Abstract
In Aedes aegypti females, the ammonia released during blood meal digestion is partially metabolized to facilitate the disposal of excess nitrogen. In this study, we used low- and high-resolution liquid chromatography-mass spectrometry (LC/MS) techniques to investigate the role of glucose during ammonia detoxification. Mosquitoes were fed a blood meal supplemented with [1,2-13C2]glucose, and downstream metabolites were measured for 24 h. Quantification of [13C] amino acids in the entire mosquito body was conducted without sample derivatization using selected reaction monitoring of mass transitions that are indicative of the structural position of [13C] atom incorporation. Identification of unlabeled and [13C] isotopologs of 43 compounds, including amino acids, amino acid derivatives, and organic acids, was performed by high-resolution LC/MS techniques. Blood-fed mosquitoes synthesized [13C] metabolites in mainly 2 carbon positions from [1,2-13C2]glucose. [13C2]Ala and [13C2]Pro were the most abundant and rapidly labeled amino acids synthesized. Additional [13C] amino acids, [13C] amino acid derivatives, and [13C] organic acids in 1 or 2 carbon positions were also identified. Two kinetic routes were proposed based on the incorporation of a [13C] atom at position 1 in specific amino acids. Our findings provide evidence that glucose is used for ammonia detoxification and [13C] uric acid synthesis through multiple metabolic pathways, uncovering a metabolic link at the carbon atomic level in ammonia metabolism of A. aegypti.—Horvath, T. D., Dagan, S., Lorenzi, P. L., Hawke, D. H., Scaraffia, P. Y. Positional stable isotope tracer analysis reveals carbon routes during ammonia metabolism of Aedes aegypti mosquitoes.
Keywords: mass spectrometry, nitrogen waste, metabolomics
Aedes aegypti is a mosquito species that has recently generated a lot of attention in global health because of the Zika virus outbreaks (1–3). However, A. aegypti is also an efficient vector of viruses causing yellow fever, dengue hemorrhagic fever, and chikungunya fever (4). For more effective mosquito control, a better understanding of mosquito metabolism is needed. This challenge could be met by the application of novel, metabolism-based strategies and modern analytical technologies.
Studies performed with [14C] radioactive isotopes revealed that 10% of the amino acids released from protein meal catabolism in A. aegypti females resides in their eggs, 20% serves as maternal reserve synthesis, and ∼70% is completely oxidized for energy production and excreted as waste (5), which supported previous, classic studies (6). That tremendous rate of oxidation generates high ammonia concentrations, which must be directly excreted or metabolized efficiently to avoid death (the term “ammonia” refers to NH3, NH4+, or both). The application of [15N] compounds, tandem mass spectrometry, and RNA interference techniques has uncovered the presence of several metabolic pathways to detoxify ammonia in blood-fed A. aegypti. Although mosquitoes lack the urea cycle, ammonia can be directly excreted or used to synthesize amino acids and nitrogen waste (7–9). In vitro [15N]ammonia studies demonstrated that [15N]Gln and [15N]Pro were the main amino acids synthesized in fat body culture, whereas [15N]Gln and [15N]Ala were the main amino acids produced in midgut culture through distinct metabolic pathways, indicating that these amino acids act as temporary sinks of nitrogen to prevent ammonia toxicity (10). Moreover, supplementation of glucose enriched with [15N]ammonia salts in the midgut culture increased [15N]Ala synthesis (10), most likely through the oxidation of glucose to pyruvate, 1 of the substrates of alanine aminotransferase (ALT). In addition to serving as transient nitrogen carriers, Gln and Pro are also precursors of 2 of the 4 nitrogen atoms of uric acid (9), the main nitrogen waste excreted by blood-fed mosquitoes. Uric acid can be further hydrolyzed into urea, which is the nitrogen-containing end product of the uricolytic pathway in blood-fed A. aegypti (9). Urea can also be produced through argininolysis, when arginine is hydrolyzed into ornithine and urea by an arginase, independent of the urea cycle (11, 12). Both the argininolysis and the uricolysis pathways are regulated by a unique crosstalk signaling mechanism (13).
Recent studies have shown that blood-fed A. aegypti with ALT deficiency can survive by temporarily storing uric acid in the midgut; by delaying several physiologic processes, such as digestion, oviposition, and reproduction; and by up-regulating genes encoding rhesus 50 glycoprotein-1, an ammonia transporter, and xanthine dehydrogenase-1 (XDH-1), an enzyme involved in the last 2 steps of uric acid synthesis (14). Knockdown of XDH-1 is lethal to blood-fed mosquitoes (15). XDH-1 deficiency causes severe disruption in digestion, excretion, oviposition, and reproduction. In the fat body of some mosquitoes, XDH-1 deficiency also causes a significant up-regulation of genes encoding proteins involved in the fixation, assimilation, and excretion phases of ammonia metabolism, NMDA receptor, rhesus 50 glycoprotein-1, and several antioxidant genes, in response to ammonia and free radical toxicity (15). These findings highlight the complexity of the metabolic regulation of nitrogen metabolism in mosquitoes and emphasize the need for future metabolic studies.
Because most of the carbon skeletons of amino acids derived from protein meal digestion are completely oxidized for energy production (5, 6), we hypothesized that the carbon skeleton of glucose, obtained from the diet or maternal reserves, supplies the keto acids necessary for the synthesis of amino acids and other compounds during ammonia clearance in blood-fed female mosquitoes. To test that hypothesis, we fed mosquitoes with a blood meal, supplemented with [1,2-13C2]glucose and monitored the kinetics of incorporation of [13C] atoms from glucose into [13C] amino acids, [13C] amino acid derivatives, and [13C] organic acids during a specified period (0–24 h after feeding). The experiments were performed based on a methodology previously developed (16) using low-resolution (LR) as well as high-resolution, accurate-mass (HRAM) liquid chromatography-mass spectrometry (LC/MS) without sample derivatization. We discovered that the carbon skeleton of glucose supports ammonia detoxification through the synthesis of several compounds, including specific amino acids, amino acid derivatives, and organic acids. We also found that the synthesis of those compounds occurs through a complex interaction among glycolysis, decarboxylation of pyruvate, Krebs cycle and the pentose phosphate pathway (PPP), and pathways involved in ammonia fixation, assimilation, and excretion. The discovery of a link at the carbon atomic level between glucose and ammonia metabolism contributes to a deeper understanding of blood-fed mosquito metabolism and raises new questions regarding the metabolic regulation of the pathways involved.
