Abstract
BACKGROUND
Degludec is an ultralong-acting, once-daily basal insulin that is approved for use in adults, adolescents, and children with diabetes. Previous open-label studies have shown lower day-to-day variability in the glucose-lowering effect and lower rates of hypoglycemia among patients who received degludec than among those who received basal insulin glargine. However, data are lacking on the cardiovascular safety of degludec.
METHODS
We randomly assigned 7637 patients with type 2 diabetes to receive either insulin degludec (3818 patients) or insulin glargine U100 (3819 patients) once daily between dinner and bedtime in a double-blind, treat-to-target, event-driven cardiovascular outcomes trial. The primary composite outcome in the time-to-event analysis was the first occurrence of an adjudicated major cardiovascular event (death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke) with a prespecified non-inferiority margin of 1.3. Adjudicated severe hypoglycemia, as defined by the American Diabetes Association, was the prespecified, multiplicity-adjusted secondary outcome.
RESULTS
Of the patients who underwent randomization, 6509 (85.2%) had established cardiovascular disease, chronic kidney disease, or both. At baseline, the mean age was 65.0 years, the mean duration of diabetes was 16.4 years, and the mean (±SD) glycated hemoglobin level was 8.4±1.7%; 83.9% of the patients were receiving insulin. The primary outcome occurred in 325 patients (8.5%) in the degludec group and in 356 (9.3%) in the glargine group (hazard ratio, 0.91; 95% confidence interval, 0.78 to 1.06; P<0.001 for noninferiority). At 24 months, the mean glycated hemoglobin level was 7.5±1.2% in each group, whereas the mean fasting plasma glucose level was significantly lower in the degludec group than in the glargine group (128±56 vs. 136±57 mg per deciliter, P<0.001). Prespecified adjudicated severe hypoglycemia occurred in 187 patients (4.9%) in the degludec group and in 252 (6.6%) in the glargine group, for an absolute difference of 1.7 percentage points (rate ratio, 0.60; P<0.001 for superiority; odds ratio, 0.73; P<0.001 for superiority). Rates of adverse events did not differ between the two groups.
CONCLUSIONS
Among patients with type 2 diabetes at high risk for cardiovascular events, degludec was noninferior to glargine with respect to the incidence of major cardiovascular events.
Cardiovascular complications remain two to four times more common among patients with type 2 diabetes than among persons without diabetes.1 Observational studies have suggested that patients with type 2 diabetes who require insulin have increased rates of cardiovascular events.1–3 However, a large clinical trial involving patients with impaired fasting glucose levels, impaired glucose tolerance, or type 2 diabetes reported cardiovascular outcomes among those who received basal insulin glargine that were similar to outcomes among patients who received standard care.4
Degludec is an ultralong-acting, once-daily basal insulin approved for use in adults, adolescents, and children with diabetes.5–7 Previous open-label studies have shown lower day-to-day variability in the glucose-lowering effect and lower rates of hypoglycemia among the patients who received degludec than among those who received glargine.8,9 The Food and Drug Administration (FDA) required that a dedicated preapproval trial of cardiovascular outcomes be conducted to assess the cardiovascular safety of degludec, as compared with glargine. Consequently, we conducted the Trial Comparing Cardiovascular Safety of Insulin Degludec versus Insulin Glargine in Patients with Type 2 Diabetes at High Risk of Cardiovascular Events (DEVOTE).
METHODS
TRIAL DESIGN AND OVERSIGHT
Detailed methods of the trial have been published previously.10 Briefly, the trial was a treat-to-target, randomized, double-blind, active comparator–controlled cardiovascular outcomes trial that was conducted at 438 sites in 20 countries. The trial was designed to continue until the occurrence of at least 633 primary outcome events, as confirmed by central, blinded review by an independent event-adjudication committee.
The trial was conducted in accordance with the provisions of the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice Guidelines.11,12 The protocol (available with the full text of this article at NEJM.org) was approved by the independent ethics committee or institutional review board at each trial center. Written informed consent was obtained from each patient before any trial-related activities.
The trial was funded and conducted by Novo Nordisk. Statogen Consulting and Novo Nordisk both independently analyzed the data only after the database lock. The steering committee, which was composed of the authors, participated in designing the trial, analyzing the data, editing an earlier version of the manuscript, and making the decision to submit the manuscript for publication. Medical writing and editorial support were funded by the sponsor. The authors had full access to all the trial data and vouch for the completeness and integrity of the data and for the fidelity of the trial to the protocol.
A prespecified interim analysis was planned, for regulatory purposes as agreed with the FDA, to assess the noninferiority of degludec versus glargine for cardiovascular safety after the occurrence of 150 primary outcome events, as confirmed by the event-adjudication committee.10 Per regulatory guidance, the confirmation of an upper limit of the confidence interval below 1.8 at the interim analysis was required to establish non-inferiority and allow confidential FDA review.10 On the basis of the results of the submitted interim analysis, the FDA approved the use of degludec in the United States in September 2015.
