Abstract
The nanogold reaction between HAuCl4 and citrate is very slow, and the catalyst graphene oxide nanoribbon (GONR) enhanced the nanoreaction greatly to produce gold nanoparticles (AuNPs) that exhibited strong surface plasmon resonance (SPR) absorption (Abs) at 550 nm and resonance Rayleigh scattering (RRS) at 550 nm. Upon addition of the peptide of human chorionic gonadotropin (hCG), the peptide could adsorb on the GONR surface, which inhibited the catalysis. When hCG was added, peptides were separated from the GONR surface due to the formation of stable peptide–hCG complex, which led to the activation of GONR catalytic effect. With the increase in hCG concentration, the RRS and Abs signal enhanced linearly. The enhanced RRS value showed a good linear relationship with hCG concentration in the range of 0.2–20 ng/mL, with a detection limit of 70 pg/mL. Accordingly, two new GONR catalytic RRS/Abs methods were established for detecting hCG in serum samples.
Keywords: nanocatalysis, graphene oxide nanoribbon, peptide regulation, hCG, RRS
Introduction
Nanoenzyme is a kind of mimetic enzyme that has unique properties and catalytic function. It has the characteristics of high efficiency, stability, economy and large-scale preparation and has been applied in different fields of materials science, physics, chemistry, biology, medicine, and environment.1–3 The nanomaterials with nano analog enzyme activity have important significance in analytical chemistry. At present, the analytical application is mainly involved in the detection of heavy metal ions and biological molecules.3–6 Seok and Jongsoo3 detected mercury ions using ssDNA magnetic nanoparticles to inhibit the oxidation of H2O2 o-phenylenediamine to make no color change; the detection range was 5–75 μmoL/L. Li et al4 used the principle that mercury ions inhibited the color reaction of oxidation of tetramethyl benzidine catalyzed by nano lead mimetic enzyme to analyze Hg2+ in water samples, with a detection limit (DL) of 7.2 nmoL/L. Peng et al5 detected mercury and copper using the principle of fluorescence quenching based on the specific binding of the fluorescent silver nanoclusters and the heavy metal ions; the detection ranges were 6–160 nmoL/L and 6–240 nmoL/L, respectively. Lien et al6 used fluorescence enhancement to detect thrombin due to amplex reagent (AR) for hydrogen peroxide oxidation catalyzed by thrombin transforming protein-mediated Bi-gold nanoparticles (AuNPs). Wang et al7 reduced the DL of H2O2 to 30 nmoL/L successfully by resonance Raman scattering with the oxidation of tetramethyl benzidine catalyzed by Au@Ag nanoparticles. Jiang et al8 achieved a low concentration using the glucose detection method, which was based on the catalytic effect of nanogold by spectrophotometry, with a detection range of 2–10 nmoL/L. Wang et al9 used Cu2+-ion-modified graphene oxide (GO) nanoparticles as a heterogeneous catalyst mimicking functions of horseradish peroxidase (HRP), and a simple colorimetric method was proposed to detect 30–140 μM ascorbic acid.10 Our research group has established some sensitive resonance Rayleigh scattering (RRS) and surface enhanced Raman scattering methods for rapid detection of melamine,11 PDGF12 and Cu,13 based on the nanoparticle probe aggregation and catalysis. Graphene oxide nanoribbons (GONRs) were prepared by using the method of facile unzipping of nitrogen-doped multiwalled carbon nanotubes (N-MWCNTs) with the help of microwave energy,14,15 which exhibited good water solubility and has been used in nanoanalysis. Zhang et al16 reported a GONR-modified electrochemical biosensor for the detection of 0.25–1.25 mmol/L amino acid. Dong et al17 developed a GONR biosensor for determination of 5–100 μmol/L ATP. Zhu et al18 fabricated an MWCNT@GONR electrochemical sensor for 8–500 nmol/L polycyclic aromatic amines. In recent years, nanoplasmonic sensors have become widely used in medical, biotechnology and environmental science. One of the most promising directions has been surface-based nanoplasmonic sensors, and the potential of such technologies is still emerging.19–21 To date, there have been no reports about GONR catalytic gold nanoplasmon RRS/absorption (Abs) analytical platform for human chorionic gonadotropin (hCG), based on peptide reaction-modulating nanoenzyme activity, without preparation of nanoprobes and nanoparticle aggregation.
hCG is a glycoprotein secreted by the placenta trophoblast cells, and it is an important medical diagnostic marker of pregnancy. hCG also is one of the important markers of clinical disease. Its content is closely related to some diseases, such as gestational trophoblastic disease, germ cell tumors and Down syndrome.22–24 In addition, the quantitative detection of hCG is of great significance to the analysis of clinical medicine and the abuse of stimulants. At present, the detection methods of hCG are mainly electrochemical immunoassay, radioimmunoassay, chemiluminescent immunoassay, fluorescence resonance energy transfer method and optical immunoassay.25–30 Among these methods, radioimmunoassay is widely used, but there are radiation hazards. The electrochemical immunoassay method has a high sensitivity, but the operation is complex. The cost of fluorescence immunoassay is low, but there is a fluorescence quenching effect. Immunogold method is the most mature and widely used method for rapid detection of hCG, but nanogold aggregation caused unstability. Most of these hCG immune analysis methods are based on the antigen–antibody reaction; the biggest disadvantage is that the antibody biological protein is easily affected by the environment deterioration and then affects the detection effect. Recently, some new assays were developed for the detection of hCG.31–36 Zhao et al31 reported a microplate magnetic chemiluminescence enzyme immunoassay for the determination of hCG using the alkaline phosphatase (ALP) as a labeled reagent and 3-(2′-spiroadamantane)-4-methoxy-4-(3″-phosphoryloxy) phenyl-1,2-dioxetane (AMPPD) as a chemiluminescence reagent. Chang et al32 combined catalytic gold nanoparticles with an hCG-specific peptide aptamer to create a simple, sensitive, label-free colorimetric assay for hCG. Ding and Yang33 reported an antibody-free and label-free mechanism for detecting hCG using a short oligopeptide as an hCG receptor to bind hCG, with a DL of 2 nmol/L hCG. Lei et al34 reported a strategy of chemiluminescence resonance energy transfer for detection of 0.12 nmol/mL hCG using graphene as an efficient long-range energy acceptor and the magnetic nanoparticles for magnetic separation and immobilization of HRP-labeled anti-hCG antibody. Chen et al35 reported an immunomagnetic assay for quantitative analysis of total β-subunit of hCG concentration in urine. The peptide of hCG was more stable than hCG antibody. Thus, Xia et al36 found a new and stable method to detect hCG by using the polypeptide nano silver electrochemical sensor with voltammetry, with a DL of 0.4 mIU/mL. In this article, a new, simple, sensitive and low-cost nanoplasmon RRS/Abs analytical platform was developed, which was based on the polypeptide–hCG reaction regulation of GONR catalytic nanogold particle reaction.