MATERIALS AND METHODS
Reagents, chemicals, and others
LC/MS-grade water and acetonitrile were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Four labeled internal standards (ISs), [13C3,15N1]Ala, [13C5,15N1]Glu, [13C5,15N2]Gln, and [13C5,15N1]Pro, and [1,2-13C2]glucose were obtained from Cambridge Isotope Laboratories, Inc. (Tewksbury, MA, USA). Bovine whole blood was purchased from Pel-Freez Biologicals (Rogers, AR, USA). Upon arrival to the laboratory, the blood was immediately divided into aliquots and stored without cryoprotectives at −80°C. Because the freeze-thaw process is known to lyse blood cells, blood cells were considered to be lysed after thawing for feeding procedures. All other authentic reference standards and chemicals were purchased from MilliporeSigma (Billerica, MA, USA). A Supelco Ascentis Express hydrophilic interaction liquid chromatography (HILIC) analytical column (2.1 × 150 mm, 2.7-µm particle size) was purchased from MilliporeSigma and was used for the LR-LC/tandem mass spectrometry (MS-MS) quantitation of Glu, Gln, Ala, and Pro. An Intrada amino acid analytical column (2.1 × 150 mm, 3-µm particle size) was purchased from Imtakt USA (Portland, OR, USA) and was used for high-resolution, accurate-mass (HRAM)-LC/MS screening of the amino acids and amino acid derivatives. A SeQuant ZIC-pHILIC analytical column (2.1 × 150 mm, 5-µm particle size) was purchased from MilliporeSigma and was used for HRAM-LC/MS screening of the organic acids, and the amino acids Asp and Glu.
Analyte and labeled internal-standard solution preparations
Individual aqueous stock solutions were prepared for Ala, [13C3,15N1]Ala, Gln, [13C5,15N2]Gln, Pro, and [13C5,15N1]Pro, and individual Glu and [13C5,15N1]Glu stock solutions were prepared in an aqueous 0.1% formic acid solution. Individual intermediate-concentration solutions were prepared at 10 mM for each compound using consistent solvents. Individual combined intermediate solutions were prepared for the analyte and labeled IS compounds at concentrations of 100 and 50 µM, respectively, in an aqueous 0.1% formic acid solution. A single-point calibration standard was prepared by adding 10 µl each of the analyte and the IS combined intermediate solutions, followed by 180 µl of an acetonitrile solution containing 0.2% formic acid, resulting in final analyte and IS concentrations of 5 and 2.5 µM, respectively, in the calibration standard.
Mosquitoes and blood-feeding procedures
A. aegypti mosquitoes (NIH–Rockefeller strain; National Institutes of Health, Bethesda, MD, USA) were reared and maintained as previously described (13, 14). Females were fed on a 3% sucrose solution for the first 3 d and starved for 24 h. At 5 d old, females were fed for 15 min with the following diets: 1) bovine blood meal plus 5 mM ATP (BM), 2) BM supplemented with 100 mM glucose, and 3) BM supplemented with 100 mM [1,2-13C2]glucose, using an artificial blood feeder connected to a 37°C water bath. At different times after feeding, which varied between 0 and 24 h, mosquitoes were immersed into liquid nitrogen and then stored at −20°C. We previously reported that during a 72-h period, no behavioral phenotypes or mortality were observed in the group of mosquitoes fed with BM + 10 mM glucose or BM + 100 mM glucose when compared with BM alone (17).
Mosquito sample preparation
At each individual time, during the time course studied (0–24 h), 5 fully engorged females were homogenized in a Potter-Elvehjem tissue grinder (VWR, Radnor, PA, USA) in 500 µl LC/MS-grade water. The suspension was boiled for 2 min, centrifuged at 14,000 g for 10 min twice, and the supernatant was collected. For the quantification of unlabeled and labeled amino acids, 5 µl of supernatant was mixed with 5 µl of 1 mM of each of the following ISs: [13C3,15N1]Ala, [13C5,15N1]Glu, [13C5,15N2]Gln, and [13C5,15N1]Pro, yielding a total sample volume of 25 µl. The sample was dried using a vacuum centrifuge and stored at −20°C until analysis. Samples were reconstituted in 200 µl of a 0.1% formic acid solution, vortex-mixed, and centrifuged at 17,000 g for 5 min. A 20-µl aliquot of the supernatant was mixed with 180 µl of an acetonitrile solution containing 0.2% formic acid, and the resulting sample was vortex-mixed and centrifuged at 17,000 g for 5 min. The supernatant was then transferred to polypropylene injection vials. The overall sample dilution factor for the mosquito study samples was 400-fold. The single-point calibration standard and all study samples were analyzed in triplicate. The LR-LC/MS-MS injection volume for each analysis was 10 µl. Experiments were replicated with 3 separate cohorts of mosquitoes.
Targeted LR-LC/MS-MS instrumentation and method
Measurements were made to quantify the time-dependent levels of each individual unlabeled compound and [13C] atom positional isotopomer for each isotopolog contained in the mass isotopomer distributions of Ala, Pro, Gln, and Glu in [1,2-13C2]glucose–fed mosquito whole-body samples. The LR measurements were performed using a Agilent Technologies (Santa Clara, CA, USA) LR-LC/MS-MS system composed of an Infinity 1290 Ultra-HPLC system coupled to a 6460 triple-quadrupole mass spectrometer operated in the dynamic multiple reaction monitoring (dMRM) scan mode (see Supplemental Tables S1–4 for MS-MS transitions). MassHunter Workstation LC/MS Data Acquisition (v.B.06.00) and MassHunter Quantitative Analysis (v.B.06.00) software packages were used for data acquisition and peak integration. Quantitative analysis of the study samples was performed using a single-point calibration standard method. Targeted amino acid separations were performed with an Ascentis Express HILIC analytical column with mobile phase A (MPA) and mobile phase B (MPB) solution compositions of 95/5 acetonitrile/200 mM ammonium formate with 2% formic acid, and 50/45/5 acetonitrile/water/200 mM ammonium formate with 2% formic acid, respectively. The chromatographic method included a column temperature of 30°C, autosampler tray chilled to 4°C, a mobile phase flow rate of 0.200 ml/min, and a gradient elution program specified as follows: 0–2.5 min, 15% MPB; 2.5–10.5 min, 15–60% MPB; 10.5–13.0 min, 60% MPB; 13.0–14.0 min, 60–15% MPB; and 14.0–17.0 min, 15% MPB. The Agilent Jet Stream electrospray ionization (ESI) source parameters were specified as follows: ionization mode polarity, positive mode; gas temperature, 325°C; gas flow, 6 L/min; nebulizer, 40 psi; sheath gas temperature, 350°C; sheath gas flow, 9 L/min; capillary voltage, +1500 V; and nozzle voltage, 0 V.
HRAM-LC/MS instrumentation
Measurements were made to screen the mass isotopomer distribution of [13C] atom incorporation into 43 semitargeted metabolites (38 amino acids and amino acid derivatives and 5 organic acids from 2 separate chromatographic methods). That work was performed with a Thermo Fisher Scientific HRAM-LC/MS system comprising a Dionex Ultimate 3000 Rapid Separation Liquid Chromatography system coupled to an Orbitrap Fusion mass spectrometer. Xcalibur (v.3.0.49) and Chromeleon (v.6.8) were used for system operation and data acquisition, and TraceFinder (v.3.3.350.0) was used for HRAM-data analysis (all from Thermo Fisher Scientific).
