Abstract
This study is a secondary analysis from a randomized clinical trial of computerized vs. in-person brief intervention (BI) for illicit drug misuse among adult primary care patients (N=359; 45% Female; 47% Hispanic) with moderate-risk illicit drug misuse as measured by the World Health Organization’s Alcohol, Smoking, and Substance Involvement Screening Test (ASSIST). This study examined differences in response to the two brief intervention strategies (both based on motivational interviewing) on the basis of gender and ethnicity, comparing non-Hispanic males, non-Hispanic females, Hispanic males, and Hispanic females. Participants were assessed at baseline, 3-, 6-, and 12-month follow-up with the ASSIST. Trajectories in Global Continuum of Illicit Drug Risk Scores were examined using a generalized linear mixed model. There were significant differences in response to computerized vs. in-person BI over time on the basis of gender-ethnic subgroups (Gender X Ethnicity X Condition X Time interaction; p= .03), with Hispanic males tending to respond more favorably to the computerized BI and Hispanic females tending to respond more favorably to the in-person BI. There was no clear differentiation in response to the two BIs among non-Hispanic males, while among non-Hispanic females the pattern of change converged following baseline differences. Consideration of gender and ethnic differences in future studies of BI is warranted.
Keywords: Brief intervention, primary care, gender, ethnicity, Hispanic
1. Introduction
Illicit drug use remains highly prevalent in the US and epidemiological surveillance surveys estimate that in 2015 over 27 million individuals (10.1% of the US population) 12 years of age or older used illicit drugs in the past 30 days. These substances included marijuana (8.3%), non-medical use of psychotherapeutic medications including opioid analgesics, stimulants, sedatives and tranquilizers (2.4%), and cocaine (0.7%) (SAMHSA, 2016a). Substance use disorders (SUDs), characterized by the recurrent use of alcohol or drugs leading to significant clinical or functional impairment, continue to be a prevalent and serious public health problem in the US. In 2015, approximately 20.8 million individuals (7.8% of the US population) 12 years of age or older met diagnostic criteria for abuse or dependence related to alcohol or illicit drug use in the past year (SAMHSA, 2016a). Substance use has been associated with a range of adverse health outcomes, including increased risk of mental disorders, injury, cardiovascular disease, HIV infection, stroke, and premature death, to name a few (Deganhardt & Hall, 2012; Rehm et al., 2006).
1.1 Screening, Brief Intervention, and Referral to Treatment
The screening, brief intervention, and referral to treatment (SBIRT) service model is increasingly being used in healthcare settings to identify and intervene with individuals with substance use problems, particularly the large number of people with unhealthy substance use who may not fully meet SUD diagnostic criteria (Agerwala & McCance-Katz, 2012; Babor et al., 2007). Primary care in particular has been considered a promising setting in which to identify and engage patients with unhealthy substance use, based on findings that most individuals with substance use problems do not seek treatment (SAMHSA, 2016a), that most people (~80%) see a healthcare provider on a yearly basis (Blackwell, Lucas, & Clarke, 2012), and the strong evidence base for brief interventions in reducing alcohol and tobacco use (Jonas, et al., 2012; Siu et al., 2015). Moreover, primary care settings often serve diverse patient populations, including members of racial and ethnic minority communities (Manuel et al., 2015). Racial/ethnic minorities have previously been found to be more likely to receive substance use services in non-specialty facilities (e.g., primary care) compared to non-Hispanic Whites, who are more likely to access SUD treatment in specialty facilities (Lo & Cheng, 2011). Thus, primary care can be an important access point for substance use services, particularly for racial/ethnic minority communities.
There is good empirical support regarding both the efficacy and cost-effectiveness of screening and brief intervention (SBI), a key component of SBIRT, to reduce risky alcohol use in healthcare settings (Babor et al., 2007; Jonas et al., 2012; Saitz, 2007), although evidence is lacking that SBI can successfully address alcohol dependence (Saitz, 2010). The corresponding empirical support regarding SBI for drug misuse has been mixed. For example, several randomized trials found that SBI is effective in reducing illicit drug use and associated risks (Bernstein et al., 2005; Gelberg et al., 2015; Gelberg et al., in press; Humeniuk et al., 2012; Ondersma, Svikis, & Schuster, 2007; Ondersma et al., 2014). However, other studies have not found SBI to be effective for drug use, including two large randomized trials conducted in primary care settings (Roy-Byrne et al., 2014; Saitz et al., 2014). Moreover, brief intervention was not successful in securing referral to SUD treatment (Kim et al., 2017). The international World Health Organization trial of BI (Humeniuk et al., 2012) found that BI was effective in reducing drug use risks in Brazil, India, and Australia, but not in the US, which may be due to research design or implementation factors, or to population differences.
Given the mixed findings for SBI for illicit drug use, questions remain whether particular sub-populations (including women and minorities) may benefit from brief interventions, or respond more positively to certain approaches.
1.2 Women and Hispanics
Epidemiological surveys in the US have found higher prevalence of illicit drug use among males as compared to females (SAMHSA, 2016b). However, even though women initially engage in lower levels of illicit drug use, they tend to experience an accelerated progression of drug use behaviors resulting in regular use, addiction, and first treatment episode, known as telescoping (Greenfield et al., 2010; Greenfield et al., 2007; Hernandez-Avila, Rounsaville, & Kranzler, 2004). There are important differences between males and females across virtually all aspects of substance use, with women initiating substance use at lower doses, developing addiction more quickly, and being more likely to relapse after ceasing substance use (Becker & Hu, 2008). Hence, it is plausible that women would exhibit different patterns of response to substance use interventions than men.
