The lung is the site most commonly affected by infectious and non-infectious complications of HIV infection [1], even in individuals on effective antiretroviral therapy (ART). HIV infection is associated with lymphocytic alveolitis [2], a condition characterised by the influx of CD8+ T-cells into the alveolar space. This is thought to occur in response to HIV antigens and forms part of the host response to the presence of HIV in the lung [2, 3]. Excessive influx of inflammatory cells in the lung probably leads to tissue damage, disruption of immune cell homeostasis, impaired gas exchange [4] and predisposition to HIV-associated lung complications.
Short abstract
Disruption of lung cytokine networks during chronic HIV infection is incompletely restored in individuals on antiretroviral therapy http://ow.ly/klkF30gTtwf
To the Editor:
The lung is the site most commonly affected by infectious and non-infectious complications of HIV infection [1], even in individuals on effective antiretroviral therapy (ART). HIV infection is associated with lymphocytic alveolitis [2], a condition characterised by the influx of CD8+ T-cells into the alveolar space. This is thought to occur in response to HIV antigens and forms part of the host response to the presence of HIV in the lung [2, 3]. Excessive influx of inflammatory cells in the lung probably leads to tissue damage, disruption of immune cell homeostasis, impaired gas exchange [4] and predisposition to HIV-associated lung complications.
The cytokine microenvironment in the lung plays a crucial role in shaping the cellular composition and immune response in this compartment [5]. We have previously shown that antigen-specific CD4+ T-cell responses to respiratory pathogens differ both in quality and magnitude between alveolar and peripheral blood CD4+ T-cells [6]. Furthermore, impairment of CD4+ T-cell responses to Mycobacterium tuberculosis and influenza virus in HIV-infected adults was more pronounced in alveolar than peripheral blood cells [6], indicating that immune responses are compartmentalised and are impacted differentially by HIV infection.
Cytokines function in clusters of organised integrated networks that maintain homeostasis and immune surveillance in the systemic and tissue compartments. While the integrity of cytokine networks in plasma was shown to impact HIV disease progression during acute HIV infection, with rapid disease progressors having more dysregulated cytokine networks than slow progressors [7], the impact of chronic HIV infection or ART on the cytokine microenvironment and immune cell homeostasis in the lung is incompletely understood.
We hypothesised that chronic HIV infection alters the lung cytokine microenvironment in a manner that promotes accumulation of lymphocytes in the alveolar space. We explored this hypothesis in a prospective cross-sectional study that recruited healthy HIV-1-uninfected and asymptomatic HIV-1-infected ART-naïve and ART-treated adults (aged ≥18 years) for assessment of lung immunity. Clients attending the HIV voluntary counselling and testing (VCT) and ART clinics at Queen Elizabeth Central Hospital in Blantyre, Malawi, were invited to join the study. Exclusion criteria were current or previous history of smoking, use of immunosuppressive drugs, severe anaemia (Hb <8 g·dL−1) and known or suspected pregnancy. The research ethics committee of the Malawi College of Medicine approved the study and all participants provided written informed consent.
Participants underwent bronchoscopy for bronchoalveolar lavage (BAL) fluid sampling [3]. The levels of 34 cytokines (interleukin (IL)-12, IL-23, IL-27, monocyte chemoattractant protein (MCP)-1 (CCL2), RANTES (CCL5), GRO-α (CXCL1), stromal cell-derived factor (SDF)-1α (CXCL12), interferon-γ-inducible protein (IP)-10 (CXCL10), Eotaxin, granulocyte–macrophage colony-stimulating factor (GM-CSF), interferon (IFN)-α, IFN-γ, IL-1α, IL-1β, IL-1RA, IL-10, IL-13, IL-15, IL-17A, IL-18, IL-2, IL-21, IL-22, IL-31, IL-4, IL-5, IL-6, IL-7, IL-8 (CXCL8), IL-9, macrophage inflammatory protein (MIP)-1α, MIP-1β, tumour necrosis factor (TNF)-α and TNF-β) were measured in concentrated cell-free BAL fluid using a ProcartaPlex 34-plex Human Cytokine and Chemokine Panel 1A (Affymetrix eBioscience, USA) and reported as the cytokine concentration per mL of epithelial lining fluid following standardisation using the urea dilution method [8]. Statistical analysis and graphical presentation were performed using GraphPad Prism 5 (GraphPad Software, USA) or R statistical software version 3.2.4 (www.r-project.org). We performed intergroup comparisons using the Kruskal–Wallis test with Dunn's multiple comparisons test or with the Mann–Whitney U-test. Associations were analysed using Spearman's test. Differences were considered statistically significant when p<0.05.