Materials and methods
Materials
A DXR SmartRaman Spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) with a laser wavelength of 633 nm and a power of 3.0 mW, Cary Eclipse Fluorescence Spectrophotometer (Varian Company, Salt Lake City, UT, USA), TU-1901 double beam ultraviolet (UV)–visible spectrophotometer (Beijing Purkinje General Instrument Co., Ltd., Beijing, China) and a thermostatic bath were used.
Reagents
A 0.50 mg hCG (Beijing Boosen Biotechnology Co., Ltd., Beijing, China) freeze-dried powder was mixed with 1.0 mL water and diluted to 10 mL; hCG polypeptide (HP; Biological Engineering Shanghai Limited by Share Ltd., Shanghai, China) was prepared by using Pro–Pro–Leu–Arg–Ile– Asn–Arg–His–Ile–Leu–Thr–Arg (PPLRINRHILTR), 1.0% HAuCl4⋅4H2O (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China), 1.0% trisodium citrate (TCA; Guangdong Shantou Timong Chemical Factory, Guangzhou, China) and 0.1 mol/L HCl. All reagents were analytically pure, and water was double distilled.
GONR was prepared by the modified KMnO4 procedure. Briefly, 50 mg of MWCNT powder was added into a 50 mL flask containing 10 mL concentrated H2SO4 solution for 1 hour. Then, 500 mg of KMnO4 was added and heated at 60°C for 2 h. The product was poured into a 5 mL 30% H2O2 solution that was cooled by ice water. The mixture was dispersed by ultrasonic treatment for 10 min and centrifuged at 7,000 rpm for 10 min to obtain the supernatant containing 230 μg/mL GONR that was calculated by adding MWCNT. Before use, it was neutralized with 50 mmol/L NaOH and then was diluted to the desired concentration.
Methods
A moderate amount of hCG and 100 μL of 1.4 μmol/L HP were added to a 5 mL test tube for ~15 min and then 480 μL of 1 μg/mL GONR was added and mixed well. After ~10 min, 75 μL of 3.4 mmol/L TCA, 150 μL of 0.01 mol/L HCl and 100 μL of 0.1% (84 μmol/L) HAuCl4 were put into the test tube, diluted to 1.5 mL, mixed well, incubated at 60°C in a water bath for ~12 min and cooled with tap water. Finally, the mixture was diluted to 2 mL with water. The mixture was transferred into a quartz cell, and its RRS spectra were recorded. The RRS peak intensity at 550 nm (I) and the I0 blank without hCG were recorded, and the value of ΔI = I − I0 was calculated.
Results and discussion
Analysis principle
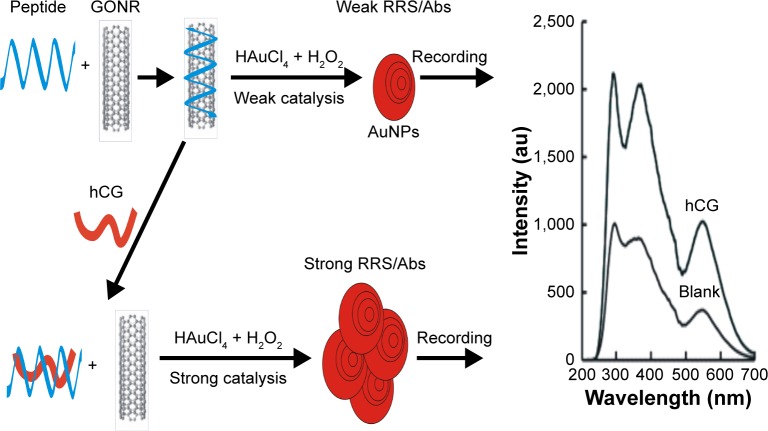
Nanocatalytic reaction is an important way for signal amplification and enhancing sensitivity. The new nanocatalytic particle reaction of citrate–HAuCl4 nanoparticles was investigated and used in nanoplasmon molecular spectral analysis. The AuNP reaction was very slow in the absence of nanocatalyst. Hence, in the non-catalytic reaction system, there was a low concentration of the produced AuNPs with weak surface enhanced Raman scattering signal due to small amount of VB4r molecular probes being adsorbed on the AuNPs. The GONRs with abundant surface hydroxyl groups had a strong catalytic effect on the AuNP reaction between citrate and HAuCl4. The AuCl4− and citrate could adsorb on the GONR catalyst surface, and redox reaction took place to form Au. The surface electrons of GONR enhanced the electron transfer in the redox reaction and speeded up the redox reaction. The produced AuNPs exhibited surface plasmon resonance (SPR)– RRS and Abs, and both increased with GONR concentration. HP had a high affinity with GONR and a high specificity for targeting hCG. It could easily adsorb to the GONR surface through electrostatic attraction that weakened GONR catalysis. When the hCG was present in the solution, the HP selectively recognized and tightly bound to hCG to form a stable HP–hCG complex that escaped the nanosurface to recover the nanocatalysis. With the increase in hCG concentration, the catalysis enhanced due to the increase in the free GONR concentration. Thus, we could establish a new nanogold plasmon RRS/Abs platform for the determination of hCG (Figure 1) with high sensitivity, high selectivity and simplicity.
