Abstract
Leishmania mexicana infection causes localized skin lesions that can lead to tissue damage and permanent disfigurement if not resolved. Currently, recommended treatments include intravenous administration of Amphotericin B, which is undesirable due to the associated cost and patient burden related to receiving regular injections. In this study, we evaluated the effect of topical treatment with a nanoliposomal formulation of Amphotericin B that is penetrable to the skin (SinaAmphoLeish 0.4%) in mice infected with L. mexicana by using ulcerated (BALB/c) and non-ulcerated (129SVE) models. BALB/c mice received a 4 week treatment following ulcerated lesion development, while 129SVE mice received a 10 week treatment beginning at week 5 post-infection. Although mice from both models showed comparable susceptibility to L. mexicana infection after topical treatment with SinaAmphoLeish relative to controls, 129SVE mice displayed a transient decrease in lesion sizes which eventually became similar to control mice. On other hand this treatment resulted in no reduction in the lesion sizes in BALB/c mice. 129SVE treated mice exhibited greater IFN-γ, IL-4, and IL-10 cytokine levels and higher T-cell proliferation in re-stimulated draining lymph node cells. BALB/c mice showed no differences in cytokine responses between treated and control mice. These findings indicate that topical SinaAmphoLeish treatment is not likely to be effective in the treatment of cutaneous leishmaniasis caused by L. mexicana.
Keywords: Nanoliposomal amphotericin B, IFN-γ, IL-4, IL-10
1. Introduction
Leishmaniasis is a zoonotic, intracellular parasitic infection caused by members of the protozoan parasite Leishmania. Leishmania species cause infection in humans when transmitted via bites from its sandfly vector, Phlebotomus papatasi. Currently, over 12 million people are infected with leishmaniasis, with about 0.7–1.2 million people infected annually. Leishmaniasis is endemic in third world countries of the tropical and subtropical regions, and is especially prevalent in communities of lower socioeconomic classes. Because of its extensive impact and consequential annual economic cost, it has been designated as a neglected tropical disease by the Center for Disease Control and Prevention (CDC). The most common presentation of the disease is cutaneous leishmaniasis (CL), which causes painless, localized skin lesions. Without treatment, these lesions may resolve, or persist resulting in severe tissue damage and socially stigmatized disfigurement. The causative species of CL varies, and is dependent on the geographical location of transmission (Old World encompasses Asia and Africa, and New World consists of Central and South America). Leishmania mexicana is a prevalent cause of CL in the New World, and is a viable candidate for the study of new anti-leishmanial drugs and treatments.
Despite the prevalence of CL worldwide, there is currently no FDA approved drug indicated for the disease. For the past sixty years, pentavalent antimonials have been the leading treatment for CL cases. These drugs are known to cause liver, kidney, and cardiac toxicity, and other severe side effects (Veiga et al., 1985; Ribeiro et al., 1999). Treatment with pentavalent antimonials (Pentostam™ and Glucantime™), as well as other anti-leishmanial drugs, is usually via parenteral routes, often intravenously (Croft et al., 2006; Croft and Yardley, 2002). However, parenteral drug administration can create barriers to treatment in areas with insufficient medical care providers. Compounding these difficulties in treating CL is the fact that some strains of Leishmania have begun to exhibit resistance to pentavalent antimonial compounds (Chakravarty and Sundar, 2010). Liposomal amphotericin B is a newer drug that has been shown to be effective in the treatment of visceral leishmaniasis when administered intravenously. It has specific chemical properties that enable it to bind to ergosterol, a major component of the Leishmania parasite membrane, and causes the formation of pores, thereby inducing death by osmotic lysis (Ramos et al., 1996; Balasegaram et al., 2012). Other studies have shown that intravenous treatment of liposomal amphotericin B resulted in reduced lesion sizes in experimental Leishmania major infected BALB/c mice (Yardley and Croft, 1997). In addition, clinical studies have shown that liposomal amphotericin B treatment via the intravenous route is effective in the treatment of Leishmania tropica (Solomon et al., 2010) as well as other strains of old world and new world CL (Wortmann et al., 2010; Mushtag et al., 2016). Due to the endemic nature of CL in populations with limited access to medical care, effective and affordable drugs with patient-accessible delivery methods are desperately needed. Current treatments for CL are invasive and toxic. A topical treatment method is therefore desirable, because the ease of use and non-invasive nature of this form of treatment can potentially increase treatment accessibility and yield high levels of patient adherence and satisfaction, thereby resulting in a higher rate of resolution.
The Drugs for Neglected Diseases initiative (DNDi) recently started developing short, safe, efficacious and affordable treatments for CL caused by L. tropica and L. braziliensis (www.dndi.org). As a short-term strategy, DNDi initiated the process of developing a topical treatment based on amphotericin B aiming to improve the treatment strategies and combinations available. DNDi aspires to deliver a new topical treatment of Amphotericin B by 2020, which would ideally be at least 95% effective against all Leishmania species, easy to use with a short duration of 14–28 days, affordable and safe to use in pregnant and breastfeeding women.