We recruited 21 HIV-uninfected (median age (range) 25 (18–40) years), 33 ART-naïve HIV-infected (35 (20–52) years) and 20 ART-treated HIV-infected (36 (20–52) years) adults. The male:female ratio was 3:1 in HIV-uninfected, 2:3 in ART-naïve and 1:1 in ART-treated HIV-infected participants. Median (interquartile range, IQR) CD4+ T-cell counts were 647 (536–757) cells/µL in HIV-uninfected, 331 (256–428) cells·L−1 in ART-naïve and 383 (194–627) cells·L−1 in ART-treated HIV-infected participants. The median (IQR) plasma HIV viral load was 25 638 (11 140–251 011) copies·mL−1 in ART-naive and 2304 (295–87 436) copies·mL−1 in 4 ART-treated HIV-infected individuals (the remaining 16 had HIV viral loads of <150 copies·mL−1, the lower limit of detection of the assay). ART consisted of tenofovir, lamivudine and efavirenz, with a median duration of treatment of 5.5 years (range 0.1–10 years).
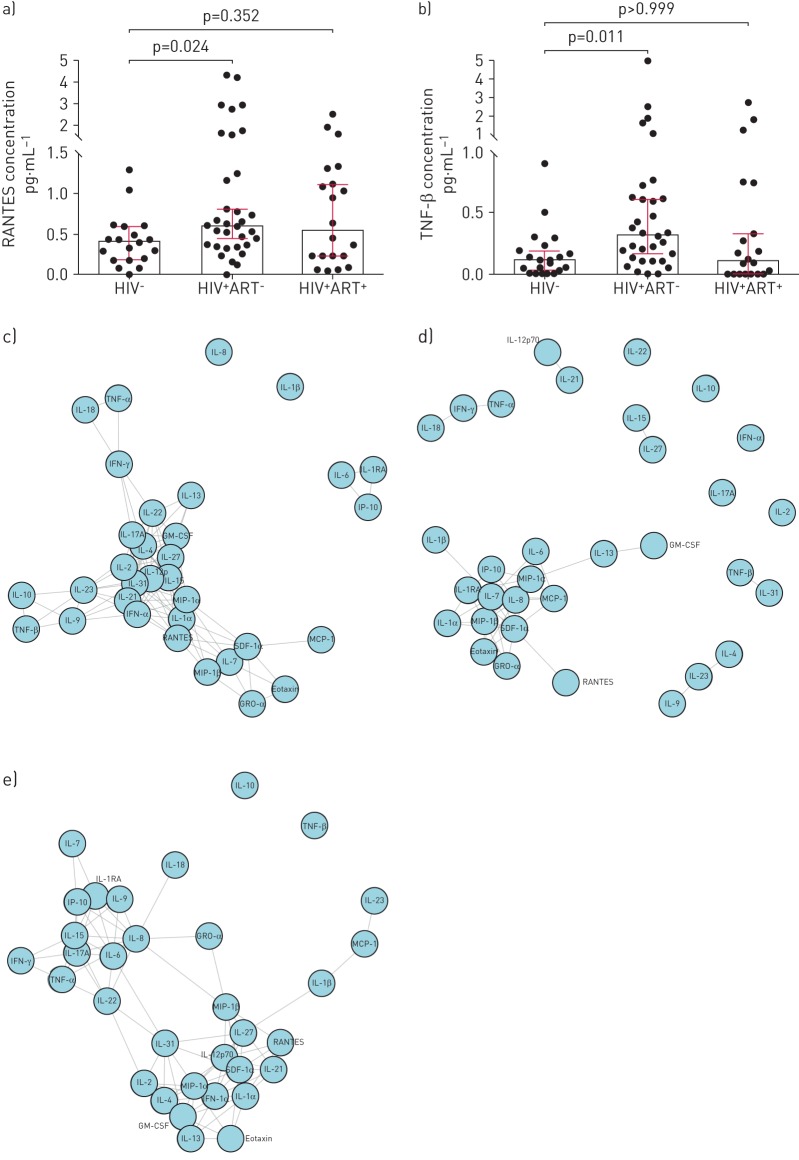
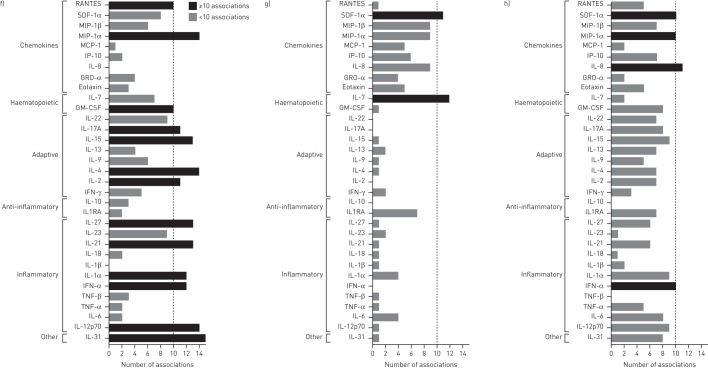
Analysis of BAL fluid cytokine levels showed significantly higher concentrations of RANTES and TNF-β in ART-naïve HIV-infected than in HIV-uninfected participants (all p<0.05; figure 1a and b). Cytokine levels were not significantly different between HIV-uninfected and ART-treated HIV-infected participants (figure 1a and b). We next assessed the inter-cytokine relationships to identify the key cytokine networks disrupted during chronic HIV infection. We constructed cytokine networks by pooling data from only those associations that showed strong correlations (r≥0.80, p<0.05) and found that fewer cytokines strongly correlated with each other in ART-naïve HIV-infected than in HIV-uninfected individuals (figure 1c–e). We then grouped the cytokines based on their functional profiles into inflammatory, anti-inflammatory, adaptive, haematopoietic and chemokines (figure 1c–e). Compared with HIV-uninfected individuals, ART-naïve HIV-infected participants had disrupted cytokine networks, with predominance of chemokine-driven networks (comprising SDF-1α, MIP-1α, MIP-1β, MCP-1, IP-10, GRO-α, Eotaxin and IL-8) and a marked reduction in the other cytokine networks (figure 1f–h). Furthermore, the RANTES-driven network was severely depleted in ART-naïve HIV-infected adults but remained strongly correlated with SDF-1α (figure 1c–h). Interestingly, the SDF-1α-driven network was the most dominant in ART-naïve HIV-infected individuals and showed strong associations with MIP-1α, MIP-1β, MCP-1, IP-10, GRO-α, Eotaxin and IL-8 (figure 1c–h). ART-treated HIV-infected individuals had re-emergence of cytokine networks that partially resembled those observed in HIV-uninfected individuals (figure 1c–h).
FIGURE 1.