Targeted HRAM-LC/MS amino acid and amino acid derivative method
Targeted amino acid and amino acid derivative separations were performed using an Intrada amino acid analytical column with MPA and MPB solution compositions of acetonitrile containing 0.1% formic acid and 50 mM ammonium formate, respectively. The chromatographic method included a column temperature of 35°C, autosampler tray chilled to 8°C, a mobile phase flow rate of 0.300 ml/min, and a gradient elution program specified as follows: 0–3.0 min, 17% MPB; 3.0–20.0 min, 17–30% MPB; 20.0–30.0 min, 30–100% MPB; 30.0–40.0 min, 100% MPB; 40.0–42.0 min, 100–17% MPB; and 42.0–45.0 min, 17% MPB. The heated ESI source parameters were specified as follows: ionization mode polarity, positive mode; probe spray voltage, static at +3500 V; sheath gas (arbitrary units), 60; auxiliary gas (arbitrary units), 2; sweep gas (arbitrary units), 0; ion transfer tube temp, 375°C; and vaporizer temperature, 150°C. Global MS acquisition parameters were specified as follows: pressure mode, standard; default charge state, 1; internal mass calibration, true; internal mass calibration, Easy-IC (Thermo Fisher Scientific). HRAM data acquisition for the amino acids and amino acid derivatives was performed with a series of timed single-ion monitoring (tSIM) experiments that bracketed both the retention time of interest in the chromatographic time domain and a MS scan range that encompassed all potential [13C] isotopologs (defined range, 2 Da less than the fully unlabeled metabolite to 2 Da greater than the fully [13C]-labeled metabolite) in the mass-to-charge ratio domain (Supplemental Table S5). The acquisition parameters for the individual tSIM experiments were specified as follows: multiplex ions, false; isolation mode, quadrupole; detector type, Orbitrap; Orbitrap resolution, 240,000 (at 200 m/z); radio frequency lens, 50%; automatic gain control target, 200,000; injection ions for all available parallelizable time, true; maximum injection time, 100 ms; microscans, 1; data type, profile; polarity, positive; source fragmentation, disabled; use Easy-IC, true; include start and end times, false; scan cycle time, 3 s.
Targeted HRAM-LC/MS organic acid method
Separations were performed using a Sequant ZIC-pHILIC analytical column (MilliporeSigma) and MPA and MPB solution compositions of 90/10 acetonitrile/200 mM ammonium acetate (pH 5.8), and 50/40/10 acetonitrile/water/200 mM ammonium acetate (pH 5.8), respectively. The chromatographic method included a column temperature of 30°C, an autosampler tray chilled to 8°C, a mobile phase flow rate of 0.400 ml/min, and a gradient elution program specified as follows: 0–2.5 min, 5% MPB; 2.5–16.0 min, 5–60% MPB; 16.0–17.0 min, 60–90% MPB; 17.0–20.0 min, 90% MPB; 20.0–20.1 min, 90–5% MPB; and 20.1–25.0 min, 5% MPB. The heated ESI source parameters in the negative ionization mode were specified as follows: ionization mode polarity, negative mode; probe spray voltage, static at −1500 V; sheath gas (arbitrary units), 40; auxiliary gas (arbitrary units), 2; sweep gas (arbitrary units), 2; ion transfer tube temp, 375°C; and vaporizer temperature, 150°C. Global MS acquisition parameters were specified as follows: pressure mode, standard; default charge state, 1; internal mass calibration, true; and internal mass calibration, Easy-IC. HRAM-LC/MS data acquisition for the organic acids, Asp, and Glu was performed using a similar tSIM experimental approach, and acquisition parameters used were similar to those previously described (Supplemental Table S6).
Quantification of amino acid levels in mosquito whole-body samples
Quantification for the total level (naturally abundant, isotopically labeled, background amino acids plus enriched [13C] amino acids) of each unlabeled and each isotope-labeled positional isotopomer for each isotopolog of Ala, Glu, Gln, and Pro was performed using a single-point calibration method that used Eq. 1:
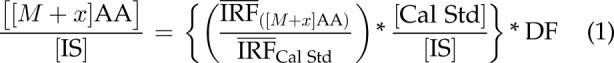 |
where [[M + x]AA] is the measured level of a particular isotope-labeled ([13C], [2H], [15N], or [18O]) amino acid (AA) isotopolog in the mosquito whole-body sample, and x is the labeling state (number of atoms labeled) of each amino acid (e.g., 0, unlabeled; 1, 1 isotope-labeled atom). The [Cal Std], [IS], and DF (see Eq. 1) are defined as the nominal concentrations of the calibration standard, the IS, and the dilution factor, respectively. The mean instrument response factor (IRF) is defined as the ratio of the integrated amino acid peak area for a specific MS-MS transition over the integrated IS peak area for a specific IS MS-MS transition.
Mathematical corrections for naturally occurring isotopes for labeled isotopologs of Ala, Pro, Gln, and Glu using LR-LC/MS-MS
To determine the level of enrichment of [13C] atoms in Ala, Glu, Gln, and Pro isotopologs in the [1,2-13C2]glucose-fed mosquito whole-body extracts, corrections were made to subtract the naturally occurring background ensemble of [13C], [15N], [18O], or [2H] amino acids present in the sample. For example, the natural occurrence of [13C] is 1.1%; therefore, quantitating carbon-containing metabolites must account for a 1.1% probability that each carbon position is labeled with a [13C] atom. Before the correction of the experimental data, there were 3 individual, experimental parameters that were determined from the mosquitoes that were fed BM and BM supplemented with glucose for each amino acid studied. First, the total amino acid content in each mosquito whole-body extract was calculated by summing the concentrations of the unlabeled isotope to the fully isotope-labeled species (e.g., [ALA]Total = [[M + 0]Ala] + [[M + 1]Ala] + [[M + 2]Ala] + [[M + 3 ]Ala]). Next, the natural abundance proportion (PNA) of each successive level of isotope labeling (e.g., PNA,([M+1]Ala) = [[M + 1]Ala]/[Ala]Total) was calculated. To properly account for the distribution of specific carbon positions among the product ions and various neutral loss fragments, fragmentation-specific, positional-probability factors were determined for each individual [13C] amino acid isotopolog MS-MS transition. The naturally occurring background ensemble of [13C], [15N], [18O], or [2H] amino acids in the mosquito whole-body extracts was subtracted from the empirically determined concentrations using the following equation:
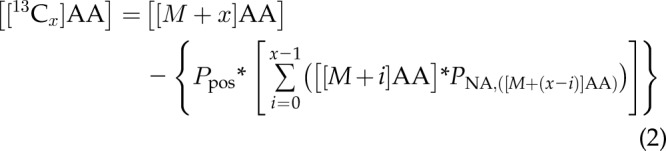 |
where the variable x represents the number of isotope labels present on an amino acid isotopolog and ranges from a single [13C] atom (x = 1) to the equivalent of fully labeled with [13C] atoms [x = 3 (Ala), 5 (Glu), 5 (Gln), and 5 (Pro)], and [[M + x]AA] is the amino acid isotopolog level calculated using Eq. 1. These corrections allow for the accurate determination of the rate, extent, and position of the [13C] atom incorporation into Ala, Glu, Gln, and Pro in the mosquito whole body.
Statistical analysis
One-way ANOVA was used to analyze the data (15). A value of P < 0.05 was considered significant. The statistical analyses were performed with GraphPad Prism (version 6.0 for Mac OS X; GraphPad Software, La Jolla, CA, USA).