A recent study has compared the efficacy of topical Amphotericin B versus intralesional Meglumine Antimoniate (Glucantime) in the treatment of CL caused by L. tropica, and L. major, in outpatient settings. The authors determined that topical administration of liposomal Amphotericin B has the same efficacy as intralesional administration of Glucantime (Layegh et al., 2011). In another recent study by Jaafari et al., topical treatment using nanoliposomal Amphotericin B (SinaAmphoLeish 0.1%, 0.2% and 0.4%) successfully reduced lesion sizes as well as splenic and lesion parasitic burdens in L. major infected BALB/c mice (US Patent application number: US 20150147382A1). In this present study, we determined the efficacy of daily topical treatment of SinaAmphoLeish (0.4%) in resolving CL caused by L. mexicana using ulcerative BALB/c and non-ulcerative 129SVE murine models. SinaAmphoLeish is an 80 nm semisolid nanoliposomal Amphotericin B gel, which allows for topical administration. Nano-liposomal preparations of Amphotericin B are non-toxic and are able to cross the biological barriers of the skin, targeting macrophages in the dermis and epidermis. This is the first report evaluating the efficacy of this safe, affordable and convenient treatment method against CL caused by L. mexicana.
2. Materials and methods
2.1. Mice
BALB/c female mice were purchased from Envigo (Harlan laboratories) Indianapolis, IN, USA. 129SVE female mice were purchased from Taconic Farms, Cambridge City, IN, USA. All experimental mice were maintained at an OSU-ULAR facility in compliance with OSU-IACUC regulations. All experiments were performed in accordance with IACUC approved protocols and methods.
2.2. Parasites and infection
L. mexicana (MNYC/BZ/62/m379) parasites were obtained and maintained as described previously (Cummings et al., 2012). L. mexicana amastigotes were aspirated from a previously infected stock mouse and cultured in vitro in M199 medium supplemented with 10% FBS, 1% Penicillin/streptomycin at 25 °C to generate metacyclic promastigotes. Promastigotes were maintained, passaged, and subsequently injected into experimental mice (5 × 106 parasites) subcutaneously into their shaven rumps.
2.3. Topical drug treatments and lesion measurements
BALB/c mice were infected with L. mexicana parasites for 8 weeks after which they developed ulcerated lesions on their back rumps. After 8 weeks infection, mice with similar lesion sizes were selected and randomized into control and treatment groups. Treatment groups were administered with SinaAmphoLeish 0.4% gel (Exir Nano Sina, Tehran, Iran) twice a day for 4 weeks. Control groups received Vaseline petroleum jelly. 129SVE mice were infected with L. mexicana parasites for 5 weeks. Following the 5 week infection period, mice developed lesions on their back rumps. We selected mice with similar lesion sizes and randomized them into control and treatment groups. Control group mice were treated with Vaseline petroleum jelly and the treatment group with SinaAmphoLeish 0.4% gel on their lesions twice every day for 10 weeks. Lesion sizes were measured by using a small animal caliper weekly and the area of the lesion was calculated by L × W, where L is the length and W is the width.
2.4. Quantitation of parasitic load
Mice were euthanized at week 15 post infection. Back rump lesions and draining lymph nodes were harvested from all experimental mice, mashed in Schneider’s medium supplemented with 10% FBS and 1% Penicillin/streptomycin. The suspensions were centrifuged at 3000 rpm and resuspended in 0.4 ml of Schneider’s media. A limiting dilution assay was performed as previously described (Rosas et al., 2005). Viability of parasites was determined at 3–7 days of parasite culture. The values reported represent the highest log dilution with viable parasites.
2.5. Cell proliferation
All experimental mice were euthanized at 15 weeks post infection and draining lymph nodes were harvested. Single cell suspensions were made at 3 × 106/ml and stimulated with 20 μg/ml freeze thawed L. mexicana Antigen (LmAg) in RMPI1640 medium supplemented with 10% FBS, 1% Penicillin/streptomycin and 1% HEPES for 72 h at 37 °C with 5% CO2. Proliferation of T cells was measured by an Alamar Blue assay method. Briefly, 10% Alamar Blue solution was added to the unstimulated control and LmAg stimulated wells at 60 h of incubation and absorbance was measured at 72 h at 570 nm and 600 nm. Percent reduction of Alamar Blue was calculated according to the manufacturer’s instructions based on the absorbance values at 570 and 600 nm.
