Abstract
Background
Our previous study found that lower lymphocyte-to-monocyte ratio (LMR) is an independent risk factor of clinical outcome of acute ischemic stroke (AIS). However, whether lower LMR is independently associated with adverse prognosis of AIS treated with thrombolysis has not been determined. In this study, we explored the relationship between LMR and prognosis of AIS treated with thrombolysis.
Material/Methods
We retrospectively enrolled 108 patients treated with thrombolysis. LMR was calculated according to lymphocyte count and monocyte count on admission. Patients were classified into 3 groups according to LMR values on admission (group 1 LMR >4.34, group 2 LMR 2.79 to 4.34, group 3 LMR <2.79). Neurologic impairment was estimated by use of the National Institute of Health Stroke Scale. Clinical prognosis at 3 months was assessed by modified Rankin Scale. The relationship between LMR and neurologic impairment was analyzed by Spearman rank correlation. Receiver operating characteristic curve (ROC) was used to evaluate the ability of LMR to predict outcome.
Results
Patients in group 3 had lower lymphocyte counts and LMR values and higher monocyte counts (P<0.001). LMR value was negatively correlated with the degree of neurologic impairment (r=−0.372, P<0.001). The ROC suggested a moderate sensitivity (71.6%) and specificity (80.5%) of LMR for predicting prognosis with an optimal cut-off point at 3.48. Higher LMR value was an independent protective factor against adverse prognosis (odds ratio 0.683, 95% confidence interval 0.490−0.952, P=0.024).
Conclusions
A lower LMR value is an independent predictor of poor prognosis of AIS treated with thrombolytic therapy.
MeSH Keywords: Lymphocyte Count, Monocytes, Prognosis, Stroke, Thrombolytic Therapy
Background
Stroke is a major cause of disability and death worldwide [1]. In China, stroke has become the leading cause of death, and the prevalence of stroke in adults aged 40 years or older is 2.21%, with ischemic stroke representing approximately 80% of all strokes [2]. Current therapy with intravenous (IV) recombinant tissue plasminogen activator (rt-PA) is the only treatment approved for acute ischemic stroke (AIS) within 4.5 h after onset [3]. However, the risk of fatal symptomatic intracranial hemorrhage (sICH) after treatment with rt-PA is a major threat. Therefore, it is essential to identify the potential risk factors for sICH and the prognosis of patients with thrombolysis.
Recently, there has been much evidence indicating a causal link between the inflammatory response and poor outcome of patients with AIS [4]. A higher monocyte count and increased neutrophil numbers have been found to be independently associated with adverse post-stroke prognosis, whereas low lymphocyte count suggests this is a cause-effect association [5–7]. In addition, neutrophil-to-lymphocyte ratio (NLR) independently predicts poor outcome of AIS [8], and high neutrophil counts and evaluated NLR values before thrombolysis are associated with worse outcome at 3 months [9].
Recent studies reported that a lower lymphocyte-to-monocyte ratio value (LMR) is independently associated with severity of coronary artery disease and is related to poor outcome of various solid tumors [10,11]. Inspired by previous studies, in the present study we focused on the potential relationship between LMR and the outcome of AIS and found that lower LMR on admission is independently correlated with worse outcome of patients with AIS [12]. However, the considerable influence of LMR on prognosis before thrombolytic therapy has not been addressed. Thus, the present study was conducted to identify the relationship between LMR on admission and outcome at 3 months after AIS with thrombolytic treatment.
Material and Methods
Study Population
All patients with AIS who received IV rt-PA therapy were hospitalized in the Department of Neurology, the Affiliated Hospital of Chengde Medical University and enrolled from January 2014 to April 2017. Patients were included if they met the guidelines modified by the American Heart Association/American Stroke Association for the early management of AIS regarding thrombolysis treatment [3]. Exclusion criteria were: (1) history of infection within 2 weeks before admission; (2) dying or having infections during the period of admission; (3) fatal renal or kidney dysfunction; (4) adverse tumor or immune diseases; (5) taking immunosuppressants or steroids; (6) history of AIS with a modified Rankin Scale (mRS) score 3 to 6; and (7) receiving endovascular thrombectomy after thrombolysis.
Our institution’s Committee on Medical Ethics gave approval for this study. All patients or their relatives gave informed consent.