To mitigate the potential risk that an interim analysis posed to the overall integrity of the trial, a data-access management plan was developed before the interim analysis and is described in detail in the Supplementary Appendix, available at NEJM.org.10 The conduct of the trial was overseen by a steering committee that consisted of academic investigators and Novo Nordisk employees. In addition, an independent external data and safety monitoring committee was established to review accumulated data and evaluate the risk–benefit balance at planned intervals. An external independent statistics group, Statistics Collaborative, provided unblinded data to the data and safety monitoring committee, which could recommend to continue, modify, or terminate the trial prematurely on the basis of criteria developed before the initiation of the trial. Operational advice for the trial was provided by the global expert panel throughout the trial.
PATIENTS AND TREATMENTS
Patients with type 2 diabetes who were at high risk for cardiovascular events were randomly assigned in a 1:1 ratio to receive either degludec or glargine (both in identical 10-ml vials containing 100 U per milliliter), with each drug added to standard care and administered once daily between dinner and bedtime. Eligible patients included those who were being treated with at least one oral or injectable antihyperglycemic agent. Also required was a glycated hemoglobin level of 7% or more while the patients were receiving the anti-hyperglycemic agent; if the level was less than 7%, treatment with at least 20 units of basal insulin per day was required. Two groups of patients were eligible for the trial: those who were 50 years of age or older who had at least one coexisting cardiovascular or renal condition and those who were 60 years of age or older who had at least one cardiovascular risk factor. A complete list of inclusion and exclusion criteria is provided in the Supplementary Appendix.
Patients could continue their pretrial antihyperglycemic therapy except for basal and premix insulins, which were discontinued. Patients adjusted their dose of basal insulin weekly on the basis of the lowest of three self-measured blood-glucose values, as measured before breakfast 2 days before and on the day of dose adjustment, with the aim of reaching a target of 71 to 90 mg per deciliter (4.0 to 5.0 mmol per liter) (Table S1 in the Supplementary Appendix). To safeguard vulnerable patients, an alternative blood-glucose target of 90 to 126 mg per deciliter (5.0 to 7.0 mmol per liter) was available for these patients. Bolus insulin (aspart) was provided by Novo Nordisk for patients who were either continuing or initiating bolus treatment during the trial, with weekly adjustments based on the lowest of three preprandial or bedtime self-measured blood-glucose values measured on the 3 days before dose adjustment and aiming to reach a target of 71 to 126 mg per deciliter (Table S1 in the Supplementary Appendix). Higher targets were allowed at the discretion of the investigator.
The following events were adjudicated by the event-adjudication committee in a blinded manner: acute coronary syndrome (defined as myocardial infarction or unstable angina pectoris leading to hospitalization), stroke, death, and severe hypoglycemia. The definitions that were used for the clinical-event adjudication are provided in the Supplementary Appendix. Neoplasms were classified by a blinded independent committee as malignant, benign, or not classifiable. For neoplasms that were classified as malignant, a further subclassification was performed to assess the primary organ site.
OUTCOMES
All outcomes were prespecified unless otherwise stated. The primary composite outcome in the time-to-event analysis was the first occurrence of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke. The multiplicity-adjusted confirmatory secondary outcomes were the number and incidence of adjudicated events of severe hypoglycemia, which was defined by the American Diabetes Association as an episode requiring the assistance of another person to actively administer carbohydrate or glucagon or to take other corrective actions.13 According to this definition, plasma glucose levels may not be available during an event, but neurologic recovery after the return of plasma glucose to a normal level is considered to be sufficient evidence that the event was induced by a low plasma glucose level.
Other secondary outcomes included an expanded composite cardiovascular outcome (the primary composite outcome or unstable angina leading to hospitalization) and the time from randomization to death from any cause, along with serious adverse events or adverse events leading to discontinuation of the intervention, levels of glycated hemoglobin and fasting plasma glucose, blood pressure, pulse, lipid measurements, weight, body-mass index, estimated glomerular filtration rate, nocturnal severe hypoglycemia (occurring between 12:01 a.m. and 5:59 a.m.), and basal and bolus insulin dose. Glycated hemoglobin was measured at randomization, at months 3, 6, 9, and 12, and yearly thereafter. Other laboratory tests were performed at randomization and yearly thereafter.
STATISTICAL ANALYSIS
The statistical analysis plan is available in the Supplementary Appendix. Details regarding the sample-size estimates and statistical analyses have been published previously.10 We estimated that the follow-up of 7500 patients for approximately 5 years with an assumed event rate of 2.1 per 100 patient-years of exposure would produce 633 events and hence a power of 91% to rule on the null hypothesis. A Cox proportional-hazards regression model was used to analyze the intention-to-treat population for the primary composite outcome to test for the noninferiority of degludec as compared with glargine. Noninferiority would be confirmed if the upper boundary of the 95% confidence interval was less than 1.3. If noninferiority was established, we then tested for superiority with respect to severe hypoglycemic episodes using a negative binomial-regression model that was adjusted for observation time and treatment group to test for the number of events and a logistic-regression model that was adjusted for treatment group to test for incidence. Superiority of these secondary outcomes would be confirmed if the upper boundary of the 95% confidence interval was less than 1.0. Selected sensitivity analyses, including the per-protocol analysis, were performed to address the robustness of the results. The rationale for the use of a noninferiority threshold of 1.3 in the primary analysis and a threshold of 1.8 in the interim analysis is described in the Supplementary Appendix.