Figure 1.
Scheme of peptide-controlling GONR catalytic reaction–RRS detection of hCG.
Abbreviations: Abs, absorption; AuNP, gold nanoparticle; au, atomic unit; GONR, graphene oxide nanoribbon; hCG, human chorionic gonadotropin; RRS, resonance Rayleigh scattering.
RRS spectra
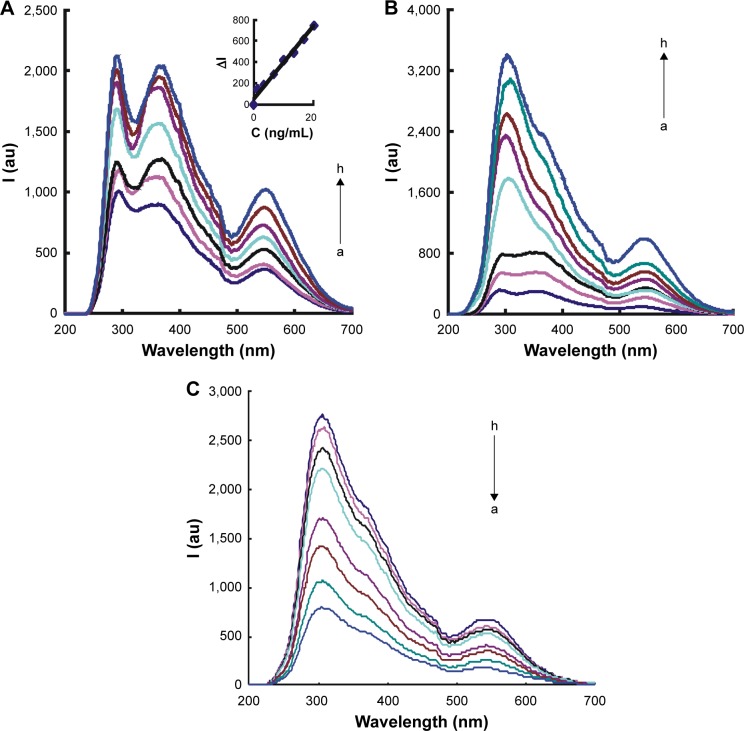
RRS is a simple and sensitive molecular spectral technique. It is suitable for nanoparticle reaction and is selected for the study of the nanocatalytic system. To date, there have been no reports about RRS study of the GONR catalytic nanogold reaction and its coupling with immunoreactions without nanoparticle aggregation that could cause instability of the analytical system. Thus, RRS was selected for the study of the analytical system. The catalysis of GONR and GO in the redox reaction was considered; as the GONR is stronger than the GO, GONR was selected as the nanoenzyme. For the HP–hCG–GONR–HAuCl4–TCA analytical system, the generated AuNPs exhibited three RRS peaks at 290, 370 and 550. The RRS peak at 550 nm had a good linear relationship between the signal and hCG concentration and was selected for assay of hCG (Figure 2A). The nanocatalytic system of GONR–HAuCl4–TCA exhibited three RRS peaks at 290 and 545 nm (Figure 2B), and the RRS peak intensity increased with GONR concentration. Upon addition of HP, HP could inhibit the catalytic activity of GONR nanoenzyme; the catalytic activity decreased and the two RRS peaks at 300 and 550 nm decreased with the increasing HP concentration (Figure 2C).
Figure 2.
RRS spectra of the GONR systems.
Notes: (A) HP–hCG–GONR–HAuCl4–TCA system – a: 35 nmoL/L HP +240 ng/mL GONR +0.167 mmoL/L HCl +0.34 mmoL/L TCA +5.6 μmoL/L HAuCl4; b: a+0.33 ng/mL hCG; c: a+1 ng/mL hCG; d: a+2 ng/mL hCG; e: a+4 ng/mL hCG; f: a+6 ng/mL hCG; g: a+10 ng/mL hCG; and h: a+13.33 ng/mL hCG. The inset graph was the working curve. (B) GONR–HAuCl4–TCA system – a: 0.167 mmoL/L HCl +0.34 mmoL/L TCA+ 5.6 μmoL/L HAuCl4; b: a+16 ng/mL GONR; c: a+40 ng/mL GONR; d: a+80 ng/mL GONR; e: a+120 ng/mL GONR; f: a+160 ng/mL GONR; g: a+240 ng/mL GONR; and h: a+320 ng/mL GONR. (C) HP–GONR–HAuCl4–TCA system – a: 240 ng/mL GONR +0.167 mmoL/L HCl +0.34 mmoL/L TCA +5.6 μmoL/L HAuCl4; b: a+1.4 nmoL/L HP; c: a+3.5 nmoL/L HP; d: a+7 nmoL/L HP; e: a+10.5 nmoL/L HP; f: a+14 nmoL/L HP; g: a+21 nmoL/L HP; and h: a+28 nmoL/L HP.
Abbreviations: C, concentration; I, intensity; RRS, resonance Rayleigh scattering; GONR, graphene oxide nanoribbon; HP, hCG polypeptide; hCG, human chorionic gonadotropin; TCA, trisodium citrate.
