Abstract
Background
Mesial temporal epilepsy (MTLE) is the most common type of focal epilepsy in adults, and is often drug-resistant. This study investigated the effects of aquaporins (AQP) inhibitor on multi-drug-resistant protein expression in an MTLE rat model.
Material/Methods
The MTLE rat model was established by injecting pilocarpine into rats. The MTLE rats were divided into an MTLE-6 h group, an MTLE-12 h group, and an MTLE-24 h group, together with a normal saline group (NS), to examine the AQP4 expression by using Western blot assay and immunohistochemistry assay. The other 18 MTLE model rats were used to observe the effects of the AQP4 inhibitor, acetazolamide, on the multi-drug-resistant protein 1 (MRP1) and P-glycoprotein (Pgp) by using Western blot and immunohistochemistry assays, respectively.
Results
AQP4 expression was enhanced in hippocampal tissues of MTLE model rats compared to NS rats (P<0.05). More positively stained AQP4 was discovered in hippocampal tissues of MTLE model rats. AQP4 inhibitor significantly decreased multi-drug-resistant protein MRP1 and Pgp expression in the AQP4 inhibitor Interfere group and the AQP4 inhibitor Therapy group compared to the TMLE model group (P<0.05).
Conclusions
The present findings confirm that the AQP4 inhibitor, acetazolamide, effectively inhibits the multi-drug-resistant protein, MRP1, and Pgp, in the MTLE rat model.
MeSH Keywords: Acetazolamide; Aquaporins; Epilepsy, Temporal Lobe; Tuberculosis, Multidrug-Resistant
Background
In clinical practice, focal epilepsy is considered as an acquired disease secondary to partial lesions, including trauma, hippocampal sclerosis, tumors, malformations, and vascular malformations of cortical development [1]. According to previous studies [2,3], there are 2 main forms of focal epilepsy: mesial temporal epilepsy (MTLE) and extratemporal epilepsy. MTLE is the most common type of focal epilepsy in adults, and it is drug-resistant partial epilepsy in approximately 60% of adults [4]. Extratemporal epilepsy is also a common form of focal epilepsy, and is also treatment-resistant [5]. However, MTLE has become refractory to medical therapy in approximately 60% of patients. Few studies have investigated the pathogenic mechanism of MTLE. Therefore, we performed a preliminary study of MTLE-associated mechanisms.
Previous studies reported that MTLE always results from the damaged or impaired hippocampal network induced by a few initial brain insults [4,6,7]. There are a series of processes mediating the epilepsy-induced changes of hippocampal physiology [8–10]. Generally, a prolonged generalized seizure induces neuron injury or neuron death in brain tissues, whereas a generalized seizure damages the hippocampus and impairs memory. Baik et al. [11] reported that neuronal loss and damage in the temporal lobe and hippocampus play important roles in the long-term sequela of recurrent seizures, which has been proved to be related to the progression of MTLE. Kahane and Bartolomei [12] found that seizures derive from a series of multi-structural epileptogenic zones, including the amygdala, entorhinal cortex, and hippocampus, and limbic structures are altered in the temporal lobe.
According to the above descriptions, MTLE mainly damages the hippocampus, and some MTLE is drug-resistant in clinical practice. Therefore, discovering an anti-drug-resistant and hippocampus-protective medicine is critical for MTLE therapy. In recent years, many multi-drug transporters have been discovered, among which the family of multi-drug resistance-related proteins (MPRs) and P-glycoprotein (Pgp) are the most frequently studied [13,14]. The overexpression or up-regulation of the MPRs, including MPR1 and MPR2, have recently been reported in many human refractory epilepsy patients [15]. Tishler et al. [16] found that hippocampal sclerosis-related epilepsy mainly involves the overexpression of MRP1 and Pgp protein in a few regions of the hippocampus.
Aquaporins (AQP) are a series of specific water channels, among which AQP4 is the most important member of the AQP family [17]. AQP4 mainly is located in brain tissues; it plays a critical role in regulating water transportation, and participates in central nervous system (CNS) physiological processes [18–20]. Previous studies also showed that the dysregulation of AQP4 expression is correlated with a few forms of epilepsy [21–23]. Song et al. [24] proved that the inhibition of AQP4 expression relieves symptoms of epilepsy. The AQP4 plays a critical role in the activation of astrocytes [25], which can also up-regulate the expression of resistance-related proteins MRP1 and P-gp [26]. Therefore, we speculated that AQP4 participates in the occurrence and development of MTLE, which may be of clinical importance for treating multi-drug-resistant MTLE. Many AQP4 inhibitors have recently been discovered, including acetazolamide, sumatriptan, and rizatriptan. Recent studies [27,28] reported that acetazolamide is an effective inhibitor. The AQP4 inhibitors are important factors regulating AQP4 expression. Therefore, we speculated that the investigation of AQP4 inhibitors regulating AQP4 expression may help to develop a novel drug for treatment of MTLE.