2.6. Cytokine ELISA analysis
Culture supernatants were collected after 72 h incubation of lymph node cells with LmAg, and analyzed for the production of IL-4, IL-10, IFN-γ and TNF-α cytokines using the Sandwich ELISA method. Briefly, 96 well plates were coated with Capture antibodies for overnight, blocked and culture supernatants or standards were added for overnight. Plates were washed, respective biotinylated secondary antibodies were added, streptavidin-AKP and PNPP (p-nitrophenyl phosphate) substrates were used for detecting secondary antibodies. Capture and detection antibodies were obtained from BioLegend, San Diego, CA and cytokine standards were obtained from BD biosciences, San Jose, CA. The cytokine production was quantified by measuring the absorbance at 405 nm using Spectramax microplate reader and Softmax Pro software (Molecular Devices LLC, Sunnyvale, CA, USA).
2.7. Antibody ELISA
Mice were tail bled at weeks 8, 12 and 15 (for BALB/c mice) and weeks 8, 11 and 15 (for 129SVE mice) post infection, and sera were collected after centrifuging the blood at 6000 rpm for 7 min. L. mexicana specific IgG1 and IgG2a antibodies were detected by ELISA as previously described (Rosas et al., 2005) using HRP conjugated anti IgG1 and IgG2a antibodies and Streptavidin conjugated alkaline phosphatase enzyme (BD Biosciences, San Diego, CA). Briefly, 96 well plates were coated with 5 ug/ml freeze thawed L. mexicana antigen for overnight; plates were washed, blocked with 5% milk solution prepared in PBS- tween for an hour. Plates were washed serum samples were serially diluted at 1:100 ratio in PBS-Tween and incubated for 2 h. HRP-conjugated antibodies against mouse IgG1 and IgG2a were added, peroxidase substrate and 5% phosphoric acid were used as substrate and stopping solution respectively. Detection of IgG1 and IgG2a antibody titers were quantified by measuring the absorbance at 450 nm using Spectramax microplate reader and Softmax Pro software (Molecular Devices LLC, Sunnyvale, CA, USA).
2.8. Detection of nitric oxide levels by Greiss assay
Culture supernatants were collected after 72 h incubation of lymph node cells with LmAg, and analyzed for nitric oxide release by Griess Method. Briefly, triplicates of each sample or sodium nitrite standard were plated, equal volumes of Griess reagent was added to detect the formation of nitric oxide. The formation of nitrite was quantified by measuring the absorbance at 570 nm using Spectramax microplate reader and softmax Pro software (Molecular Devices LLC, Sunnyvale, CA, USA).
2.9. Statistical analysis
All data presented were acquired from 2 independent experiments with 3–5 mice per group. All data are represented as the average of 2 similar experiments combined. Unpaired Student’s t test was performed to compare statistical significance in back rump lesion sizes, parasite loads and cytokine levels. p value < 0.05 was considered significant. Antibody titers were analyzed by using Mann–Whitney U prime test.
3. Results
3.1. Transient decrease in lesion sizes of L. mexicana infected 129SVE mice after topical treatment with SinaAmphoLeish
To evaluate the effect of topical treatment of nanoliposomal amphotericin B in vivo, we utilized two mouse models of L. mexicana infection: a BALB/c model of ulcerative CL which best represents human disease, as well as a 129SVE infection model which does not form ulcerated lesions, but permits an extended treatment regimen with SinaAmphoLeish. BALB/c mice infected with stationary phase L. mexicana promastigotes developed ulcerated lesions at week 8 post-infection, at which time the mice were randomized and treatment with SinaAmpholeish or vehicle control began for 4 weeks. Lesions were followed for another 3 weeks after the end of treatment (Fig. 1A). Both treatment and control mouse groups showed increased lesion sizes and we observed no significant differences in the lesion sizes between the groups (Fig. 2A and B). Similarly, the parasitic burdens in the lesions as well as in the draining lymph nodes at time of sacrifice were found to be similar between the treatment and control groups (Fig. 2C and D). In contrast, 129SVE mice infected with L. mexicana promastigotes developed non ulcerative lesions, and at week 5, once the lesions were developed, the mice were randomized into treatment and control groups. Treatment with SinaAmphoLeish or vehicle control occurred twice daily for 10 weeks (Fig. 1B). Beginning at 3 weeks post-treatment (8 weeks post infection), the SinaAmphoLeish treated mice showed significant reduction in their lesion sizes. This lesion size reduction in the SinaAmphoLeish treated group continued for about 4–5 weeks, after which their lesions became comparable to those of the control mice (Fig. 2E and F). Parasitic burdens were determined to be comparable in both treatment and control groups at the time of sacrifice in the lesions and lymph nodes (Fig. 2G and H). Based on these data, we conclude that while topical application of nanoliposomal amphotericin B was not effective in inhibiting lesion development following a 4 week treatment period in an ulcerative BALB/c mouse L. mexicana infection model of CL, extended treatment for up to 10 weeks in a non-ulcerative 129SVE mouse L. mexicana infection model shows minimal efficacy as evidenced by a transient decrease in lesion sizes.
