Abstract
Solitary fibrous tumor is a mesenchymal neoplasm exhibiting a broad spectrum of biological behavior and harboring the NAB2–STAT6 fusion. Clinicopathologic parameters are currently used in risk prediction models for solitary fibrous tumor, but the molecular determinants of malignancy in solitary fibrous tumors remain unknown. We proposed that the activation of telomere maintenance pathways confers a perpetual malignant phenotype to these tumors. Therefore, we investigated telomerase reverse transcriptase (TERT) reactivation induced by promoter mutations as a potential molecular mechanism for aggressive clinical behavior in solitary fibrous tumor. The retrospective study included tumor samples from 94 patients with solitary fibrous tumor (31 thoracic and 63 extra-thoracic). Follow-up information was available for 68 patients (median, 46 months). TERT promoter mutation analysis was performed by PCR and Sanger sequencing, and TERT expression was assessed by real-time quantitative reverse transcription PCR. Patients were stratified into clinicopathologic subgroups [high-risk (n=20), moderate-risk (n=28), and low-risk (n=46)] according to the risk stratification model proposed by Demicco et al. TERT promoter mutations were identified in 26 of 94 (28%) solitary fibrous tumors: −124C>T in 23 tumors (88%), −124C>A in 1 tumor (4%), and −146C>T in 2 tumors (8%). Real-time quantitative reverse transcription PCR revealed that TERT mRNA expression was higher in all solitary fibrous tumors with the mutant TERT promoter than those with the wild-type TERT promoter. TERT promoter mutations were strongly associated with high-risk clinicopathologic characteristics and outcome. An adverse event (relapse, death) occurred in 16 of 68 (24%) patients, 12 with solitary fibrous tumors with TERT promoter mutations and 4 with the wild-type TERT promoter. TERT promoter mutations were strongly associated with older age (P=0.006), larger tumor size (P=0.000002), higher risk classifications (P=2.9×10−9), and a worse event-free survival (P=0.0082). Thus, TERT promoter mutations in solitary fibrous tumor influence gene expression and are associated with adverse patient outcome. Integrating TERT promoter mutational status with existing multivariable risk prediction models might improve risk prediction in patients with solitary fibrous tumor.
Keywords: TERT promoter mutations, prognostic marker, TERT expression, solitary fibrous tumor, real-time quantitative reverse transcription PCR
INTRODUCTION
Solitary fibrous tumor is a rare mesenchymal neoplasm of fibroblastic origin that can occur at any anatomic site. Solitary fibrous tumor occurs primarily in adults and exhibits a wide spectrum of morphologic features and biologic behavior.1 The classical solitary fibrous tumor consists of fibroblast-like tumor cells arranged in a “patternless” pattern in a collagenous stroma with staghorn, hyalinized blood vessels and diffuse CD34 expression.1–3 Unconventional subtypes showing distinct morphologic features, such as the lipomatous, myxoid, or dedifferentiated variants, have also been described.1,4–6 According to the 2013 World Health Organization classification of soft tissue tumors, solitary fibrous tumor is defined as an intermediate (rarely metastasizing) tumor.1 Most solitary fibrous tumors follow a favorable course, but 10%–20% of tumors recur or metastasize.7–10 Although most of the clinically aggressive solitary fibrous tumors are histologically malignant, a definitive correlation between morphology and behavior has not been established and the clinical course can be unpredictable.2,7,9–12 In multivariate analyses that included several clinical and histological parameters such as increased mitotic activity, hypercellularity, nuclear atypia, and pleomorphism, the most reliable prognostic indicators for solitary fibrous tumor were patient age, tumor size, and mitotic activity.8,13 To date, however, genetic changes underlying the clinicopathologic determinants of outcome have not been determined.
Next-generation sequencing studies have recently identified that the NAB2–STAT6 fusion genes, with highly variable breakpoints derived from an intra-chromosomal inversion at chromosome 12q13, are the genetic hallmark and the putative driver oncogene of solitary fibrous tumor.14,15 NAB2–STAT6 chimeric transcripts have been identified in both histologically benign and malignant solitary fibrous tumors at every anatomic site, which supports the concept of a unified biologic entity for these tumors despite their clinicopathologic heterogeneity.14–17 The variation of breakpoints in fusion genes is thought to contribute to the morphologic diversity of solitary fibrous tumors, and in some studies the fusion variants have been associated with certain clinicopathologic features. 18–21 However, the genetic alteration underpinning the malignant behavior in a subset of patients with solitary fibrous tumor has not yet been identified.
Telomeres are repetitive stretches of DNA at the ends of chromosomes that stabilize the integrity of the genome by protecting the chromosome ends from degradation and end-to-end fusions.22 Each time a cell divides, a portion of telomeres is lost until telomeres shorten below a critical point, which results in cell death or replicative senescence.23 Cancer cells have the ability to overcome telomere shortening mainly through the activity of telomerase, whose active protein component is encoded by the telomerase reverse transcriptase (TERT) gene.24 TERT is normally active in fetal tissue and stem cells and physiologically silenced in terminally differentiated somatic cells.25 In 85%–90% of cancers, TERT is upregulated again, which enables immortalization and promotes cancer progression.26 Transcriptional activating point mutations in the core promoter of the TERT gene have recently been recognized as a mechanism for TERT reactivation in cancer.27,28 These mutations were first discovered in melanoma27,28 and subsequently in several other cancer types.29,30 TERT promoter mutations contribute to the upregulation of TERT expression by creating de novo binding motifs for E-twenty-six transcription factors,27,30 including the multimeric GA-binding protein transcription factor that is specifically recruited to the binding sites.31
TERT promoter mutations are highly recurrent in myxoid liposarcoma but relatively rare in other soft tissue sarcomas.32–34 In some soft tissue sarcomas, telomere maintenance is more commonly regulated by alternative lengthening of telomeres than by TERT reactivation.35 TERT promoter mutations, however, have also been reported in a subset of solitary fibrous tumors, more frequently in meningeal tumors36 and less commonly in extracranial tumors.21,33,37
In a recent study on biologically indeterminate spitzoid melanocytic neoplasms, we found that the presence of TERT promoter mutations predicted a highly aggressive form of spitzoid tumors with metastatic potential.38 Given the important role of telomerase in cancer development, coupled with the identification of TERT promoter mutations in a subset of solitary fibrous tumors in previous studies,21,33,37 we proposed that these mutations might be responsible for the clinically malignant behavior exhibited by a subset of solitary fibrous tumors. Therefore, we studied tumor samples from a large cohort of patients with solitary fibrous tumors across the entire biologic spectrum to determine the prevalence of TERT promoter mutations and their association with TERT expression, clinicopathologic parameters, and patient outcome.
