Abstract
Nine cases of fatal infection with Babesia odocoilei were confirmed in reindeer (Rangifer tarandus tarandus) and elk (Cervus canadensis) housed in zoological institutions located in southern Quebec, Ontario, and Manitoba, Canada between 2013 and 2016. All animals died of a hemolytic crisis. Frequent postmortem findings were extensive hemorrhage, pigmenturia, and intrahepatic cholestasis. The described ante- and postmortem signs are consistent with those of previously reported cases in the United States. Diagnosis was confirmed in all cases by polymerase chain reaction performed on DNA extracted from whole blood or frozen spleen. We propose that babesiosis is an emerging disease of cervids in multiple Canadian provinces, most likely as a result of climate change and the northward range expansion of Ixodes scapularis, the primary tick vector for B. odocoilei. The role of captive animals as sentinels for wildlife health is also highlighted.
Résumé
Babesia odocoilei, une cause de la mortalité chez les cervidés captifs au Canada. Entre 2013 à 2016, neuf cas d’infection fatale par Babesia odocoilei ont été détectés chez des caribous (Rangifer tarandus tarandus) et des wapitis (Cervus canadensis) gardés dans des établissements zoologiques situés dans le sud du Québec, de l’Ontario et du Manitoba, Canada. Les animaux sont morts suite à une crise hémolytique. Hémorragies, pigmenturie et cholestase intrahépatique ont fréquemment été identifiées à l’examen postmortem. Les signes ante- et postmortem décrits correspondent avec ceux des cas précédemment signalés aux États-Unis. Le diagnostic de babésiose fut confirmé par réaction en chaîne par polymérase sur l’ADN extrait d’échantillons de sang ou de rate congelée. Nous proposons que la babésiose des cervidés est une maladie émergente au Canada, et ce probablement en conséquence du réchauffement climatique et du mouvement vers le nord de la tique Ixodes scapularis, le principal vecteur de B. odocoilei. La valeur des animaux captifs comme sentinelles pour la santé de la faune est également discutée.
(Traduit par les auteurs)
Introduction
Babesia odocoilei (Apicomplexa, Piroplasmida, Babesiidae) is a tick-borne intraerythrocytic protozoal parasite originally described in white-tailed deer (Odocoileus virginianus) (1,2). Ixodes scapularis is the organism’s only proven vector (3–5). Dermacentor albipictus, Amblyomma americanum, and Ixodes pacificus ticks have been found on, respectively, elk (Cervus elaphus canadensis), white-tailed deer, and bighorn sheep (Ovis canadensis nelsoni) infected with B. odocoilei, but have not been confirmed as vectors (1,6,7).
Babesiosis has been described in wild white-tailed deer and desert bighorn sheep, as well as in captive elk, woodland caribou (Rangifer tarandus caribou), reindeer (Rangifer tarandus tarandus), muskoxen (Ovibos moschatus), markhor (Capra falconeri), yaks (Bos grunniens), and muntjacs (Muntiacus reevesi) in the United States (1,6–12) (Figure 1). However, clinical disease has only been reported in elk, reindeer, and caribou (6–8). Clinical signs reported in these cervids include lethargy, pyrexia, icterus, hemoglobinuria, and sudden death (8,13,14). Disease can develop following infection of naïve animals or can manifest as a recrudescence of a latent infection in persistently infected animals (13). The stressors responsible for this recrudescence are often unknown, but may include concurrent disease, poor nutrition, rutting season, calving, high population density, and transportation (13,15,16). In persistently infected immunocompetent white-tailed deer, a mild transient decrease in hematocrit or clinical disease manifested by pyrexia, anemia, and emaciation can occur in association with low-grade parasitemia (11).
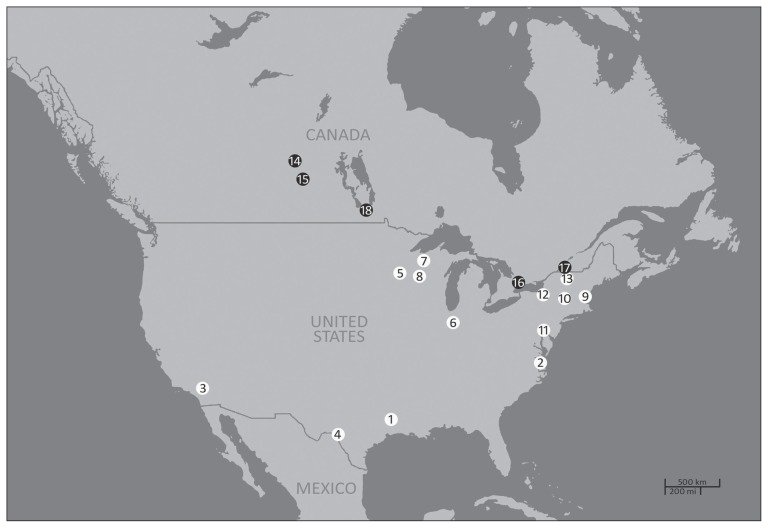
Figure 1.