Fig. 1.
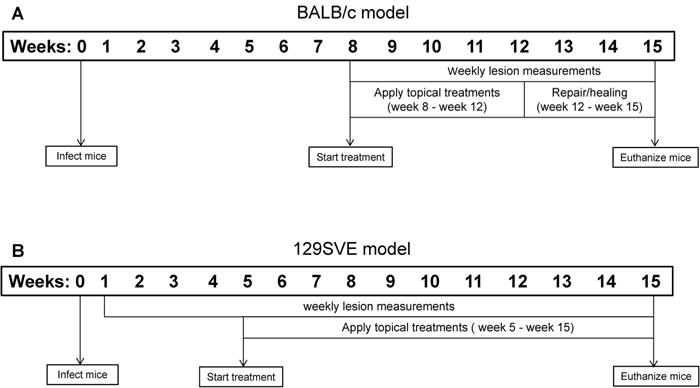
Timeline of study. (A) For the ulcerated model, BALB/c mice were infected with L. mexicana promastigotes subcutaneously at their back rumps. After establishment of ulcerated lesions (week 8 post infection) mice were randomized into two groups and treated for 4 weeks. Serum was collected at weeks 8, 12 and 15. Mice were euthanized at week 15 and lesions and lymph node cells were collected for analysis. (B) For the non-ulcerated model, 129SVE mice were infected with L. mexicana promastigotes subcutaneously at their back rumps. After development of visible lesions (week 5 post infection) mice were randomized into two groups treatments were applied for 10 weeks (weeks 5–15 post infection). Serum was collected at weeks 8, 11 and 15. Mice were euthanized at week 15 and lesions and lymph node cells were collected for analysis.
Fig. 2.
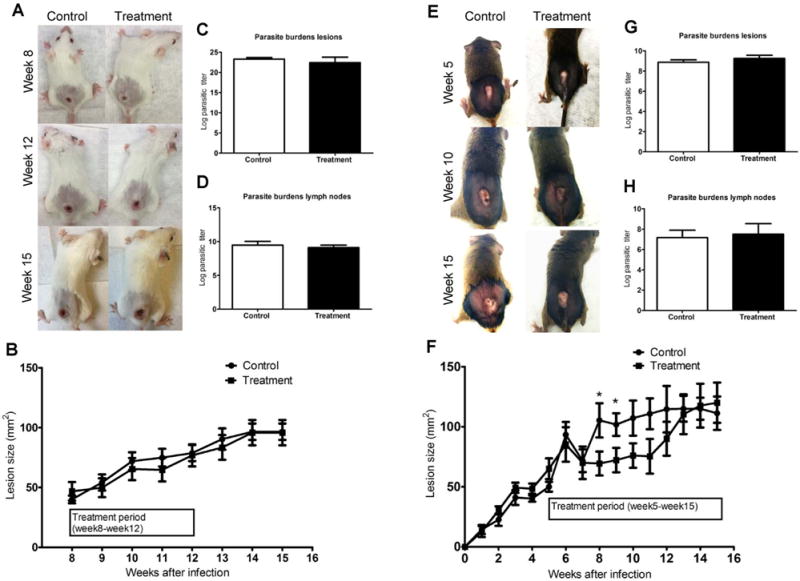
Evaluation of efficacy of topical nanoliposomal Amphotericin B treatment on L. mexicana infected BALB/c and 129SVE mice. BALB/c and 129SVE mice were infected with 5 × 106 stationary phase L. mexicana promastigotes via subcutaneous injection in their shaven back rumps. After mice developed ulcerated lesions (BALB/c at week 8 and 129SVE at week 5), mice with similar sized lesions were randomized and treated with SinaAmphoLeish 0.4% or Vaseline (control) twice a day. Representative images BALB/c (A) and 129SVE (E) mice with back rump lesions from treatment and control groups. Lesion sizes of BALB/c (B) and 129SVE (F) mice expressed as mean lesion sizes (millimeters2) ± SE. Parasitic loads expressed as the largest log dilution that represents live parasites in cultures obtained from lesions of BALB/c (C) and 129SVE (G) mice. Parasitic loads expressed as the largest log dilution that represents live parasites in cultures obtained from lymph nodes of BALB/c (D) and 129SVE (H) mice. Data represents the means ± SE from one of the two similar experiments (n = 5 mice per group). * p < 0.05 (Students unpaired t-test).