Recent findings from the National Survey on Drug Use and Health indicate that rates of past 30-day illicit drug use were 12.5% among African Americans, 9.2% among Hispanics, and 10.2% among Whites (SAMHSA, 2016b). Research indicates that Hispanics often experience considerable disparities in SUD treatment length and completion, as well as indicators of quality care (Alegria et al., 2006; Alvarez et al., 2007; Guererro, Marsh, Khachikian et al., 2013). Hispanics and other racial/ethnic minorities are less likely to complete SUD treatment compared to Whites (Guerrero, Marsh, Duan et al., 2013), and may respond differently to SUD treatment based on social, cultural, and environmental factors that influence drug use (Alegria et al., 2006; Alvarez et al., 2007; Amaro et al., 2006; Guerrero, Marsh, Khachikian et al., 2013). Moreover, Hispanics are underrepresented in clinical trials and, thus, may not fully benefit from substance use treatment advances (Burlew et al., 2011).
Research also suggests a possible interaction between gender and ethnicity with respect to substance use, SUD treatment services, and patient outcomes. For example, persistence of SUDs over the longer-term has been found to vary markedly across gender and race/ethnic subgroups (Evans et al., 2017). A national study found that the link between SUD treatment services and drug use outcomes varied considerably by gender and ethnicity, and that gender differences in this relationship were particularly prominent in the Hispanic subgroup (Guerrero, Marsh, Cao et al., 2014). Thus, an examination of response patterns to different substance use interventions by gender and ethnicity is warranted to better identify the types of interventions that are most likely to be beneficial for different patient groups.
1.3 Gender and Ethnic Differences in SBI
Recently, researchers and practitioners have emphasized adaptation of SBI to patient populations from varied racial and ethnic backgrounds, as research indicates that substance use patterns and consequences vary by ethnicity (Alvarez et al., 2007; Manuel et al., 2015; Mukku et al., 2012; Pacek, Malcolm, & Martins, 2012). Research in the Veterans Affairs system has found that BIs are more likely to be delivered to minority group members compared to non-Hispanic Whites (Dobscha et al., 2009; Manuel et al., 2015; Williams et al., 2012). Although many BI studies have included substantial numbers of female and Hispanic participants, there has not been much research on how different population subgroups respond to different BI approaches. With respect to gender and racial/ethnic considerations specifically, only a few studies have reported on differential outcomes (Manuel et al., 2015). For example, in subgroup analyses of the Quit Using Drugs Intervention Trial (QUIT), there was some evidence to suggest that female participants experienced greater reductions in drug use after BI than male participants (Gelberg et al., 2015). More research is needed to determine the most effective intervention strategies for different patients groups because of a lack of focus in these areas (Manuel et al., 2015).
1.4 Brief Intervention Delivery Methods
Typically, BIs are conducted in-person by trained physicians, counselors, social workers, or other service providers (Agerwala & McCance-Katz, 2012; Babor et al., 2007). However, the use of computerized brief interventions (CBIs) has been growing in recent years (Carey et al., 2009; Ondersma et al., 2007; Ondersma et al., 2014; Schwartz et al., 2014). An important potential advantage of CBIs is that they may reduce some of the implementation challenges with respect to conducting in-person BIs (as physician and staff time is often limited) and decrease costs related to staff education and technical training, while potentially increasing patient disclosure of substance use problems and improving BI reliability (Babor et al., 2007; Gryczynski et al., 2015; Newman et al., 2002; Schwartz et al., 2014). Empirical evidence is emerging regarding the efficacy of using CBIs for both alcohol and drug use, with positive findings reported regarding reductions in alcohol misuse (Carey et al., 2009) and illicit drug use (Gilbert et al., 2008; Ondersma et al., 2007; Ondersma et al., 2014). Although many BI studies have included substantial numbers of female and Hispanic participants, there has not been much research on how different population subgroups respond to different BI delivery formats, such as in-person versus computerized delivery.
1.5 The Present Study
The present study is a secondary analysis from a randomized trial comparing a computerized BI (CBI) vs. an in-person BI (IBI) delivered by a behavioral health counselor for medical patients with moderate-risk illicit drug use (Schwartz et al., 2014). Previously, we reported findings from this study at a 3-month endpoint (Schwartz et al., 2014), and through 12 months of follow-up (Gryczynski et al., 2015), with results indicating no significant differences between CBI and IBI regarding global ASSIST drug scores or drug-positive hair tests, the primary outcomes under examination. The focus of the present study is to examine the relationship between two specific patient factors - Gender (male vs. female) and ethnicity (non-Hispanic vs. Hispanic) - and responsivity to different BI strategies (in-person vs. computerized BI). We sought to examine outcome trajectories for these different BI strategies on the basis of Gender-Ethnic subgroups, comparing non-Hispanic Males, non-Hispanic Females, Hispanic Males, and Hispanic Females.
2. Methods
2.1 Design
This study was a randomized clinical trial in which 360 participants with moderate-risk illicit drug use, who were not seeking substance use treatment, were randomly assigned to either a single session of CBI or IBI (Schwartz et al., 2014). Participants were provided similar content in both conditions. However, CBI was delivered by a computer whereas IBI was delivered by a master’s-level behavioral health counselor. The behavioral health counselors had multiple years of experience delivering IBIs in the clinics as part of the state of New Mexico’s SBIRT initiative. The study was conducted in collaboration with the Sangre de Cristo Community Health Partnership (SDCCHP), a non-profit organization established to assist community organizations in improving medical and behavioral health services for underserved populations in Northern New Mexico. SDCCHP was the organization responsible for administering the SAMHSA-funded SBIRT initiative in the state. The study was approved by the Institutional Review Boards of Friends Research Institute and Christus Health.
2.2 Settings
The study was conducted at two federally qualified health centers in rural New Mexico. Both health centers were community clinics with primary care and dental services, and were the largest medical service providers for their respective communities.
2.3 Participants
Study participants were adult primary care or dental patients 18 years of age or older, mean age of 36.2 years (SD = 14.6), of whom 45% were female and 47% were Hispanic. The racial breakdown of the sample was 90% White, 3% American Indian/Alaska Native, 1% Black, 1% Pacific Islander, and 5% multiracial. The majority of participants finished high school or equivalent education (78%) and owned a computer at home (66%). Most were unemployed (59%) and less than a quarter were married (22%). There were no significant differences between participants in the two study conditions (i.e., CBI vs. IBI) with respect to demographics or computer ownership (Schwartz et al., 2014).
