Abstract
A 6-month-old intact female giant schnauzer dog fed a nutritionally unbalanced homemade diet was evaluated because of a 1-month history of lameness and difficulty walking. Abnormalities identified on ancillary tests, in conjunction with the dog’s clinical improvement following diet change, suggested a diagnosis of vitamin D deficiency and nutritional secondary hyperparathyroidism. This report underlines the importance of appropriate feeding management, especially during the vulnerable growth phase.
Résumé
Déséquilibres alimentaires chez un chiot de grande race causant des fractures de compression, une carence en vitamine D et de l’hyperparathyroïdisme soupçonné secondaire à la nutrition. Une chienne Schnauzer géante intacte âgée de 6 mois qui consommait une diète maison qui n’était pas équilibrée sur le plan nutritionnel a été évaluée en raison d’une anamnèse de 1 mois de boiterie et de difficultés ambulatoires. Les anomalies identifiées sur des tests ancillaires, de concert avec l’amélioration clinique du chien après le changement de diète, suggéraient un diagnostic de carence en vitamine D et d’hyperparathyroïdisme nutritionnel secondaire. Ce rapport souligne l’importance d’une gestion appropriée de l’alimentation, particulièrement durant la phase de croissance vulnérable.
(Traduit par Isabelle Vallières)
Growing dogs have more demanding dietary requirements than adult dogs (1–3). Unbalanced diets can lead to nutritional deficiencies or excesses, resulting in detrimental health consequences, especially during growth (4). These clinical consequences are most evident in puppies (5,6) with adult weights > 23 kg (6), as they are more prone to diseases related to rapid growth. Such diseases include developmental orthopedic diseases (DODs) (e.g., osteochondritis dissecans, osteochondrosis, retained cartilaginous core, panosteitis, hypertrophic osteodystrophy, canine elbow dysplasia, and hip dysplasia) (7) and dilated cardiomyopathy (8). Previous studies demonstrated malnutrition, especially energy excess, suboptimal concentrations of dietary vitamin D (VitD), calcium (Ca), and phosphorus (P), as well as a reverse Ca to P ratio (Ca:P), to be a risk factor for the development of DODs in large breed puppies (6,9). This report presents an assessment of the nutritional requirements for growth of a large breed puppy as a result of clinical implications due to feeding an unbalanced homemade diet and accounts for the key nutritional factors mentioned above when designing the diet plan.
Case description
A 6-month-old, intact female giant schnauzer dog was presented to the Ontario Veterinary College Health Sciences Centre (OVC-HSC) Neurology Service with an acute onset of generalized pain and paraparesis. Medical history revealed lameness, difficulty walking, and yelping/painful episodes with an unknown trigger during the past 2 mo. One month before consultation at the OVC-HSC, the dog was presented to the referring veterinarian with suspected urinary infection — a urine pH of 8, elevated leukocytes (30 to 50/400 × field), and a small amount of bacteria on urinalysis (urine sample collected via free catch). The patient was treated initially with amoxicillin/clavulanic acid [12.5 mg/kg body weight (BW), PO, q12h], based on a first-line drug choice approach (10). On physical examination at the OVC-HSC the puppy was tense and rigid, especially on hind limb palpation. Lumbar pain was noted particularly with extension of the hips. The dog was tachypneic and in pain upon abdominal palpation. She was non-ambulatory in her hind legs, lacked proprioception, had a hunched posture, and was knuckling bilaterally.