3.2. Topical treatment of amphotericin B leads to increased th1 and th2 cytokines in L. mexicana infected 129SVE mice
Both Th1 and Th2 immune responses play an important role in CL. To determine the possible immunological effects of nanoliposomal amphotericin B treatment on ulcerated (BALB/c model) and non-ulcerated (129SVE model) L. mexicana lesions, we collected the draining lymph node cells from both groups of mice at week 15 post-infection and stimulated them with L. mexicana antigen (LmAg) in vitro for 72 h. The cytokines IL-4, IL-10, IFN-γ and TNF-α were subsequently measured by ELISA. SinaAmphoLeish treated 129SVE mice showed significantly higher levels of IFN-γ, IL-4 and IL-10 compared to the control mice (Fig. 3A–C), which indicates that treatment with SinaAmphoLeish leads to the increased production of Th1 and Th2 cytokines in antigen re-stimulated lymph node cells of L. mexicana infected 129SVE mice. On the other hand we did not observe any significant differences in cytokine levels in SinaAmphoLeish treated BALB/c mice compared to vehicle controls (data not shown). No detectable levels of TNF-α were observed in both models (data not shown).
Fig. 3.

Topical treatment of nanoliposomal Amphotericin B leads to increased Th1, Th2 cytokines and T cell proliferation in L. mexicana infected 129SVE mice. Draining lymph nodes were collected from control and SinaAmphoLeish treated 129SVE mice infected with L. mexicana at week 15 and re-stimulated with 20 ug/ml L. mexicana antigen for 72 h. Cytokines produced in the culture supernatants were determined by ELISA. Concentrations of (A) IFN-γ, (B) IL-4 and (C) IL-10 produced by L. mexicana re-stimulated lymph node cells of infected 129SVE mice treated with SinaAmphoLeish or vehicle control. (D) T cell proliferation and (E) nitric oxide production were determined by alamar blue assay and Greiss assay respectively. Data represents the means ± SE from one of the two similar experiments (n = 3 to 5 mice per group). * p < 0.05 (Students unpaired t-test).
3.3. Effect of SinaAmphoLeish on T cell proliferation and nitric oxide production in L. mexicana infected mice
Since activation and proliferation of T cells play an important role in the production of cytokines and stimulation of macrophages to clear L. mexicana infection, we analyzed the proliferation of draining lymph node T cells after L. mexicana antigen re-stimulation in vitro. Our results show that SinaAmphoLeish treatment significantly increased antigen specific T cell proliferation compared to control mice in non-ulcerated 129SVE mice at 10 week of treatment (Fig. 3D). In contrast, in the BALB/c ulcerated model of L. mexicana infection, the 4 week treatment with SinaAmphoLeish did not significantly affect antigen specific T cell proliferation compared to control mice (data not shown). Next, we examined whether SinaAmphoLeish treatment affected production of the leishmanicidal compound, nitric oxide (NO), by L. mexicana infected macrophages re-stimulated in vitro. Levels of NO were measured in culture supernatants of draining lymph node cells of treated and untreated L. mexicana infected BALB/c and 129SVE mice that were re-stimulated with L. mexicana antigen. We observed comparable levels of NO production in the treatment and control groups of both 129SVE (Fig. 3E) BALB/c (data not shown) infected mice. This result is in agreement with the comparable parasitic loads and lesion sizes observed between treated and untreated mice in both models.
3.4. SinaAmphoLeish treatment results in temporal increase in IgG1 but not IgG2a antibody production in L. mexicana infected 129SVE mice
Next, we examined whether SinaAmphoLeish treatment of L. mexicana infected 129SVE and BALB/c mice affected the levels of L. mexicana specific antibody isotype production by these mice. The antibody isotypes IgG1 and IgG2a are reflective of the host Th2 and Th1 immune responses to CL respectively, which in turn contribute to host susceptibility or resistance to L. mexicana (Rosas et al., 2005). 129SVE mice were bled at 8, 11 and 15 weeks post-infection while BALB/c mice were bled at 8, 12 and 15 weeks post-infection. In L. mexicana infected 129SVE mice, IgG1 antibody titers were higher in the treatment group at week 11 compared to the control group. At weeks 8 and 15, similar IgG1 titers between both groups were observed (Fig. 4A). This difference in IgG1 antibody titer at week 11 appears to coincide with the period following the observed lesion size differences between treated and control 129SVE mouse groups (Weeks 8–10 post-infection). After this time, lesions in SinaAmphoLeish treated mice begin to grow much more rapidly and are eventually comparable to the vehicle treated control group. The higher IgG1 levels in SinaAmphoLeish treated mice at week 11 therefore support the notion that a predominant Th2 response is developing in these mice at this time. This predominant Th2 response, which is reflected by the higher levels of IgG1 in SinaAmphoLeish treated 129SVE mice, explains the increased rate of lesion growth observed in this mouse group at weeks 11–13. The levels of IgG2a, a Th1 indicator, were similar in treated and control groups of L. mexicana infected 129SVE mice at all time points (Fig. 4B). We observed similar levels of IgG2a and IgG1 antibody titers in the sera of both control and treatment groups in our ulcerated (BALB/c) L. mexicana infection model (data not shown).
