Introduction
Granulomatosis with polyangiitis (GPA), known as Wegener’s granulomatosis, is characterized by systemic necrotizing vasculitis in small and medium sized vessels affecting the upper and lower respiratory tracts, paranasal sinuses, and kidneys (1). We here describe a patient who presented with acute congestive heart failure (CHF) and progressive renal failure requiring hemodialysis and was later diagnosed with GPA based on positive renal biopsy.
Case Report
A 69-year-old man presented with 2–3 weeks’ duration of progressive dyspnea. His exercise capacity was unlimited until 2 months ago, but lately, he was able to walk only 1-2 blocks before experiencing shortness of breath along with leg edema. System review was overall negative, except for dry cough for 3–4 weeks and chronic sinusitis for which he was taking antihistamines.
On examination, he was in mild respiratory distress with inspiratory crackles, elevated JVP of 7-8 cm (S3 gallop sound), and palpable liver. Electrocardiogram (ECG) showed sinus rhythm with a nonspecific intraventricular conduction delay and congested pulmonary vessels on chest X-ray. Laboratory tests revealed a WBC count of 13.6 (normal range, 4.00–11.0 ×109/L), serum creatinine level of 0.9 (normal range, 0.6–1.2 mg/dL), BNP of 2844 (normal range, 100–300 pg/mL), and troponin I level of 0.08 at admission with a peak level of 0.1 (normal range, <or=0.04 ng/mL). As the clinical presentation was consistent with acute CHF, he was promptly treated with intravenous diuretic and oxygen via nasal cannula. Transthoracic echocardiogram (TTE) showed severely reduced left ventricular ejection fraction (LVEF, 15%) and dilated cardiomyopathy (DCM) with left ventricular end-diastolic diameter of 6.6 cm (normal range, 4.2–5.9), interventricular septum diastolic thickness of 1 cm, left atrium systolic diameter of 5.2 cm (normal range, 3–4 cm), and pulmonary artery systolic pressure of 53 mm Hg (Fig. 1). Coronary artery catheterization was performed, and it revealed normal coronary vasculature (Fig. 2).
Figure 1.
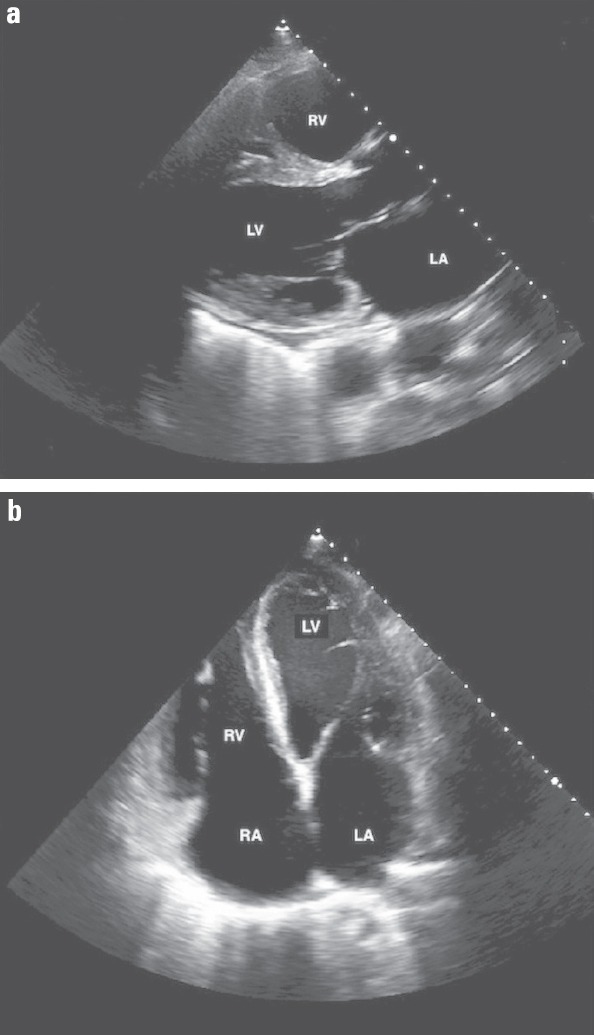
Transthoracic echocardiogram: left parasternal (a) and apical 4-chamber (b) views demonstrating dilated cardiomyopathy and severely reduced global left ventricular systolic function with an ejection fraction of 15%
LA - left atrium; LV - left ventricle; RA - right atrium; RV - right ventricle
Figure 2.