Geolocations of documented cases of Babesia odocoilei infection in cervids and bovids in the US and in Canada from 1968 to 2016. White and black points represent, respectively, cases in the USA and Canada, in chronological order of publication.
1 — wild white-tailed deer in Texas (1); 2 — wild white-tailed deer in Virginia (11); 3 — wild desert bighorn sheep in California (7,12); 4 — captive elk in Texas (6); 5 — captive reindeer, muskoxen, and woodland caribou in Minnesota (6,7,14); 6 — captive elk in Indiana (13); 7 — captive reindeer in Wisconsin (34); 8 — captive elk in Wisconsin (36); 9 — captive elk in New Hampshire (7); 10 — captive reindeer in New York (7); 11 — captive reindeer in Pennsylvania (7); 12 — captive reindeer, yak, muntjac, and markhor in New York (8); 13 — captive elk in New York (19); 14 — captive elk in Saskatchewan (18); 15 — wild white-tailed deer in Saskatchewan (17); 16 — captive reindeer and elk in Ontario; 17 — captive elk in Quebec; 18 — captive reindeer in Manitoba.
Knowledge of the epidemiology of B. odocoilei in North America is limited despite a number of published reports. Insufficient targeted surveillance for this pathogen in wildlife might explain the lack of geographic continuity between reported endemic regions; only sporadic reports exist regarding B. odocoilei infections in wildlife despite the widespread distribution of Ixodes spp. ticks in the eastern half of the United States (4). Serological evidence of B. odocoilei has been demonstrated in free-ranging white-tailed deer in southern Virginia, eastern Texas, Oklahoma, and Saskatchewan, as well as in desert bighorn sheep in southern California (1,2,7,11,16,17). Babesia odocoilei DNA was also detected in I. scapularis ticks found in Maine, Massachusetts, and Wisconsin (3). Reports of disease in captive susceptible hosts in ranches, farms, or zoos may provide further insight into the geographic range of the disease. Captive cervids and bovids infected with B. odocoilei have been identified in New Hampshire, New York, Pennsylvania, Indiana, Texas, Minnesota, and Wisconsin (6,7,13,14). Cervid babesiosis was reported for the first time in Canada in ranched elk in central Saskatchewan in 2012 (18). Although the source of the infection has rarely been investigated, several authors have proposed that cases in captive animals reflect endemicity in local wildlife or tick populations (8,13,18,19).
This is an overview of the diagnosed cases of cervid babesiosis in Canada following its first recognition in 2012 until 2016. This report highlights the emergent nature of the disease in Canada, as well as the role of captive animals in wildlife disease surveillance.
Materials and methods
Case histories
Medical files of cervid babesiosis cases diagnosed by the authors were gathered and data regarding signalment, antemortem clinical signs, clinical pathology findings, ancillary diagnostic test results, and postmortem findings were collated. Clinical presentation was defined as peracute or acute. Animals that died without overt prodromal signs were classified as peracute cases, while animals that showed clinical signs for a few days before death were classified as acute. Tissue and blood samples were collected during necropsy and held at −20°C for later DNA extraction. Data were reviewed for trends and patterns, but no statistical analysis was performed.
DNA Extraction, piroplasm-specific PCR and sequencing
Molecular genetic analysis of the cervid spleen and blood samples collected during necropsies at the Toronto Zoo was performed by the Department of Pathobiology, Ontario Veterinary College, University of Guelph, Guelph, Ontario, using the following protocol. DNA was extracted from the samples using a DNAzol kit according to the manufacturer’s protocol (Molecular Research Center, Cincinnati, Ohio, USA). After isolation, DNA was quantified spectrophotometrically using a Nanodrop 2000 instrument (Thermo Fisher Scientific, Wilmington, Delaware, USA). Standard polymerase chain reaction (PCR) was performed in a T100 thermal cycler (Bio Rad, California, USA) in a 50-μL reaction containing 1× PCR buffer, 2U Platinum® Taq polymerase (Invitrogen, Carlsbad, California, USA), 0.8 mM dNTPs, 3 mM MgCl2, 0.5 μM of each amplification primer (Table 1) and 100 to 200 ng DNA template (mixed cervid/parasite DNA). The PCR reaction conditions consisted of an initial melt at 94°C for 3 min followed by 35 amplification cycles (denature at 94°C for 30 s, anneal at ~59°C for 45 s, extend at 72°C for 1.5 min), and then terminate with a final extension of 72°C for 5 min to complete any partial products. Annealing temperatures were chosen based on Primer3 implemented from within the Geneious bioinformatics software (Version 6.1 and later, available from http:\\www.geneious.com) (22). Polymerase chain reaction (PCR) products were separated electrophoretically using a submarine 1.4% agarose gel with 1× TAE buffer (100 mL) and 4 μL of ethidium bromide dye (10 mg/mL, w/v). The GeneRuler 1 kb Plus DNA size ladder (Thermo Fisher Scientific) was used to determine product fragment lengths. Gels were examined using an ultraviolet transilluminator and DNA bands of expected sizes were excised using a sterile scalpel. DNA was extracted from the gel slice using the QIAquick Gel Extraction Kit (QIAGEN, Toronto, Ontario) according to the manufacturer’s instructions. Purified PCR amplicons were then submitted for sequencing in both directions with forward and reverse amplification primers using an ABI Prism 7000 Sequence Detection System (Applied Biosystems, Foster City, California, USA) by the Molecular Biology Unit of the Laboratory Services Division, University of Guelph, Guelph, Ontario. Chromatograms received from sequencing reactions were imported into Geneious for analyses.