We observed the expression of AQP4 in rats undergoing treatment with acetazolamide in brain tissues of an established MTLE rat model. Then, the MTLE model rats were injected with the AQP4 inhibitor to regulate AQP4 expression and to evaluate its effects on the expression of multi-drug-resistance-associated proteins in MTLE model rats.
Material and Methods
Animals and MTLE
Thirty-two Sprague-Dawley (SD) rats (6–8 weeks old) weighing 220–250 g were purchased from the Animal Center of Nanjing Medical University. The SD rats were maintained under the conditions of 24–25°C, 50–60% humidity, and light/dark cycle of 12/12 h, with free access to rat chow and water. All animal experiments in this study were approved by the Ethics Committee of Nanjing Medical University, Nanjing, China.
Model establishment and trial grouping
The SD rats were divided into a normal saline group (NS, n=8) and an experimental group (n=24). In the experimental group, the SD rats were pre-treated with the lithium chloride (125 mg/kg, i.p.) to enhance the effects of the pilocarpine. At 18–20 h later, scopolamine (1 mg/kg, i.p.) was injected into the rats to decrease the peripheral effects of the pilocarpine injection. Then, 30 min after the scopolamine injection, pilocarpine was administered i.p. at a dose of 10 mg/kg every 30 min until the occurrence of MTLE. The SD rats were observed and monitored for seizure behaviors at least for 3 h. Seizures were scored and classified according to the Racine score [29]. Criteria for determining the successful establishment of the MTLE model were continuous limbic seizures with scores of 3–5 and lasting for at least 1 h. After 60–90 min of continuous seizures, 10% chloral hydrate (0.3 ml/100 g) was injected to terminate the continuous seizures.
To study changes in MTLE mode at different time points, the experimental group was divided into 3 groups: seizures for 6 h (MTLE-6h, n=8), 12 h (MTLE-12h, n=8), and 24 h (MTLE-24h, n=8).
Acetazolamide therapy trial and grouping
To observe the effects of acetazolamide on the MTLE model rats, acetazolamide was applied. Firstly, the MTLE rat model was established as detailed above and the MTLE model was successfully established in 18 rats. The MTLE model rats were divided into 3 groups: the MTLE model group (injected with 35 mg/kg saline, n=6), the acetazolamide interfered group (Interfere group, injected with acetazolamide 35 mg/kg for 7 continuous days, n=6), and the acetazolamide therapy group (Therapy group, injected with acetazolamide 35 mg/kg per day for 28 continuous days, n=6). The normal saline-treated MTLE group (NS group, injected with 35 mg/kg saline, n=6) was defined as the normal control group.
Sample preparation
The MTLE model and NC rats were anesthetized using 10% chloral hydrate (0.3 ml/100 g) and decapitated. The hippocampus tissues were isolated and fixed with 4% paraformaldehyde, and sliced into the DG, CA1, and CA3 regions. Finally, the hippocampus was stored at −80°C. A portion of the fixed hippocampus tissue (n=4 for every group) was cut into a series of 4-μm-thick horizontal sections for the following immunohistochemistry assays.
Western blot assay
The hippocampal tissues in DG, CA1, and CA3 regions were lysed by using a whole-protein extraction kit (catalog No. KGP250; KeyGen BioTech., Jiangsu, China), and centrifugated at 10 000×g for 30 min. Then, the supernatant (50 μg) was separated using an SDS-PAGE synthesis kit (catalog No. KGP113; KeyGen BioTech., Jiangsu, China), and then transferred onto PVDF membranes (catalog No. KGP1201; KeyGen BioTech., Jiangsu, China) by using a Trans-Blot SD cell instrument (Bio-Rad, USA). The PVDF membrane was blocked using 5% defatted milk at room temperature for 1.5–2 h, followed by rabbit anti-rat AQP4 polyclonal antibody (catalog No. sc-20812; Santa Cruz Biotech, Santa Cruz, CA, USA), rabbit anti-rat GAPDH polyclonal antibody (catalog No. KGAA002; KeyGen BioTech., Jiangsu, China), rabbit anti-rat MRP1 polyclonal antibody (catalog No. BA0567; Boster Biol., Wuhan, China), and rabbit anti-rat P-glycoprotein 1 polyclonal antibody (catalog No. PB0162; Boster Biol., Wuhan, China) at 4°C overnight. Membranes were washed 3 times using PBST buffer for 5 min each time. Membranes were incubated with goat anti-rabbit HRP-labeled IgG (catalog No. KGAA35; KeyGen BioTech., Jiangsu, China) at room temperature for 1 h. Finally, the ECL reagent (catalog No. KGP1123; KeyGen BioTech., Jiangsu, China) was used to treat the membrane for 2 min in the dark. The relative grey density of the bands was observed using the G: BOX Chemi XR 5 gel imaging system (Model: GBCXR50313; Syngene Frederick, MD, USA) and analyzed using Gel-Pro32 software (version: 4.0; Media Cybernetics Inc., Rockville, MD, USA).
