on cardiac macrophages to tissue repair; however, the contribution of precursor monocyte phagocytic receptors, which are the first to interact with the cardiac parenchyma, is unclear. The scavenger receptor cluster of differentiation (CD)36 protein was detected on cardiac Ly6cHI monocytes, and bone marrow–derived Cd36 was essential for both early phagocytosis of dying cardiomyocytes and for smaller infarct sizes in female and male mice after permanent coronary ligation. Cd36 deficiency led to reduced expression of phagocytosis receptor Mertk and nuclear receptor Nr4a1 in cardiac macrophages, the latter previously shown to be required for phagocyte survival. Nr4a1 was required for phagocytosis-induced Mertk expression, and Nr4a1 protein directly bound to Mertk gene regulatory elements. To test the overall contribution of the Cd36-Mertk axis, MI was induced in Cd36−/− Mertk−/− double-knockout mice and led to increases in myocardial rupture. These data implicate monocyte CD36 in the mitigation of early infarct size and transition to Mertk-dependent macrophage function. Increased myocardial rupture in the absence of both Cd36 and Mertk underscore the physiologic significance of phagocytosis during tissue injury.—Dehn, S., Thorp, E. B. Myeloid receptor CD36 is required for early phagocytosis of myocardial infarcts and induction of Nr4a1-dependent mechanisms of cardiac repair.
Keywords: myocardial infarction, inflammation, monocyte
Heart failure secondary to myocardial infarction (MI) is the most common form of heart disease in the United States (1). Reduced blood flow and depressed oxygen supply lead to cardiac cell necrosis and apoptosis (2, 3). Repair of cardiac injury depends on efficient debridement of the cardiac wound; inefficiencies in this process prolong inflammation and lead to infarct expansion, adverse cardiac remodeling, and worsened contractile performance (4). Strategies that enhance phagocytic clearance and its associated organ-reparative signaling have the potential to limit both infarct size and the progression to heart failure.
In the early hours after MI, innate immune cell neutrophils and monocytes are recruited from the circulation and also mobilized in situ to clear necrotic and apoptotic cardiomyocytes (5–7). During experimental murine MI, Ly6cHI CCR2+ monocytes enter the myocardium within the first 3 d (5), where they secrete proteases and other inflammatory factors to facilitate wound debridement (8). Days later, surface monocyte Ly6c levels are reduced during differentiation into F4/80HI,MHCII+,Ly6cLO macrophages (5, 9). This second phase is important for phagocytic clearance by molecules such as myeloid epithelial reproductive receptor tyrosine kinase (MERTK) and milk fat globule-EGF (MFGE)-8 (10, 11) and is also associated with molecular processes that promote collagen production and cardiac remodeling (5, 9). A key factor in the transition from phases I to II is the nuclear receptor subfamily 4, group A, member 1 (NR4A1), which is necessary for survival of Ly6cLO phagocytes (7, 12–14). The activating triggers of NR4A1 are unclear.
We hypothesized that phase II polarization of cardiac macrophages by NR4A1 is activated by early cluster of differentiation (CD)36-dependent phagocytosis. To this end, we identified the scavenger receptor CD36 as necessary for early cardiomyocyte clearance and further linked this pathway to NR4A1-mediated phagocyte differentiation.
MATERIALS AND METHODS
Human blood
Peripheral blood was stained with antibodies against CD16 and CD14 according to institutional review board approval IRB-STU00075325 at Northwestern University. Human peripheral blood was collected from healthy controls and patients diagnosed with ST-segment elevation MI (often referred to as STEMI) via electrocardiography 1 d after being admitted to the cardiac care unit at Northwestern Memorial Hospital (Chicago, IL, USA). Subjects were excluded from the study if they were pregnant or unable to consent.
Mice
Mice were maintained in a pathogen-free environment, and all animal protocols and studies were conducted under Institutional Animal Care and Use Committee approval at Northwestern University. In this study, C57BI/6J mice were used as wild-type (WT) controls. Cd36 −/− JAX 019006 and αMHC-mCherry B6;D2-tg JAX 021557 mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA) (15). MerTK −/− mice have been described in Scott et al. (16).
Left anterior descending artery ligation
MI was induced as described in Wan et al. (11). In brief, the left anterior descending coronary artery (LAD) was ligated with a nylon 8-0 suture. Ligation was confirmed with blanching of the anterior wall. Sham operations were performed where the suture was passed completely underneath the LAD; however, the vessel was not tied off. For Nr4a1 activation (17), mice were administered cytosporone B (C2997; Millipore-Sigma, St. Louis, MO, USA), 10 mg/kg per mouse in 200 μl DMSO or 200 μl DMSO control, via oral gavage. Mice were treated with cytosporone B on post-MI d 0, 3, and 5.
Infarct size
At 2, 4, and 24 h after MI, mice were anesthetized with isoflurane and intubated. The diaphragm was cut and the heart was exposed. The myocardial area at risk (AAR) was labeled via intraventricular injection of fluorescent microspheres (F8834; Thermo Fisher Scientific). After allowing for perfusion of the microspheres, hearts were removed and sliced in 1-mm transverse cross sections and then incubated with a 1% solution of 2,3,5-triphenyltetrazolium chloride (T8877; Millipore-Sigma) in saline at 37°C for 5 min. The infarct size was normalized to AAR and expressed as a percentage of the total left ventricular myocardium.
Echocardiography
Two-dimensional echocardiograms were measured on a 25 MHz probe as described in Wan et al. (11). In brief, 28 d after coronary ligation short- and long-axis views of the left ventricle were taken just below the papillary muscles and used to calculate end-diastolic and -systolic dimensions, as well as wall and septum thicknesses. The Teichholz rule was applied for echocardiographic analyses.
Bone marrow transplant
Four- to 6-wk-old recipient mice were irradiated with 1000 rads from a cesium source before transplantation. Donor bone marrow cells from Cd36+/+ or Cd36−/− mice were injected into irradiated recipients through the tail vein. Mice were kept on acidified water containing neomycin after transplantation. Cd36 chimeras were achieved after bone marrow transplantation, as described in Nicholls et al. (18).
Flow cytometry
Hearts were processed for flow cytometry (11). Peripheral blood was collected by retro-orbital bleeding. Total cells were counted via hemocytometer and normalized to milligrams of infarct isolated or volume of blood collected. Cells were stained with antibodies against CD45 (clone 30-F11; 1:200 dilution), CD11b (M1/70, 1:200), CD115 (AFS98, 1:200), Ly6G (IA8, 1:200), CCR2 (475301, 1:40), F480 (BM8, 1:200), CD11c (HL3, 1:200), MHCII (M5/114.15.2, 1:200), and Ly6C (AL-21, 1:200) (BioLegend, San Diego, CA, USA, and R&D Systems, Minneapolis, MN, USA). For fluorescence-activated cell sorting (FACS) experiments, cells were sorted on a FACSAria (BD, Franklin Lakes, NJ, USA) directly into Trizol reagent (Thermo Fisher Scientific) for downstream RNA isolation. Human cells were stained with antibodies against CD11b, CD16, CD14, and CD36 (BD Biosciences, San Jose, CA, USA, and R&D). For in vivo cardiac efferocytosis, transgenic Myh6-driven mCherry mice received bone marrow transplanted from Cd36+/+ or Cd36−/− mice. After MI surgery, flow cytometry of myocardial extracts was used to identify CD11b+, Ly6G−, F4/80HI and -LO, Ly6cHI, and mCherry+ cells.