RESULTS
Selected reaction-monitoring transitions, products, and their [13C] representation
A series of selected reaction monitoring (SRM) MS-MS experiments was performed to analyze whole-body mosquito extracts. The SRM transitions used for Glu, Gln, Pro, and Ala (the main amino acids involved in mosquito ammonia metabolism) are summarized in Supplemental Tables S1–4. Those transitions were chosen based on a previous study (16) in which we used ESI–collision-induced dissociation MS-MS to measure amino acids and their isotopologs. The selected transitions for the amino acids monitored included protonated molecular ions of the unlabeled amino acids and their [13C] isotopologs as precursors. The product ions selected were usually at lower mass-to-charge ratio values (26–60 m/z), chosen to distinguish the specific structural location of [13C] atom incorporation (isotopomer specificity) into the amino acids. For example, the ion at 130 m/z (or 131 and 132 for the isotopologs) for Glu or Gln includes labeling at any of the 5 carbons, including 1-C (the backbone carbon of the amino acid), whereas lower mass-to-charge ratio ions represent products that do not include 1-C. In Glu and Gln, 29 m/z ions (or 30 or 31 for the isotopologs) were chosen for their specific indication of 2-C labeling. Those product ions may, nevertheless, represent 0, 1, or 2 [13C] atom incorporation into different metabolites, making them less useful for carbon-origin distinction (16). A typical total ion current chromatogram of Glu, Ala, Pro, and Gln, which is representative of mosquito whole-body extracts, is shown in Fig. 1 and Table 1. Overall, 70 SRM transitions were monitored and split into 4 partially overlapping time windows. The 4 amino acids were baseline resolved chromatographically, and each transition was quantified with no apparent interferences. For the isotopologs, the LC retention times were similar to the unlabeled amino acids, but all transitions had different precursors (and, in some cases, they also had different product ions), depending on the number of [13C] atoms incorporated into the amino acids and into the MS-MS product ion. Therefore, the [13C] amino acid isotopologs were easily distinguished and quantified.
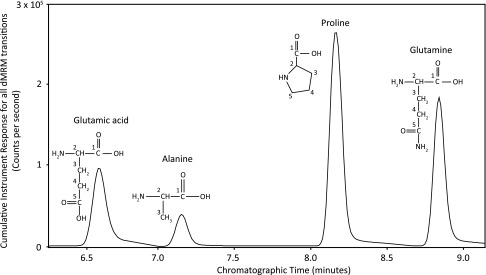
Figure 1.
A representative total ion current chromatogram for the cumulative instrument response for all unlabeled and [13C] isotopologs measured for glutamic acid, alanine, proline, and glutamine present in mosquito whole-body extracts. Each peak is labeled with the name and structure (with numbered carbon atoms) for each corresponding amino acid, and Table 1 lists the number of transitions monitored in the acquisition method, the retention time of interest, and the dMRM (a specific time-shared MRM mode utilized by Agilent triple-quadrupole mass spectrometers) acquisition window specified for each amino acid and its corresponding isotopologs. Mosquitoes were fed on a BM, BM supplemented with glucose, or BM supplemented with [1,2-13C2] glucose. Data are representative of 3 independent experiments.
TABLE 1.
Number of transitions monitored in the acquisition method, the retention time of interest, and the dMRM acquisition window specified for each amino acid and its corresponding isotopologs
| Amino acid | Transitions monitored (n) | Retention time (min) | dMRM window (min) |
|---|---|---|---|
| Glutamic acid | 21 | 6.6 | 6.1–7.1 |
| Alanine | 10 | 7.1 | 6.6–7.6 |
| Proline | 18 | 8.2 | 7.7–8.7 |
| Glutamine | 21 | 8.8 | 8.2–9.2 |
Kinetics of incorporation of [13C] atoms from [1,2-13C2]glucose into [13C]Ala, [13C]Glu, [13C]Gln, and [13C]Pro
As expected, [13C] amino acids are not observed above naturally abundant background levels in the whole body of mosquitoes fed a blood meal or a blood meal supplemented with unlabeled glucose. The kinetic study of [13C]Ala, [13C]Glu, [13C]Gln, and [13C]Pro in whole body of A. aegypti fed a blood meal supplemented with [1,2-13C2]glucose is shown in Figs. 2 and 3. The concentrations, expressed in nanomoles per insect, reflect the sum of [13C] amino acid isotopologs containing the relevant number of [13C] atoms per transition at 0, 0.25, 1, 6, 12, and 24 h after feeding. The [13C] atoms may be located at 1 or several positions within the amino acid backbone/side chain, as indicated in the specific transition monitored. For instance, the transition 150–58 m/z for Glu reflects the [13C2]Glu, where both [13C] atoms are in the 58 m/z product ion; the isotopic carbon atoms are located at positions 2, 3, or 4 of the Glu structure (but not at the backbone 1-C or 5-C positions). The rise time to maximum, the full decay time for the various SRM transitions, and the possible carbon positions of the [13C] atom incorporation in the amino acid structures are summarized in Table 2. Our data indicated the following:
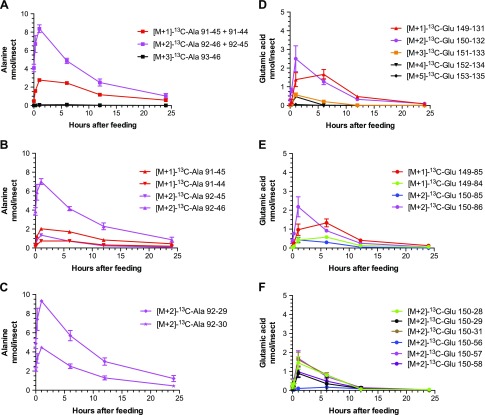
Figure 2.
Time-course dependence (0–24 h) of selected SRM transitions for [13C]alanine (A–C) and [13C]glutamic acid (D–F) in mosquito whole-body extracts. Mosquitoes were fed a blood meal supplemented with [1,2-13C2]glucose. The amino acids were analyzed in mosquito whole-body extracts by LR-LC/MS-MS. SRM transitions were from the [M + 1]+ to [M + 5]+ precursor isotopologs to various product isotopologs. Data are presented as means ± sem of 3 independent samples. N = 15 mosquitoes for each time point.
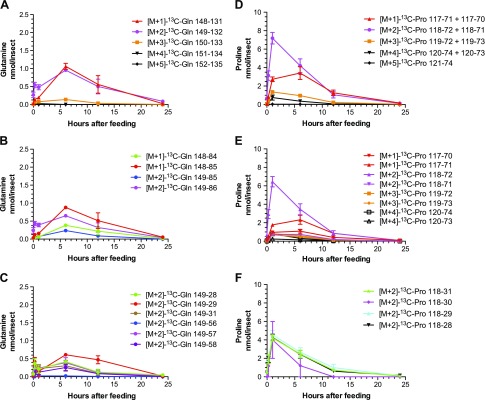
Figure 3.
Time dependence (0–24 h) of selected SRM transitions for [13C]glutamine (A–C) and [13C]proline (D–F) in mosquito whole-body extracts. Mosquitoes were fed on a blood meal supplemented with [1,2-13C2]glucose. The amino acids were analyzed in mosquito whole-body extracts by LR-LC/MS-MS. SRM transitions were from the [M + 1]+ to [M + 5]+ precursor isotopologs to various product isotopologs. Data are presented as means ± sem of 3 independent samples. N = 15 mosquitoes for each time point.