Immunohistochemistry assay and image analysis
The paraformaldehyde-fixed sections were embedded in paraffin, and sectioned into 4-μm-thick sections. The paraffin was removed by using the regular method [30], and the sections were used for immunohistochemical analysis. The sections were blocked with 5% BSA for 20 min, and incubated with rabbit anti-rat AQP4, GAPDH, MRP1, and P-glycoprotein 1 polyclonal antibody at 4°C overnight. Then, slices were incubated with goat anti-rabbit HRP-labeled IgG at room temperature for 1 h. The immunohistochemistry was conducted using a commercial SP immunohistochemistry kit (KeyGen BioTech., Jiangsu, China). Finally, the sections were immersed in alkaline phosphatase-labeled diaminobenzidine (DAB, KeyGen BioTech., Jiangsu, China), and the images were captured using a biological inverted fluorescence microscope (Model: IX51; Olympus, Japan).
The brown granules in the immunohistochemistry images were considered as the AQP4-, MRP1-, and P-glycoprotein 1-positively stained cells. Image Pro Plus 6.0 software (Media Cybernetics, Inc., Bethesda, MD, USA) was used to quantitatively analyze the images. The images were captured at the amplification of 400×, and 5 visual fields were selected randomly for every section. Then, the sections were stained with Ponceau-Victoria blue B, and the integrated optical density (IOD) values of AQP4, MRP1, and P-glycoprotein 1 were evaluated. The mean optical density of 5 visual fields was calculated by the following formula: IOD Sum (total IOD in 5 visual fields)/5 (visual fields).
Carbonic anhydrase levels measurement
We excluded the inhibitive effects of acetazolamide (carbonic anhydrase inhibitor) on the carbonic anhydrase levels in rats. Serum carbonic anhydrase levels were evaluated using a specific ELISA kit (B&D Systems, Minneapolis, MN, USA). In brief, the rabbit anti-carbonic anhydrase antibody was pre-coated onto the micro-titer wells. Aliquots of the serum were added to every well, followed by peroxidase-conjugated antibody to carbonic anhydrase. The color was developed with the hydrogen peroxide and tetramethylbenzidine peroxidase and the absorbance at 450 nm was measured. Wavelength correction was also performed by using the absorbance at 570 nm. Finally, the concentration of carbonic anhydrase was evaluated by interpolation from the standard curve.
Statistical analysis
All data were analyzed with SPSS software 19.0 (SPSS Inc., Chicago, IL, USA). Data are described as mean ±SD of at least 3 independent experiments. The t test was used for statistical comparison between 2 groups, and Tukey’s post hoc test was used for multiple-group comparisons. P value less than 0.05 was regarded as a significant difference.
Results
AQP4 expression was enhanced in hippocampal tissues of MTLE model rats
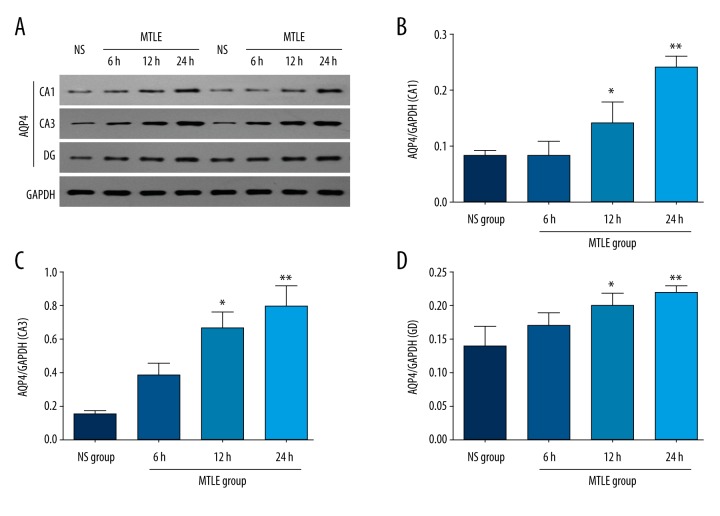
We examined AQP4 expression in DG, CA1, and CA3 regions of the hippocampus by Western blot assay. The results indicated that the AQP4 expression was obviously enhanced in the MTLE model group compared to the NS group at both 12 h and 24 h after the occurrence of seizures (Figure 1A). According to the results of the different hippocampal regions, AQP4 was significantly enhanced in the CA1 region (Figure 1B), CA3 region (Figure 1C), and DG (Figure 1D) of MTLE model rats compared to the NS group at both 12 h (P<0.05) and 24 h (P<0.01) after the occurrence of seizures.