Primers for quantitative PCR were as follows (F, forward; R, reverse): SRA F: GGCTGGAGGGAAGTTGTCAATAC, SRA R: CCACTGGTGATGTAAAAGTTCTTG; CD36 F: TCCTCTGACATTTGCAGGTCTATC, CD36 R: AAAGGCATTGGCTGGAAGAA; MARCO F: TCCCAGGTCTTGTAGGCAGA, MARCO R: TAACCGAGCATGCGACAGAA; MerTK F: GAGGACTGCTTGGATGAACTG TA, MerTK R: AGGTGGGTCGATCCAAGG; Axl F: CATATCGAGGCCAGGACACC, Axl R: ATCCCCAGCCGAGGTATAGG; Nr4a1 F: CGGACAGACAGCCTAAAAGG, Nr4a1 R: TAACGTCCAGGGAACCAGAG; and B2M F: CTGCTACGTAACACAGTTCCACCC, B2M R: CATGATGCTTGATCACATGTCTCG.
Primary cell culture
Bone marrow–derived monocytes/macrophages were isolated from experimental mice and cultured for 7 d in normoglycemic DMEM (5.5 mM glucose) containing 20% L929-conditioned medium (19).
Chromatin immunoprecipitation
Chromatin for chromatin immunoprecipitation (ChIP) was prepared with a commercially available kit (17-600; Millipore-Sigma). Cross-linked chromatin was incubated overnight with 5 μg of antibodies against Nr4a1 (clone E-6) or isotype control. PCR primers were designed to amplify a 298 bp region flaking a nerve growth factor-induced gene B response element (NBRE) site 5 kb downstream of the murine Mertk promoter predicted using Matinspector (http://www.genomatix.de/matinspector) (Supplemental Fig. 5): NBRE-F: GGGTGCCTAAGTCCTTTGCT, NBRE-R: CTACTGGGAAAGCCTGCT CC.
Statistics
Data were analyzed with Prism 5.0 software (GraphPad Software, La Jolla, CA, USA), using unpaired 2-tailed Student’s t tests for 2 groups, as appropriate. Significance was accepted at P < 0.05. Data are expressed as means ± sem.
RESULTS
Cd36 is expressed on cardiac Ly6cHI monocytes in mice and in humans after MI
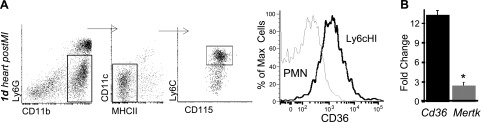
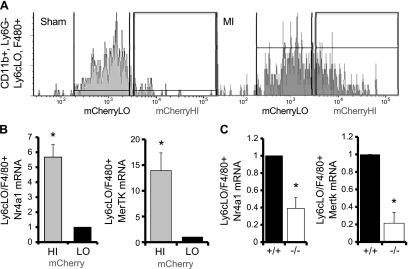
To examine relationships between innate immune cells and cardiac repair, we permanently ligated the LAD artery to induce MI in mice (11). We first sought to determine the expression of candidate phagocytosis receptors on cardiac Ly6cHI monocytes. Cardiac CD11b+,Ly6g−,Ly6cHI CD115+ monocytes were isolated 1 d after MI. The scavenger receptor CD36 was specifically detected on Ly6cHI cardiac monocytes and not on cardiac neutrophils 1 d after MI (Fig. 1A). CD36 was also found on CD14+ monocytes in circulating blood from patients with ST segment elevation MI (STEMI) (Supplemental Fig. 1). Quantitative PCR of cardiac monocyte receptors revealed that Cd36 expression was specific, in contrast to phagocytosis receptor Mertk (20), the latter of which is predominantly expressed on Ly6cLO, F4/80+ cardiac macrophages (Fig. 1B). Given the extent of cardiomyocyte necrosis after MI (2), we decided to examine CD36 in closer detail, given the link between CD36 and necrotic cell phagocytosis (21).
Figure 1.
Scavenger receptor CD36 is specifically expressed on cardiac Ly6cHI CDC115+ monocytes 1 d after MI. A) Left panels: infarct tissue was harvested 1 d after MI, and Ly6cHI monocytes were gated as CD11b+, Ly6G−, MHCII-LO, CD11c−, and CD115+. Right panel: histogram of CD36 on monocytes vs. CD11b+ Ly6g+ polymorphonuclear neutrophils. B) Fold change of Cd36 vs. MerTK gene expression over 18S control mRNA in cardiac Ly6cHI CD115+ monocytes (n = 6).
Cd36 is essential in bone marrow–derived cells and contributes to infarct size
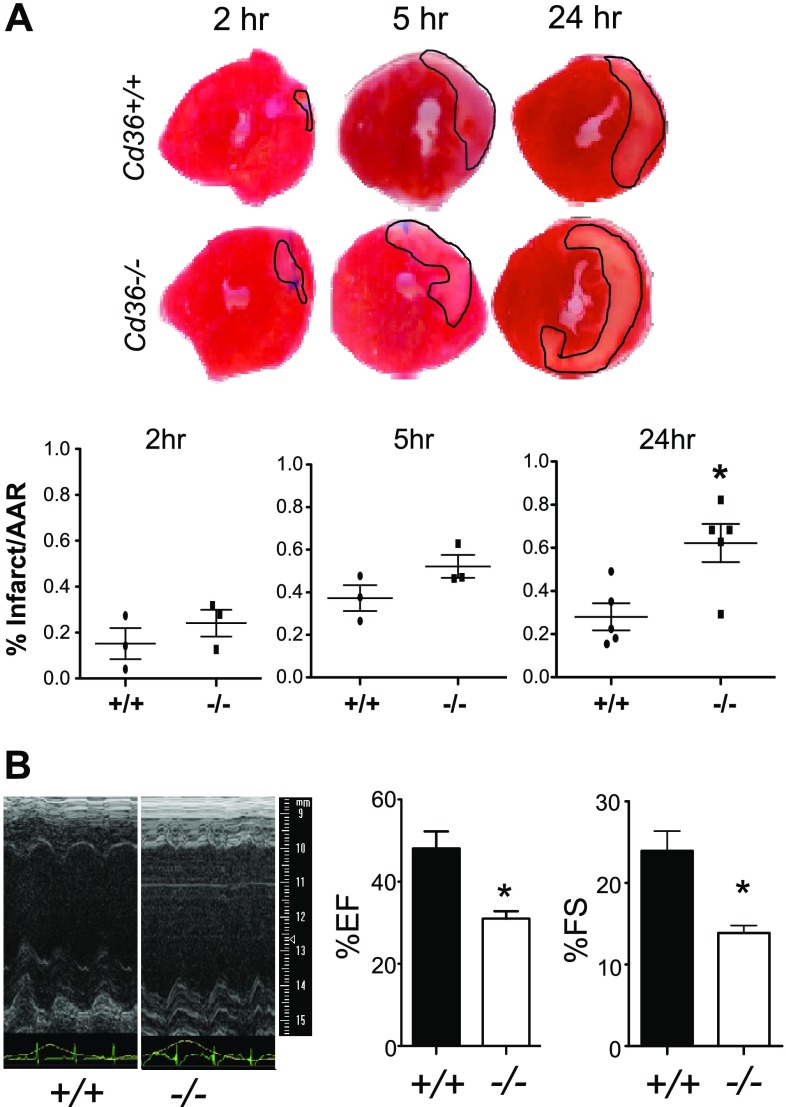
To focus our studies on hematapoietically derived Cd36, Cd36+/+ B6 mice were irradiated and received bone marrow transplanted from Cd36+/+ and Cd36−/− congenic donors. Efficiency of engraftment was confirmed by flow cytometry (Supplemental Fig. 2). Investigators have examined Cd36 whole-body knockouts after MI, which included cardiomyocytes (22), as well as after CD36 blockade (23); however, the contribution of CD36 to infarct repair by innate immune cells is unclear. To focus on acute CD36-dependent events, we examined infarct size within 1 d after MI, during the height of Ly6cHI CD115 monocyte accumulation in the infarcted heart (5) and during which time F4/80+ macrophages die off during ischemia (24). Infarcted hearts were stained with tetrazolium after perfusion with fluorescent beads (25) to label the infarct and AAR at the 2-, 5-, and 24-h time points. By 24 h after MI, hearts from Cd36−/− mice had significantly larger infarcts, relative to the AAR, compared to Cd36+/+ mice (Fig. 2). This increased myocardial death in the Cd36−/− recipients also preceded increased left ventricular dysfunction after MI, as measured by suppressed ejection fraction and fractional shortening (Fig. 2 and Supplemental Fig. 3). These data are consistent with functionally important Cd36 requirements early after MI, to limit infarct size.