Scatter plots showing the concentration of a) RANTES (CCL5) and b) tumour necrosis factor (TNF)-β in bronchoalveolar lavage (BAL) fluid from HIV-uninfected adults compared to untreated and treated HIV-infected individuals. Bars represent the median and error bars are 95% confidence intervals. Data were analysed using the Kruskal–Wallis test with Dunn's multiple comparisons test. Network graphs of the cytokine associations among 33 BAL fluid cytokines for c) HIV-uninfected individuals, d) antiretroviral therapy (ART)-naïve HIV-infected individuals and e) HIV-infected individuals on long-term ART. The shorter the connecting line between the circles, the greater the magnitude of the correlation. Bar graphs showing the number of associations per cytokine in BAL fluid for f) HIV-uninfected individuals, g) ART-naïve HIV-infected individuals and h) HIV-infected individuals on ART. Only correlations >0.80 with p<0.05 were considered in this analysis (HIV−, n=21; HIV+ ART−, n=33; HIV+ ART+, n=20). SDF: stromal cell-derived factor; MIP: macrophage inflammatory protein; MCP: monocyte chemoattractant protein; IP: interferon-γ-inducible protein; IL; interleukin; GM-CSF: granulocyte–macrophage colony-stimulating factor; IFN: interferon; IL1RA: IL-1 receptor antagonist.
Dysregulated BAL fluid cytokine profiles have been reported in respiratory conditions such as asthma [9] and IgG4-related respiratory disease [10]. Here, we show that chronic HIV infection was associated with disruption of key cytokine networks involved in immune defence and maintenance of immune cell homeostasis in the lung. RANTES and SDF-1α are major lymphocyte chemoattractants that have been shown to play a crucial role in recruitment of CD8+ T-cells to the lung in murine models of respiratory syncytial virus (RSV) infection [11] and obliterative bronchiolitis [12]. CD8+ T-cells and alveolar epithelial cells (AECs) are among the main producers of RANTES in the lung, while SDF-1α, a potent inducer of HIV-specific CD8+ T-cell chemotaxis, is produced mainly by AECs and fibroblasts [13]. HIV-specific CD8+ T-cells are known producers of TNF-β upon stimulation with HIV antigens [14]. The influx of HIV-specific CD8+ T-cells into the alveolar space may explain the increase in TNF-β levels in BAL fluid of ART-naïve HIV-infected individuals. RANTES and SDF-1α are ligands for the CCR5 and CXCR4 receptors, respectively. Both chemokines have antiviral properties against HIV by blocking attachment and entry of HIV into cells through the CCR5 and CXCR4 HIV co-receptors [15]. The strong positive association between RANTES, SDF-1α and other known cell chemoattractants (IL-8, MIP-1α, MIP-1β, MCP-1, IP-10 and Eotaxin) is evidence of the tightly integrated networks in which these chemokines function. The predominance of the chemokine-driven networks in the alveolar space during chronic HIV infection is probably a double-edged sword; on the one hand it is a beneficial antiviral response against HIV infection, but on the other it disrupts immune cell homeostasis by promoting excessive recruitment of CD8+ T-cells to the lung.