Figure 1.
Observation for the AQP4 expression in MTLE model rats at 6 h, 12 h, and 24 h after the seizure. (A) Western blot bands for AQP4 expression in each group. (B) Statistical analysis for AQP4 expression in CA1 region. (C) Statistical analysis for AQP4 expression in CA3 region. (D) Statistical analysis for AQP4 expression in DG region. * P<0.05 and ** P<0.01 represent the AQP4 levels in MTLE model rats at 12 h and 24 h compared to the NS group.
More positively stained AQP4 was discovered in hippocampal tissues in MTLE model rats
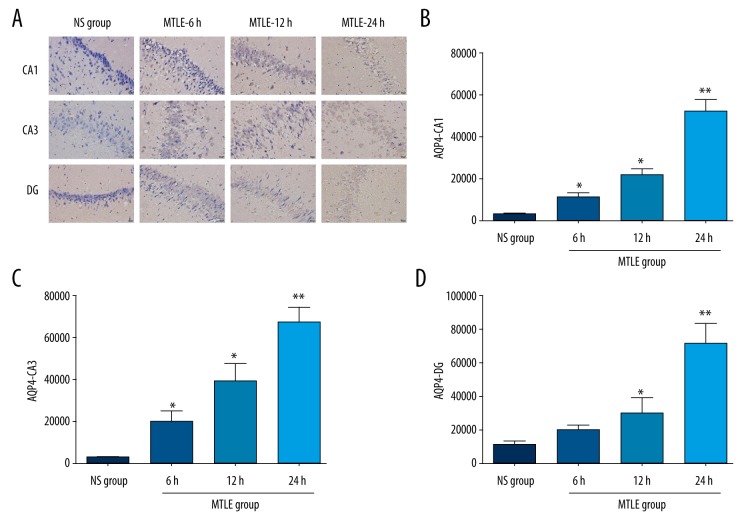
To observe the distribution of APQ4 protein in different regions of the hippocampus, we used immunohistochemistry assay. The results showed that the distribution of positively stained AQP4 protein in the MTLE group was obviously increased compared to the NS group (Figure 2A). Based on the statistical analysis, the positively stained AQP4 was significantly increased in the CA1 region (Figure 2B), CA3 region (Figure 2C), and DG (Figure 2D) of MTLE model rats compared to the NS group (P<0.05).
Figure 2.
AQP4 expression examined by using immunohistochemistry assay in MTLE model rats at 6 h, 12 h, and 24 h after the seizure. (A) Immunohistochemistry assay images for AQP4 staining. (B) Statistical analysis for AQP4 staining in CA1 region. (C) Statistical analysis for AQP4 staining in CA3 region. (D) Statistical analysis for AQP4 staining in DG region. * P<0.05 and ** P<0.01 represent the AQP4 staining in MTLE model rats at 6 h, 12 h, and 24 h compared to the NS group.
AQP4 inhibitor decreases multi-drug-resistant protein MRP1
First, we evaluated the inhibitive effects of acetazolamide (carbonic anhydrase inhibitor) on serum carbonic anhydrase levels in rats. The results indicated that there were no significant differences among the 4 groups (Supplementary Figure 1). Therefore, we used acetazolamide in the following experiments.
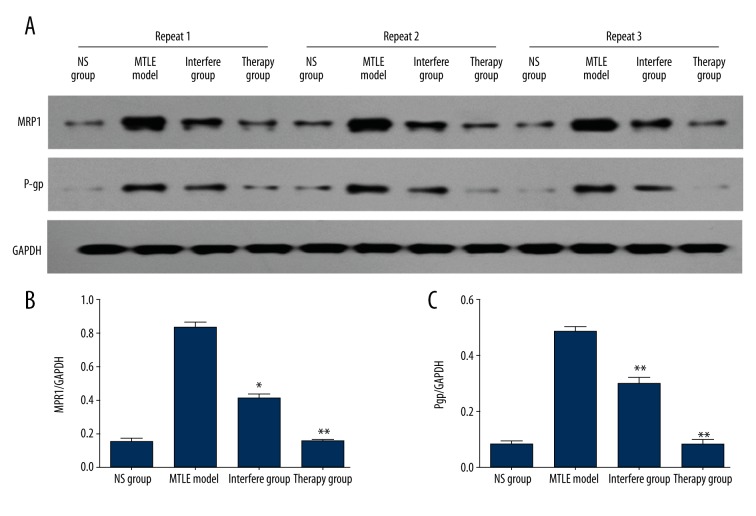
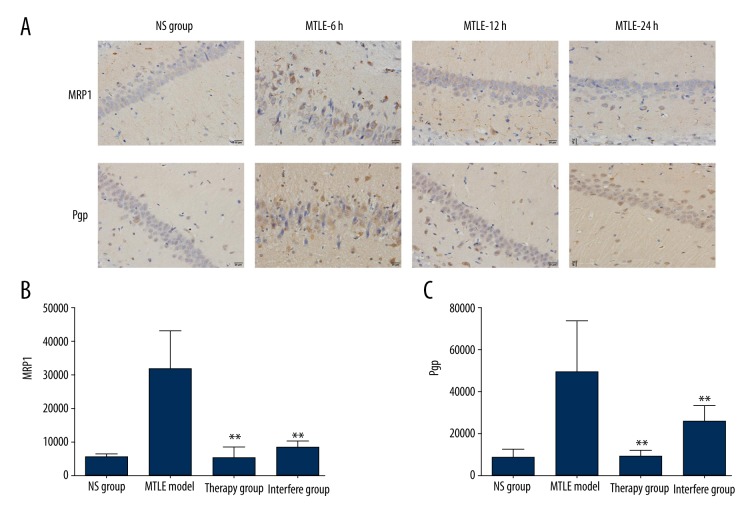
In this study, we injected the AQP4 inhibitor, acetazolamide, into the MTLE model rats. Western blot assay (Figure 3A) results indicated that MRP1 expression was significantly decreased in the Interfere group (P<0.05) and Therapy group (P<0.01) compared to the NS group (Figure 3B). The immunohistochemistry assay (Figure 4A) also showed that the positively stained MRP1 was also obviously decreased in the Interfere group and Therapy group. Statistical analysis showed significantly different levels of positively stained MRP1 in the Interfere group (P<0.01) and Therapy group (P<0.01) compared to the NS group (Figure 4B).
Figure 3.
Multi-drug-resistant protein, MRP1 and Pgp, observation in AQP4 inhibitor-treated MTLE rats. (A) Western blot bands for the MRP1 and Pgp expression in each group. (B) Statistical analysis for MRP1 expression. (C) Statistical analysis for Pgp expression. * P<0.05 and ** P<0.01 represent the MRP1 or Pgp expression in the Interfere group or Therapy group compared to the MTLE rat model.