Figure 2.
Bone marrow–derived Cd36 is necessary to limit early MI size and lessen systolic dysfunction. A) MI size. Representative triphenyltetrazolium chloride staining of hearts to identify infarcts at indicated time points after MI. Infarct size was normalized to AAR, as measured by fluorescent bead injection (n = 5 per genotype). B) Echocardiography at d 28 after MI in mice deficient for the scavenger receptor Cd36. B6 mice with Cd36+/+ vs. Cd36−/− bone marrow were subjected to permanent LAD artery ligation, and echocardiography (representative images) was assessed 28 d later. EF, ejection fraction; FS, fractional shortening. Heart rate was maintained at 500 beats per minute and respiration rate at 150 breaths per minute (n = 5/genotype). *P < 0.05.
Bone marrow–derived Cd36 is needed for acute uptake of cardiomyocytes within the infarct
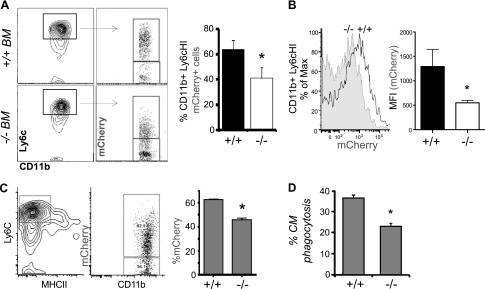
Inefficient clearance of cellular debris prolongs inflammation and leads to further tissue damage, potentially explaining the enlarged infarcts in Cd36−/− hearts. In vivo clearance of dying cardiomyocytes was measured after intravenous injection of bone marrow from Cd36+/+ or Cd36−/− mice into irradiated recipients expressing cardiomyocyte-specific mCherry (15) under the myosin heavy chain promoter (mCherry Cd36+/+ or Cd36−/−). This combination allowed us to associate fluorescent cardiomyocyte debris with recruited cardiac phagocytes. Clearance of dead cardiac cells was measured as a percentage of mCherry+ CD11b+, Ly6G−, CCR2+, Ly6cHI, and MHCII-LO cells in the infarct (Fig. 3), as well as total mCherry mean fluorescence intensity. Note, control experiments in which the phagocytosis inhibitor cytochalasin D was added during preparation of cardiac extracts indicated that postextract phagocytosis accounted for <19% of mCherry association. Relative to mice with sham surgery, mCherry Cd36+/+ mice had an acute increase in the percentage and total number of mCherry+ cells, consistent with recruited monocytes acting to clear dead cardiomyocytes after MI. Phagocytic clearance was dependent on Cd36 as Cd36−/− αMHC-mCherry mice had a significant decrease in cardiomyocyte engulfment.
Figure 3.
Cd36 is required for early (d 1) cardiomyocyte uptake by Ly6cHI and MHCII-LO phagocytes after MI. A). Myh6-mCherry Cd36+/+ and Cd36−/− bone marrow chimeras were subjected to permanent LAD occlusion, and cardiac extracts were sorted for CD11b+, Ly6cHI cells 1 d after MI and as a function of mCherry association. Representative dot plots are quantified at right to indicate percentage of cardiac CD11b+ Ly6cHI mCherry+ cells. B) Data are plotted to indicate CD11b+ Ly6cHI mean fluorescence intensity. C) Ly6cHI, MHCII-LO monocytic cells were gated from heart, and the percentage of mCherry association was enumerated in infarcted mice with Cd36+/+ and Cd36−/− bone marrow, 1 d after MI (n = 3/group). D) Cardiomyocyte phagocytosis required Cd36 in bone marrow–derived macrophages. *P < 0.05.
Cd36 is necessary for emergence of cardiac Ly6cLO macrophages
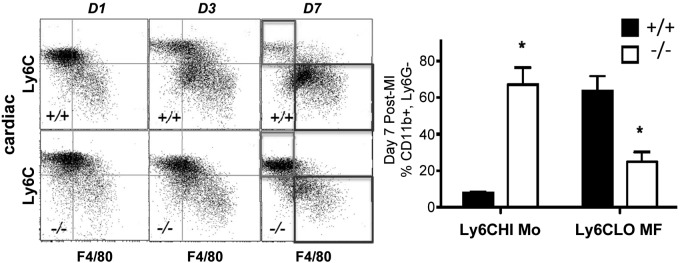
Innate inflammation after MI consists of a biphasic transition from Ly6cHI monocytes to Ly6cLO cardiac macrophages (9). Cardiac macrophages were gated as CD11b+, Ly6g−, F4/80-HI, Ly6cLO cells (Supplemental Fig. 4). We examined the kinetics of the monocyte and macrophage response in Cd36−/− mice after MI vs. control. In Cd36+/+ mice, by d 7, Ly6cHI monocytes were largely replaced by cardiac Ly6cLO macrophages (Fig. 4). Although there was no difference in blood or heart myeloid counts at baseline, Cd36−/− recipients exhibited reduced accumulation of cardiac Ly6cLO macrophages and increased Ly6cHI, F4/80LO cells at d 7 after MI (Fig. 4). Reductions in Ly6cLO macrophage accumulation were not associated with diminutions of peripheral blood Ly6cHI monocytes in either Cd36+/+ or Cd36−/− mice (Supplemental Fig. 5).
Figure 4.
Kinetics of cardiac CD11b+, Ly6cg−, Ly6cHI F4/80-LO monocytic cells and Ly6cLO, F4/80 macrophages after MI in mice deficient for bone marrow–derived Cd36. Analyses were performed at indicated days after permanent coronary artery ligation (n = 3/group). *P < 0.05.
Phagocytic Ly6cLO macrophages express elevated Cd36-dependent genes that are necessary for Ly6cLO macrophage function
Given the delayed accumulation of Ly6cLO cardiac phagocytes, we hypothesized that Cd36-dependent phagocytosis is a signal to promote the maturation of Ly6cLO phagocytes. Indeed, ex vivo, phagocytosis has been shown to activate nuclear receptors, including Nr4a1 (26), and separately, Nr4a1 is necessary for the survival of Ly6cLO cardiac phagocytes (9). To examine relationships between phagocytosis and Ly6cLO cell maturation, we separated cardiac Ly6cLO populations into those with high levels of mCherry vs. those with low levels of mCherry. In comparison to Ly6cLO mCherryLO macrophages, Ly6cLO mCherryHI macrophages expressed increased Nr4a1 (Fig. 5). Also increased was expression of Mertk, an important phagocytic receptor on Ly6cLO cardiac macrophages (11). Both gene inductions required Cd36.
Figure 5.
CD36-dependent phagocytic engulfment of dying cardiomyocytes is linked to Ly6cLO F4/80+ macrophage expression of MerTK and Nr4a1. A, B) At d 7 after MI or sham surgery, Ly6CLO macrophages were sorted by FACS from the infarcts of myh6-mCherry bone marrow recipients based on mCherry expression (A) and probed for Nr4a1 and MerTK mRNA (B). C) Nr4a1 and MerTK transcript expression in Ly6CLO macrophages sorted from Cd36+/+ and Cd36−/− mice (n = 6/group). *P < 0.05.