Table 1.
Polymerase chain reaction (PCR) amplification primers used for amplification of Babesia odocoilei from cervid tissue samples.
| Amplified fragment | Product size (bp) | Primer names | Primer sequences (5′–3′) |
|---|---|---|---|
| Ribosomal 18S rDNAa | 1687 | Medlin A (F)b | AACCTGGTTGATCCTGCCAGT |
| Piro_18S_1688_Rc | CGACTTCTCCTTCCTTTAAGTGATAAG | ||
| Ribosomal 18s rDNAd | 681 | Piro_144_S | ACCGTGCTAATTGTAGGGCTAATACA |
| BCOMMON2R | TGCTTTCGCAGTAGTTCGTC |
PCR performed by the Department of Pathobiology, Ontario Veterinary College, University of Guelph, Guelph, Ontario, Canada.
Primer A of Medlin et al (20) less polylinker region.
Equivalent to primer BN1700 of Ramos et al (21).
PCR performed by the Vector Borne Disease Laboratory, College of Veterinary Medicine, North Carolina State University, Raleigh, North Carolina, USA, using primers described by Schoelkopf et al (7).
Molecular genetic analysis of the blood and spleen samples collected from the elk housed at the Parc Safari in Hemmingford, Quebec was performed by the Department of Veterinary Pathobiology, College of Veterinary Medicine, Texas A&M University, College Station, Texas, USA using primers and methods described by Schoelkopf et al (7).
The sample of spleen collected from the female reindeer that died in November 2014 at the Toronto Zoo (Z167-14) was submitted to the Department of Veterinary Pathology, Western College of Veterinary Medicine, University of Saskatchewan, and molecular genetic analysis was performed following the protocol described by Pattullo etal (18). The blood sample collected from the reindeer housed at the Assiniboine Park Zoo in Winnipeg, Manitoba, was sent to the Vector Borne Disease Laboratory, College of Veterinary Medicine, North Carolina State University, Raleigh, North Carolina, USA, for molecular genetic analysis. The DNA extraction was performed using QIAsymphonySP (Qiagen, Valencia, California, USA) with QIAsymphony DNA Mini Kit (192) (Qiagen). Standard PCR was performed in a thermal cycler (Mastercycler EP gradient aluminum block thermocycler, Eppendorf North America, Hauppauge, New York, USA) in a 25-μL reaction containing 12.5 μL of Mytaq Red Mix 2X (Bioline, London, UK), 0.5 μM of each amplification primer (Table 1) and 150 ng DNA template (mixed cervid/parasite DNA). The PCR reaction conditions consisted of an initial melt at 94°C for 5 min followed by 35 amplification cycles (denature at 95°C for 20 s, anneal at 58°C for 30 s and extend at 72°C for 1 min) and then terminate with a final extension of 72°C for 5 min to complete any partial products. The PCR products were separated by electrophoresis through a 2% (w/v) agarose gel. The unpurified PCR product was directly sent for Sanger sequencing through GENEWIZ (Research Triangle Park, North Carolina, USA). Sequences were aligned and compared with GenBank sequences using the AlignX software (Vector NTI Suite 6.0, InforMax, Bethesda, Maryland, USA).
Results
Nine confirmed cases of fatal infection by B. odocoilei were identified in captive reindeer and elk in 3 Canadian provinces (Manitoba, Ontario, and Quebec) subsequent to the first report of clinical babesiosis in Canada in 2 ranched elk in central Saskatchewan in 2012 (18). Signalment and geographic location of all 9 cases are detailed in Table 2 and Figure 1. Clinical disease was not reported in any of the other susceptible species housed in these zoos (i.e., white-tailed deer, muskox, markhor, yak); however, PCR was not performed to identify asymptomatic carriers. More females than males were affected. Diseased animals ranged in age from 2.5 to 14 y. Cases occurred between the months of June and December.
Table 2.
