Abstract
Intrahepatic portosystemic shunt was encountered in 2 cats (10 and 5 months old) exhibiting neurological symptoms and general deterioration. Both cats were treated with coil embolization using a hybrid surgical technique combining conventional open surgery and interventional radiology techniques, achieving good postoperative outcomes (follow-up: 22 and 10 months, respectively).
Résumé
Traitement chirurgical hybride pour deux cas félins de shunt intrahépatique. Un shunt portosystémique intrahépatique a été observé chez deux chats (âgés de 10 et de 5 mois) manifestant des symptômes neurologiques et une détérioration générale. Les deux chats ont été traités par une embolisation utilisant des spires à l’aide d’une technique chirurgicale hybride combinant une chirurgie ouverte conventionnelle et des techniques de radiologie d’intervention afin d’obtenir de bons résultats postopératoires (suivi : 22 et 10 mois, respectivement).
(Traduit par Isabelle Vallières)
Unlike cases of portosystemic shunt (PSS) in canines, cases of feline PSS are rare (1). Ideal methods of treatment have yet to be established in dogs or cats. Feline PSS is usually identified at a young age, with a mean age at diagnosis of 4 to 9 mo (1–3). The mean body weight (BW) at diagnosis is also low, reportedly ranging from 1.2 to 2.9 kg for intrahepatic cases (mean: 2.3 kg) (4,5) and from 1.1 to 6.2 kg for extrahepatic cases (mean: 2.9 kg) (6,7). Because cats with PSS and neurological symptoms treated medically are reported to die within 2 y following diagnosis (8), surgery is often required at an early age.
The main method of treatment for feline PSS is surgical ligation (3), but dissection of the liver parenchyma to identify the shunting vessel can be challenging with intrahepatic PSS (IHPSS), in which a direct approach to the shunting vessel is anatomically difficult. Such operative stresses can occasionally prove fatal for kittens (9). Previous methods of treating extrahepatic portosystemic shunts in cats have included the use of surgical ligation (1,3,10,11), ameroid constrictors (12), and cellophane banding (7,13). With the exception of cellophane banding, these methods are also used for intrahepatic shunt vessels (1,3,4,14).
Microhepatia is seen in half of feline cases of intra- and extrahepatic PSS (2), and large-diameter shunts usually show a higher volume of blood flow. Attempting to achieve complete occlusion of the shunt in a single procedure under these circumstances sometimes causes portal hypertension, which in turn can cause ascites, postoperative development of multiple shunts, intestinal injury, or even death. Stepwise closure of the shunting vessel is therefore recommended in such cases (12). In our experience, when attempting surgical ligation, multiple approaches to the shunting vessel are sometimes difficult because of adhesions caused by the earlier approaches. An approach other than conventional surgery is thus desirable.
Percutaneous transvenous coil embolization (PTCE) has been used for both dogs (11,15) and cats (5) in an attempt to resolve the issues associated with conventional surgery. In those previous reports, jugular, femoral, or saphenous veins were used as approach sites. Size of the device in cats is restricted by the smaller body conformation and vascular diameter, so PTCE has been applied in only a limited number of cases. In addition, based on our personal experience, measuring portal vein (PV) pressure and performing angiography after shunt vessel occlusion in PTCE is sometimes difficult in cats.
The hybrid surgical technique (HST) represents a combination of conventional open surgery and interventional radiology. This approach was chosen for the following reasons based on our experience with canine and feline cases of PSS requiring stepwise closure. The HST requires a surgical approach only around the main trunk of the PV or mesenteric vein. The incision for the surgical site is relatively small, and the risk of adhesion is minimal. In addition, no dissection of the hepatic parenchyma is required. The distance between the sheath and shunt vessel is short, facilitating more accurate maneuvering of the catheter tip. Because this procedure is conducted under direct visualization of the organs inside the abdomen, intestinal color and peristaltic movement after coil implantation are easily evaluated, and liver biopsy can be readily and safely conducted. In addition, the sheath acts not only for placement of the device, but also as a catheter for the measurement of PV pressure and the injection of contrast agent.