Figure 4.
Immunohistochemistry assay examination for MRP1 and Pgp expression in AQP4-treated MTLE rats. (A) Immunohistochemistry assay images for MRP1 and Pgp staining. (B) Statistical analysis for MRP1 staining. (C) Statistical analysis for Pgp staining. * P<0.05 and ** P<0.01 represent the MRP1 or Pgp staining in the Interfere group or Therapy group compared to the MTLE rat model.
AQP4 inhibitor inhibits multi-drug-resistant protein Pgp expression
The other multi-drug-resistant protein, Pgp, was also evaluated. The results indicated that Pgp expression was significantly inhibited during treatment with AQP4 inhibitor (acetazolamide, both Interfere group and Therapy group) compared to the NS group, according to Western blot results (Figure 3C, P<0.01). Moreover, the immunohistochemistry assay also proved that Pgp expression was decreased in the Interfere group (P<0.01) and Therapy group (P<0.01) compared to the NS group (Figure 4C).
Discussion
Many studies [31–33] have confirmed that multi-drug-resistant proteins are extensively over-expressed during epilepto-genesis, both in the MTLE model and in epilepsy patients, especially in hippocampal brain tissues. Therefore, the inhibition of multi-drug-resistant proteins in the MTLE model or epilepsy patients may enhance the therapeutic effect of anti-epilepsy drugs, which is a critical strategy for the development of drugs for treatment of epilepsy.
AQP4 is mainly expressed in the hippocampal brain tissues, which includes M1 and M23 subtypes [17,34]. AQP4 mediates transportation of water in brain tissues, which maintains physiological functions of the CNS. A previous study [35] reported that AQP4 expression is increased during the process of epilepsy, and is considered as an inducer of cytotoxic cerebral edema in epilepsy. Therefore, we believe that the AQP4 protein plays a protective function in preserving homeostasis in the CNS. The epileptic rat model induced by lithium chloride combined with pilocarpine could be considered as the pathological model to investigate the effects of AQP4 inhibitor on AQP4 expression and regulation of multi-drug-resistance proteins. In our study, AQP4 levels were significantly increased in the MTLE rat model compared to the normal rats, which is consistent with previous studies reported [36,37]. Therefore, epilepsy may be associated with the AQP4-mediated water transportation in the MTLE model. Previous studies [38,39] proved that deficiency or down-regulation of AQP4 expression relieves cerebral edema and improves conditions of local cerebral ischemia caused by toxic water molecule accumulation. Therefore, we applied the AQP4 inhibitor to the MTLE models to suppress the expression of AQP4, and observed the related mechanism for the protective role of AQP4 inhibitor in the MTLE rat model.