2.4 Recruitment and eligibility
Potential participants were approached by the study research assistant (RA) in the waiting rooms of the two health centers. The RAs invited patients to participate in a health study. If a potential participant expressed interest, the RA met with them in a private room and screened them for eligibility using the Alcohol, Smoking, and Substance Involvement Screening Test (ASSIST; Humeniuk et al., 2008). Study inclusion criteria were adult clinic patients ages 18 or older, who scored between 4 and 26 (i.e., moderate risk) for any illicit drug class (including marijuana, cocaine, amphetamine-type stimulants, hallucinogens, sedatives, and opioids). Patients who scored higher than 26 (i.e., high risk) for alcohol or any drug except tobacco were excluded from study participation and referred to meet with the behavioral health counselor outside of the study. After determining eligibility based on the ASSIST score, the RA assessed exclusion criteria via a self-report checklist. Exclusion criteria were: no illicit drug use within the past 3 months (in some cases it is possible to score as “moderate-risk” on the ASSIST based on substance use problems in the more distant past); receipt of substance use disorder treatment within the past year; receipt of an IBI from the clinic behavioral health counselor within the past month; plans to move out of New Mexico in the next year; or inability to comprehend written English (Schwartz et al., 2014). Patients who met study criteria completed a written informed consent process, in which they were provided an explanation of study purpose, procedures, risks, and benefits. English reading ability was confirmed by asking participants to read aloud the first line of the consent form. The consent process included obtaining participants’ permission to use their ASSIST screening as part of the research record.
2.5 Random assignment
Following the completion of informed consent and a brief baseline assessment, study participants were randomly assigned within site to either CBI or IBI conditions using a block randomization procedure. RAs at each site were provided opaque envelopes that contained condition assignment information.
2.6 Study conditions
2.6.1 Computerized brief intervention (CBI)
The CBI was written using an intervention authoring software platform developed by Ondersma and colleagues (2005). CBIs written using this software platform had proven to be both acceptable and efficacious with respect to reducing perinatal and postpartum substance use and smoking during pregnancy (Ondersma et al., 2005; Ondersma et al., 2012; Ondersma et al., 2007; Ondersma et al., 2014). The CBI in the current study was designed to provide content that mirrored an ideal form of the IBI. It was written in collaboration with SDCCHP clinical supervisors and reviewed by the BHCs. The CBI delivered tailored content based on the participant’s choice of substance, motivation to change, and self-efficacy with respect to reducing or eliminating substance use. The CBI included gender-specific normative feedback regarding substance use quit rates based on national survey data (Gryczynski et al., 2015; Schwartz et al., 2014). (The same gender-specific normative data was also provided to the BHCs delivering the IBI intervention.) The CBI was self-directed and study participants could complete up to two substance-specific modules (for more detailed information see Schwartz et al., 2014). For participants assigned to the CBI condition, the RA gave the participant a tablet computer and headphones, provided a brief tutorial on using the software, and allowed them to complete the CBI in private. The average duration of CBI completion was 7 minutes, as clocked internally by the software platform.
2.6.2 In-person brief intervention (IBI)
Based on motivational interviewing techniques, the IBI was the standard of care that had been used by the participating clinics over the past several years. Each clinic had a masters-level, licensed BHC that worked on-site and delivered the IBI. These BHCs were employees of the SDCCHP organization but were integrated into the clinics. The BHCs had been trained in motivational interviewing and received weekly supervision from a Ph.D.-level psychologist. Both BHCs had been providing alcohol and drug BIs for many years at their respective clinics, prior to their involvement in the study, as part of the SAMHSA-funded SBIRT initiative in New Mexico. Participants assigned to the IBI condition were accompanied to the office of the behavioral health counselor (BHC), who delivered the IBI after a brief introduction by the RA. Following the principles of standard BIs and motivational interviewing techniques, the IBI lasted approximately 14 minutes on average, based on the BHC report on a standardized brief form that they completed for each IBI (Schwartz et al., 2014).
2.7 Measures
2.7.1 ASSIST
The ASSIST was used to determine study eligibility, and to track change in drug-related risks over the study period. The ASSIST assesses use and related problems for all psychoactive substances including alcohol, tobacco, and specific categories of illicit drugs, including marijuana, cocaine, amphetamine-type stimulants, sedatives, hallucinogens, and opioids (Humeniuk et al., 2008). Derived from ASSIST responses, the Global Continuum of Illicit Drug Risk (GCIDR) score was used as the primary outcome measure for the parent study, as well as in the current study. This score essentially sums scores across illicit drug categories for each item on the ASSIST, which includes measures of frequency of use as well as various problems related to each substance (e.g., failure to fulfill obligations, cravings, etc.). Thus, the ASSIST GCIDR represents a general measure of the severity of illicit drug risks.
2.7.2 Gender and Ethnicity
Gender and ethnicity were measured via self-report, using a study-specific questionnaire. Participants were asked whether they identified as male, female, or transgender (no one identified as transgender). A separate question asked participants whether they identified as Hispanic (yes or no). Participants were then asked to select their race (conceptualized separately from Hispanic ethnicity) from a list of multiple choice items.
2.8 Follow-up
Follow-up assessments were conducted with study participants at 3, 6, and 12 months post-study enrollment and included the ASSIST (as well as other measures that are beyond the scope of the present analysis). Follow-up rates at 3-, 6-, and 12-months were 91%, 89%, and 86%, respectively (Gryczynski et al., 2015).