MATERIALS AND METHODS
This retrospective study was approved by the institutional review boards of participating institutions. From one of the authors’ (CDF) consultation files and the pathology archives at the participating institutions, 96 patients with solitary fibrous tumor for whom sufficient material was available for analysis were identified. Cases were identified during variable periods across the participating institutions. The diagnostic period for the entire cohort spanned from 2004 to 2012. Each case was reviewed by at least 2 of the pathologists who participated in this study, and only cases for which consensus was obtained were included for analysis. Samples from 2 patients were excluded from analysis: DNA could not be detected in one sample and the primary tumor was not available for analysis in the other case. Clinical and follow-up data were retrospectively collected from institutional medical records or obtained from referring pathologists (see Acknowledgments). Hematoxylin and eosin–stained sections were reviewed and the following histologic features were documented: mitotic rate [number of mitotic figures per 10 high-power fields (HPFs)], presence of histologic features suggestive of malignancy (high cellularity, increased mitotic activity, pronounced nuclear atypia, and necrosis), dedifferentiated areas (defined as morphologically distinct, sharply demarcated sarcoma-like areas, often CD34-negative, within conventional solitary fibrous tumors), atypical features (foci of hypercellularity and nuclear pleomorphism), cellular features, and special variants (e.g., lipomatous and myxoid).1,4,6 For risk stratification of patients, the 3-tiered assessment model proposed by Demicco et al. was used, which takes into account patient age, tumor size, and mitotic rate.8 The total score for each patient was tabulated by using age (<55 years vs. ≥55 years), tumor size (<5 cm, 5 to <10 cm, 10 to <15 cm, or ≥15 cm), and mitotic figures (0, 1–3 mitotic figures per 10 HPFs, or ≥4 mitotic figures per 10 HPFs), and the patients were assigned to the low-, moderate-, and high-risk categories.
Immunohistochemical Analysis
Antibodies specific to CD34 (QBEnd-10; Ventana Medical Systems), STAT6 (S-20, SC-621; Santa Cruz Biotechnology, Inc.), and p53 (DO-7; Zeta Corporation) were applied on sections of formalin-fixed paraffin-embedded tissue (4 μm) by using the BenchMark ULTRA automated staining platform (Ventana Medical Systems/Roche). Immunohistochemical studies were performed according to the manufacturer’s instructions for antigen retrieval and detection conditions by using the iVIEW or ultraView DAB detection kit (Ventana Medical Systems/Roche). The nuclear expression of p53 in solitary fibrous tumor samples was scored on the basis of the percentage of tumor cells with strong or moderate staining intensity as follows: negative (0% of cells stained), 1+ (rare to 25% of cells stained), 2+ (≥26% to 50% of cells stained), 3+ (>51% to 75% of cells stained), and 4+ (≥76% of cells stained). Scores of ≥2+ were marked as p53 nuclear accumulation (overexpression); scores of <2+ or weak-intensity nuclear staining regardless of the staining distribution were marked as low expression; and complete absence of expression was marked as negative. Immunoreactivity for CD34 and STAT6 was recorded as negative or positive on the basis of previously described criteria.39
TERT Promoter and TP53 Mutation Analysis
Tumor-rich sections containing more than 50% tumor cell content were selected for each sample. Genomic DNA was extracted from 12-micron slide-mounted formalin-fixed paraffin-embedded sections by using the Maxwell® 16 FFPE Plus LEV DNA Purification Kit (AS1135, Promega) according to the manufacturer’s protocol. Mutations in the TERT promoter region from positions −47 to −243 from the ATG start site (HG 19 coordinates, chr5: 1295151–1295347) were identified by direct sequencing. PCR for the TERT promoter was carried out using 5′ CAG CGC TGC CTG AAA CTC G 3′ as the forward sequencing primer and 5′ CCA CGT GGC GGA GGG ACT 3′ as the reverse sequencing primer. The PCR reaction was performed in a total volume of 50 μL, using the GoTaq® Long PCR Master Mix (M4021, Promega) and 0.2-μM primers. The PCR product was sequenced by Sanger sequencing (ABI Prism 3730XL DNA Analyzer). TERT hotspot mutations were recognized on sequencing electropherograms by using CLC Main Workbench sequence analysis software version 6.0.2 (CLC bio, Cambridge, MA). In addition, mutations in the coding regions of the TP53 gene (exons 2–11) were screened in a subset of samples by direct sequencing, according to the International Agency for Research on Cancer (IARC) TP53 database (http://p53.iarc.fr), as previously described.40
TERT mRNA Expression Analysis
Relative TERT mRNA expression was assessed by real-time quantitative reverse transcription PCR. Total RNA was isolated from the same formalin-fixed paraffin-embedded tumor samples that were used to extract DNA, by using the Maxwell® 16 LEV RNA FFPE Purification Kit (Promega, AS1260) according to the manufacturer’s protocol. To quantify TERT mRNA expression levels, 2 μg of total RNA from each sample was converted to cDNA by using the SuperScript® VILO cDNA Synthesis Kit (Invitrogen, 11754–010). Real-time quantitative reverse transcription PCR was performed in separate groups for extra-thoracic (soft tissue) and thoracic (pleural) solitary fibrous tumors. Real-time quantitative reverse transcription PCR was conducted in triplicate by using the TaqMan® Gene Expression Assays and gene-specific primers (Life Technologies) for TERT (Hs00972656_m1) and GAPDH (Hs02758991_g1), a housekeeping gene used as the endogenous standard. TERT expression levels were measured by using GAPDH expression as a reference, and relative quantification was determined by using the ΔΔCt method and log2 transformation.
Statistical Analyses
The Kruskal–Wallis test was used to evaluate the association of TERT promoter mutations with patient age and tumor size. The Fisher’s exact test was used to evaluate the association of TERT promoter mutations with site, risk classification, gender, tumor size score, and mitotic rate score. Event-free survival was defined as the time elapsed from diagnosis until death, resistant disease, progressive disease, or relapse observed with surviving event-free patients censored at the date of last follow-up. The Kaplan–Meier method was used to estimate event-free survival, and the log-rank test was used to compare event-free survival according to TERT promoter mutation status. The exact Cochran–Mantel–Haenzel test was used to evaluate the association of TERT promoter mutations with site (thoracic or extra-thoracic) while adjusting for the Demicco risk classification. Cox regression models were used to explore the association of TERT promoter mutations, age, mitotic rate, risk group, and tumor size with event-free survival. The Akaike Information Criterion41 was used to select the best Cox regression model as predictor of event-free survival. Analyses were performed by using R software (www.r-project.org) version 3.2.2 for Windows.
RESULTS
Clinicopathologic Characteristics
A total of 94 primary solitary fibrous tumors from 50 women and 44 men [age 24−88 years (median 60 years)] were studied. Of these, 31 (33%) solitary fibrous tumors arose in the thorax (pleura, lung, or mediastinum) and 63 (67%) solitary fibrous tumors arose at an extra-thoracic soft tissue site (Supplementary Table 1). Tumors ranged in size from 1 cm to 34 cm (median 6.7 cm). Mitotic activity ranged from 0 to 45 mitotic figures per 10 HPFs (median, 3 mitotic figures per 10 HPFs). A subset of tumors was histologically classified further as malignant in 28, atypical in 8, cellular in 5, myxoid in 2, and lipomatous in 4 solitary fibrous tumors. Dedifferentiated areas were identified in 2 tumors (patients S13, S29). Of the 94 solitary fibrous tumors examined, immunohistochemical studies showed STAT6 expression in 86 (91%), CD34 expression in 89 (95%), and ≥2+ p53 nuclear expression in 8 and complete absence of p53 expression in 2 solitary fibrous tumors.
As per the proposed risk stratification model by Demicco et al.,8 46 patients (49%) were stratified in the low-risk group, 28 patients (30%) in the moderate-risk group, and 20 patients (21%) in the high-risk category. Limited treatment data were available for 76 patients. Tumor resection was performed in 75 patients, and radiation and/or chemotherapy (at any point in the disease course) was given to 11 patients. Outcome data were available for 68 patients with a median follow-up of 46 months (interquartile range, 25.5–70 months). At last follow-up, 50 of 68 patients were alive with no evidence of disease, 8 were alive with disease or history of recurrence, 8 were dead of disease, and 2 were dead of other causes (Figure 1). Local recurrence occurred in 9 and distant metastasis in 3 patients. Supplementary Table 1 gives details of clinicopathologic features.