Case descriptions
Case 1
A 10-month-old neutered male exotic shorthaired cat was referred to the Tokyo University of Agriculture and Technology Animal Medical Center (TUATAMC) because of drooling after meals that had appeared 2 mo previously. Two days before the initial hospital visit, the animal exhibited loss of both vigor and appetite, altered consciousness, and convulsive seizures. Plasma ammonia (PA) concentration taken at the referring clinic had been elevated (> 587 μmol/L), and the animal was brought to TUATAMC for further investigation (Day 1).
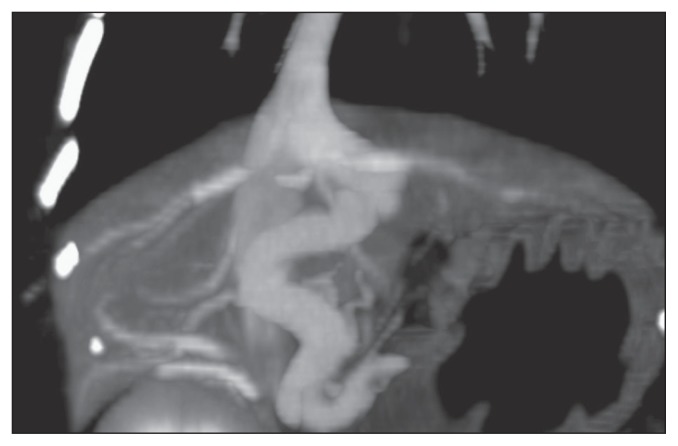
On initial examination, decreased level of consciousness and generalized seizures were evident. Body weight (BW) was 2.8 kg. The irises of both eyes showed a copper coloration, which has previously been reported in cats with PSS (16). Systolic/diastolic (mean) arterial pressure was 109/63 (80) mmHg. Complete blood cell count revealed anemia (hematocrit: 21.1%), and PA was 72.8 μmol/L. Plasma albumin concentration (29 g/L) was within normal limits. Computed tomography (CT) revealed the tortuous passage of a PV in the left hepatic segment through the liver before forming a direct shunt into the caudal vena cava via the hepatic vein (Figure 1). This shunt represented left divisional intrahepatic portosystemic shunt, which has been reported previously (17). The vascular diameter of the shunt was 5.5 to 6.8 mm, with a maximum vascular length of approximately 15 mm. Only 1 shunt vessel was present, and no other intrahepatic vascular anomalies were identified.
Figure 1.
Computed tomography in Case 1 reveals tortuous passage of a portal vein in the left hepatic segment through the liver before forming a direct shunt into the caudal vena cava via the hepatic vein.
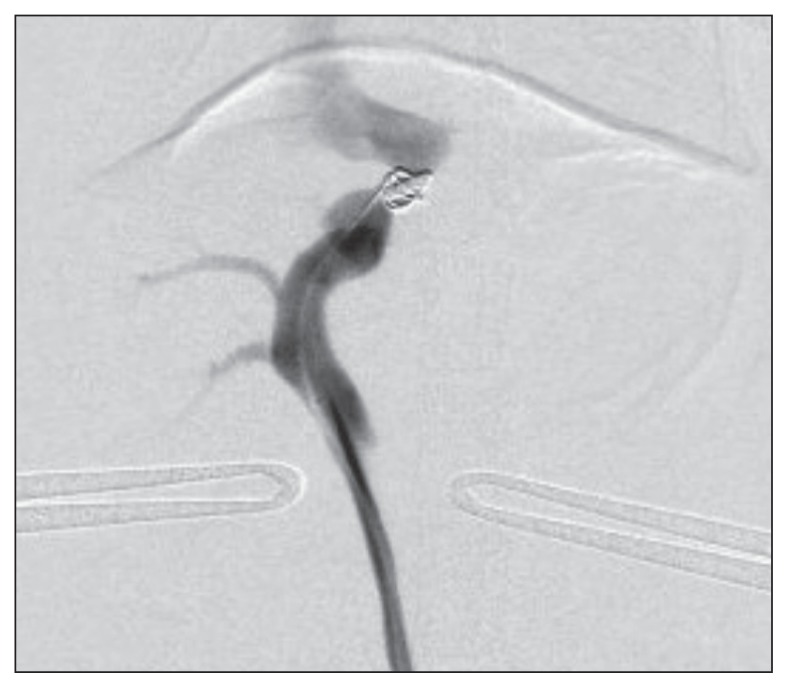
An attempt was made to control the symptoms with pharmacotherapy using levetiracetam (Otsuka Pharmaceutical, Tokyo, Japan), 20 mg/kg BW, q12h, and zonisamide (Sumitomo Dainippon Pharma, Tokyo, Japan), 2.5 mg/kg BW, q12h. However, no clear improvements were identified, and the PA concentration remained elevated (73.4 μmol/L). Coil embolization of the shunt vessel by HST was performed on Day 21. First, ampicillin sodium (injectable ampicillin Na; Kyoritsu Seiyaku, Tokyo, Japan), 30 mg/kg BW, IV, buprenorphine