Baseline information
We recorded the demographic data and clinical characteristics of patients listed below: age, sex, time from onset to hospital admission, hypertension, coronary heart disease (CHD), atrial fibrillation, diabetes mellitus, stroke history, hyperlipidemia, smoking, and alcohol drinking. Laboratory values were measured on admission before thrombolysis treatment, including lymphocyte count, monocyte count, fasting blood glucose (FBG), serum creatinine, total cholesterol (TC), low-density lipoprotein cholesterol, triglyceride (TG), and high-density lipoprotein cholesterol. LMR was evaluated using the lymphocyte and monocyte count. According to LMR values on admission, patients were divided into group 1 (LMR >4.34), group 2 (LMR 2.79–4.34), and group 3 (LMR <2.79) and each group contained 36 patients. Stroke subtypes were assorted according to the TOAST classification, including large-artery atherosclerosis, small-artery occlusion, cardioembolism, stroke of other determined etiology, and stroke of undetermined etiology [13].
Imaging
On admission, all enrolled patients underwent a CT or MRI of the head before thrombolysis. All patients underwent the aforementioned exams again 24 h after IV rt-PA treatment, or earlier if the neurological symptoms worsened in case of sICH.
Treatment
All patients were treated by IV rt-PA (alteplase) with a standard dose of 0.9 mg/kg (maximum 90mg). All patients were given 10% of the dose as a bolus within 1 min and the remainder was infused over 60 min [14].
Definition of neurologic impairment and clinical outcome
Neurologic impairment on admission was evaluated by use of the National Institute of Health Stroke Scale (NIHSS). Moderate to severe stroke was defined as an NIHSS score higher than 5 [15]. The 3-month outcome was evaluated by the mRS determined via telephone interview. An mRS score of 3 or higher at 3 months represented an adverse prognosis [16].
Statistical analysis
Baseline characteristics were compared among groups using the Kruskal-Wallis and Mann-Whitney U tests. Baseline characteristics of continuous data are expressed as mean ± standard deviation or median (interquartile range). Categorical data were compared among groups using the chi-square test. The Fisher exact test was applied in case the expected frequency was 5 or less. The relationship between LMR and neurologic impairment was analyzed by Spearman rank correlation. The area under the receiver operating characteristic curve (ROC) was used to assess the ability of LMR in predicting outcome and calculate the cut-off point. Possible risk factors of neurologic impairment and clinical outcome were performed using binary logistic regression analysis. A 2-tailed P value less than 0.05 was defined as statistical significance. All analyses were performed with SPSS 19.0 software (SPSS Inc., Chicago, IL).
Results
Baseline characteristics
This study cohort included 117 patients with AIS treated by thrombolysis according to the inclusion criteria. Among 117 patients, 5 patients were excluded because of loss to follow-up and 4 patients underwent endovascular thrombectomy after IV rt-PA administration. Finally, we enrolled a total of 108 patients in this study. Baseline characteristics of eligible patients are shown in Table 1. The study population consisted of 76 males and 32 females, with a median age of 56.0 years (range, 52.0–69.0 years). According to LMR values on admission, patients were divided into tertiles and each group contained 36 patients. Age, NIHSS score on admission, monocyte count, and percentage of atrial fibrillation were higher in patients who had higher LMR values (P<0.05). TG concentration, lymphocyte counts, and LMR values in group 3 were significantly lower compared with the other 2 groups (P<0.05). The remainder of baseline data among subgroups showed no significant difference (P>0.05).
Table 1.
Baseline characteristics of the study population.
| Total | LMR on admission | P value | |||