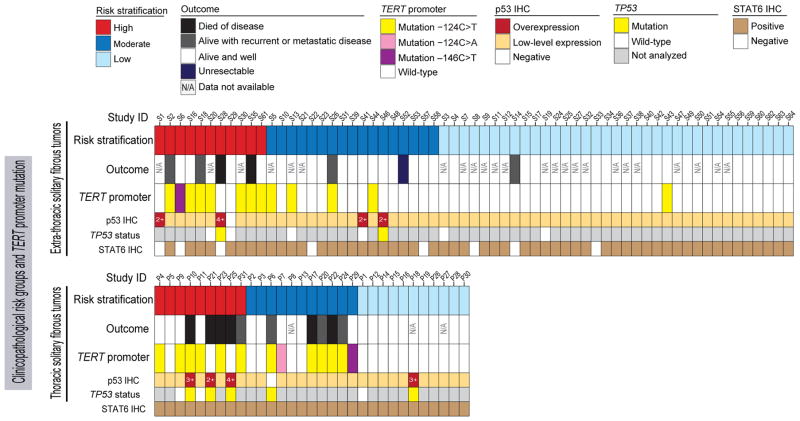
Figure 1. Association of TERT promoter mutations with risk group and outcome in 63 patients with extra-thoracic solitary fibrous tumors and 31 patients with thoracic solitary fibrous tumors.
Solitary fibrous tumors with TERT promoter mutations in both the soft tissue and pleural subgroups were clustered in the high- and moderate-risk clinicopathologic categories. IHC, immunohistochemistry.
TERT Promoter Mutations in solitary fibrous tumor
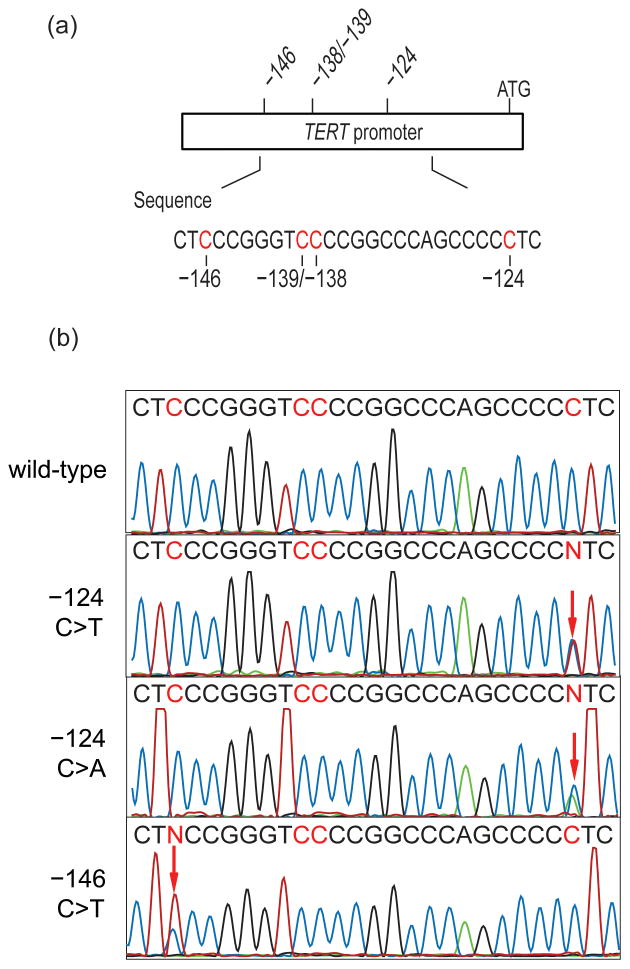
TERT promoter mutations were identified in 26 of 94 (28%) solitary fibrous tumors (Figure 1), including the −124C>T mutation in 23 (88%), the −146C>T mutation in 2 (8%), and the −124C>A mutation in 1 (4%) (Figure 2). Overall, TERT promoter mutations were identified in 1 of 46 (2%) low-risk solitary fibrous tumors, 11 of 28 (40%) moderate-risk solitary fibrous tumors, and 14 of 20 (70%) high-risk solitary fibrous tumors (Figure 3). TERT promoter mutations were identified in 14 of 28 (50%) histologically malignant solitary fibrous tumors, 1 of 8 atypical solitary fibrous tumors, 1 of 2 dedifferentiated solitary fibrous tumors, 1 of 2 myxoid solitary fibrous tumors, 3 of 5 cellular solitary fibrous tumors (Figure 3), and none of the 4 lipomatous solitary fibrous tumors. Only 10% of solitary fibrous tumors in patients <55 years old, as compared to 40% of solitary fibrous tumors in patients ≥55 years old, harbored TERT promoter mutations. TERT promoter mutations were present in 64% of solitary fibrous tumors ≥15 cm, 54% of solitary fibrous tumors 10 cm to <15 cm, 25% of solitary fibrous tumors 5 cm to <10 cm, and 6% of solitary fibrous tumors <5 cm in size.
Figure 2. TERT promoter mutations in solitary fibrous tumor.
(a) Schematic of the TERT promoter showing the position of mutations in solitary fibrous tumor samples in relation to the ATG start site. (b) Sequence chromatogram of a wild-type and mutated −124C>T, −124C>A, and−146C>T sequence from top to bottom. The mutated sequences are heterozygous at the chr5, 1,295,228 or the chr5, 1,295,250 residue, which is indicated as an N in the printed sequence (red arrow) and represents 2 overlapping peaks: a C (wild-type allele) and a T or A (mutant allele).
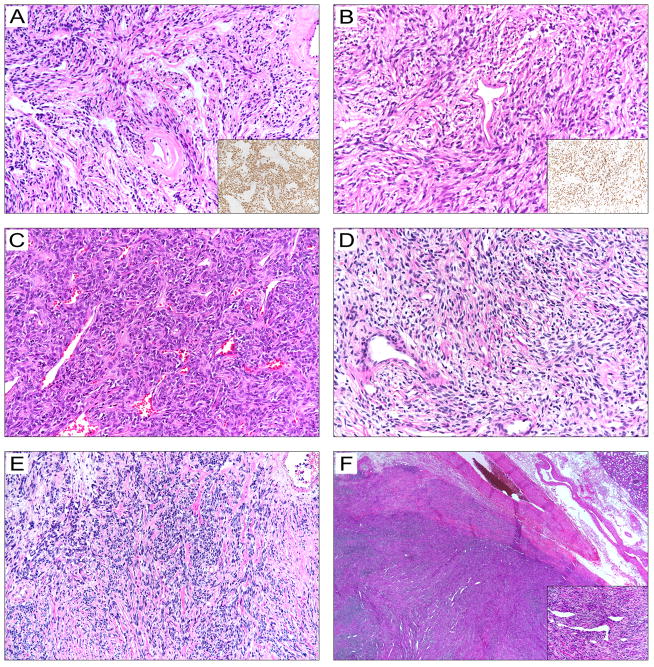
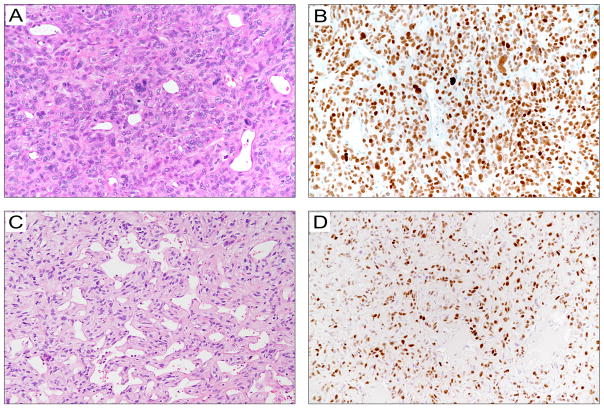
Figure 3. Histopathological features of solitary fibrous tumors with the −124C>T TERT promoter mutation.