Signalment and geographic location of captive elk (Cervus canadensis) and reindeer (Rangifer tarandus) that died of infection with Babesia odocoilei.
| ID | Species | Gender | Age class | City | Province | Latitude (°N) | Longitude (°W) | Date of death |
|---|---|---|---|---|---|---|---|---|
| Z165-12 | Elk | Female | Adulta | Scarborough | Ontario | 43° 49′ | 79° 11′ | October 2012 |
| Z175-12 | Elk | Female | Adult | Scarborough | Ontario | 43° 49′ | 79° 11′ | November 2012 |
| Z176-12 | Elk | Female | Adult | Scarborough | Ontario | 43° 49′ | 79° 11′ | November 2012 |
| Z137-13 | Elk | Female | Adult | Scarborough | Ontario | 43° 49′ | 79° 11′ | October 2013 |
| Z174-13 | Reindeer | Female | Adult | Scarborough | Ontario | 43° 49′ | 79° 11′ | December 2013 |
| K00075 | Reindeer | Male | Adult | Winnipeg | Manitoba | 49° 52′ | 97° 14′ | June 2014 |
| D24259 | Elk | Female | Adult | Hemmingford | Quebec | 45° 02′ | 73° 31′ | June 2014 |
| Z167-14 | Reindeer | Female | Adult | Scarborough | Ontario | 43° 49′ | 79° 11′ | November 2014 |
| Z147-15 | Reindeer | Female | Adult | Scarborough | Ontario | 43° 49′ | 79° 11′ | November 2015 |
Over 2.5 years of age.
Reported antemortem clinical syndromes and major postmortem findings are displayed in Table 3. All reindeer cases were acute, while elk cases were either peracute or acute. Postmortem lesions in peracute cases included jaundice, hematochezia, and pigmenturia. Clinical signs in acutely affected animals included depression, dysorexia, respiratory distress, jaundice, pigmenturia, hematochezia, pyrexia, and separation from herd-mates, and were identified between 0 to 3 days before death. The 4 animals for which clinical pathology results were available were anemic and showed evidence of hemolysis and hyperbilirubinemia. Blood smears were performed in 7 cases, and a presumptive diagnosis of babesiosis based on visualization of intraerythrocytic piroplasms was made in 5 of these (Table 3, Figure 2). Necropsies were performed on all cases; the most consistent gross findings were extensive subcutaneous and muscular hemorrhages and the presence of deep red-brown urine (Table 3). On histopathologic examination, the most consistent finding was the presence of intrahepatic cholestasis. Other microscopic lesions described included hepatic necrosis (1/9), centrilobular hepatocyte degeneration (1/9), hepatic lipidosis (1/9), extravascular erythrophagia (2/9), and reactive spleen (2/9).
Table 3.
Major clinical and pathologic findings in captive elk (Cervus canadensis) and reindeer (Rangifer tarandus) that died of infection with Babesia odocoilei.
| ID | Species | Clinical syndrome | Piroplasms on blood smear | Hemorrhage | Pigmenturia | Intrahepatic cholestasis | Tissue(s) PCR positive for B. odocoilei |
|---|---|---|---|---|---|---|---|
| Z165-12 | Elk | Acute | −a | − | +b | + | Spleen |
| Z175-12 | Elk | Peracute | − | − | + | + | Spleen |
| Z176-12 | Elk | Peracute | NAc | + | + | + | Spleen |
| Z137-13 | Elk | Peracute | NA | + | + | + | Spleen |
| Z174-13 | Reindeer | Acute | + | − | − | + | Spleen |
| K00075 | Reindeer | Acute | + | + | + | + | Whole blood |
| D24259 | Elk | Acute | + | + | + | + | Whole blood, spleen |
| Z167-14 | Reindeer | Acute | + | + | + | − | Spleen |
| Z147-15 | Reindeer | Acute | + | + | + | − | Whole blood, spleen |
Absent.
Present.
NA — data not available.
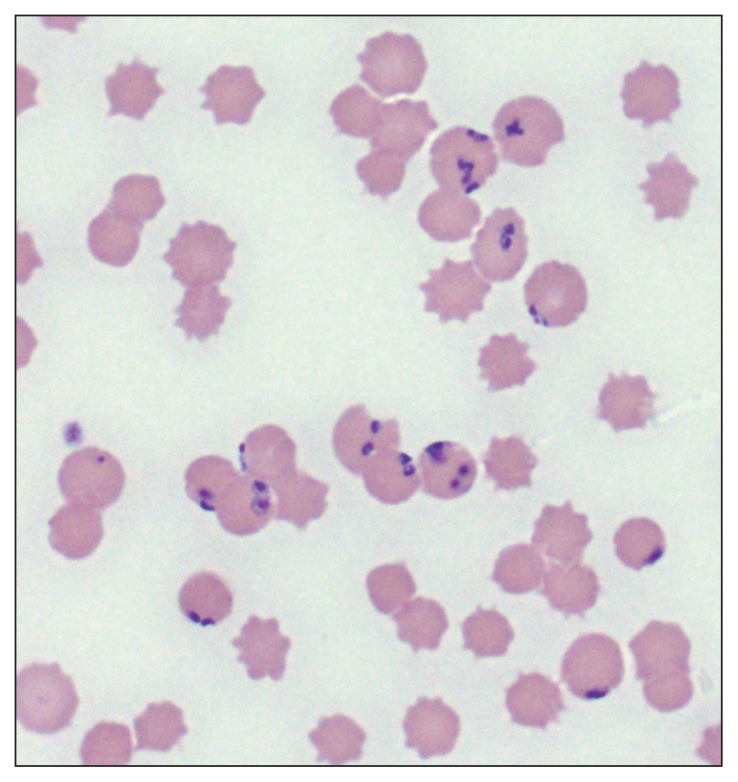
Figure 2.