RESULTS
PATIENTS
From November 2013 through November 2014, a total of 7637 patients were randomly assigned to receive either degludec (3818 patients) or glargine (3819 patients) once daily (Fig. S1 in the Supplementary Appendix). Of these patients, 98% completed the final follow-up visit or died during the trial (Fig. S2 in the Supplementary Appendix). The vital status was known for 99.9% of the patients. Five patients (0.06%) were lost to follow-up, and three patients (0.04%) had withdrawn consent at the time of the database lock. The median observation time was 1.99 years, and the median exposure time was 1.83 years.
The characteristics of the patients at baseline were similar in the two groups (Table S2 in the Supplementary Appendix). Of the 7637 patients, 6509 (85.2%) had established cardiovascular disease or moderate chronic kidney disease. The mean age was 65.0 years, the mean duration of diabetes was 16.4 years, and the mean (±SD) glycated hemoglobin level was 8.4±1.7%. Of the 6409 patients (83.9%) who were receiving insulin at baseline, 3515 (54.8%) were receiving a basal–bolus regimen.
CARDIOVASCULAR OUTCOMES
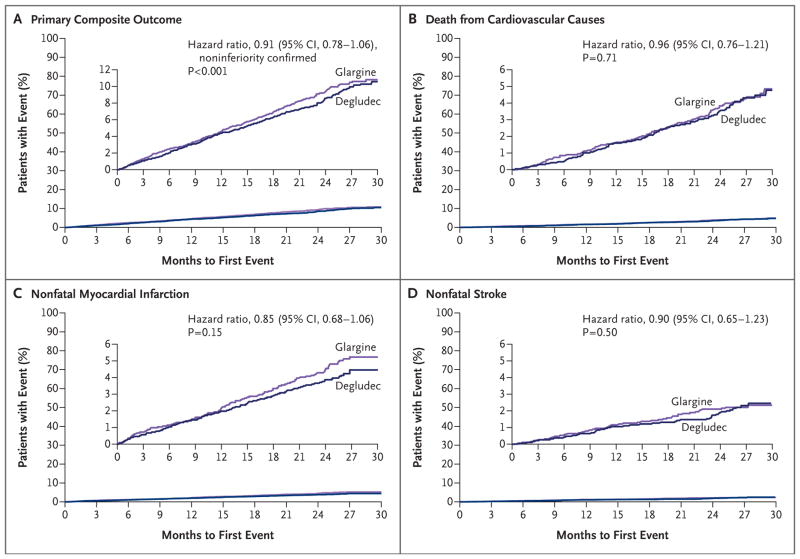
The primary composite outcome occurred in 325 patients (8.5%) in the degludec group and in 356 patients (9.3%) in the glargine group (hazard ratio, 0.91; 95% confidence interval [CI], 0.78 to 1.06; P<0.001 for noninferiority in a one-sided test) (Table 1 and Fig. 1A). Individual components of the composite cardiovascular outcome are provided in Table 1 and Figures 1B, 1C, and 1D. There was no significant difference in the incidence of death in the degludec and glargine groups (202 patients [5.3%] vs. 221 patients [5.8%]; hazard ratio, 0.91; 95% CI, 0.76 to 1.11; P = 0.35).
Table 1.
Primary Outcomes.*
| Outcome | Degludec (N = 3818) | Glargine (N = 3819) | Hazard Ratio (95% CI) | P Value | ||
|---|---|---|---|---|---|---|
| Patients no. (%) |
Event Rate no./100 patient-yr |
Patients no. (%) |
Event Rate no./100 patient-yr |
|||
| Primary composite cardiovascular outcome | 325 (8.5) | 4.29 | 356 (9.3) | 4.71 | 0.91 (0.78–1.06) | <0.001† |
|
| ||||||
| Expanded composite cardiovascular outcome‡ | 386 (10.1) | 5.10 | 419 (11.0) | 5.54 | 0.92 (0.80–1.05) | 0.22 |
|
| ||||||
| Component outcomes | ||||||
|
| ||||||
| Death from any cause | 202 (5.3) | 2.67 | 221 (5.8) | 2.92 | 0.91 (0.76–1.11) | 0.35 |
|
| ||||||
| Noncardiovascular death | 66 (1.7) | 0.87 | 79 (2.1) | 1.05 | 0.84 (0.60–1.16) | 0.28 |
|
| ||||||
| Cardiovascular death | 136 (3.6) | 1.80 | 142 (3.7) | 1.88 | 0.96 (0.76–1.21) | 0.71 |
|
| ||||||
| Cardiovascular death excluding undetermined cause of death | 97 (2.5) | 1.28 | 106 (2.8) | 1.40 | 0.91 (0.69–1.20) | 0.52 |
|
| ||||||
| Nonfatal myocardial infarction | 144 (3.8) | 2.27 | 169 (4.4) | 2.47 | 0.85 (0.68–1.06) | 0.15 |
|
| ||||||
| Nonfatal stroke | 71 (1.9) | 0.98 | 79 (2.1) | 1.16 | 0.90 (0.65–1.23) | 0.50 |
|
| ||||||
| Unstable angina leading to hospitalization | 71 (1.9) | 1.04 | 74 (1.9) | 1.10 | 0.95 (0.68–1.31) | 0.74 |
The primary composite outcome was analyzed with the use of a Cox proportional-hazards regression model with treatment group as a factor in the intention-to-treat population with testing for noninferiority. All P values are two-sided unless otherwise stated.