Fig. 4.
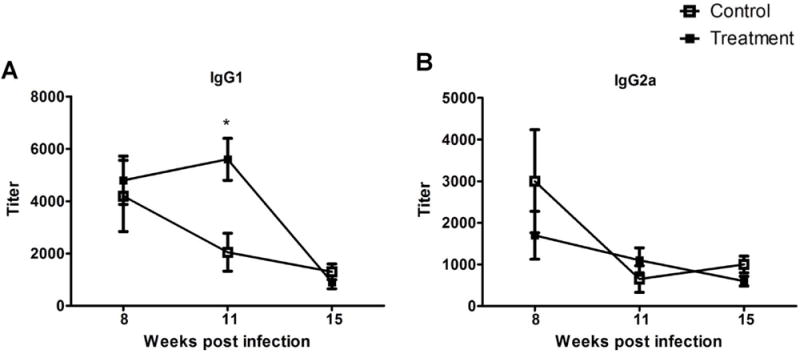
Evaluation of IgG1 and IgG2a antibody responses after topical nanoliposomal Amphotericin B administration. Control and Amphotericin B treated 129SVE mice were bled at weeks 8, 11 and 15 to determine antigen specific (A) IgG1 and (B) IgG2a antibody production by ELISA. Data represents the means ± SE from one of the two similar experiments (n = 3 to 5 mice per group). * p < 0.05 (Students unpaired t-test).
4. Discussion
Our current study determined the effect of topical treatment of nanoliposomal amphotericin B (SinaAmphoLeish) in CL caused by L. mexicana. SinaAmphoLeish was previously shown to be effective against L. major using a BALB/c back rump infection model (US Patent application number: US 20150147382A1). We decided to evaluate the efficacy of topical SinaAmphoLeish by using two murine models of experimental L. mexicana infection. The ulcerated BALB/c infection model with short term (4 weeks) SinaAmphoLeish treatment more closely resembles clinical disease, where L. mexicana infection produces ulcerative lesions in humans. Further, in this model, SinaAmphoLeish treatment began after the development of ulcerated lesions at week 8 post infection. In contrast, the non-ulcerative 129SVE infection model with long-term SinaAmphoLeish treatment does not closely resemble human disease, but it allows for a long term treatment regimen in order to extensively evaluate the chemotherapeutic potential of this drug formulation and its impact on the host immune response. In this model, treatment with SinaAmphoLeish began much earlier (at week 5) and continued for 10 weeks. Our results indicate that topical treatment with SinaAmphoLeish did not have an impact on lesion development and parasitic burdens in the BALB/c infection model. In the 129SVE model we observed a transient decrease in lesion sizes beginning at 3 weeks post treatment (week 8 post infection), but this inhibitory effect was not maintained as lesion sizes eventually increased to levels comparable to vehicle treated mice. In summary, topical SinaAmphoLeish treatment was unable to clear the infection in both murine L. mexicana infection models.
Although intravenous administration of liposomal amphotericin B has been shown to be effective against old and new world CL (Solomon et al., 2010; Wortmann et al., 2010; Mushtag et al., 2016), topical administration of SinaAmphoLeish in the murine L. mexicana infection model which closely resembles clinical disease (BALB/c model) did not result in a reduction in parasite burdens. There could be a number of reasons for this result. The virulence of this parasite strain might require a higher treatment dose, an earlier treatment time or a more prolonged period of intervention. Topical treatment with SinaAmphoLeish might still be effective in a low-dose L. mexicana infection model. Future studies which examine these possibilities should shed more light on the potential therapeutic efficacy of this nanoliposomal amphotericin B formulation. The urgent need for non-toxic, patient-compliant anti-leishmanial drugs highlights the importance and practical value of these studies.
The potential efficacy of topical SinaAmphoLeish against new world CL caused by L. mexicana can be seen in the transient decrease in lesion sizes in L. mexicana infected 129SVE mice at weeks 8–10 post-infection. In this model, intervention with SinaAmphoLeish began just after the establishment of lesions (week 5), which was much earlier than in the BALB/c model. However, as in the BALB/c model SinaAmphoLeish failed to resolve lesions and reduce parasite burdens.