In conclusion, the lung cytokine microenvironment was profoundly perturbed in individuals with untreated chronic HIV-1 infection and was incompletely restored by long-term ART. This contributes to the high risk of HIV-associated lung complications such as tuberculosis and pneumococcal pneumonia. RANTES, SDF-1α and associated chemokines are the likely key drivers of lymphocyte accumulation in the alveolar space during chronic HIV infection.
Acknowledgements
The authors thank all study participants, Marie Kunkeyani, Felistas Kanyandula, Elizabeth Jalisi and staff of MLW and QECH for their support and cooperation during the study.
K.C. Jambo, H.C. Mwandumba, D.L. Tembo, R.S. Heyderman, D.G. Russell and T.J. Allain conceived and designed the study; all authors analysed and interpreted that data; K.C. Jambo, H.C. Mwandumba, D.L. Tembo, D.G. Russell and R.S. Heyderman drafted the manuscript for important intellectual content; and all authors gave final approval of the manuscript for publication.
Footnotes
Support statement: This work was supported by Wellcome Trust and Bill and Melinda Gates Foundation awards 088696/Z/09/Z and OPP1125279, to H.C. Mwandumba, US National Institutes of Health awards AI118582 and HL100928 to D.G. Russell, and Wellcome Trust award 105831/Z/14/Z to K.C. Jambo. A Strategic award from the Wellcome Trust supports the Malawi–Liverpool–Wellcome Trust Clinical Research Programme. Funding information for this article has been deposited with the Crossref Funder Registry.
Conflict of interest: None declared.
References
- 1.Gingo MR, George MP, Kessinger CJ, et al. Pulmonary function abnormalities in HIV-infected patients during the current antiretroviral therapy era. Am J Respir Crit Care Med 2010; 182: 790–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neff CP, Chain JL, MaWhinney S, et al. Lymphocytic alveolitis is associated with the accumulation of functionally impaired HIV-specific T cells in the lung of antiretroviral therapy-naive subjects. Am J Respir Crit Care Med 2015; 191: 464–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jambo KC, Banda DH, Kankwatira AM, et al. Small alveolar macrophages are infected preferentially by HIV and exhibit impaired phagocytic function. Mucosal Immunol 2014; 7: 1116–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crothers K, McGinnis K, Kleerup E, et al. HIV infection is associated with reduced pulmonary diffusing capacity. J Acquir Immune Defic Syndr 2013; 64: 271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yosef N, Regev A. Writ large: genomic dissection of the effect of cellular environment on immune response. Science 2016; 354: 64–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jambo KC, Banda DH, Afran L, et al. Asymptomatic HIV-infected individuals on antiretroviral therapy exhibit impaired lung CD4+ T-cell responses to mycobacteria. Am J Respir Crit Care Med 2014; 190: 938–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang X, Liu X, Meyers K, et al. Cytokine cascade and networks among MSM HIV seroconverters: implications for early immunotherapy. Sci Rep 2016; 6: 36234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rennard SI, Basset G, Lecossier D, et al. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J Appl Physiol 1986; 60: 532–538. [DOI] [PubMed] [Google Scholar]
- 9.Hosoki K, Ying S, Corrigan C, et al. Analysis of a panel of 48 cytokines in BAL fluids specifically identifies IL-8 levels as the only cytokine that distinguishes controlled asthma from uncontrolled asthma, and correlates inversely with FEV1. PLoS One 2015; 10: e0126035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamamoto H, Yasuo M, Ichiyama T, et al. Cytokine profiles in the BAL fluid of IgG4-related respiratory disease compared with sarcoidosis. ERJ Open Res 2015; 1: 00009–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Culley FJ, Pennycook AM, Tregoning JS, et al. Role of CCL5 (RANTES) in viral lung disease. J Virol 2006; 80: 8151–8157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu J, Torres E, Mora AL, et al. Attenuation of obliterative bronchiolitis by a CXCR4 antagonist in the murine heterotopic tracheal transplant model. J Heart Lung Transplant 2008; 27: 1302–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meditz AL, Schlichtemeier R, Folkvord JM, et al. SDF-1α is a potent inducer of HIV-1-specific CD8+ T-cell chemotaxis, but migration of CD8+ T cells is impaired at high viral loads. AIDS Res Hum Retroviruses 2008; 24: 977–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jassoy C, Harrer T, Rosenthal T, et al. Human immunodeficiency virus type 1-specific cytotoxic T lymphocytes release gamma interferon, tumor necrosis factor alpha (TNF-α), and TNF-β when they encounter their target antigens. J Virol 1993; 67: 2844–2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oberlin E, Amara A, Bachelerie F, et al. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature 1996; 382: 833–835. [DOI] [PubMed] [Google Scholar]