This one-sided P value confirmed noninferiority. The two-sided P value testing for a significant between-group difference was 0.21.
The expanded composite cardiovascular outcome (a secondary outcome) consisted of the primary composite outcome plus unstable angina leading to hospitalization.
Figure 1. Kaplan–Meier Analysis of the Composite Primary Outcome.
Shown are plots of time until the primary outcome (Panel A) and its composite events — death from cardiovascular causes (Panel B), nonfatal myocardial infarction (Panel C), and nonfatal stroke (Panel D) — in the degludec group and the glargine group. The noninferiority of degludec as compared with glargine was confirmed because the upper limit of the two-sided 95% confidence interval for the hazard ratio was less than 1.3. The results were determined by the event-adjudication committee on the basis of Cox proportional-hazards regression analysis in the intention-to-treat population. Data for patients without an event were censored at the time of the last contact (telephone or visit). The inset graphs show the same data on expanded y axes.
The results of various sensitivity analyses that used alternative censoring methods were aligned with the findings of the primary analysis and are shown, along with the subgroup analyses, in Figures S3 and S4 in the Supplementary Appendix. Findings for the remaining adjudicated cardiovascular outcomes and the expanded composite outcome are shown in Figure S5 in the Supplementary Appendix.
SEVERE HYPOGLYCEMIA
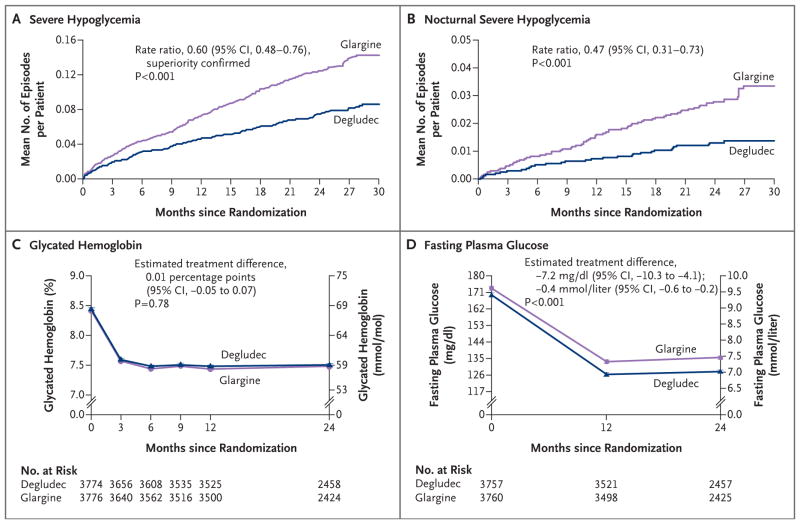
A total of 752 severe hypoglycemic events occurred, 280 events in 187 patients in the degludec group and 472 events in 252 patients in the glargine group; the rate was 3.70 events per 100 patient-years in the degludec group and 6.25 events per 100 patient-years in the glargine group (rate ratio, 0.60; 95% CI, 0.48 to 0.76; P<0.001 for superiority) (Table 2 and Fig. 2A). One or more events of severe hypoglycemia13 occurred in 187 patients (4.9%) in the degludec group and in 252 (6.6%) in the glargine group, for an absolute difference of 1.7 percentage points (odds ratio, 0.73; 95% CI, 0.60 to 0.89; P<0.001 for superiority) (Table 2). Of the 752 severe hypoglycemic events that occurred in the two groups, blood-glucose measurements were available for 637 events (84.7%) (Fig. S6 in the Supplementary Appendix). In addition, there was a lower rate of nocturnal severe hypoglycemia in the degludec group than in the glargine group (0.65 vs. 1.40 events per 100 patient-years) for a rate ratio of 0.47 (95% CI, 0.31 to 0.73; P<0.001) (Table 2 and Fig. 2B).
Table 2.
Secondary Outcomes.*
| Outcome | Degludec (N = 3818) | Glargine (N = 3819) | Rate Ratio (95% CI) | P Value | ||||
|---|---|---|---|---|---|---|---|---|
| Patients | Events | Patients | Events | |||||
| no. (%) | no. | rate/100 patient-yr | no. (%) | no. | rate/100 patient-yr | |||
| Severe hypoglycemia | 187 (4.9) | 280 | 3.70 | 252 (6.6) | 472 | 6.25 | 0.60 (0.48–0.76) | <0.001† |
|
| ||||||||
| Unconsciousness or coma | 54 (1.4) | 60 | 0.79 | 63 (1.6) | 75 | 0.99 | 0.81 (0.55–1.19) | 0.28 |
|
| ||||||||
| Seizure | 9 (0.2) | 11 | 0.15 | 10 (0.3) | 11 | 0.15 | 1.02 (0.38–2.73) | 0.97 |
|
| ||||||||
| Nocturnal severe hypoglycemia | 38 (1.0) | 49 | 0.65 | 73 (1.9) | 106 | 1.40 | 0.47 (0.31–0.73) | <0.001 |
|
| ||||||||
| Frequency of severe hypoglycemia | ||||||||
|
| ||||||||
| ≥1 event | 187 (4.9) | — | — | 252 (6.6) | — | — | 0.73 (0.60–0.89)‡ | <0.001† |
|
| ||||||||
| 1 event | 141 (3.7) | — | — | 168 (4.4) | — | — | — | — |
|
| ||||||||
| 2 events | 22 (0.6) | — | — | 43 (1.1) | — | — | — | — |
|
| ||||||||
| ≥3 events | 24 (0.6) | — | — | 41 (1.1) | — | — | — | — |
|
| ||||||||
| No events | 3631 (95.1) | — | — | 3567 (93.4) | — | — | — | — |
The number of severe hypoglycemic episodes was tested for superiority with the use of a negative binomial-regression model adjusted for observation time as offset (100 patient-years) and treatment group, and the incidence was tested with the use of a logistic-regression model adjusted for treatment group. All P values are two-sided unless otherwise stated.