TABLE 2.
Summary of the kinetic experiments for [13Cx] amino acids by LR-LC/MS-MS
| [13Cx] amino acid | Rise to maximum (h) | Decay to baseline (h) | Possible carbon positions involved |
|---|---|---|---|
| [13C1]Ala, not 1-C | 1–6 | >24 | 2 or 3 |
| [13C2]Ala | <1 | >24 | 2, 3 mainly |
| [13C1]Glu, 1-C | 1–6 | 12 | 1 |
| [13C1]Glu, not 1-C | 1 | 12 | 2, 3, 4, 5 |
| [13C2]Glu, 1-C | 1–6 | 12 | 1, 2, 3, 4, 5 |
| [13C2]Glu, not 1-C | 1 | 12 | 2, 3, 4, 5 |
| [13C3-4]Glu, 1-C | 1 | 12 | 1, 2, 3, 4, 5 |
| [13C1]Gln, 1-C | 6 | >12 | 1 |
| [13C1]Gln, not 1-C | 6 | >12 | 2, 3, 4, 5 |
| [13C2]Gln, 1-C | 1–6 | >12 | 1, 2, 3, 4, 5 |
| [13C2]Gln, not 1-C | 1–6 | >12 | 2, 3, 4, 5 |
| [13C3]Gln, 1-C | 6 | >12 | 1, 2, 3, 4, 5 |
| [13C1]Pro, 1-C | 1–6 | >12 | 1 |
| [13C1]Pro, not 1-C | 1–6 | >12 | 2, 3, 4, 5 |
| [13C2]Pro, 1-C | 1 | >12 | 1, 2, 3, 4, 5 |
| [13C2]Pro, not 1-C | 1 | >12 | 2, 3, 4, 5 |
| [13C3–4]Pro, 1-C | 1–6 | >12 | 1, 2, 3, 4, 5 |
| [13C3–4]Pro, not 1-C | 1–6 | >12 | 2, 3, 4, 5 |
Most Ala contained 2 [13C] atoms (mainly at positions 2 and 3), which were formed almost immediately after feeding and had a very long decay (possibly monoexponential) with an estimated half-life of >6 h and measurable levels even after 24 h.
Glu, Gln, and Pro isotopologs containing 1, 2, and 3 [13C] atoms were quantified; lower concentrations of Glu and Pro isotopologs containing 4 [13C] atoms were also measured.
In the first kinetic route, Glu, Gln, and Pro exhibited similar kinetic behavior, with a rise time to maximum of 6 h (still slower than Ala formation) and a fast, complete decay to baseline in <24 h, which suggests that Glu, Gln, and Pro are conjugated in their metabolic cycle and have the same rate-limiting reactions in both formation and disappearance.
In the second kinetic route, a different kinetic behavior was observed, with a fast rise time to maximum of ∼1 h for Glu, not involving 1-C; Pro (with 2 [13C] atoms), not involving 1-C; and Ala (with 2 [13C] atoms), not involving 1-C, which suggests a separate pathway with unique kinetics involving Glu, Pro, and Ala.
Kinetics of incorporation of [13C] atoms from [1,2-13C2]glucose into additional [13C] amino acids, [13C] amino acid derivatives, and [13C] organic acids
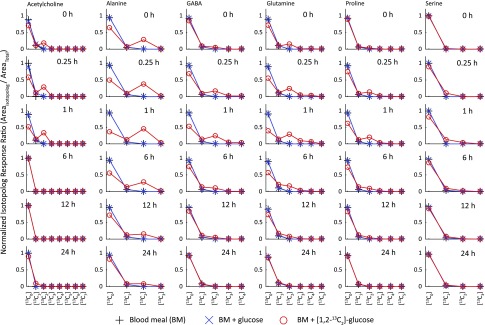
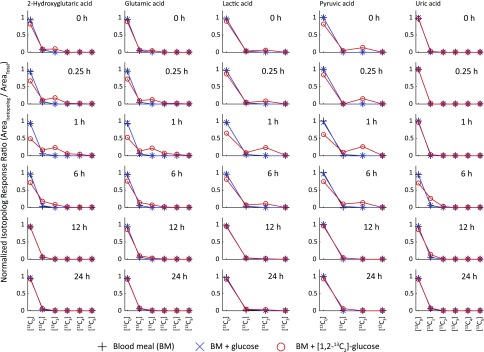
To investigate whether [13C] atoms from [1,2-13C2]glucose can be incorporated into other metabolic products, we performed Orbitrap-based experiments to explore the kinetics of the 20 proteogenic amino acids, 18 amino acid derivatives, and 5 organic acids, including uric acid. We studied the kinetics of 43 compounds altogether (Supplemental Tables S5 and S6) using the same mosquito whole-body extracts (without the inclusion of IS). Many of the explored compounds did not exhibit any [13C] atom incorporation above the naturally occurring background, but several exhibited notable rises and decays for [13C] atom incorporation. In addition to the amino acids mentioned previously ([13C]Ala, [13C]Glu, [13C]Gln, and [13C]Pro), the carbon skeleton of [1,2-13C2]glucose was found to be incorporated into [13C]acetylcholine, [13C]GABA, [13C]2-hydroxyglutaric acid (2-HG), [13C]lactic acid (Lac), [13C]pyruvic acid (Pyr), [13C]Ser, and [13C] uric acid, as shown in Figs. 4 and 5. Table 3 contains a summary of the kinetics for the additional compounds that had [13C] isotopologs in the mosquito whole-body extracts. [13C] uric acid exhibited a relatively slow rise time to maximum (6 h) and a very slow decay (24 h). Most other compounds exhibited a very fast rise time to maximum of ∼1 h and decay times of ∼6 h (except for [13C]GABA and [13C]Ser, which both had decay times of ∼12 h). The fast rise time to maximum for many of the compounds shown in Table 3 suggests that they are formed within the second, fast kinetic route proposed in the Results section.
Figure 4.
Unlabeled and [13C] isotopologs of amino acids and amino acid derivatives present in mosquito whole-body extracts. Measurements were made using an Orbitrap HRAM-LC/MS instrument. Plots are of the normalized mass isotopomer distributions against the degree of [13C] labeling for metabolites that exhibited enrichment of [13C] labeling greater than the isotopically labeled background. Each column corresponds to a single metabolite isotopolog series, and each individual data plot corresponds to an individual time point during the time course studied. Three groups of mosquitoes were fed on 3 different diets: BM, BM supplemented with glucose, and BM supplemented with [1,2-13C2]glucose, as indicated in Materials and Methods.
Figure 5.
Unlabeled and [13C] isotopologs of 2-HG, glutamic acid, Lac, Pyr, and uric acid present in mosquito whole-body extracts. Measurements were made using an Orbitrap HRAM-LC/MS instrument. Plots are of the normalized mass isotopomer distributions against the degree of [13C] labeling for metabolites that exhibited enrichment of [13C] labeling greater than the isotopically labeled background. Each column corresponds to a single metabolite isotopolog series, and each individual data plot corresponds to an individual time point during the time course studied. Three groups of mosquitoes were fed on 3 different diets: BM, BM supplemented with glucose, and BM supplemented with [1,2-13C2]glucose, as indicated in Materials and Methods.