It is known that, in addition to direct effects on the Leishmania parasite, Amphotericin B potentiates the anti-parasitic activity of immune cells either directly or through the production of cytokines (Cleary et al., 1992; Wilson et al., 1991; Wolf et al., 1991). For example, recent studies showed that intravenous administration of Amphotericin B induced Delayed Type Hypersensitivity (DTH) responses, proliferation of lymphocytes, and production of IFN-γ in L. donovani infected mice (Banerjee et al., 2008), as well as induction of Th1 immune responses in patients treated for mucocutaneous leishmaniasis (Cuna et al., 2007). It appears that our topical application of nanoliposomal amphotericin B induced both protective Th1 (evidenced by elevated IFN-γ and enhanced T cell proliferation) and non-protective Th2 (characterized by increased IL-4 production) and anti-inflammatory (IL-10 production) immune responses in the non-ulcerated 129SVE model. But in contrast short term treatment in ulcerated BALB/c model resulted in no changes in the immune responses which could be because of the strain difference and also duration of the treatment. It is possible that changes in the cytokine responses we have seen in 129SVE mice are due to the extended duration of the treatment, however the induction of both Th1 and Th2 immune responses which exert suppressive activity on each other resulted in no outcome of the treatment. In addition to this, SinaAmphoLeish treatment also resulted in induction IL-10 which possibly down-regulated both Th1 and Th2 inflammatory responses. Further, levels of IgG1, which are indicative of Th2 responses, were significantly higher in SinaAmphoLeish treated 129SVE mice compared to control mice. This is in contrast with the selective increase in Th1 immune responses seen in the above mentioned studies using Amphotericin B. It must be noted that in these studies, Amphotericin B was administered via the intravenous route, either liposomal or empty, while in our study Amphotericin B was administered in a topical nanoliposomal formulation. These findings suggest that SinaAmphoLeish exhibits potentially non-specific immunomodulatory properties during L. mexicana infection which could have counterproductive effects on parasite clearance, as observed in this model.
Our results also revealed similar levels of nitric oxide (NO) production in the culture supernatants obtained from lymph node cells of both BALB/c and 129SVE mice compared to their respective controls. Although we observed elevated amounts of IFN-γ in the SinaAmphoLeish treated group from 129SVE mice, it is likely that the increased levels of IL-4 and IL-10 in these mice suppressed IFN-γ induced NO production. This could explain the inability of SinaAmphoLeish to resolve L. mexicana infection using this murine model and treatment regimen.
In summary, we show that in topical treatment with nanoliposomal Amphotericin B transiently decreased lesion sizes in a 10 week treatment study of non-ulcerated cutaneous lesions (129SVE mice), but not in a 4 week treatment study of ulcerated cutaneous lesions (BALB/c mice). In both murine models of CL caused by L. mexicana, topical SinaAmphoLeish treatment resulted in no resolution of infection using the infectious dose and treatment regimen described in this study. 129SVE treated mice mounted elevated Th1 and Th2 responses, but these responses did not ultimately lead to resolution of infection. SinaAmphoLeish treatment resulted in no significant changes in parasite burdens, lesion sizes or immunological responses to L. mexicana infection in BALB/c mice. It is important to note that infection caused by L. mexicana is much more virulent compared to other strains of CL. The lack of disease resolution in SinaAmphoLeish treated mice in our study may not necessarily speak to the complete lack of efficacy of this nanoliposomal amphotericin B formulation, but may indicate the limitations of this drug for use as treatment against more virulent stains of leishmaniasis such as L. mexicana. It is possible that lower L. mexicana infectious doses and a more robust treatment regimen with SinaAmphoLeish would result in significant reduction in parasite burdens, and therefore requires further investigation. However, based on this first report, it can be concluded that in BALB/c and 129SVE mouse models, topical administration of nanoliposomal amphotericin B is not likely to be an effective method of treatment for CL caused by L. mexicana.
Acknowledgments
Authors would like to thank Dr. Bijay Kumar Jha, Pablo C Alarcon, Rachel H Sperling and Junrong Zhang for their technical assistance.
Financial support
This work was supported by Ohio State University intramural grant.