|---|---|---|---|---|---|
| LMR higher than 4.34 (36, 33.3%) | LMR 2.79–4.34 (36, 33.3%) | LMR lower than 2.79 (36, 33.3%) | |||
| Age(y) | 56.0 (52.0–69.0) | 56.7±9.5 | 58.1±12.7 | 67.0 (53.3–74.8) | 0.030* |
| Male, n | 76 (70.4) | 26 (72.2) | 27 (75.0) | 23 (63.9) | 0.561 |
| Admission to hospital (h), n | 2.5 (2.0–3.0) | 2.3 (2.0–2.9) | 2.5 (2.0–3.0) | 3.0 (2.0–3.9) | 0.208 |
| Hypertension, n | 70 (64.8) | 24 (66.7) | 21 (58.3) | 25 (69.4) | 0.590 |
| Diabetes, n | 20 (18.5) | 6 (16.7) | 5 (13.9) | 9 (25.0) | 0.450 |
| Hyperlipidemia, n | 41 (38.0) | 18 (50.0) | 13 (36.1) | 10 (27.8) | 0.146 |
| AF, n | 13 (12.0) | 2 (5.6) | 2 (5.6) | 9 (25.0) | 0.025* |
| CHD, n | 20 (18.5) | 5 (13.9) | 7 (19.4) | 8 (22.2) | 0.651 |
| Stroke history, n | 19 (17.6) | 6 (16.7) | 8 (22.2) | 5 (13.9) | 0.639 |
| Smoking, n | 62 (57.4) | 22 (61.1) | 23 (63.9) | 17 (47.2) | 0.309 |
| Alcohol drinking, n | 52 (48.1) | 20 (55.6) | 18 (50.0) | 14 (48.1) | 0.354 |
| Ongoing oral anticoagulant drugs | 6 (5.6) | 3 (8.3) | 1 (2.8) | 2 (5.6) | 0.869** |
| Anti-platelet therapy | 16 (14.8) | 6 (16.7) | 5 (13.9) | 5 (13.9) | 0.929 |
| Anti-hypertension therapy | 30 (27.8) | 9 (25) | 11 (30.6) | 10 (27.8) | 0.871 |
| Stroke etiologic subtypes | |||||
| LAA | 50 (46.3) | 16 (44.4) | 17 (47.2) | 17 (47.2) | 0.969** |
| SAO | 37 (34.3) | 14 (38.9) | 12 (33.3) | 11 (30.5) | |
| CE | 13 (12.0) | 3 (8.3) | 4 (11.1) | 6 (16.7) | |
| SOE | 4 (3.7) | 2 (5.6) | 1 (2.8) | 1 (2.8) | |
| SUE | 4 (3.7) | 1 (2.8) | 2 (5.6) | 1 (2.8) | |
| NIHSS score | 7.0 (4.0–11.8) | 5.0 (3.0–8.0) | 5.5 (4.0–11.0) | 9.8±5.0 | 0.002* |
| FBG (mmol/L) | 6.1 (5.3–7.3) | 5.8 (5.3–7.2) | 6.2±1.0 | 6.3 (5.3–7.7) | 0.557 |
| TC (mmol/L) | 4.3±1.1 | 4.2 (3.8–5.5) | 4.4 (4.0–5.1) | 4.2±0.8 | 0.440 |
| TG (mmol/L) | 1.6 (1.2–2.3) | 1.8 (1.5–2.3) | 1.8 (1.4–2.7) | 1.2 (1.0–1.7) | <0.001* |
| LDL (mmol/L) | 2.2 (1.6–2.8) | 2.0 (1.5–3.0) | 2.2±0.9 | 2.2±0.7 | 0.971 |
| HDL (mmol/L) | 1.1 (1.0–1.3) | 1.1 (1.0–1.4) | 1.1±0.2 | 1.2±0.3 | 0.051 |
| SCr (μmol/L) | 70.3 (60.5–79.0) | 68.5 (61.5–78.7) | 70.5±13.8 | 70.7±15.8 | 0.966 |
| Lymphocytes (109/L) | 1.6 (1.3–2.2) | 1.6 (1.3–1.8) | 2.4±0.8 | 1.3±0.5 | <0.001* |
| Monocyte (109/L) | 0.46 (0.35–0.60) | 0.38±0.12 | 0.47±0.12 | 0.66±0.30 | <0.001* |
| LMR | 3.5 (2.5–5.4) | 6.0 (5.3–7.6) | 3.5 (3.0–4.0) | 2.2 (1.8–2.5) | <0.001* |
AF – atrial fibrillation; CE – cardioembolism; CHD – coronary heart disease; FBG – fasting blood glucose; HDL – high-density lipoprotein; LAA – large-artery atherosclerosis; LDL – low-density lipoproteins; LMR – lymphocyte to monocyte ratio; NIHSS – National Institutes of Health Stroke Scale; SAO – small-artery occlusion; SCr – serum creatinine; SOE – stroke of other determined etiology; SUE – stroke of undetermined etiology; TC – total cholesterol; TG – triglyceride.
Indicates statistically significant difference of P<0.05;
indicates Fisher exact test.
Numbers in parentheses represent percentages or ranges.
Stroke severity, incidence of sICH, and 3-month prognosis
As shown in Table 2, patients with lower LMR values in group 3 had more moderate to severe strokes and higher mRS scores than those in the other 2 groups (P<0.05). Patients with higher LMR who had either moderate to severe stroke or mild stroke were more likely to suffer poor outcome (P<0.05). Moreover, compared with the other 2 groups, incidence of sICH after thrombolysis in group 3 also was remarkably higher (P=0.027). LMR value was inversely associated with the degree of neurologic impairment on admission (r=−0.372, P<0.001; Figure 1). However, a lower LMR was not an independent risk factor of stroke severity (P=0.443; Table 3). The ROC curve of LMR values showed that the area under the curve was 0.767 (95% CI 0.674-0.859, P<0.001) and the cut-off point of LMR was 3.48 with a sensitivity of 71.6% and a specificity of 80.5% (Figure 2). An evaluated LMR was independently associated with favorable outcome at 3 months (OR 0.683, 95% CI 0.490–0.952, P=0.024; Table 4).