In this study, we injected the AQP4 inhibitor, acetazolamide, into the MTLE model rats, which has been used as the antagonist to AQP4 in a few reports [28,40,41]. Our results indicate that acetazolamide significantly inhibits the multi-drug-resistant protein, MRP1, which was first reported in the MTLE rat model. Sun et al. [42] reported that the inhibition of MRP1 enhanced the efficacy of antiepileptic drugs (AEDs) through enhancing local drug availability. Chen et al. [43] also found that the up-regulation of MRP1 protein plays an important role in drug resistance of brain cells to AEDs in the amygdale of kindling rats. Moreover, another multi-drug-resistant protein, Pgp [44,45], has also been evaluated in the MTLE rat model. These findings show that acetazolamide significantly suppresses Pgp expression in the MTLE rat model.
Although our study had some interesting results, there were also limitations. Firstly, acetazolamide is also a carbonic anhydrase inhibitor. Although our results illustrated no effects of acetazolamide on carbonic anhydrase due to the low dose, the AQP4-specific inhibitor would be better compared to acetazolamide. Secondly, the present study does not clarify the effects of acetazolamide on the pathology of MTLE and disease progression, and we did not observe the therapeutic effects of acetazolamide.
Conclusions
The AQP4 inhibitor, acetazolamide, could effectively inhibit the multi-drug-resistant proteins, MRP1 and Pgp, in the MTLE rat model. However, the specific mechanism for the AQP4 inhibitor triggered MRP1 and Pgp down-regulation has not been clarified, which would be explored in the future study.
Supplementary Figure
Serum concentrations of carbonic anhydrase in rats of each group. The carbonic anhydrase levels were measured by using an ELISA kit as described in the “Materials and Methods” section.
Footnotes
Source of support: Departmental sources
Conflict of interests
None.
References
- 1.Nguyen DK, Rouleau I, Senechal G, et al. X-linked focal epilepsy with reflex bathing seizures: Characterization of a distinct epileptic syndrome. Epilepsia. 2015;56:1098–108. doi: 10.1111/epi.13042. [DOI] [PubMed] [Google Scholar]
- 2.Pedersen M, Omidvarnia AH, Walz JM, et al. Increased segregation of brain networks in focal epilepsy: An fMRI graph theory finding. Neuroimage Clin. 2015;8:536–42. doi: 10.1016/j.nicl.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee EM, Park GY, Im KC, et al. Changes in glucose metabolism and metabolites during the epileptogenic process in the lithium-pilocarpine model of epilepsy. Epilepsia. 2012;53:860–69. doi: 10.1111/j.1528-1167.2012.03432.x. [DOI] [PubMed] [Google Scholar]
- 4.Soussi R, Boulland JL, Bassot E, et al. Reorganization of supramammillary-hippocampal pathways in the rat pilocarpine model of temporal lobe epilepsy: Evidence for axon terminal sprouting. Brain Struct Funct. 2015;220:2449–68. doi: 10.1007/s00429-014-0800-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kutsy RL. Focal extratemporal epilepsy: Clinical features, EEG patterns, and surgical approach. J Neurol Sci. 1999;166:1–15. doi: 10.1016/s0022-510x(99)00107-0. [DOI] [PubMed] [Google Scholar]
- 6.Wieser HG. ILAE commission report. Mesial temporal lobe epilepsy with hippocampal sclerosis. Epilspsia. 2004;45:695–714. doi: 10.1111/j.0013-9580.2004.09004.x. [DOI] [PubMed] [Google Scholar]
- 7.Jin SH, Jeong W, Chung CK. Mesial temporal lobe epilepsy with hippocampal sclerosis is a network disorder with altered cortical hubs. Epilepsia. 2015;56:772–79. doi: 10.1111/epi.12966. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Hernandez A, Bland BH, Facelli JC, et al. Septo-hippocampal networks in chronic epilepsy. Exp Neurol. 2010;222:86–92. doi: 10.1016/j.expneurol.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang XH, Wu XY, Xu L, et al. Tenidap is neuroprotective in a pilocarpine rat model of temporal lobe epilepsy. Chin Med J (Engl) 2013;126:1900–5. [PubMed] [Google Scholar]
- 10.Gorter JA, Lyer A, White I, et al. Hippocampal subregion-specific microRNA expression during epileptogenesis in experimental temporal lobe epilepsy. Neurobiol Dis. 2014;62:508–20. doi: 10.1016/j.nbd.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 11.Baik EJ, Kim EJ, Lee SH, et al. Cyclooxygenase-2 selective inhibitors aggravate kainic acid induced seizure and neural cell death in the hippocampus. Brain Res. 1999;843:118–29. doi: 10.1016/s0006-8993(99)01797-7. [DOI] [PubMed] [Google Scholar]