A. A 5.6-cm pleural-based, moderate-risk, classical solitary fibrous tumor in a 78-year-old female (patient P17) that resulted in multiple metastatic pleural nodules and eventually death 144 months after diagnosis. The inset shows a STAT6 immunostained section.
B. A 14-cm histologically malignant pleural solitary fibrous tumor in a 67-year-old man (patient P23). The tumor recurred 9 years later and caused death. The inset shows a STAT6 immunostained section.
C. An 11-cm histologically malignant abdominal wall solitary fibrous tumor in a 77-year-old man (patient S35) that metastasized to the femur and eventually caused death 30 months after diagnosis.
D. A 6-cm histologically classical moderate-risk solitary fibrous tumor in the posterior mediastinum of a 71-year-old woman (patient P24) that resulted in local relapse in 4 years and persistent disease at last follow-up.
E. A 22-cm histologically malignant solitary fibrous tumor in the chest wall of a 55-year-old man (patient S18), which resulted in hypoglycemia symptoms at presentation and local recurrence 66 months after resection.
F. A 2.5-cm solitary fibrous tumor with cellular features in the perirenal fat of a 35-year-old man (patient S43) with no evidence of recurrence 35 months after excision.
TP53 Mutations in solitary fibrous tumor
Mutational analysis was performed in 9 solitary fibrous tumors with aberrant p53 expression (overexpression or complete absence of expression) and 7 solitary fibrous tumors with low-levels of p53 expression. A single base pair substitution that would be expected to lead to a missense change, or a frame-shift mutation, was identified in exon 5 or exon 6 of the TP53 in 7 of 9 solitary fibrous tumors with aberrant p53 expression (Figure 1; Supplementary Table 1). The 7 solitary fibrous tumors with low-level p53 expression harbored the wild-type TP53.
Association of TERT Promoter Mutations with Clinicopathologic Characteristics
We explored the association of TERT promoter mutations with clinicopathologic characteristics and outcome. TERT promoter mutations were not associated with gender (P=0.81; Table 1) or tumor site (P = 0.17; Table 1). TERT promoter mutations were present in 13 of 31 (42%) thoracic solitary fibrous tumors and 13 of 63 (21%) extra-thoracic soft tissue solitary fibrous tumors (P=0.048). However, the difference between the prevalence of mutations in thoracic and extra-tho-racic solitary fibrous tumors was not statistically significant after adjusting for risk groups (P=0.248).
Table 1.
Association of TERT promoter mutations with clinicopathological characteristics in 94 patients with solitary fibrous tumor
| All patients (n=94) | TERT Promoter | P-value | ||
|---|---|---|---|---|
|
| ||||
| Wild-type | Mutated | |||
|
| ||||
| Gender | ||||
| Female | 50 | 37 | 13 | P = 0.8 |
| Male | 44 | 31 | 13 | |
|
| ||||
| Age (years) | ||||
| Mean ± SD | 58.4 ± 15.4 | 55.8 ± 15.8 | 65.2 ± 12.1 | P = 0.006 |
|
| ||||
| Tumor location | ||||
| Thoracic/pleural | 31 | 18 | 13 | |
| Extra-thoracic | 63 | 50 | 13 | |
| Trunk | 20 | 15 | 5 | P = 0.17 |
| Extremity | 17 | 14 | 3 | |
| Abdomen/pelvis | 13 | 9 | 4 | |
| Head and neck | 13 | 12 | 1 | |
|
| ||||
| Tumor size (cm) | ||||
| Mean ± SD | 8.1 ± 6.2 | 6.13 ± 4.2 | 13.4 ± 7.5 | P = 2 × 10−6 |
|
| ||||
| Mitotic rate | ||||
| 0 | 17 | 17 | 0 | |
| 1–3 | 36 | 31 | 5 | P = 1.3 × 10−5 |
| ≥4 | 41 | 20 | 21 | |
|
| ||||
| Risk group | ||||
| Low-risk | 46 | 45 | 1 | |
| Moderate-risk | 28 | 17 | 11 | P = 2.9 × 10−9 |
| High-risk | 20 | 6 | 14 | |
Abbreviation: SD, standard deviation.
TERT promoter mutations were significantly associated with high-risk clinicopathologic characteristics. Patients with TERT promoter mutations were significantly older (P = 0.006, Table 1), had larger tumor size (P=0.000002, Table 1), greater tumor size score (P=0.00003), greater mitotic rate score (P=0.000013), and were more often classified in the higher risk categories (P=2.9 × 10−9, Table 1) than those with the wild-type TERT promoter.
Effect of TERT Promoter Mutations on Outcome in Patients with solitary fibrous tumor
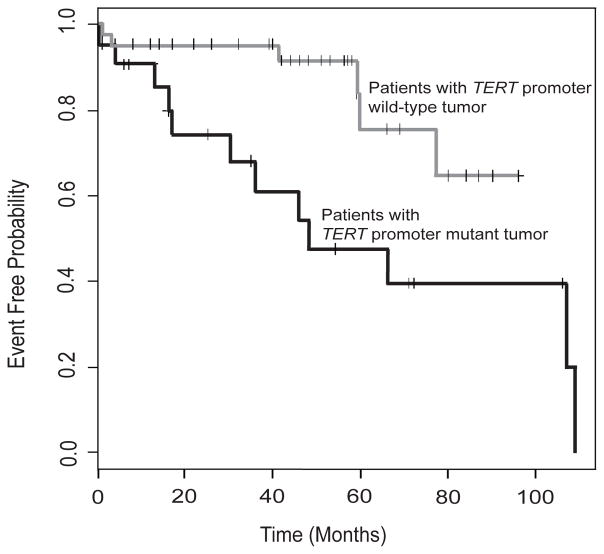
Outcome data were available for 68 patients. Kaplan-Meier analysis of event-free survival (Figure 5) revealed that patients with TERT promoter mutations had a significantly poorer event-free survival than those with the wild-type TERT promoter (P=0.0082). An adverse event (relapse, resistant disease, progressive disease, death) occurred in 16 of 68 (24%) patients, 12 patients (75%) with TERT promoter mutations and 4 patients with the wild-type TERT promoter (Figure 1). Of the solitary fibrous tumors from 4 patients with the wild-type TERT promoter who had an adverse event (Table 1), 3 were histologically malignant tumors (patients P21, P25, S28) with immunohistochemical evidence of p53 overexpression (Figure 4) and 1 was a lipomatous solitary fibrous tumor with low-risk clinicopathologic attributes (patient S14; Supplementary Table 1).
Figure 5. Association of TERT promoter mutations with event-free survival.
Kaplan–Meier event-free survival estimates showing that patients with TERT promoter mutations (black line) had a significantly poorer event-free survival than those with the wild-type TERT promoter (gray line) (P = 0.0082).