Ultraviolet Abs spectra
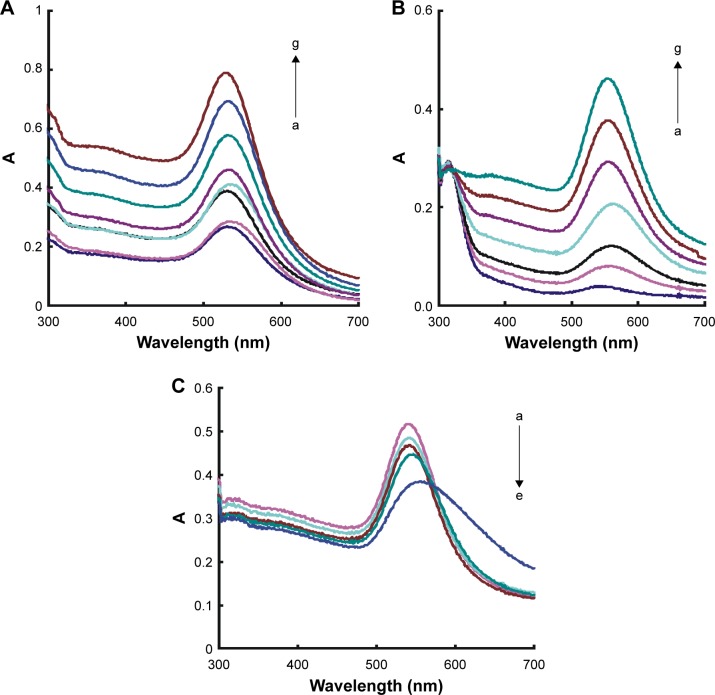
A spectrophotometer is a very simple and low-cost spectral instrument that was selected for studying the SPR Abs of nanogold nanoparticles. For the HP–hCG–GONR–HAuCl4–TCA system, the SPR Abs peak at 540 nm increased with hCG concentration and was selected for assay of hCG (Figure 3A). The GONR catalyzed the TCA reduced HAuCl4 to form AuNPs with a SPR Abs peak at 550 nm (Figure 3B) that corresponds to the RRS peak at 545 nm, in which the peak increased with the GONR increasing. HP can adsorb on the GONR surface and inhibit the catalysis; the SPR Abs peak decreased with the increasing HP concentration (Figure 3C).
Figure 3.
UV spectra of the GONR systems.
Notes: (A) HP–hCG–GONR–HAuCl4–TCA system – a: 35 nmol/L HP +240 ng/mL GONR +0.167 mmol/L HCl +0.34 mmoL/L TCA +5.6 μmoL/L HAuCl4; b: a+0.67 ng/mL hCG; c: a+1.67 ng/mL hCG; d: a+3.33 ng/mL hCG; e: a+6.67 ng/mL hCG; f: a+10 ng/mL hCG; and g: a+13.33 ng/mL hCG. (B) GONR–HAuCl4–TCA system – a: 0.167 mmoL/L HCl +0.34 mmoL/L TCA +5.6 μmol/L HAuCl4; b: a+16 ng/mL GONR; c: a+40 ng/mL GONR; d: a+80 ng/mL GONR; e: a+120 ng/mL GONR; f: a+160 ng/mL GONR; and g: a+240 ng/mL GONR. (C) HP–GONR–HAuCl4–TCA system – a: 240 ng/mL GONR +0.167 mmoL/L HCl +0.34 mmoL/L TCA +5.6 μmoL/L HAuCl4; b: a+5 nmoL/L HP; c: a+10 nmoL/L HP; d: a+20 nmoL/L HP; and e: a+30 nmoL/L HP.
Abbreviations: A, absorbance; UV, ultraviolet; GONR, graphene oxide nanoribbon; HP, hCG polypeptide; hCG, human chorionic gonadotropin; TCA, trisodium citrate.
Catalysis and inhibition
In the experimental conditions, the reduction of HAuCl4–TCA could be catalyzed by GONR or GO to form AuNPs. The RRS and Abs values increased with the increasing catalyst concentration. The catalytic action of GONR was stronger than that of the GO, because the slope of GONR is larger than the GO due to the size of GONR being smaller than the GO. When HP was added into the system, the RRS peak and Abs values decreased with increasing HP concentration (Table 1). The reason was that HP could be attached to the surface of the nanocatalyst by physical electrostatic adsorption, so as to block the exchange between the redox electrons and catalyst surface electrons.
Table 1.
Comparison of the nanocatalysis and the inhibition of HP on the reaction of TCA–HAuCl4
| System | Linear range | Linear equation | Correlation coefficient |
|---|---|---|---|
| GONR | 1.0–30 ng/mL GONR | ΔI545 nm =61.5C+203 | 0.9918 |
| AuNP (d =10 nm) | 1.0–25 ng/mL AuNP | ΔI545 nm =58.3C+150 | 0.9834 |
| HP–GONR | 2.0–30 nmoL/L HP | ΔI545 nm =47.8C+69.5 | 0.9804 |
| GO | 2.0–30 ng/mL GO | ΔI545 nm =36.9C−89.8 | 0.9621 |
Note: I is measured in au.
Abbreviations: HP, hCG polypeptide; TCA, trisodium citrate; GONR, graphene oxide nanoribbon; AuNP, gold nanoparticle; GO, graphene oxide; hCG, human chorionic gonadotropin; I, intensity; C, concentration; d, diameter.
Transmission electron microscopy
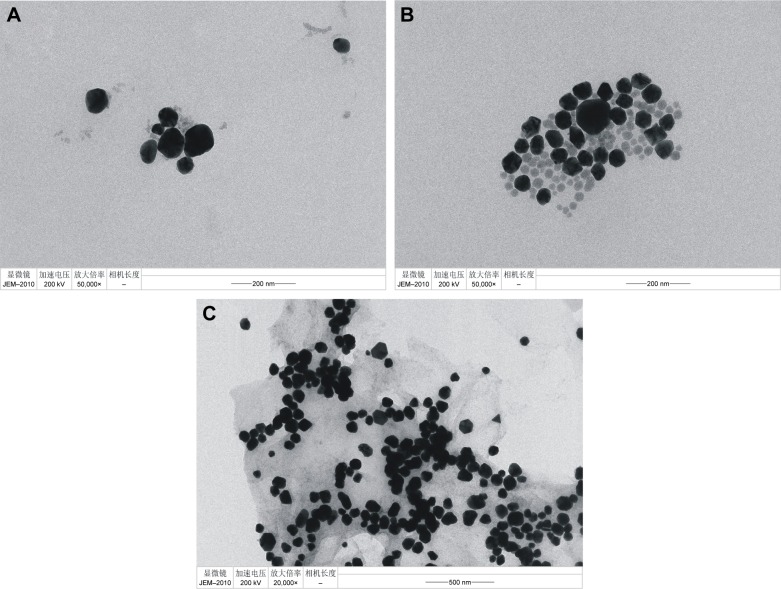
Transmission electron microscopy (TEM) is used to observe the particulate particle size and surface morphology. The blank reaction solution of HP–hCG–GONR–HAuCl4 system was obtained by the procedure, and the TEM (Figure 4A) was recorded with particle average size of 80 nm. When hCG was added into the solution, more AuNPs formed (Figure 4B and C); the particle size was 60 and 50 nm.