Photomicrograph of a blood smear from an elk with cervid babesiosis. Note the multiple Babesia odocoilei organisms located peripherally within erythrocytes.
Diagnosis of B. odocoilei infection was confirmed in all cases using PCR performed on whole blood or frozen spleen (Table 3). Partial 18S rDNA sequences from ID #Z176-12 and D24259, 2 American elk, and from ID #Z147-15, a European reindeer, were submitted to GenBank under accession numbers AB12345.6, MF045131.1 and AB12345.6, respectively. All 18S rDNA sequences had 100% sequence identity to one another and to a number of B. odocoilei sequences submitted previously to GenBank from various hosts and geographic locations: white-tailed deer in Texas (AY046577; U16369.2); elk in New Hampshire (AY661503.1), Wisconsin (AY294206.1) and Saskatchewan (KC460321); and muskox from Minnesota (AY661507.1).
Discussion
Between 2012 and 2016, there were at least 11 confirmed deaths in captive cervids in 4 Canadian provinces as a result of infection by B. odocoilei (18). Although this list is likely not exhaustive, the cases reported here suggest that cervid babesiosis is an emerging disease in Canada. Prior to the first report of the disease in a Saskatchewan elk herd, cervid babesiosis was not recognized as a clinical problem in Canada, and surveillance for the pathogen in free-ranging wildlife was not of high priority. In part, this was likely a result of the presumptive absence of the main tick vector, I. scapularis (4). While endemism in wild Canadian cervids has yet to be conclusively demonstrated, it is reasonable to assume that in Canada, as in the United States, the white-tailed deer is a natural reservoir of infection (1,5,18). Research is currently underway in Saskatchewan and Ontario to establish its prevalence in farmed and wild cervids (17).
The antemortem clinical syndromes and postmortem findings reported here are consistent with previously documented cases of cervid babesiosis. Until recently, infection with B. odocoilei in reindeer and caribou was only known to manifest as a rapidly fatal acute disease. However, subclinical infections were recently demonstrated in reindeer at the Toronto Zoo (23). Disease manifestation in elk is more variable, and ranges from peracute to asymptomatic (6,13). The variability of clinical manifestations of infection with B. odocoilei is believed to be linked to differences in host susceptibility, levels of host infection and degree of stress-induced immunosuppression (13). Documented postmortem findings in peracute and acute cases of cervid babesiosis are consistent with a hemolytic crisis, and often include marked icterus, extensive multifocal petechial hemorrhages, splenomegaly, and pigmenturia. Commonly observed histologic lesions are hemoglobinuric nephrosis with tubular degeneration, splenic hemosiderosis, and hepatic centrolobular degeneration (6,8,13,19).
Adult males are overrepresented in the literature, with most fatal cases of cervid babesiosis occurring during the rutting season (i.e., between the months of September and November) (7,8,10,13,14,19). In the present report, cases occurred mainly in adult females, and were not restricted to the rutting season. The apparent gender predilection in our study is likely biased as herds of cervids in zoos typically are mostly composed of females, but does also support the premise that stressors other than rut may be implicated in the pathogenesis of cervid babesiosis. No particular predisposing factors were identified in the cases presented here. The absence of cases in juvenile cervids may also be biased as a result of low numbers of young animals in captive collections that were available to become infected, or may be a result of an inverse age resistance, as described with bovine babesiosis (24). Cervid babesiosis has been described in woodland caribou as young as 6 to 8 mo of age (10).
The seasonality of mortality correlates with that of the tick vector’s life cycle. The development cycle of I. scapularis is controlled by temperature and day-length, with the maximum activity of nymphs and adult ticks from mid-summer through autumn in the northern United States and southern Ontario (25–28). Transtadial survival of B. odocoilei from nymph to adult, and transmission from deer to deer by the adult tick were demonstrated for I. scapularis under laboratory conditions (5). Transmission by nymphs has not been shown, but is plausible given the fact that a related Babesia species, Babesia divergens, can be transmitted to hosts by all tick life stages (29).
The recent increase in detection of B. odocoilei in Canada is the combined result of the progressive incursion of its tick vector and of an increase in disease recognition and reporting by veterinarians. Movement of asymptomatic carriers between institutions may have contributed to the spread of the pathogen. Although most animals included in this report were born on-site, all 3 institutions occasionally acquire animals from other institutions, making the introduction of infected animals from endemic areas possible. Disease vectors are sensitive to climatic factors, and the effects of global warming on the extension of vector range from tropical to more temperate areas are well-documented with other vector-borne diseases such as malaria, Lyme borreliosis, and dengue fever (30). Scanning and targeted surveillance for I. scapularis shows that its range has been expanding northward from the northern United States into southern Manitoba, Ontario, Quebec, and the Maritimes; with detection clusters around Winnipeg, Toronto, and Montreal (30,31). The locations of the cervid babesiosis cases reported here coincide with the described geographic distribution of I. scapularis. Although the presence of B. odocoilei has yet to be demonstrated in I. scapularis in Canada, carriage of the pathogen is assumed. Interestingly, I. scapularis has been identified in Saskatchewan on northern pocket gophers (Thomomys talpoides) and in a handful of submissions by the public, but there are no known established populations in this province (30,32). It was hypothesized that the ticks responsible for transmitting B. odocoilei to the Saskatchewan elk herd had been carried by birds that migrated northward from American states or westward from Canadian provinces that support endemic I. scapularis and B. odocoilei populations (18,31,33). Preliminary results of ongoing research indicate that the tick is found sporadically in Saskatchewan cervids, but no endemic foci have been identified so far (17). These findings support the hypothesis that migratory birds are randomly transporting I. scapularis along with B. odocoilei into the province. As the tick vector range continues to expand northwards, it is likely that additional cases of babesiosis will emerge in captive cervids, and possibly other ungulate species, at higher latitudes than described here, and that B. odocoilei may eventually pose a risk to the health of the wild elk and caribou populations.