Figure 4. Histopathological features of solitary fibrous tumors with the wild-type TERT promoter.
A, B. A 15-cm histologically malignant pleural solitary fibrous tumor with marked nuclear pleomorphism (A) and strong p53 expression by immunohistochemistry (B) in a 78-year-old man (patient P25). The patient succumbed to the disease 41 months after diagnosis.
C, D. A 13-cm clinicopathologicly high-risk hemangiopericytoma-like solitary fibrous tumor (C) in the buttock of a 63-year-old man (patient S28) with p53 overexpression by immunohistochemistry (D), which resulted in distant metastasis and death 6 months after diagnosis.
We also analyzed the association of TERT promoter mutations with event-free survival after adjusting for other factors. This analysis fits multiple two-predictor Cox models that use TERT promoter mutation and either age, tumor size, risk group (low risk vs. others), and mitotic rate (mitotic figures per 10 HPFs) as predictors of event-free survival. Of these models, the model using mitotic rate and TERT promoter mutations had the best fit according to the Akaike Information Criterion. In this model, TERT promoter mutation was associated with a 3.43-fold increase in the rate of failure events (P=0.01) and each unit increase in mitotic rate was associated with a 1.06-fold increase in the rate of failure events (P=0.0009). Thus, TERT promoter mutation remains an important prognostic factor after accounting for other factors.
TERT mRNA Expression Levels
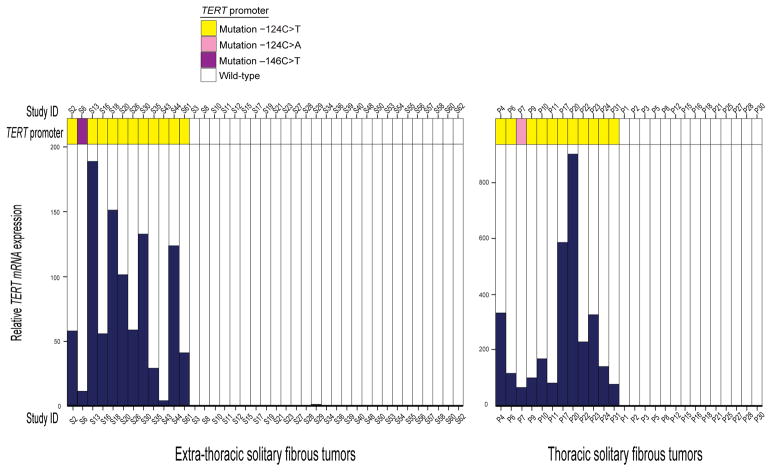
Sufficient RNA was available from 65 solitary fibrous tumors (39 soft tissue and 26 pleural) to perform real-time quantitative reverse transcription PCR. TERT mRNA was undetectable or detected at very low levels in solitary fibrous tumors with the wild-type TERT promoter. Therefore, a solitary fibrous tumor with the wild-type TERT promoter with the highest TERT mRNA expression from the soft tissue and pleural subgroups (S29 and P8) was selected as the reference. Real-time quantitative reverse transcription PCR showed higher levels of TERT mRNA expression in each of the TERT promoter mutant solitary fibrous tumors than in those with the wild-type TERT promoter, with a mean 79-fold and 260-fold increase relative to the reference values for soft tissue and pleural solitary fibrous tumors, respectively (Figure 6).
Figure 6. Relative TERT mRNA expression by real-time quantitative reverse transcription PCR in 39 soft tissue and 26 pleural/thoracic solitary fibrous tumors.
Comparison of TERT mRNA expression in solitary fibrous tumors with and without hotspot TERT promoter mutations for the soft tissue and thoracic subgroups showed higher expression in all solitary fibrous tumors with the TERT promoter mutation than those with the wild-type TERT promoter. The bar for each sample shows the average TERT mRNA expression, normalized to GAPDH expression as the endogenous standard, for 3 experiments.
DISCUSSION
We studied 94 biologically diverse thoracic and extra-thoracic solitary fibrous tumors to determine the association of TERT promoter mutations on the outcome of patients with solitary fibrous tumors. The frequency of cancer-associated TERT promoter mutations in our cohort was 28%. The most prevalent mutations were −124C>T at position −124 bp (Chr5:1,295,228 hg19 co-ordinate) from the ATG translation start site. Much less frequently, −146C>T at position −146 bp (1,295,250) or another variant mutation was present (Figure 2). TERT promoter mutations were associated with adverse patient outcome and with larger tumor size, older patient age, and mitotic rate in solitary fibrous tumor.
The difficulty in predicting the likelihood of local recurrence or the risk of distant metastasis in solitary fibrous tumor has prompted the development of several risk stratification models that are based on a multitude of clinical and pathological parameters.8,42–44 In this study, we used the model proposed by Demicco et al.,8 because it takes into account objective variables that have been shown to be predictive of outcome in independent studies across different anatomic sites.13 We found that TERT promoter mutations had a strong association with risk categories, and, except in 1 case, they occurred only in patients stratified in the high- and moderate-risk categories (Figure 1).
The prevalence of TERT promoter mutations in our cohort is slightly higher than that reported for extracranial solitary fibrous tumors in previous studies.21,33,37 TERT promoter mutations were reported in 5 of 34 (15%) extracranial solitary fibrous tumors in the study by Akaike et al.,21 4 of 31 (13%) soft tissue solitary fibrous tumors in the study by Koelsche et al,33 and 2 of 10 (20%) solitary fibrous tumors in the study by Killela et al.37 More than 50% of the solitary fibrous tumors in our cohort belonged to the moderate- or high-risk category. Therefore, it is possible that the prevalence of TERT promoter mutations in our cohort is an overestimate. Similar to our results, the TERT promoter mutations identified previously in solitary fibrous tumors were primarily −124C>T mutations.21,33,37
We also demonstrated that TERT promoter mutations correlate with TERT mRNA expression in solitary fibrous tumor. Telomerase reactivation is a hallmark of cancer cells and essential in driving cellular immortality. The association of TERT promoter mutations with reduced survival in patients with solitary fibrous tumor in our study is consistent with the finding of Akaike et al. that disease-free survival in patients with TERT promoter mutation is lower than that for patients with the wild-type TERT promoter in solitary fibrous tumor.21 Our findings are also consistent with the effect of these mutations on the prognosis of patients with other tumor types, such as bladder cancer,45 melanoma,46,47 brain tumors,48 papillary thyroid carcinoma,49 and the biologically indeterminate spitzoid neoplasms.38
However, this association of TERT promoter mutations with risk category and/or disease outcome in our study was not seen in some cases. For example, 6 of the 20 solitary fibrous tumors in the high-risk category had no TERT promoter mutation and 3 of which behaved in a clinically malignant fashion (Figure 1). The small subset of clinically malignant solitary fibrous tumors without TERT promoter mutations in our cohort shared the presence of TP53 mutations. Although the molecular mechanisms underpinning telomere maintenance among this solitary fibrous tumor subset are unknown, it appears that these tumors are under a different set of genetic constraints other than TERT promoter mutations to maintain their telomere length. Moreover, TP53 alterations were associated with pronounced nuclear pleomorphism on light microscopy evaluation and high-level or complete absence of p53 expression in immunohistochemical analysis (Figure 4). Overexpression of the p53 protein suggests the presence of a TP53 missense mutation, whereas complete absence of expression suggests biallelic loss-of-function mutations.50,51 Most solitary fibrous tumors in our study showed low levels of p53 expression and no TP53 mutations. In general, TP53 mutations are relatively uncommon in solitary fibrous tumor, but the acquisition of these mutations can contribute to dedifferentiation.5,52,53 In addition, a low-risk lipomatous solitary fibrous tumor in a patient who experienced local recurrence (patient S14) was also negative for TERT promoter mutation, but the margin status of the original resection in this patient was indeterminate. This case is seemingly an outlier in our cohort, but it shows that neither the clinicopathological criteria nor the molecular markers are entirely perfect in predicting outcome.