2.9 Statistical Analysis
Baseline differences between gender-ethnicity subgroups were examined using likelihood ratio χ2 tests. Baseline differences on the ASSIST GCIDR score, the primary outcome of interest, were examined using one-way analysis of variance (ANOVA). To answer the primary question of interest, a general linear mixed model approach was used. This approach was chosen in light of the longitudinal structure of the data, as it accounts for repeated measurement and allows for the specification of group interaction effects in modeling GCIDR scores. Fixed factors in the model were Gender (Male vs. Female), Ethnicity (non-Hispanic vs. Hispanic), Condition (IBI vs. CBI), and Assessment Time Point (baseline, 3-, 6-, 12-months). A full model that included all first-, second-, and third-order interactions was fit to the data. The four-way interaction of Gender x Ethnicity x Condition x Time represented the effect of interest, answering the primary research question of whether there was differential responsivity to the interventions based on Gender-Ethnicity subgroups. Upon identifying a significant four-way interaction suggesting differential response, we examined model-predicted Least Squares Means to probe the nature of the interaction. The model-derived Least Squares Means were plotted for each Gender-Ethnicity subgroup to characterize how ASSIST GCIDR scores change over time in each subgroup. Hence, a statistically significant four-way interaction would indicate that response trajectories for IBI vs. CBI vary uniquely between Gender-Ethnicity combinations, while model-predicted Least Squares Means are used to plot those trajectories for each subgroup. These analyses were conducted using PROC MIXED in SAS 9.2.
In order to explore the role of baseline differences in ASSIST GCIDR scores, alternative linear regression models were also fit predicting the ASSIST GCIDR score at each follow-up point. These models include a covariate for the baseline value of the ASSIST GCIDR score, as well as Gender, Ethnicity, Condition and their respective interactions. These alternative analyses were conducted using Stata SE version 13.
3.0 Results
3.1 Baseline characteristics
Table 1 shows baseline participant characteristics by gender and ethnicity. Statistically significant differences between subgroups were found for any past 3-month cocaine use (p<.001) and any past 3 month sedative use (p=.03), as reported on the ASSIST. In addition, groups differed significantly with respect to baseline ASSIST Global Drug Risk Scores (p=.03), with Hispanic Males having the highest baseline scores, followed by Hispanic Females, non-Hispanic Males, and non-Hispanic Females.
Table 1.
Baseline characteristics by gender and ethnicity
| Non-Hispanic | Hispanic | |||
|---|---|---|---|---|
|
|
||||
| Male (n= 110) | Female (n=81) | Male (n=84) | Female (n=84) | |
|
|
||||
| Background Characteristics | ||||
| Age, mean (SD) | 37.3 (15.8) | 37.4 (15.2) | 35.7 (14.2) | 33.7 (12.9) |
| White race, % | 91.8 | 90.1 | 86.9 | 90.5 |
| Currently married, % | 26.4 | 27.2 | 16.7 | 17.9 |
| Not employed, % | 61.8 | 55.6 | 52.4 | 63.1 |
| Owns a computer, % | 70.9 | 70.4 | 59.5 | 61.9 |
| Has not used a computer in past 3 months, % | 20.9 | 17.3 | 33.3 | 21.4 |
| Use a computer daily or almost daily, % | 47.3 | 51.9 | 40.5 | 48.8 |
| Baseline Substance Use, Past 3 Months | ||||
| Tobacco, % | 72.7 | 74.1 | 75.0 | 81.0 |
| Alcohol, % | 80.9 | 79.0 | 78.6 | 77.4 |
| Marijuana, % | 93.6 | 84.0 | 88.1 | 90.5 |
| Cocaine, %1 | 13.6 | 6.2 | 33.3 | 17.9 |
| Amphetamine-Type Stimulants, % | 13.6 | 8.6 | 13.1 | 8.3 |
| Sedatives, %2 | 11.8 | 9.9 | 22.6 | 22.6 |
| Opioids, % | 19.1 | 18.5 | 28.6 | 32.1 |
| ASSIST GCIDR score, mean (SD)2 | 31.1 (17.3) | 27.4 (13.7) | 35.5 (19.9) | 32.2 (18.0) |
Notes: ASSIST= Alcohol, Smoking, and Substance Involvement Screening Test. GCIDR= Global Continuum of Illicit Drug Risk. IBI= In-person Brief Intervention. CBI= Computerized Brief Intervention.
p < .001,
p = .03.
Significance tests of differences across gender-ethnicity subgroups use likelihood ratio χ2 tests (for categorical variables) or one-way analysis of variance (Age, ASSIST GCIDR score).
3.2 Differential Response to BI Condition
Table 2 shows tests for each effect in the general linear mixed model. The primary effect of interest, the four-way interaction of Gender x Ethnicity x Condition x Time, was statistically significant (p=.03), indicating unique trajectories over Time for both Gender and Ethnicity with respect to their response to the two BIs. [Because all simpler effects are contained within the four-way interaction findings, we omit discussion of any significant simpler effect.]
Table 2.
Tests of effects from the generalized linear mixed model of ASSIST GCIDR scores.
| (dfn, dfd) | χ2 | p-value | |
|---|---|---|---|
|
|
|||
| CBI | (1, 346) | 0.36 | 0.55 |
| Gender | (1, 346) | 2.81 | 0.09 |
| Ethnicity | (1, 346) | 0.15 | 0.70 |
| Time | (3, 305) | 33.99 | <.001 |
| CBI x Gender | (1, 346) | 2.82 | 0.09 |
| CBI x Ethnicity | (1, 346) | 0.15 | 0.70 |
| CBI x Time | (3, 305) | 5.80 | 0.12 |
| Gender x Ethnicity | (1, 346) | 0.03 | 0.86 |
| Time x Gender | (3, 305) | 5.33 | 0.15 |
| Time x Ethnicity | (3, 305) | 13.50 | 0.004 |
| CBI x Gender x Ethnicity | (1, 346) | 1.15 | 0.28 |
| CBI x Time x Gender | (3, 305) | 4.18 | 0.24 |
| CBI x Time x Ethnicity | (3, 305) | 0.24 | 0.97 |
| Time x Gender x Ethnicity | (3, 305) | 1.69 | 0.64 |
| CBI x Time x Gender x Ethnicity 1 | (3, 305) | 9.23 | 0.03 |
Notes: ASSIST= Alcohol, Smoking, and Substance Involvement Screening Test. GCIDR= Global Continuum of Illicit Drug Risk. IBI= In-person Brief Intervention. CBI= Computerized Brief Intervention. dfn= numerator degrees of freedom; dfd = denominator degrees of freedom. Reference categories for CBI, Gender, Ethnicity, and Time are IBI, Male, Non-Hispanic, and Baseline, respectively.