TABLE 3.
Summary of kinetic experiments for the additional [13C] amino acids, [13C] amino acid derivatives, and [13C] organic acids by HRAM LC/MS
| [13Cx] metabolite | Rise to maximum (h) | Decay to baseline (h) |
|---|---|---|
| [13C2]acetylcholine | <1 | 6 |
| [13C1]GABA (minor) | 1–6 | >12 |
| [13C2]GABA | 1 | >12 |
| [13C1]Ser | 1 | >12 |
| [13C2]Ser (minor) | 1 | >6 |
| [13C1]2-HG (minor) | 1-6 | >6 |
| [13C2]2-HG | 1 | >6 |
| [13C1]Lac (minor) | 1–6 | >6 |
| [13C2]Lac | 1 | >6 |
| [13C1]Pyr (minor) | 1–6 | >6 |
| [13C2]Pyr | 1 | >6 |
| [13C1] uric acid | 6 | >24 |
DISCUSSION
In recent years, the successful development and application of stable [15N] isotopically labeled compounds and mass spectrometry techniques to study mosquito nitrogen metabolism have demonstrated that female mosquitoes can rapidly and efficiently detoxify high ammonia concentrations released from blood meal digestion (8–10, 18, 19). Blood-fed A. aegypti females convert ammonia into specific amino acid (Gln, Glu, Ala, and Pro), and other nitrogen-containing compounds, such as uric acid, allantoin, allantoic acid, and urea, through the concerted action of multiple metabolic pathways (8–10). Additionally, argininolysis and uricolysis, 2 metabolic pathways involved in urea synthesis and excretion, are tightly regulated by a crosstalk mechanism that controls the expression of genes involved in the fixation, assimilation, and excretion of ammonia (13). Furthermore, RNA interference studies recently discovered that uric acid synthesis and excretion in blood-fed mosquitoes were closely regulated by crosstalk signaling mechanisms among genes encoding enzymes involved in ammonia and free radical detoxification (14, 15). Those findings reveal the remarkable capacity of blood-fed mosquitoes to manage an unbalanced nitrogen metabolism. In this report, we optimized and applied LR-LC/MS-MS and HRAM-LC/MS methods to identify [13C] metabolites in mosquito whole-body extracts. We obtained evidence that glucose supports ammonia detoxification pathways and facilitates the elimination of excess nitrogen. We found that the carbon skeleton of glucose is used for the synthesis of specific amino acids, amino acid derivatives, and organic acids during A. aegypti blood meal digestion.
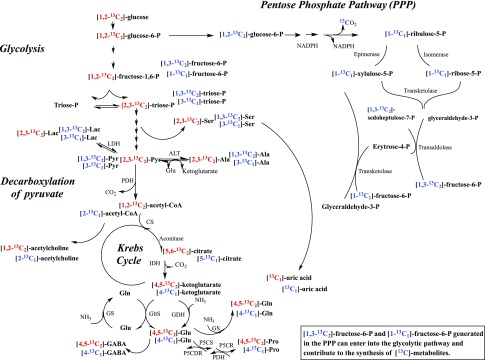
The kinetics of the incorporation of [13C] atoms from [1,2-13C2]-glucose into different [13C] amino acids in blood-fed A. aegypti whole body were fast and well correlated with studies previously performed with [15N] isotopically labeled compounds (8–10). Interestingly, only 11 of the 43 metabolites monitored in the present study exhibited [13C] atom incorporation above the naturally occurring background. Immediately after feeding female mosquitoes, it was possible to identify [13C]Ala, [13C]Glu, [13C]Gln, [13C]Pro, [13C]Ser, [13C]acetylcholine, [13C]GABA, [13C]2-HG, [13C]Pyr, and [13C]Lac labeled with 1 or 2 [13C] atoms. The metabolic routes, with their plausible [13C] atom-specific pathways, are summarized in Fig. 6. The most abundant [13C] amino acids quantified during the first 6 h after feeding were [13C]Ala and [13C]Pro. The presence of [2,3-13C2]Ala implies that oxidation of [1,2-13C2]glucose occurs through glycolysis. That pathway leads to the production of [2,3-13C2]Pyr, which is then converted into [2,3-13C2]Ala by ALT. However, [2,3-13C2]Pyr can also be converted to [2,3-13C2]Lac by lactate dehydrogenase, as shown in Fig. 6. The identification of [13C]Ala with 1 or 2 [13C] atoms which could be located in 3-C, or in 1-C and 3-C, positions also indicates that [1,2-13C2]glucose was partially metabolized through the PPP. In addition to providing the NADPH required for certain enzymatic reactions, PPP can also provide intermediates for nucleic acid synthesis and glycolysis, including [1,3-13C2]fructose-6-P or [1-13C1]fructose-6-P. Thus, the oxidation of [1,3-13C2]fructose-6-P or [1-13C1]fructose-6-P through glycolysis can lead to the production of [1,3-13C2]Pyr, [3-13C1]Pyr, [1,3-13C2]Lac, and [3-13C1]Lac. [13C]Pyr can serve as precursor for [1,3-13C2]Ala and [3-13C1]Ala synthesis. Additionally, [13C]Ser labeled with 1 or 2 [13C] atoms can be synthesized from the glycolytic intermediate 3-P-glycerate (20, 21).
Figure 6.
A proposed scheme of metabolic interactions at the [13C] atomic levels induced by glucose on ammonia metabolism. The [1,2-13C2]glucose can be converted into [1,2-13C2]glucose-6-phosphate, which can be metabolized through glycolysis ([13C] atoms are designated in red) or entered into the PPP ([13C] atoms are designated in blue). Not all possible reactions between unlabeled and [13C] metabolites are represented in the scheme. CS, citrate synthetase; GDH, glutamate dehydrogenase; GltS, glutamate synthase; GS, glutamine synthetase; IDH, isocitrate dehydrogenase; LDH, lactate dehydrogenase; P5CDH, pyrroline-5-carboxylate dehydrogenase; P5CR, pyrroline-5-carboxylate reductase; P5CS, pyrroline-5-carboxylate synthase; PDH, pyruvate dehydrogenase.
The [13C]Pyr with 1 or 2 [13C] atoms can be decarboxylated to [1,2-13C2]acetyl coenzyme A (acetyl-CoA) or [2-13C1]acetyl-CoA by pyruvate dehydrogenase. Otherwise, [13C]Pyr can be carboxylated by pyruvate carboxylase and converted into [13C] oxaloacetic acid, which can enter the Krebs cycle and produce [13C] intermediates (data not shown in Fig. 6). The observed production of [13C]acetylcholine labeled with 2 [13C] atoms indicates that a fraction of the [13C]acetyl-CoA was used to synthesize acetylcholine, probably for use as a neurotransmitter (22). Although labeled fatty acids were not monitored in this study, partial use of [13C]acetyl-CoA for fatty acids synthesis is expected (5, 23–26).