hydrochloride (Lepetan injection 0.2 mg; Otsuka Pharmaceutical), 0.01 mg/kg BW, IV, and atropine sulfate (Atropine Sulfate Injection 0.5 mg; Mitsubishi Tanabe Pharma, Osaka, Japan), 0.05 mg/kg BW, SC, were administered as premedications, and anesthesia was induced by inhalation of 5% isoflurane (Isoflurane for Animal; Intervet, Tokyo, Japan). After tracheal intubation, inhalation anesthesia was maintained using 2.0% to 3.5% isoflurane. After surgical preparation, a midline incision was made in the abdomen (~ 8 cm in length), and a 3-Fr sheath was inserted in the mesenteric vein. A 0.018-inch guide wire and microcatheter were inserted into the sheath, and the microcatheter was placed at the narrowest part of the shunt vessel under fluoroscopic guidance. A detachable coil [Platinum Coil Vascular Occlusion System (Interlocking Detachable Coil); Boston Scientific Japan, Tokyo, Japan] with a diameter of 7 mm and a length of 10 mm was deployed under fluoroscopic guidance and then detached. Systolic/diastolic PV pressure was 5/2 (mean: 3) mmHg before coil deployment and 14/11 (mean: 12) mmHg after coil occlusion. Before coil occlusion, an injection of iopamidol contrast agent (Oypalomin; Konica Minolta Japan, Tokyo, Japan) via the mesenteric vein revealed the flow of contrast agent directly into the caudal vena cava from the shunt vessel. Shunt flow was decreased after coil deployment, but as expected, some residual flow remained (Figure 2). No additional coil implantation was considered during this operation. After removal of the sheath, the hole in the mesenteric vein was closed with continuous sutures using synthetic absorbable monofilament (6/0 Prolene; Johnson & Johnson, Tokyo, Japan). The procedure took 71 min to complete.
Figure 2.
Shunt flow is decreased after coil deployment in Case 1, but is still present.
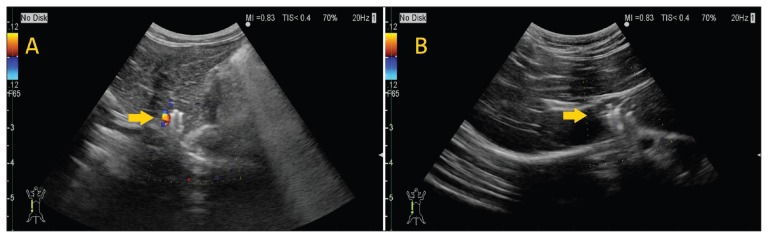
The patient recovered from anesthesia uneventfully, and showed clinical improvement in the postoperative period. On Day 154, BW had increased to 4.2 kg, no clinical symptoms were apparent, and PA concentrations had decreased to within normal limits (37.6 μmol/L) without the use of either oral medication or dietary therapy. On Day 191, ultrasonographic examination showed that shunt flow had completely disappeared (Figure 3).
Figure 3.