This one-sided P value confirmed superiority.
This comparison was calculated as an odds ratio.
Figure 2. Severe Hypoglycemia and Glucose Control.
Shown are the observed cumulative number of events of severe hypoglycemia (Panel A) and nocturnal severe hypoglycemia (Panel B) per patient in the degludec group and the glargine group. For severe hypoglycemia, the superiority of degludec over glargine was confirmed because the upper limit of the two-sided 95% confidence interval for the estimated rate ratio was below 1.0. Nocturnal severe hypoglycemia was defined as an episode with an investigator-reported onset between 12:01 a.m. and 5:59 a.m. Also shown are measures of treatment efficacy, according to the glycated hemoglobin level (Panel C) and the fasting plasma glucose level (Panel D) in the two groups, with both comparisons performed in post hoc analyses.
The results of the on-treatment analyses were similar to those in the primary analyses (Figs. S7 and S8 in the Supplementary Appendix). The results of subgroup analyses are shown in Figure S9 in the Supplementary Appendix. Treatment ratios differed significantly in subgroups defined according to sex, ethnic group (Hispanic or Latino vs. not Hispanic or Latino), cardiovascular risk group (established cardiovascular disease vs. risk factors), and trial center (United States vs. other countries in DEVOTE).
GLYCEMIC CONTROL
There was no significant between-group difference in total and bolus insulin dose levels over time (Fig. S10 in the Supplementary Appendix). For basal insulin, the estimated dose of degludec was 2 units higher than the dose of glargine (estimated treatment ratio, 1.04; 95% CI, 1.00 to 1.08; P = 0.04) at 24 months (Fig. S11 in the Supplementary Appendix). Overall initiation of concomitant antihyperglycemic medications during the trial was similar in the two groups (Table S3 in the Supplementary Appendix).
There also was no significant between-group difference in changes in glycated hemoglobin levels throughout the trial (Fig. 2C). At 24 months, the glycated hemoglobin level was 7.5% (58 mmol per mole) in the two groups, with an estimated treatment difference of 0.01 percentage points (95% CI, −0.05 to 0.07; P = 0.78 in post hoc analysis). Over 24 months, plasma glucose values that were measured by the patients before breakfast were similar in the two groups; the median value for all patients was 95 mg per deciliter (5.3 mmol per liter) (Fig. S12 in the Supplementary Appendix).
At 24 months, the mean laboratory-measured fasting plasma glucose level was significantly lower in the degludec group than in the glargine group (128±56 vs. 136±57 mg per deciliter [7.1±3.1 vs. 7.5±3.2 mmol per liter]). Laboratory-measured fasting plasma glucose levels decreased more in the degludec group than in the glargine group (−39.9 mg per deciliter vs. −34.9 mg per deciliter [−2.2 mmol per liter vs. −1.9 mmol per liter]) after 24 months (estimated treatment difference, −7.2 mg per deciliter; 95% CI, −10.3 to −4.1 [−0.4 mmol per liter; 95% CI, −0.6 to −0.2]; P<0.001 in post hoc analysis) (Fig. 2D).
CARDIOVASCULAR RISK FACTORS
The observed mean change in cardiovascular risk factors from baseline to month 24 did not differ between treatment groups for the following variables: weight, body-mass index, blood pressure, pulse, estimated glomerular filtration rate, and all blood lipid levels (high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, total cholesterol, and triglycerides) (Table S4 in the Supplementary Appendix). Changes in the overall use of cardiovascular medications during the trial were similar in the two groups (Table S3 in the Supplementary Appendix).
SAFETY AND ADVERSE EVENTS
The rate of adverse events was 44.7 events per 100 patient-years in the degludec group and 50.1 events per 100 patient-years in the glargine group; the corresponding rates of serious adverse events were 44.2 events versus 49.6 events per 100 patient-years (Table 3). The rate of events leading to permanent discontinuation of a trial drug was 3.7 events per 100 patient-years in the degludec group and 4.0 events per 100 patient-years in the glargine group. The numbers of malignant, benign, and unclassifiable neoplasms were similar in the two groups (Table 3, and Table S5 in the Supplementary Appendix). Serious adverse events that occurred in at least 1% of the patients and critical symptoms associated with severe hypoglycemic episodes (as confirmed by the event-adjudication committee) are described in Tables S6, S7, and S8 in the Supplementary Appendix. There were no confirmed fatal events associated with hypoglycemia.