Represents the primary effect of interest.
3.3 Gender-Ethnicity Trajectories
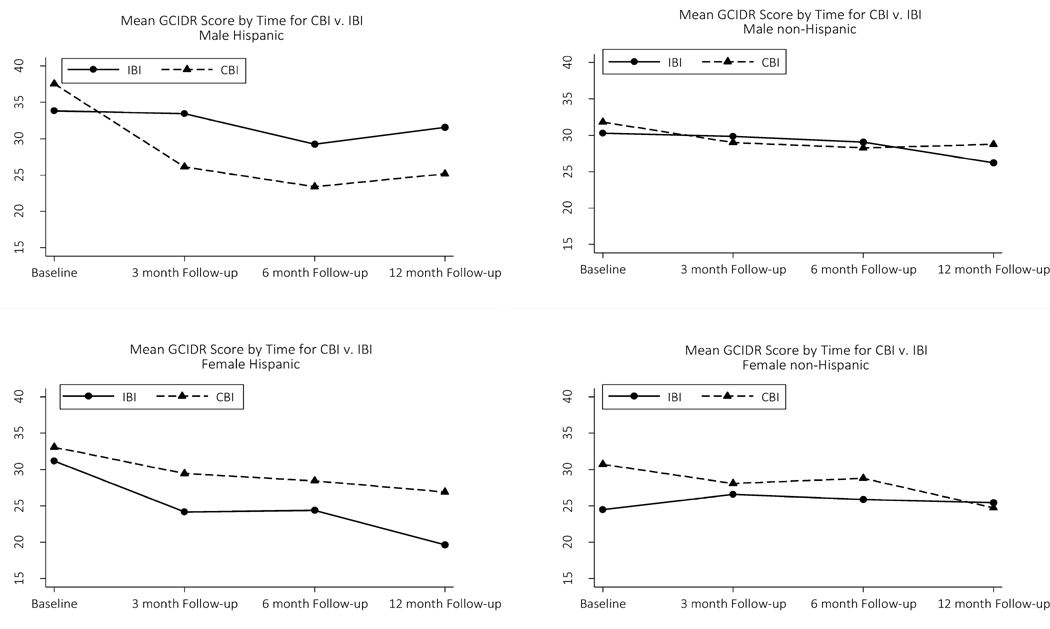
Figure 1 depicts plots of least squares means derived from the general linear mixed model showing GCIDR score trajectories over time for the four Gender-Ethnicity subgroups. Hispanic Males in the CBI condition had higher ASSIST GCIDR scores at baseline than their counterparts in the IBI condition (Δ = 3.7).
Figure 1.
ASSIST GCIDR score trajectories by gender-ethnicity subgroups.
Notes: ASSIST= Alcohol, Smoking, and Substance Involvement Screening Test. GCIDR= Global Continuum of Illicit Drug Risk. IBI= In-person Brief Intervention. CBI= Computerized Brief Intervention.
Hispanic Males in the CBI condition had a precipitous decrease in ASSIST GCIDR scores from baseline to 3-month follow-up (Δ= −11.4 points), while Hispanic Males in the IBI condition had virtually no change from baseline to 3 month follow-up (Δ= −0.3 points). Subsequently, trendlines for Hispanic Male participants had roughly parallel trajectories for IBI and CBI conditions. Hispanic Male participants in the CBI condition maintained lower ASSIST GCIDR scores than Hispanic Males in the IBI condition, by 6.4 points at 12-month follow-up (Figure 1, top left panel). In contrast, from baseline to 3-month follow-up, Hispanic Females appeared to respond more favorably to the IBI condition (Δ= −7.0 points) than the CBI condition (Δ= −3.7 points). By 12-month follow-up ASSIST GCIDR scores were 7.2 points lower for Hispanic Females in the IBI condition than Hispanic Females in the CBI condition (Figure 1, bottom left panel).
Among non-Hispanic participants, Females in the IBI condition had lower ASSIST GCIDR scores at baseline than Females in the CBI condition (by 6.2 points), with scores converging at 3-month follow-up and ending up at a difference of just 1.2 points by 12-month follow-up (Figure 1, bottom right panel). In contrast, among non-Hispanic Males, participants in IBI and CBI conditions had similar scores at baseline, with trajectories for both conditions tracking one another closely (Figure 1, top right panel).
In order to examine whether differential response to BI approaches could be attributed to baseline differences in ASSIST GCIDR scores for the Gender-Ethnicity subgroups, alternative regression models predicting ASSIST GCIDR scores at each follow-up point were fit, controlling for the baseline score. These analyses found a significant Gender X Ethnicity X Condition interaction for ASSIST GCIDR scores at 3 months (p= .03) and 12 months (p< .01), but not 6 months (p= .10). Hence, these additional analyses generally confirmed the core findings of significant differences in response to BI across the subgroups.
4. Discussion
This secondary analysis of data from a randomized trial comparing in-person vs. computerized brief intervention examined response patterns based on gender and ethnicity. As found in our prior research from this randomized trial (Gryczynski et al., 2015), the sample as a whole showed a general trend for decreased ASSIST GCIDR scores over time, which could be due to regression to the mean or to persistent intervention effects. However, the overall interaction effects found in the current study illustrate that change in these scores is not uniform across Gender-Ethnicity subgroups. This study found particularly pronounced gender differences in response to different BI approaches for Hispanic participants. Among Hispanic participants, we found the opposite pattern of response for males and females, whereby Hispanic males appeared to respond more favorably to CBI while Hispanic females responded more favorably to IBI. Among non-Hispanic participants, response patterns for IBI and CBI conditions were more closely aligned. These findings echo those of research documenting more substantive gender differences among Hispanics (as compared to other racial/ethnic groups) with respect to the link between SUD treatment services and outcomes (Guerrero, Marsh, Cao et al., 2014). These parallels with prior research are notable, although the current study’s sample of non-treatment-seeking, moderate-risk drug users recruited from primary care differs from SUD treatment samples in important ways.