A — Case 1, Day 40. Doppler examination shows evidence of blood flow in the shunting vessel. Arrow, coil. B — Case 1, Day 191. The coil deployed in the shunt vessel shows no change in position. Color Doppler ultrasonographic examination shows no blood flow around the coil in the shunt vessel. Arrow, coil.
Case 2
A 5-month-old intact male Russian blue cat was referred to TUATAMC because of loss of appetite that had appeared 2 mo previously, high serum levels of total bile acid (137.0 μmol/L preprandially, 108.2 μmol/L postprandially), and a high PA concentration (133.8 μmol/L).
On initial examination (Day 1), loss of appetite and mild wasting were observed (body condition score: 2/5). Body weight was 980 g. The mucous membranes were slightly pale, and mild dehydration was evident. The superficial lymph nodes were not enlarged and no heart murmurs were audible. Blood tests revealed mild anemia (hematocrit: 26.7%), hyperammonemia (163.8 μmol/L), hypoalbuminemia (20 g/L), and low urea nitrogen (5.9 mmol/L). Contrast-enhanced CT revealed marked microhepatia and passage of a PV branch in the left hepatic segment through the liver toward the cranial side before forming a direct shunt into the caudal vena cava. This shape of the shunt suggested patent ductus venosus, but we could not confirm the origin. The vascular diameter of the shunt ranged from a minimum of 4.1 mm to a maximum of 4.8 mm. No other vascular abnormalities were evident.
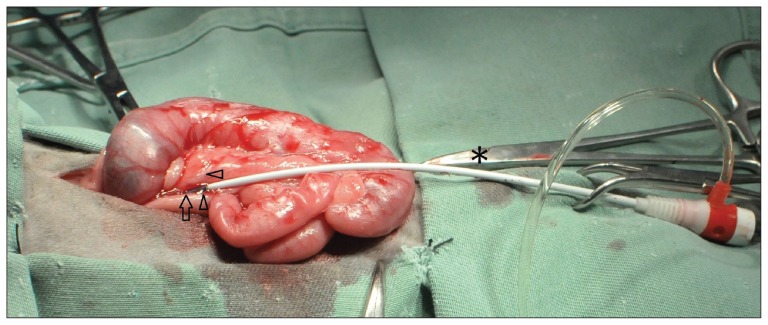
Given the underdeveloped state of the liver and the large vascular diameter of the shunt, stepwise embolizations of the shunt vessel were planned. On Day 13, general anesthesia was induced using the same method applied in Case 1. After tracheal intubation, inhalation anesthesia was maintained with 1.0% to 1.9% isoflurane. After surgical preparation, a midline incision was made in the abdomen, and a 4-Fr sheath was inserted in the main trunk of the PV. Under fluoroscopic guidance, a 0.035-inch guidewire and a 4-Fr multipurpose catheter were inserted into the sheath and placed at the cranial side of the shunt vessel. An anchor (Coil Anchor II S; Medikit, Tokyo, Japan) was deployed in the shunt vessel under fluoroscopic guidance, and coil implantation was planned for after stabilization of the anchor against the vessel wall. This coil anchor proved very useful in preventing migration of the coils added later. Unlike stents for PTCE, this coil anchor can be placed in a small space. After removal of the sheath, the hole in the PV was closed with polypropylene synthetic non-absorbable sutures (8-0 PROLENE; Johnson & Johnson). The PV pressure was 4/1 (mean: 2) mmHg before coil anchor deployment and 6/3 (mean: 4) mmHg after coil anchor deployment. After this initial anchor implantation, the appetite of the animal improved and BW increased to 1.1 kg, but PA concentration remained high (300.5 μmol/L on Day 21). On Day 71, the patient showed neurological symptoms (apparent loss of vigor after meals). We attempted medical control with phenobarbital (1.0 mg/kg BW, q12h) and lactulose (1 mL, q12h). A second HST for coil implantation was then carried out on Day 78. Sutures from the initial procedure were visible on the PV, but no adhesions were evident. A 4-Fr sheath, 0.035-inch guidewire, and 4-Fr multipurpose catheter were therefore inserted into the main trunk of the PV in a similar fashion to that of the first HST. Under fluoroscopic guidance, a pushable coil (Trufill Pushable Coil Selection Complex; Johnson & Johnson) (diameter: 7 mm; length: 60 mm) was deployed to coil around the coil anchor already in place. The PV pressure was 8/5 (mean: 4) mmHg before coil implantation and 7/5 (mean: 5) mmHg after coil implantation. Although a residual shunt was observed around the coil, thrombus formation around the coil was expected, and no additional coil implantation was conducted during the procedure. The cat recovered uneventfully from the anesthesia. A third HST was conducted on Day 102 because PA concentrations remained high (281.8 μmol/L on Day 85) and neurological symptoms were becoming worse, with the appearance of convulsions. Anesthesia and the approach to the shunt vessel were conducted as previously described, except