- 12.Kanane P, Bartolomei F. Temporal lobe epilepsy and hippocampal sclerosis: lessons from depth EEG recordings. Epilepsia. 2010;51:59–62. doi: 10.1111/j.1528-1167.2009.02448.x. [DOI] [PubMed] [Google Scholar]
- 13.Regesta S, Tanganelli P. Clinical aspects and biological bases of drug-resistant epilepsies. Epilepsy Res. 1999;34:109–22. doi: 10.1016/s0920-1211(98)00106-5. [DOI] [PubMed] [Google Scholar]
- 14.Sisodiya SM. Mechanisms of antiepileptic drug resistance. Curr Opin Neurol. 2003;16:197–201. doi: 10.1097/01.wco.0000063771.81810.6c. [DOI] [PubMed] [Google Scholar]
- 15.Dombrowski SM, Desai SY, Marroni M, et al. Overexpression of drug resistance genes in endothelial cells from patients with refractory epilepsy. Epilepsia. 2001;42:1501–6. doi: 10.1046/j.1528-1157.2001.12301.x. [DOI] [PubMed] [Google Scholar]
- 16.Tishler MD, Weinberg KI, Hinton DR, et al. MDR1 gene expression in brain of patients with medically intractable epilepsy. Epilepsia. 1995;36:1–6. doi: 10.1111/j.1528-1157.1995.tb01657.x. [DOI] [PubMed] [Google Scholar]
- 17.Song YC, Li WJ, Li LZ. Regulatory effect of miRNA 320a on expression of aquaporin 4 in brain tissue of epileptic rats. Asian Pac J Trop Med. 2015;8:807–82. doi: 10.1016/j.apjtm.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Song Z, Wang L, Liu Y, et al. A novel nonsense mutation in the MIP gene linked to congenital posterior polar cataracts in a Chinese family. PLoS One. 2015;10:e0119296. doi: 10.1371/journal.pone.0119296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bebek N, Ozdemir O, Sayitoglu M, et al. expression of analysis and clinical correlation of aquaporin 1 and 4 genes in human hippocampal sclerosis. J Clin Neurosci. 2013;20:1564–70. doi: 10.1016/j.jocn.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 20.Jin H, Li W, Dong C, et al. Effects of different dose of levetiracetam on aquaporin 4 expression in rats with brain edema following fluid percussion injury. Med Sci Monit. 2016;22:678–86. doi: 10.12659/MSM.897201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eid T, Lee TS, Thomas MJ, et al. Loss of perivascular aquaporin 4 may underlie deficient water and K homeostasis in the human epileptogenic hippocampus. Proc Natl Acad Sci USA. 2005;102:1193–98. doi: 10.1073/pnas.0409308102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsu MS, Lee DJ, Binder DK. Potential role of the glial water channel aquaporin-4 in epilepsy. Neuron Glia Biol. 2007;3:287–97. doi: 10.1017/S1740925X08000112. [DOI] [PubMed] [Google Scholar]
- 23.Medici V, Frassoni C, Tassi L, et al. Aquaporin 4 expression in control and epileptic human cerebral cortex. Brain Res. 2011;167:330–39. doi: 10.1016/j.brainres.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 24.Song YC, Li WJ, Li LZ. Regulatory effect of miRNA 320a on expression of aquaporin 4 in brain tissue of epileptic rats. Asian Pac J Trop Med. 2015;8:807–12. doi: 10.1016/j.apjtm.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Shi ZF, Zhao WJ, Xu LX, et al. Downregulation of Aquaporin 4 expression through extracellular signal-regulated kinases 1/2 activation in cultured astrocytes following scratch-injury. Biomed Environ Sci. 2015;28:199–205. doi: 10.3967/bes2015.026. [DOI] [PubMed] [Google Scholar]
- 26.Minich T, Riemer J, Schulz JB, et al. The nultidrug resistance protein 1, but not mrp5, mediates export of glutathione and glutathione disulfide from brain astrocytes. J Neurochem. 2006;97:373–84. doi: 10.1111/j.1471-4159.2006.03737.x. [DOI] [PubMed] [Google Scholar]
- 27.Tanimura Y, Hiroaki Y, Fujiyoshi Y. Acetazolamide reversibly inhibits water conduction by aquaporin-4. J Struct Biol. 2009;166:16–21. doi: 10.1016/j.jsb.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 28.Katada R, Nishitani Y, Honmou O, et al. Expression of aquaporin-4 augments cytotoxic brain edema after traumatic brain injury during acute ethanol exposure. Am J Pathol. 2012;180:17–23. doi: 10.1016/j.ajpath.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 29.Racine R, Okujava V, Chipashvili S. Modification of seizure activity by electrical stimulation. 3. Mechanisms. Electroencephalogr Clin Neurophysiol. 1972;32:295–99. doi: 10.1016/0013-4694(72)90178-2. [DOI] [PubMed] [Google Scholar]