Real-time quantitative reverse transcription PCR showed undetectable or very low TERT mRNA expression levels in high-risk solitary fibrous tumors with the wild-type TERT promoter (Figure 6). Therefore, it is possible that alternative lengthening of telomeres, a telomerase-independent mechanism, operates in a small fraction of solitary fibrous tumors in the high-risk category. The molecular events governing the maintenance of telomeres in the subset of malignant solitary fibrous tumors with the wild-type TERT promoter remain to be determined.
STAT6 expression was not detected by immunohistochemistry in several cases, even though the morphologic features of these tumors were consistent with a diagnosis of solitary fibrous tumor. Overall, 91% of the tumors in our study were STAT6 positive (Figure 1). The lack of STAT6 expression in a few cases might be due to the longevity of samples rather than an alternative diagnosis. The NAB2–STAT6 fusion product in solitary fibrous tumor is localized to the nucleus, which can be detected by using an antibody directed against the C terminus of STAT6. This antibody has been proven to be a highly specific and sensitive marker of solitary fibrous tumor.17,39,54 Although the frequency of STAT6 expression in our cohort was slightly below the rate reported in most immunohistochemical studies on solitary fibrous tumor,17,39,55–57 it is consistent with the results in the series in which older samples were examined.54
Although certain translocation breakpoints for the NAB2–STAT6 fusion gene have been associated with prognosis in solitary fibrous tumor, the prognostic implications of fusion variants are still controversial.18–21 We did not study the fusion type in our study, and it will be interesting to explore the relation of variant fusions with TERT promoter mutations in follow-up investigations. Also, evidence suggests that the effect of TERT promoter mutations on TERT expression can be modified in the presence of a common single-nucleotide polymorphism rs2853669.45,46 In our study, every sample with a TERT promoter mutation was associated with TERT expression; therefore, the potential influence of the rs2853669 polymorphism was not evaluated. Whether this polymorphism influences TERT expression in the setting of solitary fibrous tumor remains to be addressed in future studies.
Conventional chemotherapy agents have limited efficacy in solitary fibrous tumor.55,56 Currently, surgery remains the mainstay of management, 58,59 and the extent of surgical resection needs to be tailored according to the predicted clinical behavior. The relative unpredictability of the clinical behavior of solitary fibrous tumor can pose a challenge in decision making. Our results suggest that complete resection of solitary fibrous tumors harboring the TERT promoter mutation is critical and closer clinical monitoring and follow-up of such patients is probably warranted.
In conclusion, our data support the predictive value of TERT promoter mutations to identify high-risk patients with solitary fibrous tumor. TERT promoter mutations are the first potential molecular marker of prognosis in solitary fibrous tumor with promising applications in the clinic. The use of TERT promoter mutations in conjunction with existing clinicopathologic risk assessment is expected to improve the accuracy of predicting outcomes in patients with solitary fibrous tumor. The performance of TERT promoter mutations as an ancillary predictive marker for risk stratification in the clinic needs to be determined in future large-scale validation studies.
Supplementary Material
Acknowledgments
The authors thank the following colleagues who kindly submitted the cases and provided clinical follow-up data when available: Dr. R. Barber, Lower Hutt, New Zealand, Dr. E. Burks, Burlington, MA, USA, Dr. L. van Walleghem, Brussels, Belgium, Dr. S. Ries, Fountain Valley, CA, USA, Dr. A. Moran, Philadelphia, PA, USA, Dr. R. S. Aronsohn, Boca Raton, FL, Dr. G. Mazzoleni, Bolzano, Italy, Dr. E. Kay, Dublin, Ireland, Dr. A. Srivastava, Boston, MA, USA, Dr. S. Maia-Cohen, New York, NY, USA, Dr. P. de Paepe, Dr. R. N. Shah, Miami, FL, USA, Dr. Y. W. Rhee, Fall River, AM, USA Dr. M. J. Goldfischer, Hackensack, NJ, USA, Dr. H. Domanski, Skåne, Sweden, Dr. S. Zheng, Worcester, MA, USA, Dr. K. Allan, Brighton, United Kingdom, Dr. H. D. Epstein, Newport Beach, CA, USA, Dr. D. Ben-Dor, Ashkelon, Israel, Dr. D. J. Bylund, San Diego, CA, USA, Dr. J.-M. Guinebretiére, Saint Cloud, France, Dr. A. Khan, Worcester, MA, USA, Dr. V. Liu, Iowa City, IA, USA, Dr. M. Gordon, Dallas, TX, USA, Dr. C. Li, Columbus, OH, USA. Dr. D. E. Fish, Maidstone, United Kingdom, Dr. M. Paláu, Bogota, Columbia, Dr. Alvarez, Orlando, FL, USA, Dr. J. D. Wilson, Royal Oak, MI, USA, Dr. D. S. Brenner, Dover, DE, USA, Dr. J. L. Everett Jr., Dover, DE, USA, Dr. Dr. J. B. Dupont, Baton Rouge, LA, USA, Dr. B. E. Halliday, Scottsdale, AZ, USA, Dr. L. D. Wells, Lowell, MA, USA, Dr. P. Ferlisi, South Weymouth, MA, USA, Dr. B. Mario-Singh, Escondido, CA, USA, Dr. S. G. Bregman, Syracuse, NY, USA, Dr. G. Kunz, Louisville, KY, USA, Dr. J.-Y. Tan, Hackensack, NJ, USA, Dr. J. J. Ye, Bangor, ME, USA, Dr. J. Li, Hackensack, NJ, USA, Dr. P. Lysiak, Concord, MA, USA, Dr. T. E. Abbott, Newton, MA, USA, Dr. T. A. Bolton, Jupiter, FL, USA, Dr. M. E. Keohane, M.D., Auburn, CA, USA.
Funding: This study was supported in part by ALSAC.
Footnotes
This work was presented in part at the 2016 United States and Canadian Academy of Pathology Annual Meeting in Seattle, WA.