Table 2.
Comparison of outcomes among subgroups based on LMR.
| Outcomes | Total | LMR on admission | P value | ||
|---|---|---|---|---|---|
| LMR higher than 4.34 (36, 33.3%) | LMR 2.79–4.34 (36, 33.3%) | LMR lower than 2.79 (36, 33.3%) | |||
| Moderate to severe stroke(NIHSS score >5) (%) | 62 (57.4) | 16 (44.4) | 18 (20.7) | 28 (77.8) | 0.009* |
| Poor outcome(mRS score ≥3) (%) | 41 (38.0) | 5 (13.9) | 14 (38.9) | 22 (61.1) | <0.001* |
| Moderate to severe stroke with poor outcome (%) | 33 (30.6) | 3 (8.3) | 12 (33.3) | 18 (54.5) | 0.004* |
| Mild stroke with poor outcome (%) | 8 (7.4) | 2 (5.6) | 2 (5.6) | 4 (11.1) | 0.015* |
| sICH after thrombolysis (%) | 11 (10.2) | 1 (2.8) | 2 (5.6) | 8 (22.2) | 0.027* |
ICH – intracranial hemorrhage; LMR – lymphocyte-to-monocyte ratio; mRS – modified Rankin Scale; NIHSS – National Institutes of Health Stroke Scale.
P<0.05.
Numbers in parentheses represent percentages.
Figure 1.
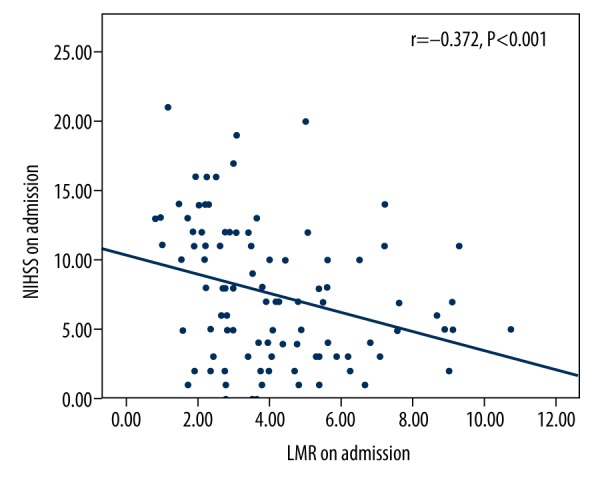
Correlation between lymphocyte-to-monocyte ratio (LMR) and NIHSS score. LMR – lymphocyte-to-monocyte ratio; NIHSS – National Institutes of Health Stroke Scale.
Table 3.
Binary logistic regression analysis predicting the stroke severity.
| Independent variable | Adjusted OR | 95% CI | P value |
|---|---|---|---|
| Age | 1.040 | 0.996–1.086 | 0.072 |
| Male | 0.697 | 0.166–2.933 | 0.623 |
| Admission to hospital | 1.236 | 0.667–2.293 | 0.501 |
| Hypertension | 1.968 | 0.715–5.414 | 0.190 |
| Diabetes | 1.157 | 0.182–7.360 | 0.877 |
| Hyperlipidemia | 0.427 | 0.134–1.355 | 0.148 |
| AF | 1.933 | 0.352–10.612 | 0.448 |
| CHD | 0.551 | 0.143–2.125 | 0.387 |
| Stroke history | 2.004 | 0.519–7.721 | 0.313 |
| Smoking | 0.582 | 0.173–1.964 | 0.383 |
| Alcohol drinking | 1.280 | 0.373–4.393 | 0.695 |
| LMR | 0.913 | 0.724–1.151 | 0.443 |
| FBG | 1.380 | 0.845–2.256 | 0.198 |
| TC | 1.454 | 0.602–3.509 | 0.405 |
| TG | 0.677 | 0.386–1.189 | 0.175 |
| LDL | 0.490 | 0.205–1.171 | 0.109 |
| HDL | 0.649 | 0.113–3.718 | 0.628 |
| SCr | 1.007 | 0.975–1.041 | 0.664 |
AF – atrial fibrillation; CHD – coronary heart disease; CI – confidence interval; FBG – fasting blood glucose; HDL – high-density lipoprotein; LDL – low-density lipoproteins; LMR – lymphocyte-to-monocyte ratio; NIHSS – National Institutes of Health Stroke Scale; OR – odds ratio; TC – total cholesterol; TG – triglyceride; SCr – serum creatinine.