Figure 4.
TEMs of analysis system.
Notes: (A) 35 nmoL/L HP +240 ng/mL GONR +0.167 mmoL/L HCl +0.34 mmoL/L TCA +5.6 μmoL/L HAuCl4. (B) A+4 ng/mL hCG. (C) A+10 ng/mL hCG.
Abbreviations: TEM, transmission electron microscopy; HP, hCG polypeptide; GONR, graphene oxide nanoribbon; TCA, trisodium citrate; hCG, human chorionic gonadotropin.
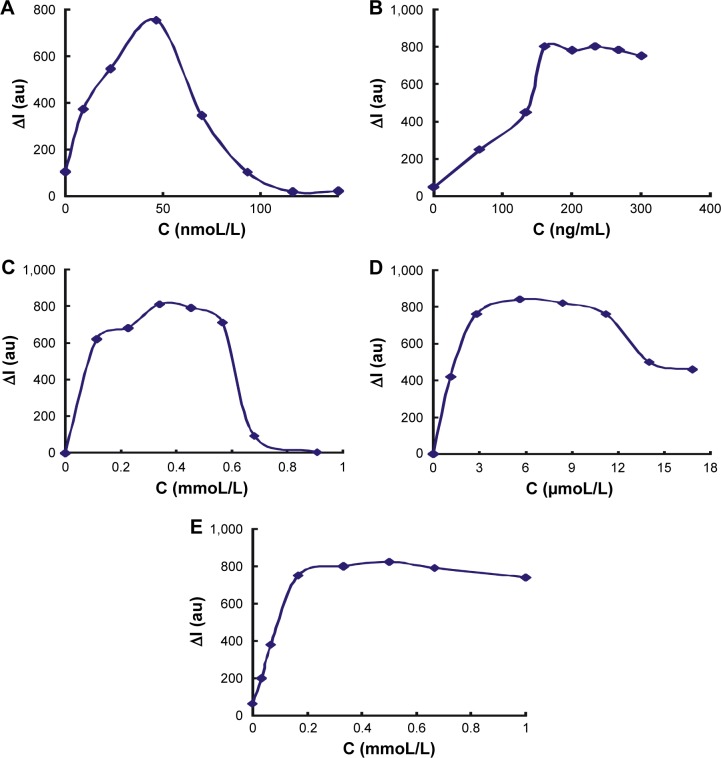
Optimization of analysis conditions
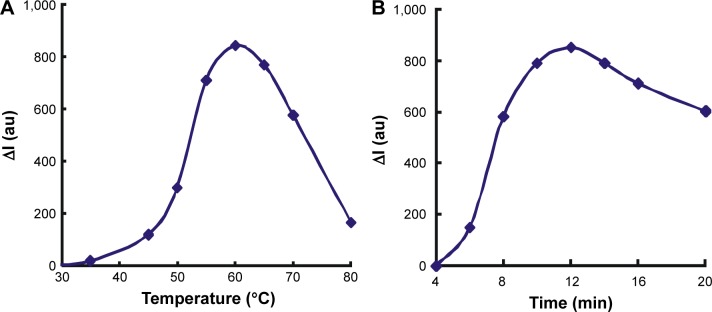
The reaction conditions of HP–hCG–GONR–TCA–HAuCl4 were optimized according to the experimental method. The effect of HP concentration on ΔI was investigated when it reached 35 nmoL/L. The value of ΔI was the largest (Figure 5A), so the selection of the concentration of HP was 35 nmoL/L. The effects of GONR concentration on the catalytic system at ΔI were investigated; according to the result, 240 ng/mL GONR was selected for use (Figure 5B). The concentration of TCA was researched; when the TCA concentration was 0.34 mmoL/L, the value of ΔI was the largest (Figure 5C); the selection of the concentration of TCA was 0.34 mmoL/L; when the HAuCl4 concentration was 5.6 μmoL/L, the ΔI value was the largest (Figure 5D); 5.6 μmoL/L HAuCl4 was selected. The dosage of HCl was optimized, and when the concentration of HCl was 0.167 mmoL/L, ΔI reached the maximum value (Figure 5E); 0.167 mmoL/L HCl was chosen; The optimization of the aforementioned reaction was carried out at 60°C water bath condition; the reaction time was 10 min. The reaction temperature and time were examined. A temperature of 60°C for 12 min was chosen for use (Figure 6).
Figure 5.
Effect of reagent concentration.
Notes: (A) HP −10 ng/mL hCG +240 ng/mL GONR +0.167 mmoL/L HCl +0.34 mmo/L TCA +5.6 μmoL/L HAuCl4. (B) GONR −35 nmoL/L HP +10 ng/mL hCG +GO +0.167 mmoL/L HCl +0.34 mmoL/L TCA +5.6 μmoL/L HAuCl4. (C) TCA – 35 nmoL/L HP +10 ng/mL hCG +240 ng/mL GONR +0.167 mmoL/L HCl +5.6 μmoL/L HAuCl4. (D) HAuCl4 −35 nmoL/L HP +10 ng/mL hCG +240 ng/mL GONR +0.167 mmoL/L HCl +0.34 mmoL/L TCA+HAuCl4. (E) HCl −35 nmoL/L HP +10 ng/mL hCG +240 ng/mL GONR+HCl +0.34 mmoL/L TCA +5.6 μmoL/L HAuCl4.