In sterile peritonitis models, Ly6cHI monocytes are replaced by Ly6cLO phagocytes and macrophages by 3 d after thioglycollate injection (27). To test whether Cd36 is necessary for Ly6cLO macrophage accumulation outside of the myocardium, apoptotic carboxyfluorescein succinimidyl ester (CFSE)–labeled thymocytes were injected i.p. into Cd36+/+ and Cd36−/− mice 6 h following thioglycollate treatment. At 48 h, peritoneal exudate was analyzed for Ly6cHI and Ly6cLO phagocyte accumulation and Nr4a1 expression. Similar to MI, Cd36−/− mice had persistent peritoneal Ly6cHI monocytes and diminished Ly6cLO macrophages compared to Cd36+/+ controls. When Ly6cLO phagocytes were sorted based on their engulfment status (CFSE+ vs. CFSE− apoptotic cells), there was decreased Nr4a1 expression in macrophages from Cd36-deficient mice (Supplemental Fig. 6), extending this mechanism to generalized inflammation.
Nr4a1 directly binds to Mertk and is required for MerTK expression after Cd36-dependent efferocytosis
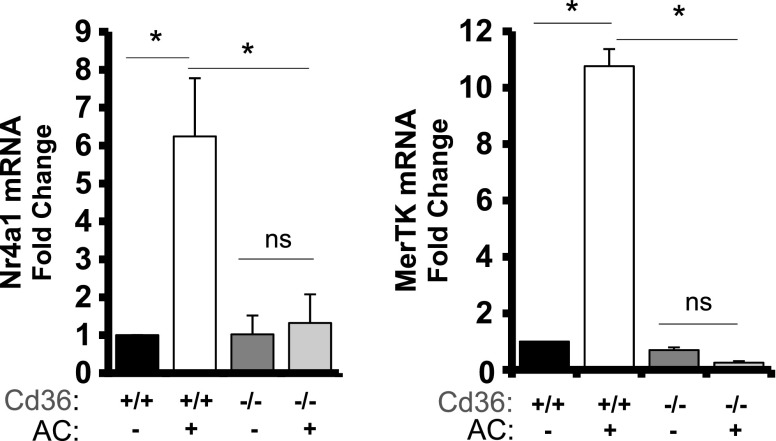
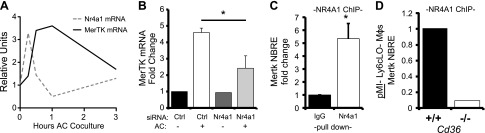
To examine the cell-intrinsic relationship between Cd36, Nr4a1, and Mertk, we next performed experiments in primary bone marrow–derived macrophages. Cd36 was essential for both Nr4a1 and Mertk gene expression during phagocytosis of apoptotic cells (Fig. 6). We next asked whether Nr4a1 can directly regulate MerTK expression. Nr4a1 is a transcription factor that binds to NBRE sites on regulatory regions of target genes, altering their expression (28). Consistent with Nr4a1 being upstream of Mertk, coculture of WT bone marrow–derived macrophages with apoptotic cells at a 5:1 ratio showed an early increase in Nr4a1 transcription, followed by MerTK induction in the minutes and hours after coculture (Fig. 7). siRNA knockdown of Nr4a1 prevented the transcriptional induction of Mertk in apoptotic cell-fed macrophages (Fig. 7). Finally, an in situ analysis of the promoter region of the murine Mertk gene identified a putative NBRE-binding site in an intronic region (Supplemental Fig. 7), downstream of the Mertk transcription start site, which was verified by Nr4a1 pulldown during ChIP (Fig. 7). Complementing our findings in vitro, ChIP pulldown of Ly6cLO macrophages sorted from Cd36+/+ and Cd36−/− mice 7 d after MI identified decreased Nr4a1 association with the NBRE-MerTK site in Cd36−/− cardiac macrophages in vivo (Fig. 7).
Figure 6.
Cd36 is necessary for Nr4a1 and Mertk gene induction. Bone marrow–derived macrophages from Cd36+/+ and Cd36−/− mice were cocultured with apoptotic targets (ACs, 5:1 ratio) for 1 h, and transcript levels of Nr4a1 and MerTK were measured by quantitative PCR analysis (n = 6/group). *P < 0.05.
Figure 7.
Nr4a1 is essential for Mertk gene expression and directly associates with Mertk gene regulatory elements. A) WT bone marrow–derived macrophages were cocultured with apoptotic cell (AC) targets (5:1 ratio), and Nr4a1 and MerTK expression was measured over time via quantitative PCR. B) Mertk expression in WT bone marrow–derived macrophages after 1 h coculture with ACs and 24 h treatment with control or Nr4a1 siRNA. C) In vitro ChIP probing for an NBRE binding site proximal to Mertk after pulldown with Nr4a1 antibody compared to IgG control (n = 3/group). D) NBRE ChIP of Ly6CLO F4/80HI cardiac macrophages sorted from Cd36+/+ and Cd36−/− mice. *P < 0.05.
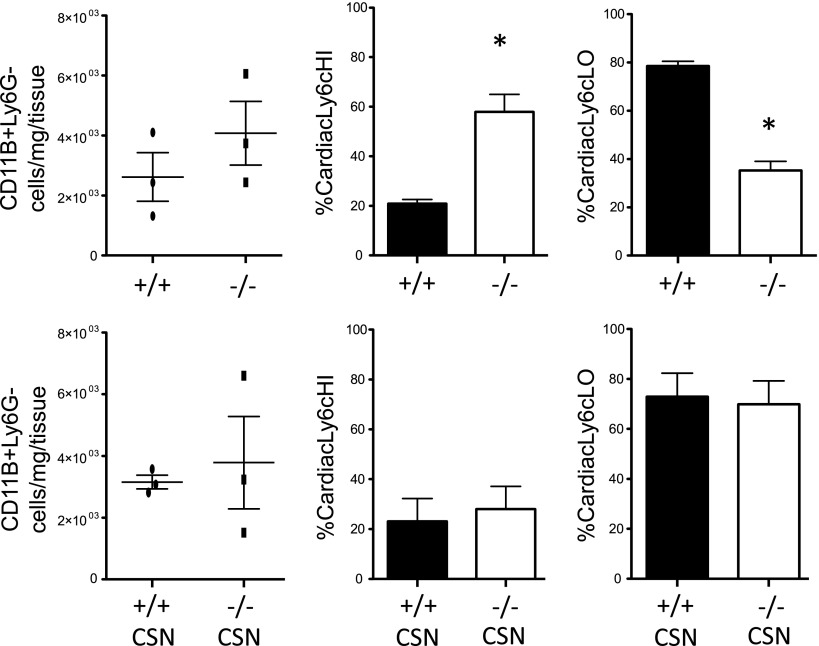
Nr4a1 agonism rescues Ly6cLO phagocytes in Cd36−/− mice after MI
To test the sufficiency of Nr4a1 to promote the accumulation of Ly6cLO macrophages in vivo, Cd36−/− mice were treated with the Nr4a1 agonist cytosporone B (CSN), or DMSO control, 0, 3, and 5 d after LAD ligation, and hearts were harvested on d 7 (Fig. 8). Although cytosporone B treatment had no effect on the total number of cells in the infarct, Nr4a1 agonism restored the ratio of monocytes to macrophages in the infarct of Cd36−/− mice 7 d after MI relative to Cd36+/+ controls, consistent with Nr4a1 activation as sufficient to bypass the defect of macrophage accumulation in Cd36-deficient mice.
Figure 8.
Nr4a1 agonist is sufficient to rescue monocyte and macrophage ratios in Cd36−/− mice. Cd36+/+ and Cd36−/− mice (n = 3/group) were treated with 3 doses of the Nr4a1 agonist cytosporone B (CSN), or DMSO control via oral gavage after MI. At 7 d after MI, hearts were harvested, and total CD11b+, Ly6G, Ly6CHI F4/80LO, and Ly6CLO F4/80HI cells were assayed. Bottom panel represents CSN-treated cells.