that this procedure used a 3-Fr sheath, 0.018-inch guidewire, and microcatheter. An additional pushable coil (Trufill Pushable Coil Selection Complex; Johnson & Johnson) (diameter: 7 mm; length: 60 mm) was implanted in the PV slightly caudad to the coil deployed in the second HST (Figure 4). Because residual shunt was still observed, another pushable coil (diameter, 5 mm; length, 40 mm) was added. The PV pressure was 5/3 (mean: 3) mmHg before additional coil implantation and increased to 27/24 (mean: 26) mmHg after additional coil implantation. Fluoroscopic observation revealed that the velocity of shunt flow was markedly reduced, as determined from the flow speed of contrast medium at the coil site. The cat recovered from the procedure uneventfully; the PA concentration was 35.2 μmol/L preprandially and 58.7 μmol/L at 2 h postprandially. Mild temporary ascites was seen after the third HST, but the general condition of the animal was markedly improved; the cat appeared energetic, showed good appetite, and was able to walk independently. Procedure time was 61 min for the first HST, 73 min for the second, and 62 min for the third. By Day 126, clinical symptoms had resolved without pharmacotherapy, and PA levels remained at approximately 58.7 μmol/L. Color Doppler ultrasonographic examination showed no blood flow around the coil in the shunt vessel on Day 144.
Figure 4.
The sheath is inserted into the portal vein (arrow). The support threads (8-0 PROLENE, arrowheads) are placed on both sides of the area of puncture. These threads are then clamped with the mosquito forceps (*) and handled to control bleeding from the puncture site. After removing the sheath, the threads are used as continuous sutures to close the puncture site.
The interval between the first and second interventions was as planned. However, the interval between the second (Day 78) and third interventions (Day 102) was short, because the cat showed neurological symptoms on day 71. At first (Day 71), loss of vigor after meals was the only neurological symptom. However, general physical condition and neurological symptoms gradually worsened, to the point where convulsions developed. We attempted medical control with phenobarbital and lactulose, but clinical symptoms did not improve. We therefore made the decision to perform a third intervention within the short period of 3.5 mo.
Discussion
Both animals in these cases were in poor general condition at presentation. An attempt was made to control the condition of the cat in Case 1 with medication, but this did not result in any apparent improvement. Waiting for the animal to gain BW was impossible in Case 2. The use of PTCE for a cat with PSS that weighed 2.9 kg at 4 mo of age has been reported (5), but the cat in Case 2 weighed only 980 g and the diameter of the jugular vein was small, so physical insertion of the catheter into this vessel for PTCE would have been difficult. Because both animals had been diagnosed with left-sided intrahepatic PSS, surgical shunt vessel ligation was also a treatment option. However, both animals were in poor condition and anemic, so HST was chosen as a less-invasive procedure to limit bleeding and avoid the need for hepatic parenchymal dissection. Bleeding mainly occurred when the mesenteric vein or PV was punctured to insert the device and during withdrawal, and hemostatic control was good. To avoid troublesome device manipulation, the sheath was inserted directly into the PV, providing good maneuverability and enabling use of a large device not only in Case 1, in which the shunt vessel followed a complex, tortuous course, but also in Case 2, in which the animal was extremely small. In Case 2, the broad shunt vessel and severe microhepatia meant that a need for staged occlusion was anticipated, but even though HST was actually performed 3 times, no problems were identified with postoperative adhesions within the peritoneum or at the PV suture site where the sheath had been inserted. The HST is thus useful for avoiding the risk of approaching an adhesion site and averting problems such as prolonged operating time and excessive bleeding that would arise from the need to detach adhesions created during multiple surgeries.