Abbreviations: HP, hCG polypeptide; hCG, human chorionic gonadotropin; GONR, graphene oxide nanoribbon; TCA, trisodium citrate; GO, graphene oxide; I, intensity; C, concentration.
Figure 6.
The effect of reaction temperature and time.
Notes: (A) Reaction temperature: 35 nmoL/L HP +10 ng/mL hCG +240 ng/mL GONR +0.167 mmoL/L HCl +0.34 mmoL/L TCA +5.6 μmoL/L HAuCl4. (B) time: 35 nmoL/L HP +10 ng/mL hCG +240 ng/mL GONR +0.167 mmoL/L HCl +0.34 mmoL/L TCA +5.6 μmoL/L HAuCl4.
Abbreviations: HP, hCG polypeptide; hCG, human chorionic gonadotropin; GONR, graphene oxide nanoribbon; TCA, trisodium citrate; I, intensity.
Working curve
The working curves for hCG were obtained according to the experimental method. For the GONR analytical system, the enhanced values of the RRS peak at 550 nm and the Abs peak at 550 nm were linear with hCG concentration in the range of 0.2–20 ng/mL and 10–15 ng/mL, respectively, with a linear equation of ΔI =91.5C +34.0 and ΔA550 nm =0.0334C+0.0223, correlation coefficient of 0.9863 and 0.9661, and DL of 0.07 and 3 ng/mL, respectively. We can see that the RRS analytical system is more sensitive than the Abs and was thus chosen for sample detection. Compared to the reported hCG methods,25–36 this RRS method is not only sensitive and selective but also facile, of low-cost and without nanoparticle aggregation. The clinical method of chemiluminescent immunoassay had a DL of 0.143 ng/mL, limit of quantitation (LQ) of 5 ng/mL and linear range of 5–540 ng/mL hCG;37 the DL and LQ of this RRS method are better than the clinical method, and the linear range of the two methods are close to each other, as the upper LQ divided by the lower LQ is 108 and 100, respectively.
Interference
The effect of the coexisting substances on the determination of 10 ng/mL hCG was investigated. The tested common interfering ions and amino acids, IgG and IgM, did not interfere with the determination when the relative error was within 10% (Table 2). This indicated that this method had a good selectivity.
Table 2.
Effect of coexisting substances
| Coexisting substance | Relative multiple | Relative error (%) | Coexisting substance | Relative multiple | Relative error (%) |
|---|---|---|---|---|---|
| K+ | 100 | −1.0 | SO32− | 100 | −5.7 |
| Ca2+ | 100 | −7.4 | NO2− | 100 | 8.0 |
| Mg2+ | 100 | −6.3 | S2O32− | 100 | −6.0 |
| Fe3+ | 100 | −5.3 | CO32− | 100 | −6 |
| IgM | 10 | −0.8 | Glycine | 100 | −4 |
| IgG | 10 | −3.5 | Lysine | 100 | −7 |
| Tryptophan | 10 | 5.0 | Aspartic acid | 100 | 8.0 |
| Glutamate | 50 | 1.2 | Valine | 100 | −4.0 |
| Phenylalanine | 50 | −1.0 | Tyrosine | 100 | −6.0 |
Analysis of samples
Seven serum samples of women were provided by the No 5 People’s Hospital of Guilin, Guangxi, China. A 100 μL sample was diluted to 10 mL with water before determination, the next detections were according to the procedure, and the content was equal to detection value multiplying dilution times of 100. In addition, recovery tests were performed, and the recovery was equal to ([Found value−Detection value]/Added value) multiplied by 100%. The results are listed in Table 3, and the recoveries were in the range of 90.1%–106%; the relative standard deviation (RSD) was in the range of 0.90%–3.97% according to five detection values. The obtained results were not obviously different from clinical diagnosis values from the No 5 People’s Hospital of Guilin,37 so the results demonstrate that the method was reliable and practical in clinical detection.
Table 3.
Analysis of hCG samples
| Sample | Detection value (ng/mL) | Content (ng/mL) | Added value (ng/mL) | Found value (ng/mL) | Recovery (%) | RSD (%) | Ref value (ng/mL) |
|---|---|---|---|---|---|---|---|
| 1 | 0.548 | 55.6 | 0.25 | 0.7842 | 94.5 | 1.05 | 56.6 |
| 2 | 0.432 | 43.7 | 0.5 | 0.9258 | 98.8 | 3.97 | 45.7 |
| 3 | 0.404 | 40.9 | 1.0 | 1.364 | 90.1 | 1.43 | 39.6 |
| 4 | 0.480 | 48.6 | 0.5 | 1.010 | 106 | 0.90 | 46.9 |
| 5a | 0.310 | 3.10 | 0.25 | 0.550 | 96.0 | 3.76 | 32.1 |
| 6 | 5.24 | 524 | 5.0 | 10.52 | 105.6 | 2.85 | 518 |
| 7 | 16.7 | 1,670 | 1.0 | 17.63 | 93.0 | 2.45 | 1,692 |
Note:
The sample dilution time was 10 min.
Abbreviations: hCG, human chorionic gonadotropin; Ref, reference; RSD, relative standard deviation.