References
- 1.Fletcher CDM, Bridge JA, Lee JC. Extrapleural solitary fibrous tumor. In: Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F, editors. WHO Classification of Tumors of Soft Tissue and Bone. 4. International Agency of Research on Cancer (IARC); Lyon: 2013. pp. 80–82. [Google Scholar]
- 2.Vallat-Decouvelaere AV, Dry SM, Fletcher CD. Atypical and malignant solitary fibrous tumors in extrathoracic locations: evidence of their comparability to intra-thoracic tumors. Am J Surg Pathol. 1998;22:1501–11. doi: 10.1097/00000478-199812000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Hanau CA, Miettinen M. Solitary fibrous tumor: histological and immunohistochemical spectrum of benign and malignant variants presenting at different sites. Hum Pathol. 1995;26:440–9. doi: 10.1016/0046-8177(95)90147-7. [DOI] [PubMed] [Google Scholar]
- 4.de Saint Aubain Somerhausen N, Rubin BP, Fletcher CD. Myxoid solitary fibrous tumor: a study of seven cases with emphasis on differential diagnosis. Mod Pathol. 1999;12:463–71. [PubMed] [Google Scholar]
- 5.Mosquera JM, Fletcher CD. Expanding the spectrum of malignant progression in solitary fibrous tumors: a study of 8 cases with a discrete anaplastic component--is this dedifferentiated SFT? Am J Surg Pathol. 2009;33:1314–21. doi: 10.1097/pas.0b013e3181a6cd33. [DOI] [PubMed] [Google Scholar]
- 6.Guillou L, Gebhard S, Coindre JM. Lipomatous hemangiopericytoma: a fat-containing variant of solitary fibrous tumor? Clinicopathologic, immunohistochemical, and ultrastructural analysis of a series in favor of a unifying concept. Hum Pathol. 2000;31:1108–15. doi: 10.1053/hupa.2000.9777. [DOI] [PubMed] [Google Scholar]
- 7.Cranshaw IM, Gikas PD, Fisher C, et al. Clinical outcomes of extra-thoracic solitary fibrous tumours. Eur J Surg Oncol. 2009;35:994–8. doi: 10.1016/j.ejso.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 8.Demicco EG, Park MS, Araujo DM, et al. Solitary fibrous tumor: a clinicopathological study of 110 cases and proposed risk assessment model. Mod Pathol. 2012;25:1298–306. doi: 10.1038/modpathol.2012.83. [DOI] [PubMed] [Google Scholar]
- 9.Gold JS, Antonescu CR, Hajdu C, et al. Clinicopathologic correlates of solitary fibrous tumors. Cancer. 2002;94:1057–68. [PubMed] [Google Scholar]
- 10.England DM, Hochholzer L, McCarthy MJ. Localized benign and malignant fibrous tumors of the pleura. A clinicopathologic review of 223 cases. Am J Surg Pathol. 1989;13:640–58. doi: 10.1097/00000478-198908000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Wilky BA, Montgomery EA, Guzzetta AA, Ahuja N, Meyer CF. Extrathoracic location and “borderline” histology are associated with recurrence of solitary fibrous tumors after surgical resection. Ann Surg Oncol. 2013;20:4080–9. doi: 10.1245/s10434-013-3241-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Houdt WJ, Westerveld CM, Vrijenhoek JE, et al. Prognosis of solitary fibrous tumors: a multicenter study. Ann Surg Oncol. 2013;20:4090–5. doi: 10.1245/s10434-013-3242-9. [DOI] [PubMed] [Google Scholar]
- 13.Doyle LA, Fletcher CD. Predicting behavior of solitary fibrous tumor: are we getting closer to more accurate risk assessment? Ann Surg Oncol. 2013;20:4055–6. doi: 10.1245/s10434-013-3243-8. [DOI] [PubMed] [Google Scholar]
- 14.Chmielecki J, Crago AM, Rosenberg M, et al. Whole-exome sequencing identifies a recurrent NAB2-STAT6 fusion in solitary fibrous tumors. Nat Genet. 2013;45:131–2. doi: 10.1038/ng.2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson DR, Wu YM, Kalyana-Sundaram S, et al. Identification of recurrent NAB2-STAT6 gene fusions in solitary fibrous tumor by integrative sequencing. Nat Genet. 2013;45:180–5. doi: 10.1038/ng.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohajeri A, Tayebwa J, Collin A, et al. Comprehensive genetic analysis identifies a pathognomonic NAB2/STAT6 fusion gene, nonrandom secondary genomic imbalances, and a characteristic gene expression profile in solitary fibrous tumor. Genes Chromosomes Cancer. 2013;52:873–86. doi: 10.1002/gcc.22083. [DOI] [PubMed] [Google Scholar]
- 17.Schweizer L, Koelsche C, Sahm F, et al. Meningeal hemangiopericytoma and solitary fibrous tumors carry the NAB2-STAT6 fusion and can be diagnosed by nuclear expression of STAT6 protein. Acta Neuropathol. 2013;125:651–8. doi: 10.1007/s00401-013-1117-6. [DOI] [PubMed] [Google Scholar]
- 18.Tai HC, Chuang IC, Chen TC, et al. NAB2-STAT6 fusion types account for clinicopathological variations in solitary fibrous tumors. Mod Pathol. 2015;28:1324–35. doi: 10.1038/modpathol.2015.90. [DOI] [PubMed] [Google Scholar]
- 19.Huang SC, Li CF, Kao YC, et al. The clinicopathological significance of NAB2-STAT6 gene fusions in 52 cases of intrathoracic solitary fibrous tumors. Cancer medicine. 2016;5:159–68. doi: 10.1002/cam4.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barthelmess S, Geddert H, Boltze C, et al. Solitary fibrous tumors/hemangiopericytomas with different variants of the NAB2-STAT6 gene fusion are characterized by specific histomorphology and distinct clinicopathological features. Am J Pathol. 2014;184:1209–18. doi: 10.1016/j.ajpath.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 21.Akaike K, Kurisaki-Arakawa A, Hara K, et al. Distinct clinicopathological features of NAB2-STAT6 fusion gene variants in solitary fibrous tumor with emphasis on the acquisition of highly malignant potential. Hum Pathol. 2015;46:347–56. doi: 10.1016/j.humpath.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 22.Blackburn EH. Structure and function of telomeres. Nature. 1991;350:569–73. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- 23.Palm W, de Lange T. How shelterin protects mammalian telomeres. Annu Rev Genet. 2008;42:301–34. doi: 10.1146/annurev.genet.41.110306.130350. [DOI] [PubMed] [Google Scholar]
- 24.Kilian A, Bowtell DD, Abud HE, et al. Isolation of a candidate human telomerase catalytic subunit gene, which reveals complex splicing patterns in different cell types. Hum Mol Genet. 1997;6:2011–9. doi: 10.1093/hmg/6.12.2011. [DOI] [PubMed] [Google Scholar]
- 25.Gunes C, Lichtsteiner S, Vasserot AP, Englert C. Expression of the hTERT gene is regulated at the level of transcriptional initiation and repressed by Mad1. Cancer Res. 2000;60:2116–21. [PubMed] [Google Scholar]
- 26.Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer. 1997;33:787–91. doi: 10.1016/S0959-8049(97)00062-2. [DOI] [PubMed] [Google Scholar]
- 27.Horn S, Figl A, Rachakonda PS, et al. TERT promoter mutations in familial and sporadic melanoma. Science. 2013;339:959–61. doi: 10.1126/science.1230062. [DOI] [PubMed] [Google Scholar]
- 28.Huang FW, Hodis E, Xu MJ, et al. Highly recurrent TERT promoter mutations in human melanoma. Science. 2013;339:957–9. doi: 10.1126/science.1229259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vinagre J, Pinto V, Celestino R, et al. Telomerase promoter mutations in cancer: an emerging molecular biomarker? Virchows Arch. 2014;465:119–33. doi: 10.1007/s00428-014-1608-4. [DOI] [PubMed] [Google Scholar]