Figure 2.
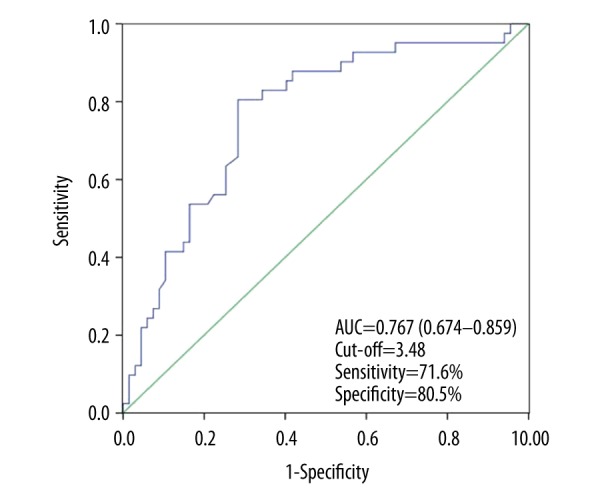
The receiver operating characteristic (ROC) curve of the lymphocyte-to-monocyte ratio (LMR) predicting values of 3-month functional outcome. AUC – area under the curve; CI – confidence interval.
Table 4.
Binary logistic regression analysis predicting outcome.
| Independent variable | Adjusted OR | 95% CI | P value |
|---|---|---|---|
| Age | 1.019 | 0.967–1.074 | 0.481 |
| NIHSS | 1.268 | 1.094–1.470 | 0.002* |
| Male | 0.718 | 0.120–4.289 | 0.623 |
| Admission to hospital | 1.236 | 0.667–2.293 | 0.636 |
| Hypertension | 0.829 | 0.261–2.633 | 0.750 |
| Diabetes | 0.874 | 0.124–6.166 | 0.892 |
| Hyperlipidemia | 0.488 | 0.117–2.162 | 0.325 |
| AF | 1.490 | 0.155–14.296 | 0.730 |
| CHD | 0.688 | 0.154–3.071 | 0.624 |
| Stroke history | 2.257 | 0.556–9.152 | 0.255 |
| Smoking | 0.330 | 0.066–1.639 | 0.175 |
| Alcohol drinking | 3.869 | 0.722–20.759 | 0.114 |
| LMR | 0.683 | 0.490–.952 | 0.024 * |
| FBG | 0.939 | 0.587–1.502 | 0.793 |
| TC | 2.081 | 0.771–5.613 | 0.148 |
| TG | 1.135 | 0.614–2.099 | 0.685 |
| LDL | 0.474 | 0.175–1.280 | 0.141 |
| HDL | 0.576 | 0.109–3.047 | 0.516 |
| SCr | 0.981 | 0.946–1.018 | 0.309 |
AF – atrial fibrillation; CHD – coronary heart disease; CI – confidence interval; FBG – fasting blood glucose; HDL – high-density lipoprotein; LDL – low-density lipoproteins; LMR – lymphocyte-to-monocyte ratio; NIHSS – National Institutes of Health Stroke Scale; OR – odds ratio; TC – total cholesterol; TG – triglyceride; SCr – serum creatinine.
P<0.05.
Discussion
Mounting evidence shows that IV rt-PA administration for AIS within the treatment time window improves neurological deficits and prognosis [17,18]. Given the increasing incidence and mortality of AIS and the heavier economic burden in China, clinical physicians and the government have paid great attention to these issues. The thrombolytic therapy for AIS was approved by the State Food and Drug Administration of China in 2001. However, only about 20% to 25% of patients with AIS were admitted to hospital within therapeutic window, and only about 2% of them agreed to be treated with thrombolysis, with a rate much lower than in developed countries [19]. Furthermore, considering the 6% fatality rate of patients with sICH after thrombolysis, some eligible patients might refuse thrombolytic therapy [20]. Therefore, it is of great importance to investigate the risk factors affecting the outcome of patients with AIS who received rt-PA administration.
Our study suggests that lower LMR is an independent risk factor for poor outcome of patients with AIS who underwent IV rt-PA administration. In addition, LMR values of 3.48 or lower are good predictors of poor prognosis. Our results show that LMR is an original marker to predict outcome after rt-PA treatment.