Conclusion
In this work, GONR exhibited strong catalysis of the AuNP nanoreaction between HAuCl4 and citrate that produced an SPR Abs peak at 550 nm and an RRS peak at 550 nm. Upon addition of HP, HP adsorbed on the surface of GONR nanoparticles, which blocked the electron transfer in the redox reaction and inhibited GONR catalytic action. When the target hCG was added, it reacted with HP to form stable HP–hCG complex and free GONR nanocatalyst that caused the SPR Abs value and the RRS intensity to increase due to the recovery of nanocatalysis. According to this principle, RRS and SPR Abs methods for detection of hCG were established based on the control of the catalytic reaction of GONR nano enzyme by the peptide reaction. With the increase in the amount of the analyte hCG, the RRS signal of the system increased gradually. The increased RRS intensity, ΔI, at 550 nm showed a good linear relationship with the concentration of hCG in the range of 0.2–20 ng/mL, with a DL of 70 pg/mL. The RRS method was used in analyzing serum samples, with a recovery of 90.1%–106% and RSD of 0.90%–3.97%.
Acknowledgments
This work is supported by the National Natural Science Foundation of China (numbers 21667006, 21767004, 21465006, and 21477025), the Doctor Scientific Research Foundation of Hezhou University (number HZUBS201608) and the Professor Scientific Research Foundation of Hezhou University (number HZUJS201613).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Gao L-Z, Zhuang J, Nie L, et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat Nanotechnol. 2007;2:577–583. doi: 10.1038/nnano.2007.260. [DOI] [PubMed] [Google Scholar]
- 2.Li J-L, Sheng A-G, Hu J-M. Research progress of nanozymes and its application analysis. Chin J Appl Chem. 2016;33(11):1245–1252. [Google Scholar]
- 3.Seok KY, Jongsoo J. A simple colorimetric assay for the detection of metal ions based on the peroxidase-like activity of magnetic nanoparticles. Sens Actuators B. 2013;176:253–257. [Google Scholar]
- 4.Li W, Chen B, Zhang H-X, et al. BSA-stabilized Pt nanozyme for peroxidase mimetics and its application on colorimetric detection of mercury(II) ions. Biosens Bioelectron. 2015;66:251–258. doi: 10.1016/j.bios.2014.11.032. [DOI] [PubMed] [Google Scholar]
- 5.Peng J, Ling J, Zhang X-Q, et al. Sensitive detection of mercury and copper ions by fluorescent DNA/Ag nanoclusters in guanine-rich DNA hybridization. Spectrochim Acta A. 2015;137:1250–1257. doi: 10.1016/j.saa.2014.08.135. [DOI] [PubMed] [Google Scholar]
- 6.Lien C-W, Huang C-C, Chang H-T. Peroxidase-mimic bismuth-gold nanoparticles for determining the activity of thrombin and drug screening. Chem Commun. 2012;48(64):7952–7954. doi: 10.1039/c2cc32833j. [DOI] [PubMed] [Google Scholar]
- 7.Wang L-H, Shen A-G, Li X-C, et al. Inclusion of guest materials in aqueous coordination network shells spontaneously generated by reacting 2,5-dimercapto-1,3,4-thiadiazole with nanoscale metallic silver. RSC Adv. 2014;4:34294–34302. [Google Scholar]
- 8.Jiang X, Sun C-J, Guo Y, Nie G-J, Xu L. Peroxidase-like activity of apoferritin paired gold clusters for glucose detection. Biosens Bioelectron. 2015;64:165–170. doi: 10.1016/j.bios.2014.08.078. [DOI] [PubMed] [Google Scholar]
- 9.Wang S, Cazelles R, Liao WC, et al. Mimicking horseradish peroxidase and NADH peroxidase by heterogeneous Cu2+-modified graphene oxide nanoparticles. Nano Lett. 2017;17(3):2043–2048. doi: 10.1021/acs.nanolett.7b00093. [DOI] [PubMed] [Google Scholar]
- 10.Fan S, Zhao M, Ding L, Li H, Chen S. Preparation of Co3O4/crumpled graphene microsphere as peroxidase mimetic for colorimetric assay of ascorbic acid. Biosens Bioelectron. 2017;89:846–852. doi: 10.1016/j.bios.2016.09.108. [DOI] [PubMed] [Google Scholar]
- 11.Liang A-H, Zhou L-P, Qin H-M, Zhang Y, Ouyang H-X, Jiang Z-L. A Highly Sensitive Aptamer-nanogold catalytic resonance scattering spectral assay for melamine. J Fluoresc. 2011;21(5):1907–1912. doi: 10.1007/s10895-011-0888-1. [DOI] [PubMed] [Google Scholar]
- 12.Luo Y-H, Zhang X-H, Yao D-M, Wen G-Q, Liang A-H, Jiang Z-L. Resonance Rayleigh scattering detection of trace PDGF based on catalysis of an aptamer-modified nanogold probe in the Fehling reaction. RSC Adv. 2014;4(53):28052–28055. [Google Scholar]
- 13.Li C-N, Ouyang H-X, Tang X-P, Wen G-Q, Liang A-H, Jiang Z-L. A surface enhanced Raman scattering quantitative analytical platform for detection of trace Cu coupled the catalytic reaction and gold nanoparticle aggregation with label-free Victoria blue B molecular probe. Biosens Bioelectron. 2017;87:888–893. doi: 10.1016/j.bios.2016.09.053. [DOI] [PubMed] [Google Scholar]
- 14.Li N, Ma HM, Cao W, et al. Highly sensitive electrochemical immunosensor for the detection of alpha fetoprotein based on PdNi nanoparticles and N-doped graphene nanoribbons. Biosens Bioelectron. 2015;74:786–791. doi: 10.1016/j.bios.2015.07.049. [DOI] [PubMed] [Google Scholar]
- 15.Tang J, Hou L, Tang DP, et al. Magneto-controlled electrochemical immunoassay of brevetoxin B in seafood based on guanine-functionalized graphene nanoribbons. Biosens Bioelectron. 2012;38:86–93. doi: 10.1016/j.bios.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Zhang TT, E YF. Preparation of bio-electrochemical sensor based on graphene oxide nanoribbon and its application in amino acid detection. Chin J Biochem Pharm. 2017;37(3):58–61. [Google Scholar]