Table 3.
Selected Adverse Events Reported during the Trial.
| Adverse Event | Degludec (N = 3818) | Glargine (N = 3819) | ||||
|---|---|---|---|---|---|---|
| Patients | Events | Patients | Events | |||
| no. (%) | no. | rate/100 patient-yr | no. (%) | no. | rate/100 patient-yr | |
| All adverse events* | 1488 (39.0) | 3381 | 44.7 | 1529 (40.0) | 3788 | 50.1 |
|
| ||||||
| Serious adverse events | ||||||
|
| ||||||
| Any | 1473 (38.6) | 3341 | 44.2 | 1517 (39.7) | 3745 | 49.6 |
|
| ||||||
| Excluding severe hypoglycemia | 1451 (38.0) | 3230 | 42.7 | 1489 (39.0) | 3643 | 48.2 |
|
| ||||||
| Events leading to permanent discontinuation of the intervention | 200 (5.2) | 276 | 3.7 | 222 (5.8) | 305 | 4.0 |
|
| ||||||
| Externally classified neoplasms | 121 (3.2) | 128 | 1.7 | 115 (3.0) | 127 | 1.7 |
|
| ||||||
| Malignant | 93 (2.4) | 100 | 1.3 | 99 (2.6) | 107 | 1.4 |
|
| ||||||
| Benign | 26 (0.7) | 26 | 0.3 | 19 (0.5) | 20 | 0.3 |
|
| ||||||
| Unclassifiable | 2 (0.1) | 2 | 0 | 0 | 0 | 0 |
Included in this category are all serious adverse events, adverse events leading to permanent discontinuation of the intervention, medication errors leading to serious adverse events, and technical complaints (i.e., any written, electronic, or oral communication that alleges defects in a medicine or device).
DISCUSSION
In this cardiovascular outcomes trial of basal insulin therapy in patients with type 2 diabetes at high cardiovascular risk, we found that degludec was noninferior to glargine in terms of cardiovascular events and superior with regard to hypoglycemia risk, with a lower rate of both severe and nocturnal severe hypoglycemia (by 40% and 53%, respectively; P<0.001 for both comparisons). These results were achieved at equivalent glycemic control in the two groups. The demonstrated safety of degludec with respect to cardiovascular outcomes was reflected in the individual components of the primary composite outcome and was consistent across multiple prespecified subgroups.
Patients with diabetes have a greater risk of cardiovascular disease and cardiovascular-related death than do persons without diabetes.1 Several trials have consequently investigated the effect of an intensive reduction in glycemic levels on the risk of cardiovascular outcomes in patients with type 2 diabetes.14–17 The results of these trials have been varied, with UKPDS, ADVANCE, and VADT showing a neutral effect of reducing glycemic levels on the risk of cardiovascular events, whereas ACCORD showed a significantly increased risk of death both from cardiovascular causes and from any cause associated with more intensive glycemic control.14–17 The focus on cardiovascular outcomes related to diabetes treatments was intensified when the FDA issued guidance in 2008 that described the need to establish the cardiovascular safety of new antihyperglycemic therapies.18 This recommendation led to the conduct of numerous cardiovascular outcomes trials involving patients with diabetes.19 Although the FDA guidance did not specifically include various types of insulin, the ORIGIN trial, which was designed before the issuing of the FDA guidance and specifically sought to evaluate the cardiovascular safety of glargine, showed no significant difference in cardiovascular outcomes with glargine as compared with standard care.4 In the context of this complex landscape of cardiovascular outcomes trials, we found that degludec was not associated with a greater risk of cardiovascular outcomes than was glargine at the same level of glycemic control.
The development of basal insulins with more stable pharmacodynamic profiles has allowed patients to aim safely for fasting glucose levels in the normal range by providing a consistent glucose-lowering effect with a half-life of more than 24 hours and thereby reducing the occurrence of hypoglycemia.20 The reduction in severe hypoglycemia that is reported in our trial and in previous trials that have compared degludec with glargine probably results from the improved pharmacodynamic profile of degludec.7–9,21
The incidence and rates of severe hypoglycemia across cardiovascular outcomes trials4,17,22,23 are difficult to compare owing to differences in the definitions that were used and to factors such as frailty, diabetes duration, frequency of insulin use, treatment regimens, and treatment targets at baseline and during the trial. Among all the patients in our trial, the incidence of severe hypoglycemia (2.90 events per 100 patient-years) and rates (4.97 events per 100 patient-years) were within the range that was evident in studies in which the use of insulin was a prominent component of therapy, which had a range of incidences from 0.53 to 5.05 events per 100 patient-years and a range of rates from 0.70 to 8.25 events per 100 patient-years.4,17,22,23 Severe hypoglycemia is associated with broad negative consequences for patients with diabetes.24,25 The number of patients who would need to be treated with degludec rather than glargine to avert 1 severe hypoglycemic event is 40.