The post-stroke inflammatory reaction has been widely recognized to be involved in infarct evolution and to exacerbate the clinical outcome of patients with AIS [21]. As 2 major participants in the inflammation response, lymphocytes and monocytes have been considered to aggravate a complicated situation that results in secondary brain lesion after stroke onset [22]. Based on the difference in process of immune response, lymphocytes are classified into B lymphocytes and T lymphocytes, and T cells rather than B cells are supposed to be pernicious in the evolvement of post-stroke brain injury [23]. The helper T (Th) cells are renowned as a main subtype of T cells and the function of Th1 cells and Th2 cells, subsets of Th cells, have been well defined [24]. Th1 cells play an stimulating inflammatory response role following stroke, with secreting pro-inflammatory cytokines including interferon-γ (IFN-γ), and tumor necrosis factor (TNF)-β, whereas Th2 cells have an inhibitory effect on inflammation response via producing anti-inflammatory cytokines like interleukin (IL)- 4, IL-5, IL-10, and IL-13 [25]. The information described above resolves the controversial issue of whether a lower lymphocyte count is independently associated with poor outcome at 3 months [7,26]. Following stroke, patients may have lymphopenia or a reduction in absolute lymphocyte count, a phenomenon of immunodepression, which might due to activation of the hypothalamic-pituitary-adrenal system, sympathetic nervous system, and parasympathetic nervous system, with the secretion and release of cortisol, catecholamines, and acetylcholine, contributing to lymphocyte apoptosis [27]. Our study found that patients in group 3 with decreased lymphocyte count were more likely to have poor outcome, which suggests that a lower lymphocyte count is correlated with adverse prognosis of patients undergoing IV rt-PA administration.
Monocytes, another important immunoregulator driving the post-ischemia inflammatory response, infiltrate the infarction zone and aggravate brain damage [28]. Traditionally, monocytes are classified into 3 subpopulations in humans according to the expressions of cell-surface markers CD14 and CD16 [29]. CD14highCD16− monocytes, defined as classical monocytes, mainly secret TNF-α, IL-6, and IL-1β to exert a pro-inflammatory effect on brain lesions after stroke onset [30]. Recent studies demonstrated that classical monocytes were increased in the acute phase of stroke and are independently associated with poor outcome of AIS [31,32]. CD14highCD16+ monocytes, regarded as intermediate monocytes, also grew and were reported to up-regulate the expression of inducible nitric oxide synthase, leading to secondary brain damage [31]. On the contrary, CD14dimCD16+ monocytes, also known as non-classical monocytes, decreased with the low expression of IL-10, and play an pivotal role in the development of anti-inflammation [33]. In view of these findings, a higher monocyte count might be associated with adverse prognosis of AIS, which was confirmed by a recent study [5]. In agreement with these previous finding, univariate analysis performed in the present study shows that patients with higher monocyte counts treated by thrombolytic therapy are more likely to have a poor outcome.
Therefore, a lower LMR value may be regarded as a novel hallmark to predict the clinical outcome of AIS treated with rt-PA administration. The ROC curve indicated that the area under the ROC curve was 0.767, and LMR lower than the cut-off value of 3.48 had a sensitivity of 71.6% and a specificity of 80.5%, which indicates ideal accuracy for prediction of outcome. In addition, although univariate analysis and the Spearman rank correlation suggested LMR values developed a negative correlation with stroke severity, a lower LMR was not an independent risk factor for neurologic impairment, inconsistent with our previous finding, which probably was due to a small study sample size.
Surprisingly, univariate analysis showed that patients with decreased LMR had increasing incidence of sICH after thrombolysis. Hemorrhagic transformation (HT) after IV rt-PA administration is mainly due to breakdown of the blood-brain barrier (BBB) [34]. Several pro-inflammatory cytokines, such as TNF-α, IL-6, and IL-1β, secreted by classical monocytes, had been identified as risk factors for BBB disruption [35]. Among these cytokines, TNF-α was viewed as a vital regulator stimulating the expression of the matrix metalloproteinases (MMPs), which are associated with BBB disruption [35]. IFN-γ also had been reported to damage the tight junction with relocalization of zonula occludens protein-1 and occluding protein [36]. TNF-β, another major pro-inflammatory cytokine produced by Th1 cells, was recently reported to be involved in down-regulating N-cadherin expression and extracellular matrix remodeling, which resulted in HT [37]. However, given the reduction in lymphocytes after stroke, sICH following thrombolytic treatment might be mainly attributed to a higher monocyte count and the relevant cytokines.
The present study has some limitations. First, it is a retrospective study conducted at a single center, the sample size was small, and we did not set up a control group for investigating the possible difference in the association between presence and absence of treatment with rt-PA. Second, not all patients had inflammatory cytokines laboratory results, which could further bias our findings. Third, as LMR is a floating index influenced by multiple factors, we simply recorded the LMR on admission and did not monitor LMR during the period of admission or at discharge. Finally, we just focused on the relatively early outcome.