Importantly, although all participants had moderate-risk drug misuse at baseline as measured by their ASSIST screening scores, participants came into the study with somewhat different substance use patterns and severities. For example, rates of cocaine use at baseline were highest for Hispanic Males (33%) and lowest for non-Hispanic Females (6%), and the GCIDR score itself differed significantly across subgroups at baseline. These are natural differences that may reflect gender and ethnic variation in substance use patterns. Hence, the differential response to the two types of brief interventions (based on magnitude of change) should be considered in the context of some natural differences in baseline substance use characteristics across gender-ethnicity subgroups. However, it is also important to note that baseline differences in GCIDR scores did not fully account for the observed differential response.
We focused on the ASSIST GCIDR score in this study because it represents a very general measure of risky illicit drug use. It has the advantage of being a meaningful metric for every participant in the sample in a way that substance-specific risks are not (i.e., because of the broad inclusion criteria, which was a moderate-risk score on any of seven drug classes). In our parent study (Gryczynski et al., 2015; Schwartz et al., 2014), as in the WHO ASSIST brief intervention trial (Humeniuk et al., 2012), we restricted substance-specific analyses to the subsample of participants who scored moderate-risk for that substance class. Due to small cell sizes that would occur for each specific substance, this approach would have been impractical for the present analysis.
Current findings suggest that the relative effectiveness of computerized versus in-person BI may differ as a function of both gender and ethnicity. The reasons why differences in preferential response to CBI and IBI were observed for Hispanic males versus females, or why Hispanics in general reported sharper reductions in GCIDR scores over time, remain to be fully elucidated. Hispanics in the US experience disparities in SUD treatment engagement and retention (Alegria et al., 2006; Alvarez et al., 2007; Guerrero, Marsh, Duan et al., 2013; Guererro, Marsh, Khachikian et al., 2013), and may underutilize substance use services based on perceived stigma associated with substance use (Manuel et al., 2015). One of the purported advantages of CBIs is that they could make some patients feel more comfortable in disclosing sensitive information regarding their substance use. However, there are many instances in which healthcare staff would be preferable to CBIs, including the need to provide more complex or nuanced counseling beyond the capability of a CBI, and to refer patients with SUDs to specialty drug treatment.
4.1 Limitations
This study has several limitations. First, data on the ASSIST was based on patient self-report, which may be subject to certain biases such as recall and social desirability. However, to increase the accuracy of self-report information, study research assistants emphasized confidentiality safeguards during the informed consent process, including the assurance that any information disclosed would not be shared with participating clinic staff and that it would be protected by a Certificate of Confidentiality. Second, as indicated above, participants came into the study with somewhat different substance use patterns and severities. Although this likely reflects real-world differences in gender-ethnicity subgroups, it complicates interpretation. Third, the study was not designed nor powered for parsing the sample by gender and ethnicity in this way. Nevertheless, it was fortunate that the sample included a well-balanced mix of gender and ethnicity. Fourth, although the CBI would deliver the intervention with fidelity to its programming every time, we did not record and assess the in-person BI for fidelity due to concerns about practicality and altering the natural dynamics of the encounter.
5. Conclusion
Despite the limitations outlined above, the study’s findings suggest the possibility of differential response to in-person vs. computerized BI based on the intersection of gender and ethnicity. For example, findings suggest Hispanic males tend to respond more favorably to CBI than IBI, while Hispanic females tend to respond more favorably to IBI than to CBI. These findings could have implications for addressing health disparities in drug use-related problems, but will require replication and extension. Future studies with larger sample sizes might examine brief intervention response across subgroups in a more granular way, first to replicate these findings and then to determine if similar patterns are found for different types of substance use problems, different geographical areas, and different service settings. Ultimately, this could lead to better tailoring of interventions.
Highlights.
This study examined gender-ethnic differences in response to BI strategies.
Response to computer and in-person BI differed by gender-ethnic subgroups.
Hispanic males tended to respond more favorably to computer BI.
Hispanic females tended to respond more favorably to in-person BI.
Differences in BI response were less pronounced for non-Hispanic participants.
Acknowledgments
The study was supported by the National Institute on Drug Abuse (NIDA) grant no. 1R01DA026003 (Principal Investigator Schwartz). NIDA or the National Institutes of Health had no role in the design and conduct of the study; data acquisition, management, analysis and interpretation of the data; and preparation, review or approval of the manuscript.
Footnotes
Declaration of Interests: The authors report no conflicts of interest.