References
- Balasegaram M, Ritmeijer K, Lima MA, Burza S, Ortiz Genovese G, Milani B, Gaspani S, Potet J, Chappuis F. Liposomal amphotericin B as a treatment for human leishmaniasis. Expert Opin Emerg Drugs. 2012;17:493–510. doi: 10.1517/14728214.2012.748036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A, De M, Ali N. Complete cure of experimental visceral leishmaniasis with amphotericin B in stearylamine-bearing cationic liposomes involves down-regulation of IL-10 and favorable T-cell responses. J Immunol. 2008;181:1386–1398. doi: 10.4049/jimmunol.181.2.1386. [DOI] [PubMed] [Google Scholar]
- Chakravarty J, Sundar S. Drug resistance in leishmaniasis. J Global Infect Dis. 2010;2:167–176. doi: 10.4103/0974-777X.62887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary JD, Chapman SW, Nolan RL. Pharmacologic Modulation of interleukin-1 expression by amphotericin B-stimulated human mononuclear cells. Antimicrob Agents Chemother. 1992;36:977–981. doi: 10.1128/aac.36.5.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft SL, Yardley V. Chemotherapy of leishmaniasis. Curr Pharm Des. 2002;8:319–342. doi: 10.2174/1381612023396258. [DOI] [PubMed] [Google Scholar]
- Croft SL, Seifert K, Yardley V. Current scenario of drug development for leishmaniasis. Indian J Med Res. 2006;123:399–410. [PubMed] [Google Scholar]
- Cummings HE, Barbi J, Reville P, Oghumu S, Zorko N, Sarkar A, Satoskar AR. Critical role for phosphoinositide 3-kinase gamma in parasite invasion and disease progression of cutaneous leishmaniasis. Proc Natl Acad Sci U S A. 2012;109:1251–1256. doi: 10.1073/pnas.1110339109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuna WR, Velasquez R, Riva J, Guachalla I, Rodriguez C. Enhancement of a TH1 immune response in amphotericin B-treated mucocutaneous leishmaniasis. J Biomed Biotechnol. 2007;00:1–4. doi: 10.1155/2007/96410. http://dx.doi.org/10.1155/2007/96410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layegh P, Rajabi O, Jafari MR, Emamgholi Tabar Malekshah P, Moghiman T, Ashraf H, Salari R. Efficacy of topical liposomal amphotericin B versus intralesional meglumine antimoniate (Glucantime) in the treatment of cutaneous leishmaniasis. J Parasitol Res. 2011;00:1–5. doi: 10.1155/2011/656523. http://dx.doi.org/10.1155/2011/656523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushtag S, Dogra D, Dogra N. Clinical response with intralesional Amphotericin B in the treatment of old world cutaneous leishmaniasis: a preliminary report. Dermatol Ther. 2016;00:1–8. doi: 10.1111/dth.12377. http://dx.doi.org/10.1111/12377. [DOI] [PubMed] [Google Scholar]
- Ramos H, Valdivieso E, Gamargo M, Dagger F, Cohen BE. Amphotericin B kills unicellular leishmanias by forming aqueous pores permeable to small cations and anions. J Membr Biol. 1996;152:65–75. doi: 10.1007/s002329900086. [DOI] [PubMed] [Google Scholar]
- Ribeiro AL, Drummond JB, Volpini AC, Andrade AC, Passos VM. Electrocardiographic changes during low-dose: short-term therapy of cutaneous leishmaniasis with the pentavalent antimonial meglumine. Braz J Med Biol Res. 1999;32:297–301. doi: 10.1590/s0100-879x1999000300008. [DOI] [PubMed] [Google Scholar]
- Rosas LE, Keiser T, Barbi J, Satoskar AA, Septer A, Kaczmarek J, Lezama-Davila CM, Satoskar AR. Genetic background influences immune responses and disease outcome of cutaneous L. mexicana infection in mice. Int Immunol. 2005;17:1347–1357. doi: 10.1093/intimm/dxh313. [DOI] [PubMed] [Google Scholar]
- Solomon M, Pavlotsky F, Leshem E, Ephros M, Trau H, Schwartz E. Liposomal amphotericin B treatment of cutaneous leishmaniasis due to Leishmania tropica. J Eur Acad Dermatol Venereol. 2010;25:973–977. doi: 10.1111/j.1468-3083.2010.03908.x. [DOI] [PubMed] [Google Scholar]
- Veiga JP, Rosa TT, Kimachi T, Wolff ER, Sampaio RN, Gagliardi AR, Junqueria LF, Jr, Costa JM, Marsden PD. Renal function in patients with mucocutaneous leishmaniasis treated with pentavalent antimony compounds. Rev Inst Med Trop Sao Paulo. 1985;27:298–302. doi: 10.1590/s0036-46651985000600002. [DOI] [PubMed] [Google Scholar]
- Wilson E, Thorson L, Speert DP. Enhancement of macrophage superoxide anion production by amphotericin B. Antimicrob. Agents Chemother. 1991;35:796–800. doi: 10.1128/aac.35.5.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf JE, Stein SH, Little KD, Abegg AL, Little JR. Amphotericin B selectively stimulates macrophages from high responder mouse strains. Immunopharmacol Immunotoxicol. 1991;13:221–235. doi: 10.3109/08923979109019702. [DOI] [PubMed] [Google Scholar]
- Wortmann G, Zapor M, Ressner R, Fraser S, Hartzell J, Pierson J, Magill A. Lipsosomal amphotericin B for treatment of cutaneous leishmaniasis. Am J Trop Med Hyg. 2010;83:1028–1033. doi: 10.4269/ajtmh.2010.10-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yardley V, Croft SL. Activity of liposomal amphotericin B against experimental cutaneous leishmaniasis. Antimicrob Agents Chemother. 1997;41:752–756. doi: 10.1128/aac.41.4.752. [DOI] [PMC free article] [PubMed] [Google Scholar]