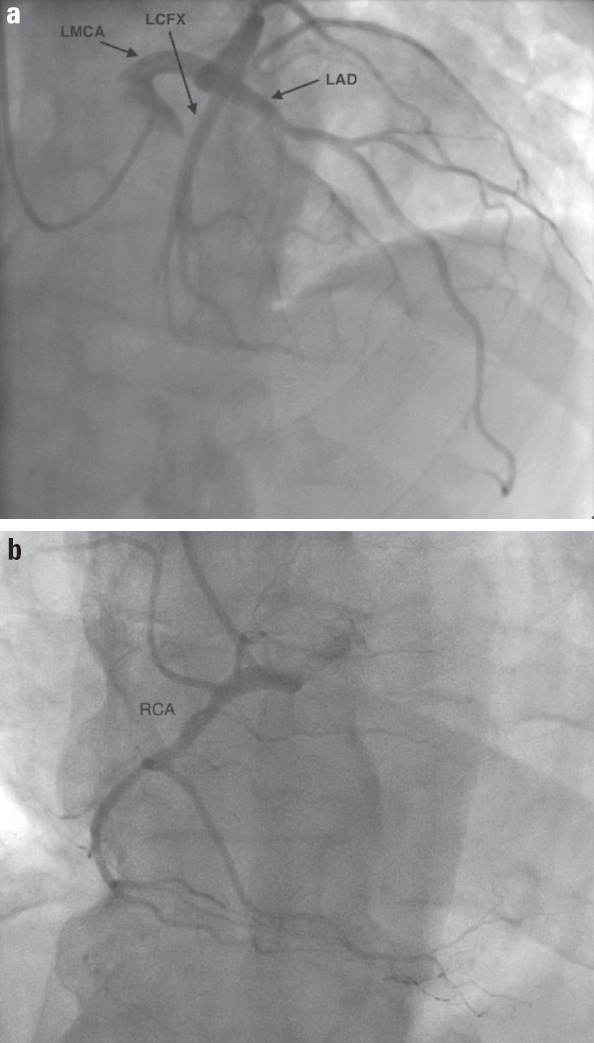
Coronary angiographic views reveal no significant stenosis in (a) left and (b) right coronary arteries
LAD - left anterior descending artery; LCFX - left circumflex artery; LMCA - left main coronary artery; RCA - right coronary artery
Heart failure symptoms had significantly improved with IV diuretic, but on hospitalization day 5, the serum creatinine level elevated to 1.7 mg/dL and WBC count to 15 × 109/L with absolute eosinophil of 0.1 K/µL (normal range, 0–0.9). Therefore, diuretic was immediately stopped, but the serum creatinine level elevated to 3.2 mg/dL on hospitalization day 10. Urinalysis revealed an RBC count of 132 (normal range, 0–3/HPF) and WBC count of 18 (normal range, 0–5/HPF) without symptoms or bacterial growth in urine culture. Renal ultrasound showed normal kidney structure without stone. Cytoplasmic antineutrophil cytoplasmic antibo- dies (cANCA) were 315 AU/mL (normal range, 0–19), ESR was 95 mm in 1 h, and CRP was 30 (normal range, <8 mg/L). Therefore, cANCA-associated vasculitis including GPA, microscopic polyangiitis, and eosinophilic granulomatosis with polyangiitis (EGPA) were considered as differential diagnoses, but renal biopsy exhibited pauci-immune glomerulonephritis (Fig. 3). Diagnosis of GPA was made, and plasmapheresis, oral cyclophosphamide, and steroid were initiated. However, renal function was getting worse, which required hemodialysis on hospitalization day 25. He was able to perform physical activities, but still experienced dyspnea with some activity (NYHA class 3). Then, the patient was discharged to a long-term care facility on cyclophosphamide, valsartan 40 mg 12 hourly, and metoprolol ER 25 mg daily.
Figure 3.
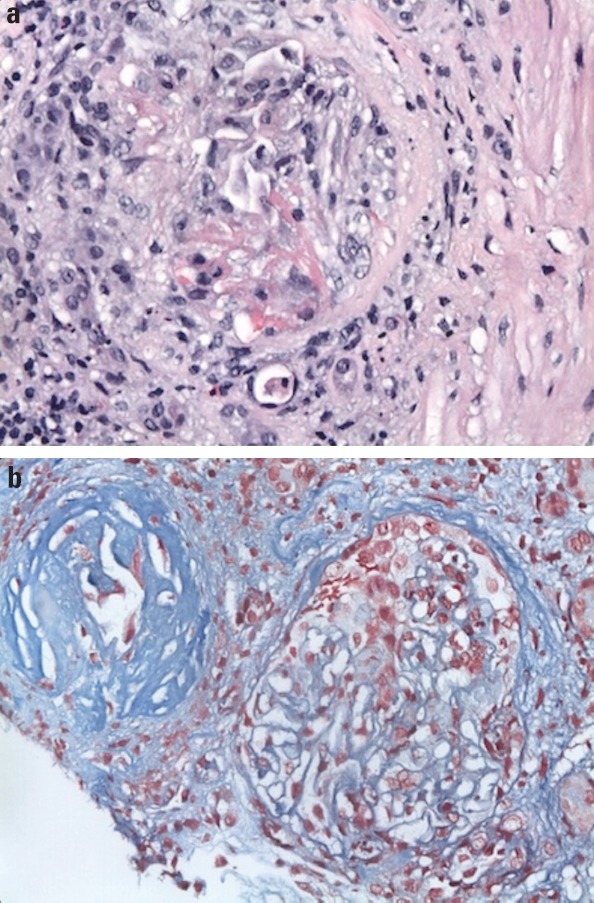
Renal biopsy demonstrates (a) sclerotic glomeruli with segmental cellular crescent formation in a background of mixed interstitial inflammation composed of mature lymphocytes, plasma cells, and eosinophils (400× hematoxylin and eosin stain) and (b) shrunken globally sclerotic glomeruli and fibrocellular crescent glomerulus with fibrin deposition-red on trichrome stain (400× trichrome)
Discussion
GPA can manifest multiple organ systems and present with a variety of symptoms. Cardiac involvement in GPA is infrequent with an estimated incidence of 3.3% in which pericarditis, myocarditis, and conduction system defects are the most frequent one (2). Furthermore, DCM with CHF related to GPA is exceedingly exceptional, and our extensive literature search revealed only eight cases reported in the past since GPA was described in 1936 by Wegener (3–9). Of the eight cases, only three presented with acute heart failure (4, 8, 9). The presence of small vessels necrotizing vasculitis could be an attributing factor of cardiac involvement in GPA, but the exact mechanism is uncertain.