Decreased functional cardiac output in engulfment receptor double-knockout mice leads to myocardial rupture
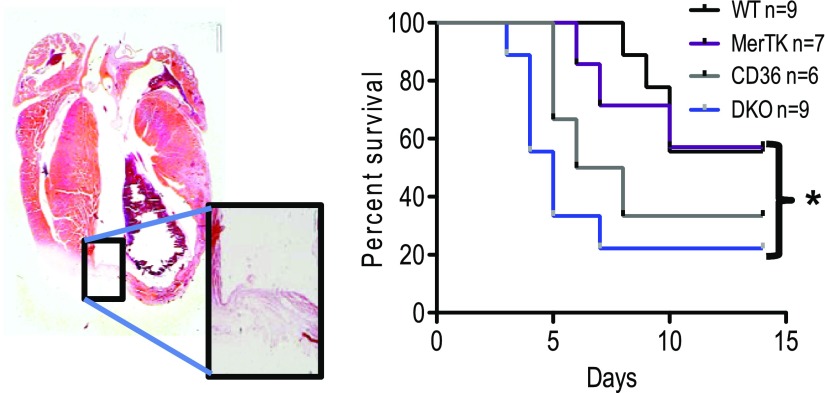
Although Cd36 deficiency delayed the emergence of Ly6cLO phagocytes, this delay was eventually overcome (i.e., the monocyte-to-macrophage ratio was returned to normal 9–10 d after MI), consistent with alternative or redundant phagocytic receptor rescue. In fact, double deficiency of Cd36 and Sra led to profound loss of Ly6cLO macrophage phagocytes (data not shown). Thus, Cd36 was not absolutely required for Mertk expression on Ly6cLO phagocytes, which permitted testing the combinatorial contribution of CD36 and Mertk (Fig. 9). In the absence of vascular reperfusion, cardiac-resident macrophages die (24). In this scenario, the recruitment of Ly6cHI monocytes is critical for sourcing phagocytic cells, and their blockade is associated with increased cardiac rupture-associated mortality (29). To test the significance of phagocytic loss by both monocytes and cardiac macrophages, we crossed Cd36−/− and Mertk−/− mice. WT mice received bone marrow transferred from Cd36+/+Mertk+/+, Cd36−/−/Mertk−/−, or single-knockout mice, and infarctions were subsequently induced. Cd36/Mertk double-knockout mice exhibited increased myocardial rupture (Fig. 9) relative to controls.
Figure 9.
Cardiac rupture in Cd36/Mertk double-deficient mice. Mortality was caused by cardiac rupture 2 wk after MI. Left: a representative image of Cd36 and Mertk double-deficient mice (DKO) after myocardial rupture. *P < 0.05.
DISCUSSION
At the height of inflammation, early molecular events actively trigger cellular programs of inflammation resolution in that the beginning programs the end (30). Consistent with this paradigm, we provide data to support a working model wherein early phagocytosis by recruited cardiac monocytes initiates cellular gene expression that is necessary for phagocyte maturation and function.
Similar to polarized cardiac macrophages (7, 31, 32), cardiac monocytes have the capacity to effect both beneficial and detrimental consequences on the injured heart. In humans, heightened levels of peripheral blood monocytes correlate with the cardiac disease severity, and in experimental rodents, an elevated number of Ly6cHI monocytes can offset cardioprotective events of wound healing (33, 34). This effect is particularly evident during hyperlipidemia, wherein high cholesterol levels are associated with increased Ly6cHI monocyte inflammation markers, such as TNF-α and myeloperoxidase (33). After coronary artery reperfusion, Ly6cHI monocytes are also linked to reperfusion injury, and strategies that inhibit CCR2-dependent monocyte cardiac influx (25, 35) or target monocyte-dependent generation of reactive oxygen intermediates, in turn, ameliorate myocardial injury (36). Relative to the aforementioned, fewer studies have examined the protective mechanisms of cardiac monocytes (29).
Phagocytic clearance of dying cells, or efferocytosis, is intricately linked to inflammation resolution (37–40). Cell intrinsic pathways secondary to apoptotic cell engulfment have long been associated with the potential to trigger anti-inflammatory cytokines (41); however, many of the mechanisms underlying these processes remain unclear. Though mature macrophages are more efficient at efferocytosis relative to their monocyte precursors, monocytes nevertheless can clear dying cells (42). Our studies herein implicate the scavenger receptor Cd36 in this context and in heart.
The class B scavenger receptor CD36 is a multifunctional membrane protein expressed by multiple cell types, including platelets (43), endothelial cells (44), adipocytes (45), myocytes (46), and monocytes and macrophages (47–49). CD36 binds several types of ligand, including oxidized LDL, long-chain fatty acids, thrombospondin-1, and modified surface membranes lipids that permit apoptotic and necrotic cell recognition (21). In the heart, most studies to date have focused on the function of CD36 during import of fatty acids during cardiomyocyte metabolism (50). However, in a study of patients with coronary heart disease and acute coronary syndromes, circulating blood CD14+ monocytes expressed increased Cd36 mRNA and CD36 protein relative to monocytes from healthy individuals (51, 52). In a mouse model of cerebral ischemia-reperfusion, Cd36 was induced in microglia and shown to promote reactive oxygen species postischemic injury, further regulating infarct size in the brain (53). Also in brain, CD36 contributes to phagocytosis during stroke resolution (54), as well as to the neurotoxic activity of infiltrating monocytes in a model of oxygen-glucose deprivation (55); myeloid CD36 has also been linked to hyperlipidemic stroke (56). Recruited monocytes in the lung express CD36 to clear infected erythrocytes in a malaria-injury model, thereby minimizing lung damage (57). In experimental hearts, pretreatment with a selective CD36 ligand agonist, EP 80317, reduced infarct area and increased ejection fraction after ischemia–reperfusion in mice (58).
In addition to our tests of the genetic requirements for Cd36, it is important to consider natural regulation of Cd36 in the post-MI milieu. For example, an inactivated form of CD36, solCD36, has been identified in both murine and human plasma after MI, implicating a natural mechanism of CD36 inactivation after infarction (59). The proteases matrix metalloproteinase 9 and ADAM17 have both been implicated in the cleavage of the CD36 ectodomain from the surface of macrophages (24, 59, 60). The soluble remnants of these cleaved products can function as decoy receptors, inhibiting interaction between surface receptors and their dead cellular targets (61). In addition, and according to our working model (Supplemental Fig. 9), CD36 cleavage would also be expected to reduce MERTK-dependent proresolving mediator biosynthesis (62), another important component of inflammation resolution. Thus, CD36 cleavage during MI may inhibit phagocytic clearance and exacerbate inflammation.
Our data suggest that the nuclear receptor Nr4a1 transduces Cd36-dependent signaling. Nr4a1 is an early response gene and a master regulator of Ly6cLO monocytes (14). Nr4a1 promotes the transcription of target genes through monomeric binding to NBREs. In addition, Nr4a1 can heterodimerize with other Nr4A family members and bind to Nur response elements (28). Our in silico analysis of the murine Mertk genomic sequence identified a putative NBRE downstream of the Mertk transcription start site in the first intronic region. The location of this binding site indicates that it may function as a cis-regulatory element, perhaps in the form of an intragenic enhancer (63). Overexpression of macrophage Nr4a1 in vitro suppressed Cd36 expression and induced Mertk in THP-1 macrophages (64). Upstream of Nr4a1, the short cytoplasmic tail of CD36 suggests partnering in signaling. In response to activating ligands oxLDL, β-amyloid, and LPS, CD36 has been shown to act as a coreceptor with TLR4 and -6 (65), as well as TLR2 (66). Studies are under way to determine whether efferocytic ligands also mimic similar signaling paradigms.
Taken together, our findings implicate CD36-mediated phagocytosis of dying cardiomyocytes in the control of early infarct size and transition to Ly6cLO-mediated cardiac repair. This pathway likely develops through Nr4a1-dependent induction of Mertk expression. In addition, increased myocardial rupture in the absence of both Cd36 and Mertk underscore the physiologic significance of phagocytosis after MI.