Nutritional screening evaluation was performed according to the World Small Animal Veterinary Association nutritional assessment guidelines (11). Growth, feeding with an unconventional diet, and the medical condition of the puppy were immediately identified as nutritional risk factors. The owner had fed the puppy a homemade diet since it was 2 mo old. The homemade diet was based on instructions provided on the Canine Life website (http://www.theskyesthelimit.com/). Some alterations were made by the owner. The recipe contained a combination of ingredients; i.e., 2 lbs of regular ground beef, 2 cups of grated carrots, 2 cups of green beans and broccoli, 2 to 3 tbsp of refined sunflower oil, 2 red apples, 3 eggs, 2 cloves of garlic, 3 to 4 cups of brown rice flour, and 1/2 a cup of oat bran per day. All ingredients were mixed together and baked into muffins. Batches of raw ingredients were prepared weekly by the owner and stored in the freezer. Four meals were fed per day. In addition to this daily portion, the dog received 1/2 to 1 cup of cooked white organic rice, apples and carrots, one capsule of cod liver oil, and 1 tsp of bone meal. The brands and manufacturers of the supplements were unknown to the owner. The patient weighed 20.36 kg with a normal body condition score (BCS) of 5/9 (12) and mild muscle wasting, likely due to disuse and potentially malnutrition (13). Previous information regarding the puppy’s BCS or weight was not obtainable. Therefore, although growth standards for various dog breeds are becoming more available, to compare an individual’s growth to a healthy reference population (14), this method was not applicable in the current case. An extended nutritional evaluation was indicated (11). The owner consented to further diagnostics and assessment of the homemade diet. The complete blood (cell) count (CBC) was unremarkable. When compared to a reference range for similarly aged dogs (15), serum total Ca and P concentrations were decreased, and serum alkaline phosphatase (ALP) and creatine kinase (CK) were elevated on the biochemistry profile (Table 1). A sterile urine sample was obtained through cystocentesis. Urinalysis showed elevated leukocytes (200 to 250/400× field), pH = 7.5, specific gravity = 1.020. Urinary culture revealed Escherichia coli, susceptible to various antibiotics including cefazolin. Treatment with cefazolin (TEVA cefazolin; Teva Canada, Scarborough, Ontario), 22 mg/kg body weight (BW), IV, every 12 h was initiated.
Table 1.
Serum biochemical panel and vitamin D profile of a 6-month-old intact female giant schnauzer dog at presentation and before discharge.
| Parameters | Blood analysis | Reference range | Units |
|---|---|---|---|
| Serum biochemical panela | |||
| Glucose | 4.6 | 5.39 to 9.22 | mmol/L |
| Total protein | 61 | 45 to 73 | g/L |
| Albumin | 3.7 | 2.6 to 3.7 | g/L |
| Globulins | 24 | 22 to 35 | g/L |
| ALP | 473 | 126 to 438 | U/L |
| ALT | 14 | ≤ 32 | U/L |
| CK | 1058 | 40 to 192 | U/L |
| GGT | 4 | ≤ 4.3 | U/L |
| Amylase | 1238 | ≤ 1683 | U/L |
| Lipase | 28 | ≤ 139 | U/L |
| Cholesterol | 8.8 | 2.6 to 12.9 | mmol/L |
| Bilirubin | 2.05 | 0.17 to 2.22 | μmol/L |
| BUN | 4.2 | 3.3 to 13.3 | mmol/L |
| Creatinine | 83.1 | 23.9 to 77.8 | μmol/L |
| Chloride | 119 | 99 to 120 | mmol/L |
| Magnesium | 1.8 | 1.4 to 5.2 | mmol/L |
| Phosphorus | 3.8 | 5.6 to 9.6 | mmol/L |
| Potassium | 3.9 | 3.9 to 6.1 | mmol/L |
| Sodium | 156 | 139 to 159 | mmol/L |
| Total calcium | 2.1 | 2.5 to 3.3 | mmol/L |
| Vitamin D profileb | |||
| iCa | 1.5 | 1.25 to 1.45 | mmol/L |
| PTH | 4.6 | 0.50 to 5.80 | pmol/L |
| 25(OH)D | 8 | 60 to 215 | nmol/L |
ALP — alkaline phosphatase; ALT — alanine aminotransferase; GGT — gamma-glutamyl transpeptidase; BUN — blood urea nitrogen; CK — creatine kinase; iCa — ionized calcium; PTH — parathyroid hormone; 25(OH)D — 25-hydroxyvitamin D.
Values at presentation. Reference range: puppies 4 to 6 months old (Reference 15).
Values at discharge (2 days following presentation). Reference range: DCPAH — Michigan State University, Diagnostic Center for Population & Animal Health.
Differential diagnoses included nutritional VitD deficiency, nutritional secondary hyperparathyroidism (NSH), discospondylitis, autoimmune meningomyelitis, spinal empyema, trauma (causing fracture/luxation), and parasitic myelitis (toxoplasma, neospora). Primary hyperparathyroidism was less likely, as hypocalcemia was evident, and not hypercalcemia (16). As there was no evidence of renal dysfunction on urinalysis or blood analysis, renal secondary hyperparathyroidism was considered unlikely (17).