Our trial has several strengths, including its double-blind design, large enrollment of patients at high cardiovascular risk, and high retention rate of patients. The primary limitation of the trial is its intermediate duration (2 years). Whether these findings can be extrapolated to longer exposure, to patients with a lower risk of cardiovascular events, or both is uncertain. Furthermore, no adjustments were made for multiplicity in the exploratory analysis beyond the prespecified hierarchical analyses of the cardiovascular outcomes and severe hypoglycemia. Overall, the exploratory analyses support the results for the primary and secondary outcomes. However, it is important to emphasize that these analyses are exploratory and have not been adjusted for multiple testing.26–28
In conclusion, we found that in patients with type 2 diabetes at high risk for cardiovascular events, degludec was noninferior to glargine in terms of the incidence of cardiovascular events.
Supplementary Material
Acknowledgments
Funded by Novo Nordisk and others; DEVOTE ClinicalTrials.gov number, NCT01959529.
Supported by Novo Nordisk, a grant (UL1TR001111, to Dr. Buse) from the National Institutes of Health (NIH), and by Clinical and Translational Science Awards to numerous trial centers from the NIH National Center for Advancing Translational Sciences.
Dr. Marso reports receiving grant support, consulting fees, and fees for executive committee membership from Novo Nordisk, fees for physician education from Abbott Vascular and Boston Scientific, and research support and travel support from and serving as steering committee member for AstraZeneca and Bristol-Myers Squibb; Dr. McGuire, receiving consulting fees and fees for serving on a clinical trial executive committee from Boehringer Ingelheim, Sanofi Aventis US, Novo Nordisk, and AstraZeneca, fees for serving on a data monitoring committee from Janssen Research and Development, advisory board fees and fees for serving on a clinical trial executive committee from Merck Sharp & Dohme, consulting fees and fees for serving on a clinical trial steering committee from Lilly USA, fees for serving on a data monitoring committee from GlaxoSmithKline, fees for serving on a clinical trial executive committee and data monitoring committee from Takeda Pharmaceuticals North America, fees for serving as a clinical trial chair and fees for serving on a clinical trial executive committee from Eisai; Dr. Zinman, receiving grant support and consulting fees from Boehringer Ingelheim, AstraZeneca, and Novo Nordisk and consulting fees from Eli Lilly, Janssen, Merck, and Sanofi Aventis; Prof. Poulter, receiving lecture fees from and serving on advisory boards for Servier, Takeda, Novo Nordisk, and AstraZeneca and grant support from Julius Clinical; Dr. Emerson, receiving fees for serving on a data monitoring committee from CTI BioPharma, Arena Pharmaceuticals, SFJ Pharmaceuticals, BioMarin, Medivation, Biom’Up, Dynavax, Genentech, GlaxoSmithKline, Janssen Research, Novartis, Novo Nordisk, Pfizer, Roche, and Xoma and statistical consulting fees from Sarepta, AstraZeneca, Celltrion, Sprout, Sanofi, Collegium Pharmaceutical, Intercept, Coherus, and Emmaus; Dr. Pieber, receiving grant support, paid to the Medical University of Graz, and consulting fees from Novo Nordisk and AstraZeneca and consulting fees from Bristol-Myers Squibb, Eli Lilly, and Roche Diabetes Care; Dr. Pratley, receiving lecture fees and consulting fees, paid to Florida Hospital, from AstraZeneca, consulting fees, paid to Florida Hospital, from Boehringer Ingelheim, GlaxoSmithKline, Hanmi Pharmaceutical, Janssen Scientific Affairs, Pfizer, and Eisai, grant support from Gilead Scienses, Lexicon Pharmaceuticals, and Sanofi-Aventis US, grant support and consulting fees, paid to Florida Hospital, from Ligand Pharmaceuticals, Lilly, and Merck, grant support, lecture fees, honoraria, and consulting fees, paid to Florida Hospital, from Novo Nordisk, and grant support, lecture fees, and consulting fees, paid to Florida Hospital, from Takeda; Drs. Haahr, Lange, Brown-Frandsen, Moses, and Skibsted, being employed by and owning stock in Novo Nordisk; Dr. Kvist, being employed by Novo Nordisk; Dr. Buse, receiving grant support, consulting fees, and travel support, paid to the University of North Carolina, from Eli Lilly, GI Dynamics, Merck, Astra-Zeneca, Sanofi, Lexicon, Orexigen, Takeda, and Novo Nordisk, consulting fees and travel support, paid to the University of North Carolina, from Elcelyx Therapeutics, Metavention, vTv Therapeutics, Dance Biopharm, Adocia, stock options, paid to the University of North Carolina, from PhaseBio Pharmaceuticals, grant support from Medtronic Minimed, Johnson & Johnson, Boehringer Ingelheim, GlaxoSmithKline, Theracos, and Bayer, grant support and consulting fees, paid to the University of North Carolina, from Intarcia Therapeutics, stock options from Insulin Algorithms, consulting fees, paid to the University of North Carolina, from Dexcom, Fractyl, Shenzhen HighTide Biopharmaceutical, and NovaTarg, and serving as a member of a nonprofit board for AstraZeneca HealthCare Foundation. No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
We thank the trial investigators, staff, and patients for their participation; Stephan Ogenstad of Statogen Consulting for independent statistical analysis; and Francesca Hemingway and Daria Renshaw of Watermeadow Medical for providing medical writing and editorial support.