Alternatively, the [13C]acetyl-CoA produced from decarboxylation by pyruvate dehydrogenase can be completely oxidized to CO2 in the Krebs cycle for energy production and/or enter the Krebs cycle for supplying [13C] keto acids for ammonia metabolism (8, 10). In the Krebs cycle, [13C]acetyl-CoA and unlabeled oxaloacetic acid lead to [5,6-13C2]citrate or [5-13C1]citrate by citrate synthetase and aconitase. [13C]citrate is converted into [4,5-13C2]α-ketoglutarate or [4-13C1]α-ketoglutarate by isocitrate dehydrogenase. Gln and [13C]α-ketoglutarate produce unlabeled Glu and [4,5-13C2]Glu or [4-13C1]Glu by glutamate synthase and generate other amino acids through reactions involved in ammonia metabolism (8, 10). Thus, [13C]Glu can be converted into [4,5-13C2]Pro or [4-13C1]Pro by pyrroline-5-carboxylase synthase and pyrroline-5-carboxylase reductase. [13C]α-ketoglutarate can also fix ammonia by glutamate dehydrogenase and generate [4,5-13C2]Glu or [4-13C1]Glu. In addition, [4,5-13C2]Gln or [4-13C1]Gln can be synthesized by [13C]Glu and ammonia through glutamine synthetase. Our data also suggest that [13C]Gln or [13C]Glu can be used to synthesize [13C]GABA, another neurotransmitter (22, 27). Both [13C]Glu and [13C]GABA exhibited similar patterns during the time analyzed in mosquito whole-body extracts. The presence of a small concentration of [13C]Glu, [13C]Gln, and [13C]Pro with 3 or 4 [13C] atoms is most likely a consequence of a reentry of certain intermediates into the Krebs cycle.
Pro is one of the most versatile amino acids in blood-fed mosquitoes. It can be used as a temporary nitrogen sink to remove excess nitrogen (7–10, 28) and as a source of energy (29, 30). The kinetics of [13C]Pro in the times studied support both roles for Pro in mosquitoes. It has been previously demonstrated that both [15N]Gln and [15N]Pro are used for [15N2] uric acid synthesis in A. aegypti (9). In this study, the identification of [13C1] uric acid in mosquito whole body at 6, 12, and 24 h after feeding concurs with the presence and quantification of uric acid in malpighian tubules of blood-fed A. aegypti females at 6, 12, and 24 h after feeding (14, 15), and, importantly, it provides evidence that the carbon skeleton of glucose contributes to the synthesis of uric acid, a key antioxidant and nitrogen waste product in blood-fed mosquitoes (14, 15). Certainly, the detection of [13C]Ser in mosquito whole body is well-correlated with its possible contribution as one of the sources of carbon for de novo purine synthesis via the production of 5, 10-methylene-tetrahydrofolate (21, 31, 32).
Although glucose can be used for the synthesis of trehalose, glycogen, and lipids to increase maternal reserves or be used to reinforce flight and mosquito reproduction (5, 23, 24, 33, 34), our data demonstrate that rapid glucose oxidation is also required to support ammonia detoxification and excess nitrogen disposal through a coordinated interaction among multiple metabolic pathways (Fig. 6). Thus, nectar ingested by females before blood feeding, or even glucose naturally present in small amounts in a blood meal, can have an important role in ammonia detoxification. The mobilization of maternal carbohydrates for the same purpose when the digestion of the blood meal begins can also contribute to ammonia clearance. In summary, we found a metabolic link at the carbon atomic level between glucose and ammonia metabolism. The mass spectrometry techniques optimized in this study can be applied to investigate the metabolic regulation of nitrogen and carbon metabolism in mosquitoes and in other vectors of diseases and, ultimately, could lead to the discovery of metabolic targets or regulators for vector control.
ACKNOWLEDGMENTS
The authors thank Dr. Lin Tan and Dr. Di Du (Proteomics and Metabolomics Core Facility, University of Texas MD Anderson Cancer Center) for technical assistance. The authors also thank Dr. Robert Swaim, Ken Miller, and Jamie Humphries (Thermo Fisher Scientific, Waltham, MA, USA) for assistance with training and use of TraceFinder software. This work was supported financially by the Corine Adams Baines Professorship Award, the U.S. National Institutes of Health (NIH) National Institute of Allergy and Infectious Diseases (Grant R01AI088092 to P.Y.S.), NIH 1S10OD012304-01, Cancer Prevention Research Institute of Texas (CPRIT; Grant RP130397), and The University of Texas M. D. Anderson National Cancer Institute Cancer Center (Grant CA016672). The authors declare no conflicts of interest.
Glossary
- 2-HG
2-hydroxyglutaric acid
- acetyl-CoA
acetyl coenzyme A
- ALT
alanine aminotransferase
- BM
bovine blood meal plus 5 mM ATP
- dMRM
dynamic multiple reaction monitoring
- ESI
electrospray ionization
- HILIC
hydrophilic interaction liquid chromatography
- HRAM
high-resolution, accurate-mass
- IS
labeled internal standard
- Lac
lactic acid
- LC/MS
liquid chromatography-mass spectrometry
- LR
low resolution
- MPA
mobile phase A
- MPB
mobile phase B
- MS-MS
tandem mass spectrometry
- PPP
pentose phosphate pathway
- Pyr
pyruvic acid
- SRM
selected reaction monitoring
- tSIM
timed single-ion monitoring
- XDH-1
xanthine dehydrogenase-1
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
P. Y. Scaraffia designed research; T. D. Horvath performed research; S. Dagan, P. L. Lorenzi, and D. H. Hawke contributed new reagents/analytic tools; T. D. Horvath, S. Dagan, P. L. Lorenzi, D. H. Hawke, and P. Y. Scaraffia analyzed data; and T. D. Horvath, S. Dagan, and P. Y. Scaraffia wrote the paper.