ACKNOWLEDGMENTS
The authors thank Matthew DeBerge, Kristofor Glinton (both from Northwestern University), and Terry Means (Massachusetts General Hospital and Harvard Medical School, Charlestown, MA, USA) for their intellectual contributions. This work was supported by Collaborative Learning and Integrated Mentoring in the Biosciences, Cellular, and Molecular Basis of Disease Program [U.S. National Institutes of Health (NIH), National Institute of General Medicine Sciences Grant T32GM08061], and NIH National Heart, Lung, and Blood Institute Diversity Supplements R01HL122309 (to S.D.) and R01HL122309 (to E.B.T.). Publication of this research was supported by the Sidney and Bess Eisenberg Memorial Fund. The authors declare no conflicts of interest.
Glossary
- AAR
area at risk
- CD
cluster of differentiation
- CFSE
carboxyfluorescein succinimidyl ester
- ChIP
chromium immunoprecipitation
- FACS
fluorescence-activated cell sorting
- LAD
left anterior descending coronary artery
- MI
myocardial infarction
- MERTK
myeloid epithelial reproductive receptor tyrosine kinase
- NBRE
nerve growth factor-induced gene B response element
- Nr4a1
nuclear receptor subfamily 4, group A, member 1
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
S. Dehn designed and conducted experiments, acquired and analyzed data, and wrote the manuscript, and E. B. Thorp designed research studies, analyzed data, and wrote the manuscript.
REFERENCES
- 1.Lloyd-Jones D., Adams R. J., Brown T. M., Carnethon M., Dai S., De Simone G., Ferguson T. B., Ford E., Furie K., Gillespie C., Go A., Greenlund K., Haase N., Hailpern S., Ho P. M., Howard V., Kissela B., Kittner S., Lackland D., Lisabeth L., Marelli A., McDermott M. M., Meigs J., Mozaffarian D., Mussolino M., Nichol G., Roger V. L., Rosamond W., Sacco R., Sorlie P., Stafford R., Thom T., Wasserthiel-Smoller S., Wong N. D., Wylie-Rosett J.; American Heart Association Statistics Committee and Stroke Statistics Subcommittee (2010) Executive summary: heart disease and stroke statistics—2010 update: a report from the American heart association. Circulation 121, 948–954; correction 21, e259 [DOI] [PubMed] [Google Scholar]
- 2.Chang J., Nair V., Luk A., Butany J. (2013) Pathology of myocardial infarction. Diagn. Histopathol. 19, 7–12 [Google Scholar]
- 3.Whelan R. S., Kaplinskiy V., Kitsis R. N. (2010) Cell death in the pathogenesis of heart disease: mechanisms and significance. Annu. Rev. Physiol. 72, 19–44 [DOI] [PubMed] [Google Scholar]
- 4.Frangogiannis N. G. (2008) The immune system and cardiac repair. Pharmacol. Res. 58, 88–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nahrendorf M., Swirski F. K., Aikawa E., Stangenberg L., Wurdinger T., Figueiredo J. L., Libby P., Weissleder R., Pittet M. J. (2007) The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J. Exp. Med. 204, 3037–3047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leuschner F., Rauch P. J., Ueno T., Gorbatov R., Marinelli B., Lee W. W., Dutta P., Wei Y., Robbins C., Iwamoto Y., Sena B., Chudnovskiy A., Panizzi P., Keliher E., Higgins J. M., Libby P., Moskowitz M. A., Pittet M. J., Swirski F. K., Weissleder R., Nahrendorf M. (2012) Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J. Exp. Med. 209, 123–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sager H. B., Hulsmans M., Lavine K. J., Moreira M. B., Heidt T., Courties G., Sun Y., Iwamoto Y., Tricot B., Khan O. F., Dahlman J. E., Borodovsky A., Fitzgerald K., Anderson D. G., Weissleder R., Libby P., Swirski F. K., Nahrendorf M. (2016) Proliferation and recruitment contribute to myocardial macrophage expansion in chronic heart failure. Circ. Res. 119, 853–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nian M., Lee P., Khaper N., Liu P. (2004) Inflammatory cytokines and postmyocardial infarction remodeling. Circ. Res. 94, 1543–1553 [DOI] [PubMed] [Google Scholar]
- 9.Hilgendorf I., Gerhardt L. M., Tan T. C., Winter C., Holderried T. A., Chousterman B. G., Iwamoto Y., Liao R., Zirlik A., Scherer-Crosbie M., Hedrick C. C., Libby P., Nahrendorf M., Weissleder R., Swirski F. K. (2014) Ly-6Chigh monocytes depend on Nr4a1 to balance both inflammatory and reparative phases in the infarcted myocardium. Circ. Res. 114, 1611–1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howangyin K. Y., Zlatanova I., Pinto C., Ngkelo A., Cochain C., Rouanet M., Vilar J., Lemitre M., Stockmann C., Fleischmann B. K., Mallat Z., Silvestre J. S. (2016) Myeloid-epithelial-reproductive receptor tyrosine kinase and milk fat globule epidermal growth factor 8 coordinately improve remodeling after myocardial infarction via local delivery of vascular endothelial growth factor. Circulation 133, 826–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wan E., Yeap X. Y., Dehn S., Terry R., Novak M., Zhang S., Iwata S., Han X., Homma S., Drosatos K., Lomasney J., Engman D. M., Miller S. D., Vaughan D. E., Morrow J. P., Kishore R., Thorp E. B. (2013) Enhanced efferocytosis of apoptotic cardiomyocytes through myeloid-epithelial-reproductive tyrosine kinase links acute inflammation resolution to cardiac repair after infarction. Circ. Res. 113, 1004–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang Y., Wang D., Wang X., Zhang Y., Liu T., Chen Y., Tang Y., Wang T., Hu D., Huang C. (2016) Abrogation of CC chemokine receptor 9 ameliorates ventricular remodeling in mice after myocardial infarction. Sci. Rep. 6, 32660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakano Y., Matoba T., Tokutome M., Funamoto D., Katsuki S., Ikeda G., Nagaoka K., Ishikita A., Nakano K., Koga J., Sunagawa K., Egashira K. (2016) Nanoparticle-mediated delivery of irbesartan induces cardioprotection from myocardial ischemia-reperfusion injury by antagonizing monocyte-mediated inflammation. Sci. Rep. 6, 29601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanna R. N., Carlin L. M., Hubbeling H. G., Nackiewicz D., Green A. M., Punt J. A., Geissmann F., Hedrick C. C. (2011) The transcription factor NR4A1 (Nur77) controls bone marrow differentiation and the survival of Ly6C- monocytes. Nat. Immunol. 12, 778–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jesty S. A., Steffey M. A., Lee F. K., Breitbach M., Hesse M., Reining S., Lee J. C., Doran R. M., Nikitin A. Y., Fleischmann B. K., Kotlikoff M. I. (2012) c-kit+ precursors support postinfarction myogenesis in the neonatal, but not adult, heart. Proc. Natl. Acad. Sci. USA 109, 13380–13385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scott R. S., McMahon E. J., Pop S. M., Reap E. A., Caricchio R., Cohen P. L., Earp H. S., Matsushima G. K. (2001) Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature 411, 207–211 [DOI] [PubMed] [Google Scholar]
- 17.Zhan Y., Du X., Chen H., Liu J., Zhao B., Huang D., Li G., Xu Q., Zhang M., Weimer B. C., Chen D., Cheng Z., Zhang L., Li Q., Li S., Zheng Z., Song S., Huang Y., Ye Z., Su W., Lin S. C., Shen Y., Wu Q. (2008) Cytosporone B is an agonist for nuclear orphan receptor Nur77. Nat. Chem. Biol. 4, 548–556 [DOI] [PubMed] [Google Scholar]