Conducting unbiased disease surveillance in free-ranging populations can be challenging. Population-based surveillance, which involves the collection of data through screening and targeted surveillance activities, is a powerful tool for monitoring infectious diseases, but is often labor-intensive and costly to implement and maintain. In the context of identifying disease in free-ranging wildlife, scanning surveillance relies on the opportunistic detection of disease events through the observation of diseased or dead animals, while targeted surveillance is based on the purposeful searching for evidence of disease or pathogens in animal populations. Sentinel surveillance can enhance detection of diseases and improve the cost-effectiveness of surveillance. In-depth data collection is performed on selected subpopulations, and the resulting analysis is used to signal disease outbreaks, identify epidemiologic trends, and monitor the burden of disease in the overall targeted population. Zoos and other collections of captive animals can be useful sentinel units for wildlife health, especially in regions in which minimal economic and human resources are dedicated to wildlife disease surveillance, or when population-based surveillance of wildlife health is limited to known enzootic pathogens (34). A telling example of how both the native and non-native species housed within zoos can act as sentinels for wildlife pathogens is the role of the Bronx Zoo in the investigation of the outbreak of West Nile viral encephalitis in New York in 1999 (35). Samples collected from wild crows and from various non-native and native captive birds found dead on zoo grounds led to the identification of the virus for the first time in the western hemisphere. Serological surveys of apparently healthy animals may also lead to the detection of endemic wildlife pathogens (34). The finding of B. odocoilei in captive cervids suggests local endemicity in wild cervid or tick populations, and reinforces the value of captive collections as sentinel units for wildlife health.
Cervid babesiosis should be included as a differential diagnosis for peracute or acute hemolytic crisis in captive cervids in Canada. Although further research is needed to clarify the epidemiology of B. odocoilei in Canadian wildlife, this case series suggests that the parasite has been introduced to southern Quebec, Ontario, and Manitoba. Cervid babesiosis is expected to become increasingly prevalent in Canada as global warming continues to alter the geographic and seasonal distributions of tick vectors.
Acknowledgments
The authors thank the veterinary and animal care staff of the Toronto Zoo, the Assiniboine Park Zoo, and Parc Safari for the exceptional care provided to the animals in this report, and for sharing their data for publication. We acknowledge Drs. Adriana M.W. Nielsen (Toronto Zoo, Ontario Veterinary College, University of Guelph; currently Wadi al Safa Wildlife Centre, Dubai, United Arab Emirates) and Doran Kirkbright (Ontario Veterinary College, University of Guelph, currently Animal Health Centre, Ministry of Agriculture, Abbotsford, British Columbia), Drs. Doris Sylvestre and Annie Deschamps (Faculté de Médecine Vétérinaire, Université de Montréal), and Dr. Brenda Bryan (Animal Health Centre, Veterinary Services Branch, Manitoba Agriculture, Food & Rural Development) for performing necropsy and histopathology of the cases described in this report. We also thank Dr. Patricia J. Holman (Texas Veterinary Medical Center, Texas A&M University) and the Vector Borne Disease Laboratory (College of Veterinary Medicine, North Carolina State University) for their assistance with the molecular diagnostics performed on Parc Safari and Assiniboine Park Zoo cases. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Emerson HR, Wright WT. The isolation of a Babesia in white-tailed deer. J Wildl Dis. 1968;4:142. doi: 10.7589/0090-3558-4.4.142. [DOI] [PubMed] [Google Scholar]
- 2.Emerson HR, Wright WT. Correction. J Wildl Dis. 1970;6:519. [Google Scholar]
- 3.Armstrong PM, Katavolos P, Caporale DA, Smith RP, Spielman A, Telford SR. Diversity of Babesia infecting deer ticks (Ixodes dammini) Am J Trop Med Hyg. 1998;58:739–742. doi: 10.4269/ajtmh.1998.58.739. [DOI] [PubMed] [Google Scholar]