References
- 1.Dailey G. Overall mortality in diabetes mellitus: where do we stand today? Diabetes Technol Ther. 2011;13(Suppl 1):S65–S74. doi: 10.1089/dia.2011.0019. [DOI] [PubMed] [Google Scholar]
- 2.Munnee K, Bundhun PK, Quan H, Tang Z. Comparing the clinical outcomes between insulin-treated and non-insulin-treated patients with type 2 diabetes mellitus after coronary artery bypass surgery: a systematic review and meta-analysis. Medicine (Baltimore) 2016;95(10):e3006. doi: 10.1097/MD.0000000000003006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li J, Tong Y, Zhang Y, et al. Effects on all-cause mortality and cardiovascular outcomes in patients with type 2 diabetes by comparing insulin with oral hypoglycemic agent therapy: a meta-analysis of randomized controlled trials. Clin Ther. 2016;38(2):372–386. e6. doi: 10.1016/j.clinthera.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 4.The ORIGIN Trial Investigators. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med. 2012;367:319–28. doi: 10.1056/NEJMoa1203858. [DOI] [PubMed] [Google Scholar]
- 5.Tresiba prescribing information. 2015 ( http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/203314lbl.pdf)
- 6.Tresiba summary of product characteristics. 2015 http://www.medicines.org.uk/emc/medicine/27360.
- 7.Heise T, Mathieu C. Impact of the mode of protraction of basal insulin therapies on their pharmacokinetic and pharmacodynamic properties and resulting clinical outcomes. Diabetes Obes Metab. 2017;19:3–12. doi: 10.1111/dom.12782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ratner RE, Gough SC, Mathieu C, et al. Hypoglycaemia risk with insulin degludec compared with insulin glargine in type 2 and type 1 diabetes: a pre-planned meta-analysis of phase 3 trials. Diabetes Obes Metab. 2013;15:175–84. doi: 10.1111/dom.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heise T, Hermanski L, Nosek L, Feldman A, Rasmussen S, Haahr H. Insulin degludec: four times lower pharmacodynamic variability than insulin glargine under steady-state conditions in type 1 diabetes. Diabetes Obes Metab. 2012;14:859–64. doi: 10.1111/j.1463-1326.2012.01627.x. [DOI] [PubMed] [Google Scholar]
- 10.Marso SP, McGuire DK, Zinman B, et al. Design of DEVOTE (Trial Comparing Cardiovascular Safety of Insulin Degludec vs Insulin Glargine in Patients with Type 2 Diabetes at High Risk of Cardiovascular Events) — DEVOTE 1. Am Heart J. 2016;179:175–83. doi: 10.1016/j.ahj.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 11.World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–4. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 12.ICH Harmonised Tripartite Guideline: Guideline for Good Clinical Practice. J Postgrad Med. 2001;47:199–203. [PubMed] [Google Scholar]
- 13.Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;36:1384–95. doi: 10.2337/dc12-2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–59. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- 16.The ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–72. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 17.Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–39. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 18.Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER) Diabetes mellitus — evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes: guidance for industry. 2008 Dec; ( http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071627.pdf)
- 19.Schnell O, Rydén L, Standl E, Ceriello A. Current perspectives on cardiovascular outcome trials in diabetes. Cardiovasc Diabetol. 2016;15:139. doi: 10.1186/s12933-016-0456-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heise T, Nosek L, Rønn BB, et al. Lower within-subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1 diabetes. Diabetes. 2004;53:1614–20. doi: 10.2337/diabetes.53.6.1614. [DOI] [PubMed] [Google Scholar]
- 21.Wysham C, Bhargava A, Chaykin L, et al. SWITCH 2: reduced risk of hypoglycaemia with insulin degludec vs insulin glargine U100 in a type 2 diabetes population on basal insulin: a randomised, double-blind, crossover trial. Diabetologia. 2016;59(Suppl 1):S43. abstract. [Google Scholar]
- 22.Zoungas S, Patel A, Chalmers J, et al. Severe hypoglycemia and risks of vascular events and death. N Engl J Med. 2010;363:1410–8. doi: 10.1056/NEJMoa1003795. [DOI] [PubMed] [Google Scholar]
- 23.Miller ME, Bonds DE, Gerstein HC, et al. The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ. 2010;340:b5444. doi: 10.1136/bmj.b5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frier BM, Schernthaner G, Heller SR. Hypoglycemia and cardiovascular risks. Diabetes Care. 2011;34(Suppl 2):S132–S137. doi: 10.2337/dc11-s220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graveling AJ, Frier BM. Hypoglycaemia: an overview. Prim Care Diabetes. 2009;3:131–9. doi: 10.1016/j.pcd.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 26.Wittes J. On looking at subgroups. Circulation. 2009;119:912–5. doi: 10.1161/CIRCULATIONAHA.108.836601. [DOI] [PubMed] [Google Scholar]
- 27.Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions, version 5.1.0. Sect. 9.6.2., 9.6.6. The Cochrane Collaboration; 2011. ( http://handbook.cochrane.org) [Google Scholar]
- 28.Wittes J. Why is this subgroup different from all other subgroups? Thoughts on regional differences in randomized clinical trials. In: Fleming TR, Weir BS, editors. Proceedings of the Fourth Seattle Symposium in Biostatistics: clinical trials. New York: Springer Science; 2013. pp. 95–115. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.