The cat in Case 2 was initially treated in an unusual stepwise manner using an anchor. The purpose of the first intervention (on Day 13) was to create an anchor, not to initiate thrombosis.
Although postoperative outcomes were uneventful in both cases reported here, issues warranting further investigation were also apparent. The thrombogenic speed of coils is difficult to predict, and protocols for staged embolization have yet to be established. In Case 2, the thrombogenic speed of coils immediately after the third HST was rapid, and mild postoperative retention of ascites occurred. Due to the rise in intraoperative PV pressure after coil deployment and the marked weakening of blood flow in the area around the coil observed on postoperative abdominal ultrasonography, the cause of ascites was inferred to have been a transient increase in PV pressure resulting from rapid thrombus formation with the introduction of the coils. By the time of the third HST (Day 102), neurological symptoms had worsened. We considered immediate occlusion of the shunt as necessary, even though the patient faced a growing risk of portal hypertension. Even though the cat in Case 2 remained in good general condition, with good appetite and energy, and the amount of ascites retained was small, this represents an important issue in HST that requires further investigation. Venous shunt vessels are also highly elastic, making the maximum diameter during coil embolization more difficult to predict than with arterial vessels. No coil standards defined by diameter of the shunt vessel have been established, and the optimal method for measuring PV pressure after coil embolization also remains to be determined.
The HST enables direct insertion of a device into the portal or mesenteric vein via open surgery, mitigating the restrictions on usable device size, permitting easy device manipulation, and allowing device manipulation while directly observing the peritoneal organs. We believe that HST offers a potentially useful new method to extend the range of options available for treating feline PSS.
Acknowledgments
We thank Drs. Toshiharu Fukayama, Kazumi Shimada, and Seijirow Goya for their assistance in providing anesthesia for these cases. CVJ
Footnotes
No financial support was received for the research, authorship, or publication of this article.
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Ruland K, Fischer A, Reese S, Zahn K, Matis U, Hartmann K. Portosystemic shunts in cats — Evaluation of six cases and a review of the literature. Berl Munch Tierarztl Wochenschr. 2009;122:211–218. [PubMed] [Google Scholar]
- 2.Lamb CR, Forster-van Hijfte MA, White RN, McEvoy FJ, Rutgers HC. Ultrasonographic diagnosis of congenital portosystemic shunt in 14 cats. J Small Anim Pract. 1996;37:205–209. doi: 10.1111/j.1748-5827.1996.tb01767.x. [DOI] [PubMed] [Google Scholar]
- 3.Lipscomb VJ, Jones HJ, Brockman DJ. Complications and long-term outcomes of the ligation of congenital portosystemic shunts in 49 cats. Vet Rec. 2007;160:465–470. doi: 10.1136/vr.160.14.465. [DOI] [PubMed] [Google Scholar]
- 4.White RN, Forster-van Hijfte MA, Petrie G, Lamb CR, Hammond RA. Surgical treatment of intrahepatic portosystemic shunts in six cats. Vet Rec. 1996;139:314–317. doi: 10.1136/vr.139.13.314. [DOI] [PubMed] [Google Scholar]