- 4.Keirans JE, Hutcheson HJ, Durden LA, Klompen JS. Ixodes (Ixodes) scapularis (Acari:Ixodidae): Redescription of all active stages, distribution, hosts, geographical variation, and medical and veterinary importance. J Med Entomol. 1996;33:297–318. doi: 10.1093/jmedent/33.3.297. [DOI] [PubMed] [Google Scholar]
- 5.Waldrup KA, Kocan AA, Barker RW, Wagner GG. Transmission of Babesia odocoilei in white-tailed deer (Odocoileus virginianus) by Ixodes scapularis (Acari: Ixodidae) J Wildl Dis. 1990;26:390–391. doi: 10.7589/0090-3558-26.3.390. [DOI] [PubMed] [Google Scholar]
- 6.Holman PJ, Craig TM, Crider DL, Petrini KR, Rhyan J, Wagner GG. Culture isolation and partial characterization of a Babesia sp. from a North American elk (Cervus elaphus) J Wildl Dis. 1994;30:460–465. doi: 10.7589/0090-3558-30.3.460. [DOI] [PubMed] [Google Scholar]
- 7.Schoelkopf L, Hutchinson CE, Bendele KG, et al. New ruminant hosts and wider geographic range identified for Babesia odocoilei (Emerson and Wright 1970) J Wildl Dis. 2005;41:683–690. doi: 10.7589/0090-3558-41.4.683. [DOI] [PubMed] [Google Scholar]
- 8.Bartlett SL, Abou-Madi N, Messick JB, Birkenheuer A, Kollias GV. Diagnosis and treatment of Babesia odocoilei in captive reindeer (Rangifer tarandus tarandus) and recognition of three novel host species. J Zoo Wildl Med. 2009;40:152–159. doi: 10.1638/2008-0011.1. [DOI] [PubMed] [Google Scholar]
- 9.Goff WL, Jessup DA, Waldrup KA, et al. The isolation and partial characterization of a Babesia sp. from desert bighorn sheep (Ovis canadensis nelsoni) J Eukaryot Microbiol. 1993;40:237–243. doi: 10.1111/j.1550-7408.1993.tb04909.x. [DOI] [PubMed] [Google Scholar]
- 10.Holman PJ, Petrini K, Rhyan J, Wagner GG. In vitro isolation and cultivation of a Babesia from an American woodland caribou (Rangifer tarandus caribou) J Wildl Dis. 1994;30:195–200. doi: 10.7589/0090-3558-30.2.195. [DOI] [PubMed] [Google Scholar]
- 11.Perry BD, Nichols DK, Cullom ES. Babesia odocoilei Emerson and Wright, 1970 in white-tailed deer, Odocoileus virginianus (Zimmermann), in Virginia. J Wildl Dis. 1985;21:149–152. doi: 10.7589/0090-3558-21.2.149. [DOI] [PubMed] [Google Scholar]
- 12.Thomford JW, Conrad PA, Boyce WM, Holman PJ, Jessup DA. Isolation and in vitro cultivation of Babesia parasites from free-ranging desert bighorn sheep (Ovis canadensis nelsoni) and mule deer (Odocoileus hemionus) in California. J Parasitol. 1993;79:77–84. [PubMed] [Google Scholar]
- 13.Gallatin LL, Irizarry-Rovira AR, Renninger ML, et al. Babesia odocoilei infection in elk. J Am Vet Med Assoc. 2003;223:1027–1032. doi: 10.2460/javma.2003.223.1027. [DOI] [PubMed] [Google Scholar]
- 14.Petrini KR, Holman PJ, Rhyan JC, Jenkins SJ, Wagner GG. Fatal babesiosis in an American woodland caribou (Rangifer tarandus caribou) J Zoo Wildl Med. 1995;26:298–305. [Google Scholar]
- 15.Holman PJ, Madeley J, Craig TM, et al. Antigenic, phenotypic and molecular characterization confirms Babesia odocoilei isolated from three cervids. J Wildl Dis. 2000;36:518–530. doi: 10.7589/0090-3558-36.3.518. [DOI] [PubMed] [Google Scholar]
- 16.Waldrup KA, Kocan AA, Qureshi T, Davis DS, Baggett D, Wagner GG. Serological prevalence and isolation of Babesia odocoilei among white-tailed deer (Odocoileus virginianus) in Texas and Oklahoma. J Wildl Dis. 1989;25:194–201. doi: 10.7589/0090-3558-25.2.194. [DOI] [PubMed] [Google Scholar]
- 17.Healthy Wildlife: The blog of the Canadian Wildlife Health Cooperative. Babesia odocoilei recently detected in Canada. [Last accessed November 9, 2017]. [updated 2014 June 18] Available from: blog.healthywildlife.ca/babesia-odocoilei-recently-detected-in-canada/
- 18.Pattullo KM, Wobeser G, Lockerbie BP, Burgess HJ. Babesia odocoilei infection in a Saskatchewan elk (Cervus elaphus canadensis) herd. J Vet Diagn Invest. 2013;25:535–540. doi: 10.1177/1040638713491746. [DOI] [PubMed] [Google Scholar]
- 19.Ameri M, Anderson WI, Holman PJ, Palmer GW. Babesia odocoilei infection in a North American elk (Cervus elaphus canadensis) Comp Clin Pathology. 2012;21:363–365. [Google Scholar]
- 20.Medlin L, Elwood HJ, Stickel S, Sogin ML. The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene. 1988;71:491–499. doi: 10.1016/0378-1119(88)90066-2. [DOI] [PubMed] [Google Scholar]