Clinicaltrials.gov Registration: NCT01131520
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agerwala SM, McCance-Katz EF. Integrating screening, brief intervention, and referral to treatment (SBIRT) into clinical practice settings: a brief review. Journal of Psychoactive Drugs. 2012;44(4):307–317. doi: 10.1080/02791072.2012.720169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alegría M, Page JB, Hansen H, Cauce AM, Robles R, Blanco C, … Berry P. Improving drug treatment services for Hispanics: Research gaps and scientific opportunities. Drug and Alcohol Dependence. 2006;84:S76–S84. doi: 10.1016/j.drugalcdep.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Alvarez J, Jason LA, Olson BD, Ferrari JR, Davis MI. Substance abuse prevalence and treatment among Latinos and Latinas. Journal of Ethnicity in Substance Abuse. 2007;6(2):115–141. doi: 10.1300/J233v06n02_08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaro H, Arévalo S, Gonzalez G, Szapocznik J, Iguchi MY. Needs and scientific opportunities for research on substance abuse treatment among Hispanic adults. Drug and Alcohol Dependence. 2006;84:S64–S75. doi: 10.1016/j.drugalcdep.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Babor TF, McRee BG, Kassebaum PA, Grimaldi PL, Ahmed K, Bray J. Screening, Brief Intervention, and Referral to Treatment (SBIRT): toward a public health approach to the management of substance abuse. Substance Abuse. 2007;28(3):7–30. doi: 10.1300/J465v28n03_03. [DOI] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Frontiers of Neuroendocrinology. 2008;29(1):36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnke M, Smith VC Committee on Substance A, Committee on F & Newborn. Prenatal substance abuse: short- and long-term effects on the exposed fetus. Pediatrics. 2013;131(3):e1009–1024. doi: 10.1542/peds.2012-3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein J, Bernstein E, Tassiopoulos K, Heeren T, Levenson S, Hingson R. Brief motivational intervention at a clinic visit reduces cocaine and heroin use. Drug and Alcohol Dependence. 2005;77(1):49–59. doi: 10.1016/j.drugalcdep.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Blackwell DL, Lucas JW, Clarke TC. Summary health statistics for US adults: National Health Interview Survey, 2012. 2014;(260):1–161. [PubMed] [Google Scholar]
- Burlew K, Larios S, Suarez-Morales L, Holmes B, Venner K, Chavez R. Increasing Ethnic Minority Participation in Substance Abuse Clinical Trials: Lessons Learned in the National Institute on Drug Abuse’s Clinical Trials Network. Cultural Diversity & Ethnic Minority Psychology. 2011;17(4):345–356. doi: 10.1037/a0025668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey KB, Scott-Sheldon LA, Elliott JC, Bolles JR, Carey MP. Computer-delivered interventions to reduce college student drinking: a meta-analysis. Addiction. 2009;104(11):1807–1819. doi: 10.1111/j.1360-0443.2009.02691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Hall W. Extent of illicit drug use and dependence, and their contribution to the global burden of disease. The Lancet. 2012;379(9810):55–70. doi: 10.1016/S0140-6736(11)61138-0. [DOI] [PubMed] [Google Scholar]
- Dobscha SK, Dickinson KC, Lasarev MR, Lee ES. Associations between race and ethnicity and receipt of advice about alcohol use in the Department of Veterans Affairs. Psychiatric Services. 2009;60(5):663–670. doi: 10.1176/ps.2009.60.5.663. [DOI] [PubMed] [Google Scholar]
- Evans EA, Grella CE, Washington DL, Upchurch DM. Gender and race/ethnic differences in the persistence of alcohol, drug, and poly-substance use disorders. Drug and alcohol dependence. 2017;174:128–136. doi: 10.1016/j.drugalcdep.2017.01.021. [DOI] [PubMed] [Google Scholar]
- Gelberg L, Andersen RM, Afifi AA, Leake BD, Arangua L, Vahidi M, et al. Project QUIT (Quit Using Drugs Intervention Trial): a randomized controlled trial of a primary care-based multi-component brief intervention to reduce risky drug use. Addiction. 2015;110(11):1777–1790. doi: 10.1111/add.12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelberg L, Andersen RM, Rico MW, Vahidi M, Rey GN, Shoptaw S, … Baumeister SE. A pilot replication of QUIT, a randomized controlled trial of a brief intervention for reducing risky drug use, among Latino primary care patients. Drug and Alcohol Dependence. doi: 10.1016/j.drugalcdep.2017.04.022. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert P, Ciccarone D, Gansky SA, Bangsberg DR, Clanon K, McPhee SJ, et al. Interactive "Video Doctor" counseling reduces drug and sexual risk behaviors among HIV-positive patients in diverse outpatient settings. PLoS One. 2008;3(4):e1988. doi: 10.1371/journal.pone.0001988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield SF, Back SE, Lawson K, Brady KT. Substance abuse in women. Psychiatric Clinics of North America. 2010;33(2):339–355. doi: 10.1016/j.psc.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield SF, Brooks AJ, Gordon SM, Green CA, Kropp F, McHugh RK, et al. Substance abuse treatment entry, retention, and outcome in women: a review of the literature. Drug and Alcohol Dependence. 2007;86(1):1–21. doi: 10.1016/j.drugalcdep.2006.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryczynski J, Mitchell SG, Gonzales A, Moseley A, Peterson TR, Ondersma SJ, et al. A randomized trial of computerized vs. in-person brief intervention for illicit drug use in primary care: outcomes through 12 months. Journal of Substance Abuse Treatment. 2015;50:3–10. doi: 10.1016/j.jsat.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero EG, Marsh JC, Cao D, Shin HC, Andrews C. Gender disparities in utilization and outcome of comprehensive substance abuse treatment among racial/ethnic groups. Journal of Substance Abuse Treatment. 2014;46(5):584–591. doi: 10.1016/j.jsat.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero EG, Marsh JC, Duan L, Oh C, Perron B, Lee B. Disparities in Completion of Substance Abuse Treatment between and within Racial and Ethnic Groups. Health Services Research. 2013;48(4):1450–1467. doi: 10.1111/1475-6773.12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero EG, Marsh JC, Khachikian T, Amaro H, Vega WA. Disparities in Latino substance use, service use, and treatment: implications for culturally and evidence-based interventions under health care reform. Drug and Alcohol Dependence. 2013;133(3):805–813. doi: 10.1016/j.drugalcdep.2013.07.027. [DOI] [PubMed] [Google Scholar]
- Hernandez-Avila CA, Rounsaville BJ, Kranzler HR. Opioid-, cannabis- and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug and Alcohol Dependence. 2004;74(3):265–272. doi: 10.1016/j.drugalcdep.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Humeniuk R, Ali R, Babor T, Souza-Formigoni ML, de Lacerda RB, Ling W, et al. A randomized controlled trial of a brief intervention for illicit drugs linked to the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) in clients recruited from primary health-care settings in four countries. Addiction. 2012;107(5):957–966. doi: 10.1111/j.1360-0443.2011.03740.x. [DOI] [PubMed] [Google Scholar]