- 5.Weisse C, Schwartz K, Stronger R, Mondschein JI, Solomon JA. Transjugular coil embolization of an intrahepatic portosystemic shunt in a cat. J Am Vet Med Assoc. 2002;22:66–67. 1287–1291. doi: 10.2460/javma.2002.221.1287. [DOI] [PubMed] [Google Scholar]
- 6.Kyles AE, Hardie EM, Mehl M, Gregory CR. Evaluation of ameroid ring constrictors for the management of single extrahepatic portosystemic shunts in cats: 23 cases (1996–2001) J Am Vet Med Assoc. 2002;220:1341–1347. doi: 10.2460/javma.2002.220.1341. [DOI] [PubMed] [Google Scholar]
- 7.Cabassu J, Seim HB, 3rd, MacPhail CM, Monnet E. Outcomes of cats undergoing surgical attenuation of congenital extrahepatic portosystemic shunts through cellophane banding: 9 cases (2000–2007) J Am Vet Med Assoc. 2011;238:89–93. doi: 10.2460/javma.238.1.89. [DOI] [PubMed] [Google Scholar]
- 8.Tobias KM. Portosystemic shunts. In: Bonagura DJ, Twedt CD, editors. Kirk’s Current Veterinary Therapy XIV. St. Louis, Missouri: Saunders; 2009. pp. 581–586. [Google Scholar]
- 9.VanGundy TE, Boothe HW, Wolf A. Results of surgical management of feline portosystemic shunts. J Am Anim Hosp Assoc. 1990;26:55–62. [Google Scholar]
- 10.Levesque DC, Oliver JE, Jr, Cornelius LM, Mahaffey MB, Rawlings CA, Kolata RJ. Congenital portacaval shunts in two cats: Diagnosis and surgical correction. J Am Vet Med Assoc. 1982;181:143–145. [PubMed] [Google Scholar]
- 11.Leveille R, Pibarot P, Soulez G, Wisner ER. Transvenous coil embolization of an extrahepatic portosystemic shunt in a dog: A naturally occurring model of portosystemic malformations in humans. Pediatr Radiol. 2000;30:607–609. doi: 10.1007/s002470000268. [DOI] [PubMed] [Google Scholar]
- 12.Vogt JC, Krahwinkel DJ, Jr, Bright RM, Daniel GB, Toal RL, Rohrbach B. Gradual occlusion of extrahepatic portosystemic shunts in dogs and cats using the ameroid constrictor. Vet Surg. 1996;25:495–502. doi: 10.1111/j.1532-950x.1996.tb01449.x. [DOI] [PubMed] [Google Scholar]
- 13.Hunt GB, Kummeling A, Tisdall PL, et al. Outcomes of cellophane banding for congenital portosystemic shunts in 106 dogs and 5 cats. Vet Surg. 2004;33:25–31. doi: 10.1111/j.1532-950x.2004.04011.x. [DOI] [PubMed] [Google Scholar]
- 14.Bright SR, Williams JM, Niles JD. Outcomes of intrahepatic portosystemic shunts occluded with ameroid constrictors in nine dogs and one cat. Vet Surg. 2006;35:300–309. doi: 10.1111/j.1532-950X.2006.00148.x. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalo-Orden JM, Altonaga JR, Costilla S, Gonzalo Cordero JM, Millán L, Recio AO. Transvenous coil embolization of an intrahepatic portosystemic shunt in a dog. Vet Radiol Ultrasound. 2000;41:516–518. doi: 10.1111/j.1740-8261.2000.tb01880.x. [DOI] [PubMed] [Google Scholar]
- 16.Vandermeulen E, Combes A, de Rooster H, et al. Transsplenic portal scintigraphy using 99mTc-pertechnetate for the diagnosis of portosystemic shunts in cats: A retrospective review of 12 patients. J Feline Med Surg. 2013;15:1123–1131. doi: 10.1177/1098612X13488594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.White RN, Burton CA, McEvoy FJ. Surgical treatment of intrahepatic portosystemic shunts in 45 dogs. Vet Rec. 1998;142:358–365. doi: 10.1136/vr.142.14.358. [DOI] [PubMed] [Google Scholar]
- 18.Weisse C, Berent AC, Todd K, Solomon JA, Cope C. Endovascular evaluation and treatment of intrahepatic portosystemic shunts in dogs: 100 cases (2001–2011) J Am Vet Med Assoc. 2014;244:78–94. doi: 10.2460/javma.244.1.78. [DOI] [PubMed] [Google Scholar]