- 30.van der Deen M, Marks H, Willemse BW, et al. Diminished expression of multidrug resistance-associated protein 1 in bronchial epithelium of COPD patients. Virchows Arch. 2006;449:682–88. doi: 10.1007/s00428-006-0240-3. [DOI] [PubMed] [Google Scholar]
- 31.Sisodiya SM, Lin WR, Harding BN, et al. Drug resistance in epilepsy: Expression of drug resistance proteins in common causes of refractory epilepsy. Brain. 2002;125:22–31. doi: 10.1093/brain/awf002. [DOI] [PubMed] [Google Scholar]
- 32.Yu W, Chen D, Wang Z, et al. Time-dependent decrease of clusterin as a potential cerebrospinal fluid cerebrospinal fluid biomarker for drug-resistant epilepsy. J Mol Neurosci. 2014;54:1–9. doi: 10.1007/s12031-014-0237-3. [DOI] [PubMed] [Google Scholar]
- 33.Balan S, Radhab SK, Sathyan S, et al. Major vault protein (MVP) gene polymorphisms and drug resistance in mesial temporal lobe epilepsy with hippocampal sclerosis. Gene. 2013;526:449–53. doi: 10.1016/j.gene.2013.05.067. [DOI] [PubMed] [Google Scholar]
- 34.Chen JQ, Zhang CC, Jiang SN, et al. Effects of aquaporin 4 knockdown on brain edema of the uninjured side after traumatic brain injury in rats. Med Sci Monit. 2016;22:4809–19. doi: 10.12659/MSM.898190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manley GT, Binder DK, Papadopoulos MC, et al. New insights into water transport and edema in the central nervous system from phenotype analysis of aquaporin-4 null mice. Neuroscience. 2004;129:983–91. doi: 10.1016/j.neuroscience.2004.06.088. [DOI] [PubMed] [Google Scholar]
- 36.Alvestad S, Hammer J, Hoddevik EH, et al. Mislocalization of AQP4 precedes chronic seizures in the kainate model of temporal lobe epilepsy. Epilepsy Res. 2013;105:30–41. doi: 10.1016/j.eplepsyres.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 37.Dixit AB, Banerjee J, Srivastava A, et al. RNA-seq analysis of hippocampal tissues reveals novel candidate genes for durg refractory epilepsy in patients with MTLE-HS. Genomics. 2016;107:178–88. doi: 10.1016/j.ygeno.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 38.Umschweif G, Alexandrovich AG, Trembovler V, et al. Hypoxia-inducible factor 1 is essential for spontaneous recovery from traumatic brain injury and is a key mediator of heat acclimation induced neuroprotection. J Cereb Blood Flow Metab. 2013;33:524–31. doi: 10.1038/jcbfm.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi WZ, Zhao CZ, Zhao B, et al. Aggravated inflammation and increased expression of cysteinyl leukotriene receptors in the brain after focal cerebral ischemia in AQP4-deficient mice. Neurosci Bull. 2012;28:680–92. doi: 10.1007/s12264-012-1281-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sturdivant NM, Smith SG, Ali SF, et al. Acetazolamide mitigates astrocyte cellular edema following mild traumatic brain injury. Sci Rep. 2016;6:33330. doi: 10.1038/srep33330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ameli PA, Madan M, Chigurupati S, et al. Effect of acetazolamide on aquaporin-1 and fluid flow in cultured choroid plexus. Acta Neurochir Suppl. 2012;113:59–64. doi: 10.1007/978-3-7091-0923-6_13. [DOI] [PubMed] [Google Scholar]
- 42.Sun Y, Luo X, Yang K, et al. Neural overexpression of multidrug resistance associated protein 1 and refractory epilepsy: A meta-analysis of 9 studies. Int J Neurosci. 2016;126:308–17. doi: 10.3109/00207454.2015.1015724. [DOI] [PubMed] [Google Scholar]
- 43.Chen YH, Wang CC, Xiao X, et al. Multidrug resistance-associated protein 1 decreases the concentrations of antiepileptic drugs in cortical extracellular fluid in amygdale kindling rats. Acta Pharmacol Sin. 2013;34:473–79. doi: 10.1038/aps.2012.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feldmann M, Koepp M. P-glycoprotein imaging in temporal lobe epilepsy: In vivo PET experiments with the Pgp substrate [11C]-verapamil. Epilepsia. 2012;53(Suppl 6):60–63. doi: 10.1111/j.1528-1167.2012.03704.x. [DOI] [PubMed] [Google Scholar]
- 45.Chen L, Feng P, Li Y, et al. Influences of “spasmolytic powder” on pgp expression of Coriaria Lactone-kindling drug-resistant epileptic rat model. J Mol Neurosci. 2013;51:1–8. doi: 10.1007/s12031-012-9923-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Serum concentrations of carbonic anhydrase in rats of each group. The carbonic anhydrase levels were measured by using an ELISA kit as described in the “Materials and Methods” section.