With an advancement of imaging modalities, MRI has been useful to evaluate the end organs involvement along with cANCA, ESR, and CRP, although tissue biopsy remains the gold standard (10). In this case, the presence of cANCA along with normal eosinophil count and positive renal biopsy confirmed GPA. Cyclophosphamide therapy in GPA could lead to a DCM, but in our case, congestive cardiomyopathy was seemingly due to GPA as he was not taking any medicines (7). Endomyocardial biopsy or cardiac MRI with contrast was not performed given biopsy-positive GPA, positive inflammatory markers, and impaired renal function, but nonetheless, it could be a limitation of this case. To the extent of our knowledge, this is the fourth case with acute CHF as the initial presentation of GPA.
Conclusion
This case reminds clinicians that acute CHF with worsening renal function could be an initial manifestation of GPA, which should be included in the differential diagnosis.
References
- 1.Schilder AM. Wegener’s granulomatosis vasculitis and granuloma. Autoimmun Rev. 2010;9:483–7. doi: 10.1016/j.autrev.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 2.McGeoch L, Carette S, Cuthbertson D, Hoffman GS, Khalidi N, Koening CL, et al. Vasculitis Clinical Research Consortium. Cardiac Involvement in Granulomatosis with Polyangiitis. J Rheumatol. 2015;42:1209–12. doi: 10.3899/jrheum.141513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fauci AS, Haynes BF, Katz P, Wolff SM. Wegener’s granulomatosis: prospective clinical and therapeutic experience with 85 patients for 21 years. Ann Intern Med. 1983;98:76–85. doi: 10.7326/0003-4819-98-1-76. [DOI] [PubMed] [Google Scholar]
- 4.Weidhase A, Gröne HJ, Unterberg C, Schuff-Werner P, Wiegand V. Severe granulomatous giant cell myocarditis in Wegener’s granulomatosis. Klin Wochenschr. 1990;68:880–5. doi: 10.1007/BF01662788. [DOI] [PubMed] [Google Scholar]
- 5.Korzets Z, Chen B, Levi A, Pomeranz A, Bernheim J. Non dilated congestive cardiomyopathy—a fatal sequelae of Wegener’s granulomatosis. J Nephrol. 1991;1:61–4. [Google Scholar]
- 6.Day JD, Ellison KE, Schnittger I, Perlroth MG. Wegener’s granulomatosis presenting as dilated cardiomyopathy. West J Med. 1996;165:64–6. [PMC free article] [PubMed] [Google Scholar]
- 7.Delevaux I, Hoen B, Selton-Suty C, Canton P. Relapsing congestive cardiomyopathy in Wegener’s granulomatosis. Mayo Clin Proc. 1997;72:848–50. doi: 10.4065/72.9.848. [DOI] [PubMed] [Google Scholar]
- 8.To A, De Zoysa J, Christiansen JP. Cardiomyopathy associated with Wegener’s granulomatosis. Heart. 2007;93:984. doi: 10.1136/hrt.2006.098038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarlon G, Durant C, Grandgeorge Y, Bernit E, Veit V, Hamidou M, et al. Cardiac involvement in Wegener’s granulomatosis: report of four cases and review of the literature. Rev Méd Interne. 2010;31:135–9. doi: 10.1016/j.revmed.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Florian A, Slavich M, Blockmans D, Dymarkowski S, Bogaert J. Cardiac involvement in granulomatosis with polyangiitis (Wegener granulomatosis), Circulation. 2011;124:e342–3. doi: 10.1161/CIRCULATIONAHA.111.030809. [DOI] [PubMed] [Google Scholar]