- 17.Dong X, Long Q, Wang J, et al. A graphene nanoribbon network and its biosensing application. Nanoscale. 2011;3(12):5156–5160. doi: 10.1039/c1nr11006c. [DOI] [PubMed] [Google Scholar]
- 18.Zhu G, Yi Y, Han Z, Wang K, Wu X. Sensitive electrochemical sensing for polycyclic aromatic amines based on a novel core-shell multiwalled carbon nanotubes@ graphene oxide nanoribbons heterostructure. Anal Chim Acta. 2014;845:30–37. doi: 10.1016/j.aca.2014.06.027. [DOI] [PubMed] [Google Scholar]
- 19.Baffou G, Quidant R. Nanoplasmonics for chemistry. Chem Soc Rev. 2014;43:3898–3907. doi: 10.1039/c3cs60364d. [DOI] [PubMed] [Google Scholar]
- 20.Jackman JA, Ferhan AR, Cho NJ. Nanoplasmonic sensors for biointerfacial science. Chem Soc Rev. 2017;46:3615–3660. doi: 10.1039/c6cs00494f. [DOI] [PubMed] [Google Scholar]
- 21.Milekhin AG, Cherkasova O, Kuznetsov SA, et al. Nanoantenna-assisted plasmonic enhancement of IR absorption of vibrational modes of organic molecules. Beilstein J Nanotechnol. 2017;8:975–981. doi: 10.3762/bjnano.8.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishizuya Y, Okusa T, Hatano K, et al. Desperation surgery for a chemorefractory lung lesion in a patient with an extragonadal germ cell tumor. Inter Cancer Conf J. 2016;5(3):154–157. doi: 10.1007/s13691-016-0248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evans J. Hyperglycosylated hCG: a unique human implantation and invasion factor. Am J Reprod Immunol. 2016;75(3):333–340. doi: 10.1111/aji.12459. [DOI] [PubMed] [Google Scholar]
- 24.Roushani M, Valipour A. Using electrochemical oxidation of Rutin in modeling a novel and sensitive immunosensor based on Pt nanoparticle and graphene–ionic liquid–chitosan nanocomposite to detect human chorionic gonadotropin. Sens Actuators B. 2016;222:1103–1111. [Google Scholar]
- 25.Li P, Bi B-X, Li O, Yao Z-H, Yu H-Z. DNA-Redox cation interaction improves the sensitivity of an electrochemical immunosensor for protein detection. Sensors (Basel) 2015;15(8):20543–20556. doi: 10.3390/s150820543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang L, Zhao H, Fan S-M, et al. Label-free electrochemical immunosensor based on gold-silicon carbide nanocomposites for sensitive detection of human chorionic gonadotrophin. Biosens Bioelectron. 2014;57:199–206. doi: 10.1016/j.bios.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 27.Park J-M, Jung H-W, Chang Y-W, Kim H-S, Kang M-J, Pyuna J-C. Chemiluminescence lateral flow immunoassay based on Pt nanoparticle with peroxidase activity. Anal Chim Acta. 2015;853:360–367. doi: 10.1016/j.aca.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 28.Mazina O, Luik T, Kopanchuk S, Salumets A, Rinken A. Characterization of the biological activities of human luteinizing hormone and chorionic gonadotropin by a Fӧrster resonance energy transfer-based biosensor assay. Anal Lett. 2015;48(17):2799–2809. [Google Scholar]
- 29.Lei J-Q, Mei S-R, Zhou Y-K, Jing T. A simple, selective and sensitive immunoassay for determination of human chorionic gonadotrophin based on chemiluminescence resonance energy transfer. J Chin Chem Soc. 2014;61(6):638–642. doi: 10.1016/j.bios.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 30.Yan X, Huang ZB, He M, et al. Detection of hCG-antigen based on enhanced photoluminescence of hierarchical ZnO arrays. Colloids Surf B Biointerfaces. 2012;89:86–92. doi: 10.1016/j.colsurfb.2011.08.027. [DOI] [PubMed] [Google Scholar]
- 31.Zhao L-X, Lin J-M, Qu F. Micro-plate magnetic chemiluminescence enzyme immunoassay for rapid, sensitive determination of human chorionic gonadotropin (hCG) Acta Chim Sin. 2004;62:71–77. [Google Scholar]
- 32.Chang CC, Chen CP, Lee CH, Chen CY, Lin CW. Colorimetric detection of human chorionic gonadotropin using catalytic gold nanoparticles and a peptide aptamer. Chem Commun. 2014;50:14443–14446. doi: 10.1039/c4cc06366j. [DOI] [PubMed] [Google Scholar]
- 33.Ding X, Yang KL. Antibody-free detection of human chorionic gonadotropin by use of liquid crystals. Anal Chem. 2013;85(22):10710–10716. doi: 10.1021/ac400732n. [DOI] [PubMed] [Google Scholar]
- 34.Lei J, Jing T, Zhou T, et al. A simple and sensitive immunoassay for the determination of human chorionic gonadotropin by graphene-based chemiluminescence resonance energy transfer. Biosens Bioelectron. 2014;54:72–77. doi: 10.1016/j.bios.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 35.Chen CY, Hwu YM, Chen CP, Chang CC. Quantitative analysis of total β-subunit of human chorionic gonadotropin concentration in urine by immunomagnetic reduction to assist in the diagnosis of ectopic pregnancy. Int J Nanomedicine. 2015;10:2475–2483. doi: 10.2147/IJN.S81201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xia N, Chen Z-H, Liu Y-D, Ren H-Z, Liu L. Peptide aptamer-based biosensor for the detection of human chorionic gonadotropin by converting silver nanoparticles-based colorimetric assay into sensitive electrochemical analysis. Sens Actuators B. 2017;243:784–791. [Google Scholar]
- 37.Gao R, Gao Y-J, Zhao J-Z, Juo S-J, Wen T. Development of chemiluminescent immunoassay for human B-hCG. Chin J Biol. 2008;21(3):240–243. [Google Scholar]