Conclusions
The present study is the first to show that a decreased LMR value is an independent predictor of poor prognosis of AIS with thrombolytic treatment. Further studies with larger cohorts are required to verify our findings.
Acknowledgements
We are grateful to all patients and their relatives for their cooperation and support.
Footnotes
Source of support: None
Conflict of interest
None.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics – 2016 update: A report from the American Heart Association. Circulation. 2016;133:e38–360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Gan Y, Wu J, Zhang S, et al. Prevalence and risk factors associated with stroke in middle-aged and older Chinese: A community-based cross-sectional study. Sci Rep. 2017;7:9501. doi: 10.1038/s41598-017-09849-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Powers WJ, Derdeyn CP, Biller J, et al. 2015 American Heart Association/American Stroke Association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46:3020–35. doi: 10.1161/STR.0000000000000074. [DOI] [PubMed] [Google Scholar]
- 4.Rodrigues SF, Granger DN. Leukocyte-mediated tissue injury in ischemic stroke. Curr Med Chem. 2014;21:2130–37. doi: 10.2174/0929867321666131228192119. [DOI] [PubMed] [Google Scholar]
- 5.Liberale L, Montecucco F, Bonaventura A, et al. Monocyte count at onset predicts post-stroke outcomes during a 90-day follow up. Eur J Clin Invest. 2017;47(10):702–10. doi: 10.1111/eci.12795. [DOI] [PubMed] [Google Scholar]
- 6.Pagram H, Bivard A, Lincz LF, et al. Peripheral immune cell counts and advanced imaging as biomarkers of stroke outcome. Cerebrovasc Dis Extra. 2016;6:120–28. doi: 10.1159/000450620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim J, Song TJ, Park JH, et al. Different prognostic value of white blood cell subtypes in patients with acute cerebral infarction. Atherosclerosis. 2012;222:464–67. doi: 10.1016/j.atherosclerosis.2012.02.042. [DOI] [PubMed] [Google Scholar]
- 8.Qun S, Tang Y, Sun J, et al. Neutrophil-to-lymphocyte ratio predicts 3-month outcome of acute ischemic stroke. Neurotox Res. 2017;31:444–52. doi: 10.1007/s12640-017-9707-z. [DOI] [PubMed] [Google Scholar]
- 9.Maestrini I, Strbian D, Gautier S, et al. Higher neutrophil counts before thrombolysis for cerebral ischemia predict worse outcomes. Neurology. 2015;85:1408–16. doi: 10.1212/WNL.0000000000002029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishijima TF, Muss HB, Shachar SS, et al. Prognostic value of lymphocyte-to-monocyte ratio in patients with solid tumors: A systematic review and meta-analysis. Cancer Treat Rev. 2015;41:971–78. doi: 10.1016/j.ctrv.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Ji H, Li Y, Fan Z, et al. Monocyte/lymphocyte ratio predicts the severity of coronary artery disease: A syntax score assessment. BMC Cardiovasc Disord. 2017;17:90. doi: 10.1186/s12872-017-0507-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ren H, Liu X, Wang L, et al. Lymphocyte-to-monocyte ratio: A novel predictor of the prognosis of acute ischemic stroke. J Stroke Cerebrovasc Dis. 2017;26:2595–602. doi: 10.1016/j.jstrokecerebrovasdis.2017.06.019. [DOI] [PubMed] [Google Scholar]
- 13.Adams HP, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 14.Lees K, Bluhmki E, von Kummer R, et al. Time to treatment with intravenous alteplase and outcome in stroke: An updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375:1695–703. doi: 10.1016/S0140-6736(10)60491-6. [DOI] [PubMed] [Google Scholar]
- 15.Kim SH, Lee JY, Kim DH, et al. Factors related to the initial stroke severity of posterior circulation ischemic stroke. Cerebrovasc Dis. 2013;36:62–68. doi: 10.1159/000351512. [DOI] [PubMed] [Google Scholar]
- 16.Wu D, Lyu Y, Zhong P, et al. Human urinary kallidinogenase promotes good recovery in ischemic stroke patients with level 3 hypertension. Brain Behav. 2017;7:e00752. doi: 10.1002/brb3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wardlaw J, Murray V, Berge E, et al. Thrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev. 2009;(4):CD000213. doi: 10.1002/14651858.CD000213.pub2. [DOI] [PubMed] [Google Scholar]