- 21.Ramos CM, Cooper SM, Holman PJ. Molecular and serologic evidence for Babesia bovis-like parasites in white-tailed deer (Odocoileus virginianus) in south Texas. Vet Parasitol. 2010;172:214–220. doi: 10.1016/j.vetpar.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Untergasser A, Cutcutache I, Koressaar T, et al. Primer3 — New capabilities and interfaces. Nucleic Acids Res. 2012:40. doi: 10.1093/nar/gks596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pastor AR, Nielsen AMW, Kirkbright DW, et al. Babesiosis (Babesia odocoilei): An emerging disease of Ontario cervids. Proc Am Assoc Zoo Vet. 2016:101–102. [Google Scholar]
- 24.Christensson DA, Thorburn MA. Age distribution of naturally occurring acute babesiosis in cattle in Sweden. Acta Vet Scand. 1987;28:373–379. doi: 10.1186/BF03548605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Belozerov VN, Naumov RL. Nymphal diapause and its photoperiodic control in the tick Ixodes scapularis (Acari: Ixodidae) Folia Parasitol. 2002;49:314–318. doi: 10.14411/fp.2002.058. [DOI] [PubMed] [Google Scholar]
- 26.Daniels TJ, Falco RC, Curran KL, Fish D. Timing of Ixodes scapularis (Acari: Ixodidae) oviposition and larval activity in southern New York. J Med Entomol. 1996;33:140–147. doi: 10.1093/jmedent/33.1.140. [DOI] [PubMed] [Google Scholar]
- 27.Ogden NH, Bigras-Poulin M, Barker IK, et al. A dynamic population model to investigate effects of climate on geographic range and seasonality of the tick Ixodes scapularis. Int J Parasitol. 2005;35:375–389. doi: 10.1016/j.ijpara.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 28.Siegel JP, Kitron U, Bouseman JK. Spatial and temporal distribution of Ixodes-dammini (Acari: Ixodidae) in a Northwestern Illinois State Park. J Med Entomol. 1991;28:101–104. doi: 10.1093/jmedent/28.1.101. [DOI] [PubMed] [Google Scholar]
- 29.Zintl A, Mulcahy G, Skerrett HE, Taylor SM, Gray JS. Babesia divergens, a bovine blood parasite of veterinary and zoonotic importance. Clin Microbiol Rev. 2003;16:622–36. doi: 10.1128/CMR.16.4.622-636.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith KR, Woodward A, Campbell-Lendrum D, et al. Climate Change 2014: Impacts, Adaptation, and Vulnerability. Cambridge, UK and New York, USA: Cambridge University Pr; 2014. Human health: Impacts, adaptation, and co-benefits; pp. 709–754. [Google Scholar]
- 31.Ogden NH, Trudel L, Artsob H, et al. Ixodes scapularis ticks collected by passive surveillance in Canada: Analysis of geographic distribution and infection with Lyme borreliosis agent Borrelia burgdorferi. J Med Entomol. 2006;43:600–609. doi: 10.1603/0022-2585(2006)43[600:ISTCBP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 32.Anstead CA, Chilton NB. Ticks feeding on northern pocket gophers (Thomomys talpoides) in central Saskatchewan and the unexpected detection of Ixodes scapularis larvae. J Vector Ecol. 2011;36:355–360. doi: 10.1111/j.1948-7134.2011.00176.x. [DOI] [PubMed] [Google Scholar]
- 33.Ogden NH, Bouchard C, Kurtenbach K, et al. Active and passive surveillance and phylogenetic analysis of Borrelia burgdorferi elucidate the process of Lyme disease risk emergence in Canada. Environ Health Perspect. 2010;118:909–914. doi: 10.1289/ehp.0901766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hidalgo E, Salgado R, Pizarro J. Zoo animals as sentinels for wild animal infectious diseases in regions without resources for wildlife health surveillance: First report of a native South American wildlife species, southern pudu (Pudu puda), BVDV persistently infected. Proc Wild Dis Assoc Annu Conf. 2016:179. [Google Scholar]
- 35.Steele KE, Linn MJ, Schoepp RJ, et al. Pathology of fatal West Nile virus infections in native and exotic birds during the 1999 outbreak in New York City, New York. Vet Pathol. 2000;37:208–224. doi: 10.1354/vp.37-3-208. [DOI] [PubMed] [Google Scholar]
- 36.Holman PJ, Bendele KG, Schoelkopf L, Jones-Witthuhn RL, Jones SO. Ribosomal RNA analysis of Babesia odocoilei isolates from farmed reindeer (Rangifer tarandus tarandus) and elk (Cervus elaphus canadensis) in Wisconsin. Parasitol Res. 2003;91:378–383. doi: 10.1007/s00436-003-0984-5. [DOI] [PubMed] [Google Scholar]