- 18.Nicholls H. T., Kowalski G., Kennedy D. J., Risis S., Zaffino L. A., Watson N., Kanellakis P., Watt M. J., Bobik A., Bonen A., Febbraio M., Lancaster G. I., Febbraio M. A. (2011) Hematopoietic cell-restricted deletion of CD36 reduces high-fat diet-induced macrophage infiltration and improves insulin signaling in adipose tissue. Diabetes 60, 1100–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dehn S., DeBerge M., Yeap X. Y., Yvan-Charvet L., Fang D., Eltzschig H. K., Miller S. D., Thorp E. B. (2016) HIF-2α in resting macrophages tempers mitochondrial reactive oxygen species to selectively repress MARCO-dependent phagocytosis. J. Immunol. 197, 3639–3649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lemke G., Rothlin C. V. (2008) Immunobiology of the TAM receptors. Nat. Rev. Immunol. 8, 327–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Böttcher A., Gaipl U. S., Fürnrohr B. G., Herrmann M., Girkontaite I., Kalden J. R., Voll R. E. (2006) Involvement of phosphatidylserine, alphavbeta3, CD14, CD36, and complement C1q in the phagocytosis of primary necrotic lymphocytes by macrophages. Arthritis Rheum. 54, 927–938 [DOI] [PubMed] [Google Scholar]
- 22.Heather L. C., Cole M. A., Lygate C. A., Evans R. D., Stuckey D. J., Murray A. J., Neubauer S., Clarke K. (2006) Fatty acid transporter levels and palmitate oxidation rate correlate with ejection fraction in the infarcted rat heart. Cardiovasc. Res. 72, 430–437 [DOI] [PubMed] [Google Scholar]
- 23.DeLeon-Pennell K. Y., Tian Y., Zhang B., Cates C. A., Iyer R. P., Cannon P., Shah P., Aiyetan P., Halade G. V., Ma Y., Flynn E., Zhang Z., Jin Y. F., Zhang H., Lindsey M. L. (2016) CD36 is a matrix metalloproteinase-9 substrate that stimulates neutrophil apoptosis and removal during cardiac remodeling. Circ. Cardiovase Genet. 9, 14–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heidt T., Courties G., Dutta P., Sager H. B., Sebas M., Iwamoto Y., Sun Y., Da Silva N., Panizzi P., van der Laan A. M., Swirski F. K., Weissleder R., Nahrendorf M. (2014) Differential contribution of monocytes to heart macrophages in steady-state and after myocardial infarction. Circ. Res. 115, 284–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leuschner F., Dutta P., Gorbatov R., Novobrantseva T. I., Donahoe J. S., Courties G., Lee K. M., Kim J. I., Markmann J. F., Marinelli B., Panizzi P., Lee W. W., Iwamoto Y., Milstein S., Epstein-Barash H., Cantley W., Wong J., Cortez-Retamozo V., Newton A., Love K., Libby P., Pittet M. J., Swirski F. K., Koteliansky V., Langer R., Weissleder R., Anderson D. G., Nahrendorf M. (2011) Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat. Biotechnol. 29, 1005–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ipseiz N., Uderhardt S., Scholtysek C., Steffen M., Schabbauer G., Bozec A., Schett G., Krönke G. (2014) The nuclear receptor Nr4a1 mediates anti-inflammatory effects of apoptotic cells. J. Immunol. 192, 4852–4858 [DOI] [PubMed] [Google Scholar]
- 27.Lee P. Y., Li Y., Kumagai Y., Xu Y., Weinstein J. S., Kellner E. S., Nacionales D. C., Butfiloski E. J., van Rooijen N., Akira S., Sobel E. S., Satoh M., Reeves W. H. (2009) Type I interferon modulates monocyte recruitment and maturation in chronic inflammation. Am. J. Pathol. 175, 2023–2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maira M., Martens C., Philips A., Drouin J. (1999) Heterodimerization between members of the Nur subfamily of orphan nuclear receptors as a novel mechanism for gene activation. Mol. Cell. Biol. 19, 7549–7557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grisanti L. A., Gumpert A. M., Traynham C. J., Gorsky J. E., Repas A. A., Gao E., Carter R. L., Yu D., Calvert J. W., García A. P., Ibáñez B., Rabinowitz J. E., Koch W. J., Tilley D. G. (2016) Leukocyte-expressed β2-adrenergic receptors are essential for survival after acute myocardial injury. Circulation 134, 153–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Serhan C. N., Savill J. (2005) Resolution of inflammation: the beginning programs the end. Nat. Immunol. 6, 1191–1197 [DOI] [PubMed] [Google Scholar]
- 31.Shiraishi M., Shintani Y., Shintani Y., Ishida H., Saba R., Yamaguchi A., Adachi H., Yashiro K., Suzuki K. (2016) Alternatively activated macrophages determine repair of the infarcted adult murine heart. J. Clin. Invest. 126, 2151–2166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lavine K. J., Epelman S., Uchida K., Weber K. J., Nichols C. G., Schilling J. D., Ornitz D. M., Randolph G. J., Mann D. L. (2014) Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc. Natl. Acad. Sci. USA 111, 16029–16034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Panizzi P., Swirski F. K., Figueiredo J. L., Waterman P., Sosnovik D. E., Aikawa E., Libby P., Pittet M., Weissleder R., Nahrendorf M. (2010) Impaired infarct healing in atherosclerotic mice with Ly-6C(hi) monocytosis. J. Am. Coll. Cardiol. 55, 1629–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaikita K., Hayasaki T., Okuma T., Kuziel W. A., Ogawa H., Takeya M. (2004) Targeted deletion of CC chemokine receptor 2 attenuates left ventricular remodeling after experimental myocardial infarction. Am. J. Pathol. 165, 439–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Getts D. R., Terry R. L., Getts M. T., Deffrasnes C., Müller M., van Vreden C., Ashhurst T. M., Chami B., McCarthy D., Wu H., Ma J., Martin A., Shae L. D., Witting P., Kansas G. S., Kühn J., Hafezi W., Campbell I. L., Reilly D., Say J., Brown L., White M. Y., Cordwell S. J., Chadban S. J., Thorp E. B., Bao S., Miller S. D., King N. J. (2014) Therapeutic inflammatory monocyte modulation using immune-modifying microparticles. Sci. Transl. Med. 6, 219ra7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Libby P., Nahrendorf M., Swirski F. K. (2016) Leukocytes link local and systemic inflammation in ischemic cardiovascular disease: an expanded “cardiovascular continuum”. J. Am. Coll. Cardiol. 67, 1091–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fadok V. A., Bratton D. L., Konowal A., Freed P. W., Westcott J. Y., Henson P. M. (1998) Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J. Clin. Invest. 101, 890–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kiss R. S., Elliott M. R., Ma Z., Marcel Y. L., Ravichandran K. S. (2006) Apoptotic cells induce a phosphatidylserine-dependent homeostatic response from phagocytes. Curr. Biol. 16, 2252–2258 [DOI] [PubMed] [Google Scholar]
- 39.Vats D., Mukundan L., Odegaard J. I., Zhang L., Smith K. L., Morel C. R., Wagner R. A., Greaves D. R., Murray P. J., Chawla A. (2006) Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab. 4, 13–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mukundan L., Odegaard J. I., Morel C. R., Heredia J. E., Mwangi J. W., Ricardo-Gonzalez R. R., Goh Y. P., Eagle A. R., Dunn S. E., Awakuni J. U., Nguyen K. D., Steinman L., Michie S. A., Chawla A. (2009) PPAR-delta senses and orchestrates clearance of apoptotic cells to promote tolerance. Nat. Med. 15, 1266–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiong W., Frasch S. C., Thomas S. M., Bratton D. L., Henson P. M. (2013) Induction of TGF-β1 synthesis by macrophages in response to apoptotic cells requires activation of the scavenger receptor CD36. PLoS One 8, e72772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Larson S. R., Atif S. M., Gibbings S. L., Thomas S. M., Prabagar M. G., Danhorn T., Leach S. M., Henson P. M., Jakubzick C. V. (2016) Ly6C(+) monocyte efferocytosis and cross-presentation of cell-associated antigens. Cell Death Differ. 23, 997–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clemetson K. J., Pfueller S. L., Luscher E. F., Jenkins C. S. (1977) Isolation of the membrane glycoproteins of human blood platelets by lectin affinity chromatography. Biochim. Biophys. Acta 464, 493–508 [DOI] [PubMed] [Google Scholar]