REFERENCES
- 1.Attar N. (2016) Zika virus circulates in new regions. Nat. Rev. Microbiol. 14, 62 [Google Scholar]
- 2.Armstrong N., Hou W., Tang Q. (2017) Biological and historical overview of Zika virus. World J. Virol. 6, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ali S., Gugliemini O., Harber S., Harrison A., Houle L., Ivory J., Kersten S., Khan R., Kim J., LeBoa C., Nez-Whitfield E., O’Marr J., Rothenberg E., Segnitz R. M., Sila S., Verwillow A., Vogt M., Yang A., Mordecai E. A. (2017) Environmental and social change drive the explosive emergence of Zika virus in the Americas. PLoS Negl. Trop. Dis. 11, e0005135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mayer S. V., Tesh R. B., Vasilakis N. (2017) The emergence of arthropod-borne viral diseases: a global prospective on dengue, chikungunya and Zika fevers. Acta Trop. 166, 155–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou G., Flowers M., Friedrich K., Horton J., Pennington J., Wells M. A. (2004) Metabolic fate of [14C]-labeled meal protein amino acids in Aedes aegypti mosquitoes. J. Insect Physiol. 50, 337–349 [DOI] [PubMed] [Google Scholar]
- 6.Briegel H. (1985) Mosquito reproduction: incomplete utilization of the blood meal protein for oogenesis. J. Insect Physiol. 31, 15–21 [Google Scholar]
- 7.Scaraffia P. Y., Isoe J., Murillo A., Wells M. A. (2005) Ammonia metabolism in Aedes aegypti. Insect Biochem. Mol. Biol. 35, 491–503 [DOI] [PubMed] [Google Scholar]
- 8.Scaraffia P. Y., Zhang Q., Wysocki V. H., Isoe J., Wells M. A. (2006) Analysis of whole body ammonia metabolism in Aedes aegypti using [15N]-labeled compounds and mass spectrometry. Insect Biochem. Mol. Biol. 36, 614–622 [DOI] [PubMed] [Google Scholar]
- 9.Scaraffia P. Y., Tan G., Isoe J., Wysocki V. H., Wells M. A., Miesfeld R. L. (2008) Discovery of an alternate metabolic pathway for urea synthesis in adult Aedes aegypti mosquitoes. Proc. Natl. Acad. Sci. USA 105, 518–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scaraffia P. Y., Zhang Q., Thorson K., Wysocki V. H., Miesfeld R. L. (2010) Differential ammonia metabolism in Aedes aegypti fat body and midgut tissues. J. Insect Physiol. 56, 1040–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Von Dungern P., Briegel H. (2001) Enzymatic analysis of uricotelic protein catabolism in the mosquito Aedes aegypti. J. Insect Physiol. 47, 73–82 [DOI] [PubMed] [Google Scholar]
- 12.Von Dungern P., Briegel H. (2001) Protein catabolism in mosquitoes: ureotely and uricotely in larval and imaginal Aedes aegypti. J. Insect Physiol. 47, 131–141 [DOI] [PubMed] [Google Scholar]
- 13.Isoe J., Scaraffia P. Y. (2013) Urea synthesis and excretion in Aedes aegypti mosquitoes are regulated by a unique cross-talk mechanism. PLoS One 8, e65393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mazzalupo S., Isoe J., Belloni V., Scaraffia P. Y. (2016) Effective disposal of nitrogen waste in blood-fed Aedes aegypti mosquitoes requires alanine aminotransferase. FASEB J. 30, 111–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isoe J., Petchampai N., Isoe Y. E., Co K., Mazzalupo S., Scaraffia P. Y. (2017) Xanthine dehydrogenase-1 silencing in Aedes aegypti mosquitoes promotes a blood feeding-induced adulticidal activity. FASEB J. 31, 2276–2286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma X., Dagan S., Somogyi A., Wysocki V. H., Scaraffia P. Y. (2013) Low mass MS/MS fragments of protonated amino acids used for distinction of their 13C-isotopomers in metabolic studies. J. Am. Soc. Mass Spectrom. 24, 622–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belloni V., Scaraffia P. Y. (2014) Exposure to l-cycloserine incurs survival costs and behavioral alterations in Aedes aegypti females. Parasit. Vectors 7, 373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Q., Wysocki V. H., Scaraffia P. Y., Wells M. A. (2005) Fragmentation pathway for glutamine identification: loss of 73 Da from dimethylformamidine glutamine isobutyl ester. J. Am. Soc. Mass Spectrom. 16, 1192–1203 [DOI] [PubMed] [Google Scholar]
- 19.Bush D. R., Wysocki V. H., Scaraffia P. Y. (2012) Study of the fragmentation of arginine isobutyl ester applied to arginine quantification in Aedes aegypti mosquito excreta. J. Mass Spectrom. 47, 1364–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang M., Vousden K. H. (2016) Serine and one-carbon metabolism in cancer. Nat. Rev. Cancer 16, 650–662 [DOI] [PubMed] [Google Scholar]
- 21.Dong W., Keibler M. A., Stephanopoulos G. (2017) Review of metabolic pathways activated in cancer cells as determined through isotopic labeling and network analysis. Metab. Eng. 3(Pt. B), 113–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin C. A., Krantz D. E. (2014) Drosophila melanogaster as a genetic model system to study neurotransmitter transporters. Neurochem. Int. 73, 71–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Briegel H., Hefti M., DiMarco E. (2002) Lipid metabolism during sequential gonotrophic cycles in large and small female Aedes aegypti. J. Insect Physiol. 48, 547–554 [DOI] [PubMed] [Google Scholar]
- 24.Zhou G., Pennington J. E., Wells M. A. (2004) Utilization of pre-existing energy stores of female Aedes aegypti mosquitoes during the first gonotrophic cycle. Insect Biochem. Mol. Biol. 34, 919–925 [DOI] [PubMed] [Google Scholar]
- 25.Alabaster A., Isoe J., Zhou G., Lee A., Murphy A., Day W. A., Miesfeld R. L. (2011) Deficiencies in acetyl-CoA carboxylase and fatty acid synthase 1 differentially affect eggshell formation and blood meal digestion in Aedes aegypti. Insect Biochem. Mol. Biol. 41, 946–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang J., Singh J., Kim S., Hockaday W. C., Sim C., Kim S. J. (2016) Solid-state NMR reveals differential carbohydrate utilization in diapausing Culex pipiens. Sci. Rep. 6, 37350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li S., Yang J., Shen J. (2007) Novel strategy for cerebral 13C MRS using very low RF power for proton decoupling. Magn. Reson. Med. 57, 265–271 [DOI] [PubMed] [Google Scholar]
- 28.Goldstrohm D. A., Pennington J. E., Wells M. A. (2003) The role of hemolymph proline as a nitrogen sink during blood meal digestion by the mosquito Aedes aegypti. J. Insect Physiol. 49, 115–121; erratum: 629 [DOI] [PubMed] [Google Scholar]
- 29.Scaraffia P. Y., Wells M. A. (2003) Proline can be utilized as an energy substrate during flight of Aedes aegypti females. J. Insect Physiol. 49, 591–601 [DOI] [PubMed] [Google Scholar]
- 30.Soares J. B., Gaviraghi A., Oliveira M. F. (2015) Mitochondrial physiology in the major arbovirus vector Aedes aegypti: substrate preferences and sexual differences define respiratory capacity and superoxide production. PLoS One 10, e0120600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fell D. A., Snell K. (1988) Control analysis of mammalian serine biosynthesis. Feedback inhibition on the final step. Biochem. J. 256, 97–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pacold M. E., Brimacombe K. R., Chan S. H., Rohde J. M., Lewis C. A., Swier L. J., Possemato R., Chen W. W., Sullivan L. B., Fiske B. P., Cho S., Freinkman E., Birsoy K., Abu-Remaileh M., Shaul Y. D., Liu C. M., Zhou M., Koh M. J., Chung H., Davidson S. M., Luengo A., Wang A. Q., Xu X., Yasgar A., Liu L., Rai G., Westover K. D., Vander Heiden M. G., Shen M., Gray N. S., Boxer M. B., Sabatini D. M. (2016) A PHGDH inhibitor reveals coordination of serine synthesis and one-carbon unit fate. Nat. Chem. Biol. 12, 452–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Foster W. A. (1995) Mosquito sugar feeding and reproductive energetics. Annu. Rev. Entomol. 40, 443–474 [DOI] [PubMed] [Google Scholar]
- 34.Hou Y., Wang X. L., Saha T. T., Roy S., Zhao B., Raikhel A. S., Zou Z. (2015) Temporal coordination of carbohydrate metabolism during mosquito reproduction. PLoS Genet. 11, e1005309 [DOI] [PMC free article] [PubMed] [Google Scholar]