- Humeniuk R, Ali R, Babor TF, Farrell M, Formigoni ML, Jittiwutikarn J, et al. Validation of the Alcohol, Smoking And Substance Involvement Screening Test (ASSIST) Addiction. 2008;103(6):1039–1047. doi: 10.1111/j.1360-0443.2007.02114.x. [DOI] [PubMed] [Google Scholar]
- Jonas DE, Garbutt JC, Amick HR, et al. Behaviroal counseling after screening for alcohol misuse in primary care: A systematic review and meta-analysis for the US Preventive Services Task Force. Annals of Internal Medicine. 2012;157:645–654. doi: 10.7326/0003-4819-157-9-201211060-00544. [DOI] [PubMed] [Google Scholar]
- Kim TW, Bernstein J, Cheng DM, Lloyd-Travaglini C, Samet JH, Palfai TP, Saitz R. Receipt of addiction treatment as a consequence of a brief intervention for drug use in primary care: a randomized trial. Addiction. 2017;112(5):818–827. doi: 10.1111/add.13701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo CC, Cheng TC. Racial/ethnic differences in access to substance abuse treatment. Journal of Health Care for the Poor and Underserved. 2011;22(2):621–637. doi: 10.1353/hpu.2011.0054. [DOI] [PubMed] [Google Scholar]
- Manuel JK, Satre DD, Tsoh J, Moreno-John G, Ramos JS, McCance-Katz EF, Satterfield JM. Adapting Screening, Brief Intervention, and Referral to Treatment for Alcohol and Drugs to Culturally Diverse Clinical Populations. Journal of Addiction Medicine. 2015;9(5):343–351. doi: 10.1097/ADM.0000000000000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukku VK, Benson TG, Alam F, Richie WD, Bailey RK. Overview of substance use disorders and incarceration of african american males. Frontiers of Psychiatry. 2012;3:98. doi: 10.3389/fpsyt.2012.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JC, Des Jarlais DC, Turner CF, Gribble J, Cooley P, Paone D. The differential effects of face-to-face and computer interview modes. American Journal of Public Health. 2002;92(2):294–297. doi: 10.2105/ajph.92.2.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondersma SJ, Chase SK, Svikis DS, Schuster CR. Computer-based brief motivational intervention for perinatal drug use. Journal of Substance Abuse Treatment. 2005;28(4):305–312. doi: 10.1016/j.jsat.2005.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondersma SJ, Svikis DS, Lam PK, Connors-Burge VS, Ledgerwood DM, Hopper JA. A randomized trial of computer-delivered brief intervention and low-intensity contingency management for smoking during pregnancy. Nicotine and Tobacco Research. 2012;14(3):351–360. doi: 10.1093/ntr/ntr221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondersma SJ, Svikis DS, Schuster CR. Computer-based brief intervention: a randomized trial with postpartum women. American Journal of Preventive Medicine. 2007;32(3):231–238. doi: 10.1016/j.amepre.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondersma SJ, Svikis DS, Thacker LR, Beatty JR, Lockhart N. Computer-delivered screening and brief intervention (e-SBI) for postpartum drug use: a randomized trial. Journal of Substance Abuse Treatment. 2014;46(1):52–59. doi: 10.1016/j.jsat.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacek LR, Malcolm RJ, Martins SS. Race/ethnicity differences between alcohol, marijuana, and co-occurring alcohol and marijuana use disorders and their association with public health and social problems using a national sample. American Journal on Addictions. 2012;21(5):435–444. doi: 10.1111/j.1521-0391.2012.00249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perron BE, Mowbray OP, Glass JE, Delva J, Vaughn MG, Howard MO. Differences in service utilization and barriers among Blacks, Hispanics, and Whites with drug use disorders. Substance Abuse Treatment, Prevention, and Policy. 2009;4:3. doi: 10.1186/1747-597X-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm J, Rehm J, Taylor B, et al. Global burden of disease from alcohol, illicit drugs and tobacco. Drug and Alcohol Review. 2006;25(6):503–513. doi: 10.1080/09595230600944453. [DOI] [PubMed] [Google Scholar]
- Roy-Byrne P, Bumgardner K, Krupski A, Dunn C, Ries R, Donovan D, et al. Brief intervention for problem drug use in safety-net primary care settings: a randomized clinical trial. Journal of the American Medical Association. 2014;312(5):492–501. doi: 10.1001/jama.2014.7860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitz R. Treatment of alcohol and other drug dependence. Liver Transplant. 2007;13(11 Suppl 2):S59–64. doi: 10.1002/lt.21339. [DOI] [PubMed] [Google Scholar]
- Saitz R, Palfai TP, Cheng DM, Alford DP, Bernstein JA, Lloyd-Travaglini CA, et al. Screening and brief intervention for drug use in primary care: the ASPIRE randomized clinical trial. Journal of the American Medical Association. 2014;312(5):502–513. doi: 10.1001/jama.2014.7862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz RP, Gryczynski J, Mitchell SG, Gonzales A, Moseley A, Peterson TR, et al. Computerized v. in-person brief intervention for drug misuse: a randomized clinical trial. Addiction. 2014;109(7):1091–1098. doi: 10.1111/add.12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu AL. Behavioral and pharmacotherapy interventions for tobacco smoking cessation in adults, including pregnant women: U.S. preventive services task force recommendation statement. Annals of Internal Medicine. 2015;163(8):622–634. doi: 10.7326/M15-2023. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration [SAMHSA], Center for Behavioral Health Statistics and Quality [CBHSQ] Key substance use and mental health indicators in the United States: Results from the 2015 National Survey on Drug Use and Health. 2016a (HHS Publication No. SMA 16–4984, NSDUH Series H-51). Retrieved July 5, 2017 at https://www.samhsa.gov/data/sites/default/files/NSDUH-FFR1-2015/NSDUH-FFR1-2015/NSDUH-FFR1-2015.pdf.
- Substance Abuse and Mental Health Services Administration [SAMHSA], Center for Behavioral Health Statistics and Quality [CBHSQ] National Survey on Drug Use and Health: Detailed Tables. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2016b. Retrieved July 5, 2017 at https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.pdf. [Google Scholar]
- Williams EC, Lapham GT, Hawkins EJ, Rubinsky AD, Morales LS, Young BA, et al. Variation in documented care for unhealthy alcohol consumption across race/ethnicity in the Department of Veterans Affairs Healthcare System. Alcohol: Clinical and Experimental Research. 2012;36(9):1614–1622. doi: 10.1111/j.1530-0277.2012.01761.x. [DOI] [PubMed] [Google Scholar]