- 30.Huang DS, Wang Z, He XJ, et al. Recurrent TERT promoter mutations identified in a large-scale study of multiple tumour types are associated with increased TERT expression and telomerase activation. Eur J Cancer. 2015;51:969–76. doi: 10.1016/j.ejca.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bell RJ, Rube HT, Kreig A, et al. Cancer The transcription factor GABP selectively binds and activates the mutant TERT promoter in cancer. Science. 2015;348:1036–9. doi: 10.1126/science.aab0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campanella NC, Penna V, Abrahao-Machado LF, et al. TERT promoter mutations in soft tissue sarcomas. Int J Biol Markers. 2015 doi: 10.5301/jbm.5000168. [DOI] [PubMed] [Google Scholar]
- 33.Koelsche C, Renner M, Hartmann W, et al. TERT promoter hotspot mutations are recurrent in myxoid liposarcomas but rare in other soft tissue sarcoma entities. J Exp Clin Cancer Res. 2014;33:33. doi: 10.1186/1756-9966-33-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saito T, Akaike K, Kurisaki-Arakawa A, et al. TERT promoter mutations are rare in bone and soft tissue sarcomas of Japanese patients. Molecular and clinical oncology. 2016;4:61–4. doi: 10.3892/mco.2015.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Henson JD, Reddel RR. Assaying and investigating Alternative Lengthening of Telomeres activity in human cells and cancers. FEBS Lett. 2010;584:3800–11. doi: 10.1016/j.febslet.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 36.Koelsche C, Sahm F, Capper D, et al. Distribution of TERT promoter mutations in pediatric and adult tumors of the nervous system. Acta Neuropathol. 2013;126:907–15. doi: 10.1007/s00401-013-1195-5. [DOI] [PubMed] [Google Scholar]
- 37.Killela PJ, Reitman ZJ, Jiao Y, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci U S A. 2013;110:6021–6. doi: 10.1073/pnas.1303607110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee S, Barnhill RL, Dummer R, et al. TERT Promoter Mutations Are Predictive of Aggressive Clinical Behavior in Patients with Spitzoid Melanocytic Neoplasms. Sci Rep. 2015;5:11200. doi: 10.1038/srep11200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doyle LA, Vivero M, Fletcher CD, Mertens F, Hornick JL. Nuclear expression of STAT6 distinguishes solitary fibrous tumor from histologic mimics. Mod Pathol. 2014;27:390–5. doi: 10.1038/modpathol.2013.164. [DOI] [PubMed] [Google Scholar]
- 40.Pinto EM, Ribeiro RC, Kletter GB, et al. Inherited germline TP53 mutation encodes a protein with an aberrant C-terminal motif in a case of pediatric adrenocortical tumor. Fam Cancer. 2011;10:141–6. doi: 10.1007/s10689-010-9392-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akaike H. Information theory as an extension of the maximum likelihood principle. In: Petrov BN, Csaki F, editors. Second International Symposium on Information Theory; Budapest: Akademiai Kiado; 1973. pp. 267–81. [Google Scholar]
- 42.de Perrot M, Fischer S, Brundler MA, Sekine Y, Keshavjee S. Solitary fibrous tumors of the pleura. Ann Thorac Surg. 2002;74:285–93. doi: 10.1016/s0003-4975(01)03374-4. [DOI] [PubMed] [Google Scholar]
- 43.Tapias LF, Mercier O, Ghigna MR, et al. Validation of a scoring system to predict recurrence of resected solitary fibrous tumors of the pleura. Chest. 2015;147:216–23. doi: 10.1378/chest.14-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tapias LF, Mino-Kenudson M, Lee H, et al. Risk factor analysis for the recurrence of resected solitary fibrous tumours of the pleura: a 33-year experience and proposal for a scoring system. Eur J Cardiothorac Surg. 2013;44:111–7. doi: 10.1093/ejcts/ezs629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rachakonda PS, Hosen I, de Verdier PJ, et al. TERT promoter mutations in bladder cancer affect patient survival and disease recurrence through modification by a common polymorphism. Proc Natl Acad Sci U S A. 2013;110:17426–31. doi: 10.1073/pnas.1310522110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nagore E, Heidenreich B, Rachakonda S, et al. TERT promoter mutations in melanoma survival. Int J Cancer. 2016 doi: 10.1002/ijc.30042. [DOI] [PMC free article] [PubMed]
- 47.Griewank KG, Murali R, Puig-Butille JA, et al. TERT promoter mutation status as an independent prognostic factor in cutaneous melanoma. J Natl Cancer Inst. 2014:106. doi: 10.1093/jnci/dju246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simon M, Hosen I, Gousias K, et al. TERT promoter mutations: a novel independent prognostic factor in primary glioblastomas. Neuro Oncol. 2015;17:45–52. doi: 10.1093/neuonc/nou158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xing M, Liu R, Liu X, et al. BRAF V600E and TERT promoter mutations cooperatively identify the most aggressive papillary thyroid cancer with highest recurrence. J Clin Oncol. 2014;32:2718–26. doi: 10.1200/JCO.2014.55.5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen X, Bahrami A, Pappo A, et al. Recurrent somatic structural variations contribute to tumorigenesis in pediatric osteosarcoma. Cell Rep. 2014;7:104–12. doi: 10.1016/j.celrep.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baas IO, Mulder JW, Offerhaus GJ, Vogelstein B, Hamilton SR. An evaluation of six antibodies for immunohistochemistry of mutant p53 gene product in archival colorectal neoplasms. J Pathol. 1994;172:5–12. doi: 10.1002/path.1711720104. [DOI] [PubMed] [Google Scholar]
- 52.Dagrada GP, Spagnuolo RD, Mauro V, et al. Solitary fibrous tumors: loss of chimeric protein expression and genomic instability mark dedifferentiation. Mod Pathol. 2015;28:1074–83. doi: 10.1038/modpathol.2015.70. [DOI] [PubMed] [Google Scholar]
- 53.Akaike K, Kurisaki-Arakawa A, Hara K, et al. Distinct clinicopathological features of NAB2-STAT6 fusion gene variants in solitary fibrous tumor with emphasis on the acquisition of highly malignant potential. Hum Pathol. 2014 doi: 10.1016/j.humpath.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 54.Demicco EG, Harms PW, Patel RM, et al. Extensive survey of STAT6 expression in a large series of mesenchymal tumors. Am J Clin Pathol. 2015;143:672–82. doi: 10.1309/AJCPN25NJTOUNPNF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cheah AL, Billings SD, Goldblum JR, et al. STAT6 rabbit monoclonal antibody is a robust diagnostic tool for the distinction of solitary fibrous tumour from its mimics. Pathology. 2014;46:389–95. doi: 10.1097/PAT.0000000000000122. [DOI] [PubMed] [Google Scholar]
- 56.Koelsche C, Schweizer L, Renner M, et al. Nuclear relocation of STAT6 reliably predicts NAB2-STAT6 fusion for the diagnosis of solitary fibrous tumour. Histopathology. 2014;65:613–22. doi: 10.1111/his.12431. [DOI] [PubMed] [Google Scholar]
- 57.Yoshida A, Tsuta K, Ohno M, et al. STAT6 immunohistochemistry is helpful in the diagnosis of solitary fibrous tumors. Am J Surg Pathol. 2014;38:552–9. doi: 10.1097/PAS.0000000000000137. [DOI] [PubMed] [Google Scholar]
- 58.DeVito N, Henderson E, Han G, et al. Clinical Characteristics and Outcomes for Solitary Fibrous Tumor (SFT): A Single Center Experience. PLoS One. 2015;10:e0140362. doi: 10.1371/journal.pone.0140362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Constantinidou A, Jones RL, Olmos D, et al. Conventional anthracycline-based chemotherapy has limited efficacy in solitary fibrous tumour. Acta Oncol. 2012;51:550–4. doi: 10.3109/0284186X.2011.626450. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.