- 44.Swerlick R. A., Lee K. H., Wick T. M., Lawley T. J. (1992) Human dermal microvascular endothelial but not human umbilical vein endothelial cells express CD36 in vivo and in vitro. J. Immunol. 148, 78–83 [PubMed] [Google Scholar]
- 45.Aitman T. J., Glazier A. M., Wallace C. A., Cooper L. D., Norsworthy P. J., Wahid F. N., Al-Majali K. M., Trembling P. M., Mann C. J., Shoulders C. C., Graf D., St Lezin E., Kurtz T. W., Kren V., Pravenec M., Ibrahimi A., Abumrad N. A., Stanton L. W., Scott J. (1999) Identification of Cd36 (Fat) as an insulin-resistance gene causing defective fatty acid and glucose metabolism in hypertensive rats. Nat. Genet. 21, 76–83 [DOI] [PubMed] [Google Scholar]
- 46.Bonen A., Luiken J. J., Liu S., Dyck D. J., Kiens B., Kristiansen S., Turcotte L. P., Van Der Vusse G. J., Glatz J. F. (1998) Palmitate transport and fatty acid transporters in red and white muscles. Am. J. Physiol. 275, E471–E478 [DOI] [PubMed] [Google Scholar]
- 47.Park Y. M., Febbraio M., Silverstein R. L. (2009) CD36 modulates migration of mouse and human macrophages in response to oxidized LDL and may contribute to macrophage trapping in the arterial intima. J. Clin. Invest. 119, 136–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moore K. J., Kunjathoor V. V., Koehn S. L., Manning J. J., Tseng A. A., Silver J. M., McKee M., Freeman M. W. (2005) Loss of receptor-mediated lipid uptake via scavenger receptor A or CD36 pathways does not ameliorate atherosclerosis in hyperlipidemic mice. J. Clin. Invest. 115, 2192–2201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Helming L., Winter J., Gordon S. (2009) The scavenger receptor CD36 plays a role in cytokine-induced macrophage fusion. J. Cell Sci. 122, 453–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Febbraio M., Silverstein R. L. (2007) CD36: implications in cardiovascular disease. Int. J. Biochem. Cell Biol. 39, 2012–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Teupser D., Mueller M. A., Koglin J., Wilfert W., Ernst J., von Scheidt W., Steinbeck G., Seidel D., Thiery J. (2008) CD36 mRNA expression is increased in CD14+ monocytes of patients with coronary heart disease. Clin. Exp. Pharmacol. Physiol. 35, 552–556 [DOI] [PubMed] [Google Scholar]
- 52.Piechota M., Banaszewska A., Dudziak J., Slomczynski M., Plewa R. (2012) Highly upregulated expression of CD36 and MSR1 in circulating monocytes of patients with acute coronary syndromes. Protein J. 31, 511–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cho S., Park E. M., Febbraio M., Anrather J., Park L., Racchumi G., Silverstein R. L., Iadecola C. (2005) The class B scavenger receptor CD36 mediates free radical production and tissue injury in cerebral ischemia. J. Neurosci. 25, 2504–2512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Woo M. S., Yang J., Beltran C., Cho S. (2016) Cell surface CD36 protein in monocyte/macrophage contributes to phagocytosis during the resolution phase of ischemic stroke in mice. J. Biol. Chem. 291, 23654–23661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou P., Qian L., Gallo E. F., Deeb R. S., Anrather J., Gross S. S., Iadecola C. (2011) The scavenger receptor CD36 contributes to the neurotoxicity of bone marrow-derived monocytes through peroxynitrite production. Neurobiol. Dis. 42, 292–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim E., Febbraio M., Bao Y., Tolhurst A. T., Epstein J. M., Cho S. (2012) CD36 in the periphery and brain synergizes in stroke injury in hyperlipidemia. Ann. Neurol. 71, 753–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lagassé H. A., Anidi I. U., Craig J. M., Limjunyawong N., Poupore A. K., Mitzner W., Scott A. L. (2016) Recruited monocytes modulate malaria-induced lung injury through CD36-mediated clearance of sequestered infected erythrocytes. J. Leukoc. Biol. 99, 659–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bessi V. L., Labbé S. M., Huynh D. N., Ménard L., Jossart C., Febbraio M., Guérin B., Bentourkia M., Lecomte R., Carpentier A. C., Ong H., Marleau S. (2012) EP 80317, a selective CD36 ligand, shows cardioprotective effects against post-ischaemic myocardial damage in mice. Cardiovasc. Res. 96, 99–108 [DOI] [PubMed] [Google Scholar]
- 59.Driscoll W. S., Vaisar T., Tang J., Wilson C. L., Raines E. W. (2013) Macrophage ADAM17 deficiency augments CD36-dependent apoptotic cell uptake and the linked anti-inflammatory phenotype. Circ. Res. 113, 52–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Santamaria M. H., Chen A. Y., Chow J., Muñoz D. C., Schmid-Schönbein G. W. (2014) Cleavage and reduced CD36 ectodomain density on heart and spleen macrophages in the spontaneously hypertensive rat. Microvasc. Res. 95, 131–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sather S., Kenyon K. D., Lefkowitz J. B., Liang X., Varnum B. C., Henson P. M., Graham D. K. (2007) A soluble form of the Mer receptor tyrosine kinase inhibits macrophage clearance of apoptotic cells and platelet aggregation. Blood 109, 1026–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cai B., Thorp E. B., Doran A. C., Subramanian M., Sansbury B. E., Lin C. S., Spite M., Fredman G., Tabas I. (2016) MerTK cleavage limits proresolving mediator biosynthesis and exacerbates tissue inflammation. Proc. Natl. Acad. Sci. USA 113, 6526–6531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kowalczyk M. S., Hughes J. R., Garrick D., Lynch M. D., Sharpe J. A., Sloane-Stanley J. A., McGowan S. J., De Gobbi M., Hosseini M., Vernimmen D., Brown J. M., Gray N. E., Collavin L., Gibbons R. J., Flint J., Taylor S., Buckle V. J., Milne T. A., Wood W. G., Higgs D. R. (2012) Intragenic enhancers act as alternative promoters. Mol. Cell 45, 447–458 [DOI] [PubMed] [Google Scholar]
- 64.Bonta P. I., van Tiel C. M., Vos M., Pols T. W., van Thienen J. V., Ferreira V., Arkenbout E. K., Seppen J., Spek C. A., van der Poll T., Pannekoek H., de Vries C. J. (2006) Nuclear receptors Nur77, Nurr1, and NOR-1 expressed in atherosclerotic lesion macrophages reduce lipid loading and inflammatory responses. Arterioscler. Thromb. Vasc. Biol. 26, 2288–2294 [DOI] [PubMed] [Google Scholar]
- 65.Stewart C. R., Stuart L. M., Wilkinson K., van Gils J. M., Deng J., Halle A., Rayner K. J., Boyer L., Zhong R., Frazier W. A., Lacy-Hulbert A., El Khoury J., Golenbock D. T., Moore K. J. (2010) CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat. Immunol. 11, 155–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Triantafilou M., Gamper F. G., Haston R. M., Mouratis M. A., Morath S., Hartung T., Triantafilou K. (2006) Membrane sorting of toll-like receptor (TLR)-2/6 and TLR2/1 heterodimers at the cell surface determines heterotypic associations with CD36 and intracellular targeting. J. Biol. Chem. 281, 31002–31011 [DOI] [PubMed] [Google Scholar]