- 18.Lees K, Bluhmki E, von Kummer R, et al. Time to treatment with intravenous alteplase and outcome. Lancet. 2010;375:1695–703. doi: 10.1016/S0140-6736(10)60491-6. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Liao X, Zhao X, et al. Using recombinant tissue plasminogen activator to treat acute ischemic stroke in China: Analysis of the results from the Chinese National Stroke Registry (CNSR) Stroke. 2011;42:1658–64. doi: 10.1161/STROKEAHA.110.604249. [DOI] [PubMed] [Google Scholar]
- 20.Xu J, Zhang Y, Wei H, et al. A comparison of rt-PA thrombolysis guidelines between China and the USA: Are changes needed? Neurol Res. 2015;37:57–63. doi: 10.1179/1743132814Y.0000000415. [DOI] [PubMed] [Google Scholar]
- 21.Lambertsen KL, Biber K, Finsen B. Inflammatory cytokines in experimental and human stroke. J Cereb Blood Flow Metab. 2012;32:1677–98. doi: 10.1038/jcbfm.2012.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murray KN, Buggey HF, Denes A, et al. Systemic immune activation shapes stroke outcome. Mol Cell Neurosci. 2013;53:14–25. doi: 10.1016/j.mcn.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Hurn PD, Subramanian S, Parker SM, et al. T- and B-cell-deficient mice with experimental stroke have reduced lesion size and inflammation. J Cereb Blood Flow Metab. 2007;27:1798–805. doi: 10.1038/sj.jcbfm.9600482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gu L, Xiong X, Zhang H, et al. Distinctive effects of T cell subsets in neuronal injury induced by cocultured splenocytes in vitro and by in vivo stroke in mice. Stroke. 2012;43:1941–46. doi: 10.1161/STROKEAHA.112.656611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ono C, Yu Z, Kasahara Y, et al. Fluorescently activated cell sorting followed by microarray profiling of helper T cell subtypes from human peripheral blood. PLoS One. 2014;9:e111405. doi: 10.1371/journal.pone.0111405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma D, Feng L, Deng F, et al. Overview of experimental and clinical findings regarding the neuroprotective effects of cerebral ischemic postconditioning. Biomed Res Int. 2017;2017:6891645. doi: 10.1155/2017/6891645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gu L, Jian Z, Stary C, et al. T Cells and cerebral ischemic stroke. Neurochem Res. 2015;40:1786–91. doi: 10.1007/s11064-015-1676-0. [DOI] [PubMed] [Google Scholar]
- 28.Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: Role of inflammatory cells. J Leukoc Biol. 2010;87:779–89. doi: 10.1189/jlb.1109766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ziegler-Heitbrock L, Ancuta P, Crowe S, et al. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116:e74–80. doi: 10.1182/blood-2010-02-258558. [DOI] [PubMed] [Google Scholar]
- 30.Boyette LB, Macedo C, Hadi K, et al. Phenotype, function, and differentiation potential of human monocyte subsets. PLoS One. 2017;12:e0176460. doi: 10.1371/journal.pone.0176460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaito M, Araya S, Gondo Y, et al. Relevance of distinct monocyte subsets to clinical course of ischemic stroke patients. PLoS One. 2013;8:e69409. doi: 10.1371/journal.pone.0069409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Urra X, Villamor N, Amaro S, et al. Monocyte subtypes predict clinical course and prognosis in human stroke. J Cereb Blood Flow Metab. 2009;29:994–1002. doi: 10.1038/jcbfm.2009.25. [DOI] [PubMed] [Google Scholar]
- 33.Jin R, Liu L, Zhang S, et al. Role of inflammation and its mediators in acute ischemic stroke. J Cardiovasc Transl Res. 2013;6:834–51. doi: 10.1007/s12265-013-9508-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang W, Li M, Chen Q, et al. Hemorrhagic transformation after tissue plasminogen activator reperfusion therapy for ischemic stroke: Mechanisms, models, and biomarkers. Mol Neurobiol. 2015;52:1572–79. doi: 10.1007/s12035-014-8952-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Enciu AM, Gherghiceanu M, Popescu BO. Triggers and effectors of oxidative stress at blood-brain barrier level: Relevance for brain ageing and neurodegeneration. Oxid Med Cell Longev. 2013;2013:297512. doi: 10.1155/2013/297512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Capaldo CT, Nusrat A. Cytokine regulation of tight junctions. Biochim Biophys Acta. 2009;1788:864–71. doi: 10.1016/j.bbamem.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jickling GC, Liu D, Stamova B, et al. Hemorrhagic transformation after ischemic stroke in animals and humans. J Cereb Blood Flow Metab. 2014;34:185–99. doi: 10.1038/jcbfm.2013.203. [DOI] [PMC free article] [PubMed] [Google Scholar]