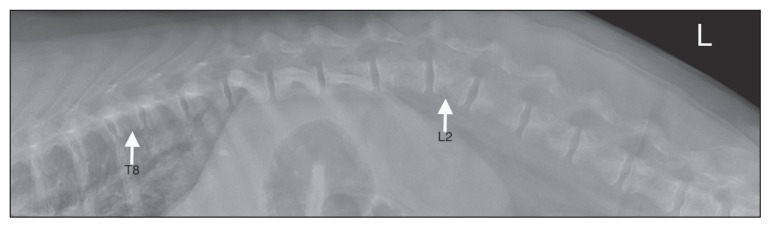
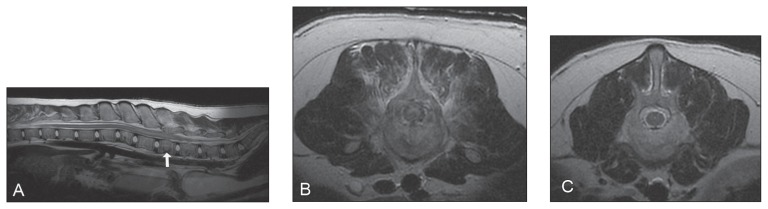
For further evaluation of the acute neurological abnormalities, thoracolumbar spinal radiographs were taken and magnetic resonance imaging (MRI) was performed. Diagnostic imaging tests were focused on the thoracic and lumbar spine based on localization of neurological deficits and normal findings on physical and orthopedic examination of the front and hind limbs. On orthogonal radiographs of the thoracic and lumbar spine the entire axial skeleton had a reduced opacity with thinning of the cortices and reduced trabecular markings. Multiple vertebral bodies were misshapen, being shortened and more cuboidal than rectangular in shape. The L2 vertebral body had a trapezoidal shape and centered at the L1-2 intervertebral disc there was mild kyphosis (Figure 1). Magnetic resonance imaging of the thoracic and lumbar spine was carried out to further evaluate the abnormalities seen on spinal radiographs and to determine the sites of and degree of spinal cord compression contributing to the acute neurological deficits (Figure 2). Sequences acquired for images included: T2*-weighted single shot fast-spin echo (FSE) in a sagittal plane; T2-weighted FSE in sagittal, dorsal, and transverse planes; T1-weighted FSE in transverse plane; short tau inversion recovery (STIR) in a sagittal plane; T1-weighted FSE in transverse and sagittal planes following intravenous contrast administration. The MRI showed compression fractures of multiple vertebrae (chronic fractures) and an intramedullary spinal cord lesion from L2 to L5 (Figure 2A). A vertebral compression at L4 was associated with marked narrowing of the vertebral canal, attenuation of the subarachnoid space, and loss of the epidural fat (Figure 2B). The muscles surrounding the L4 vertebral body had increased signal intensity on T2-weighted and STIR images, and were moderately contrast enhancing. There was no evidence of spinal cord compression at the T8 or L2 vertebrae (Figure 2C). Given the severity of the changes at this level an acute fracture was suspected and the paraparesis was attributed to this lesion. The growth plates appeared normal for a dog this age, with no indication that the compression fractures involved the endplates. The differential diagnosis for the intramedullary lesion included edema or syringohydromyelia.
Figure 1.
Lateral radiograph of the thoracolumbar vertebrae of an intact 6-month-old female giant schnauzer dog fed an unbalanced homemade diet. The thoracic and lumbar vertebrae have a reduced opacity and thin cortices. The vertebral body of L2 has a trapezoid shape. The vertebral body of T8 is shortened, being more cuboidal in shape.
Figure 2.
Magnetic resonance images of the thoracolumbar spine of an intact 6-month-old female giant schnauzer dog fed an unbalanced homemade diet. A — Sagittal T2-weighted image of the thoracolumbar spine showing an increase in size of the spinal cord from L2 to L5, with an associated increased medullary T2 signal intensity. The L4 vertebral body is shortened with a non-uniform T2 signal intensity and multifocal linear areas of low T2 signal intensity (arrow). Within the epaxial muscles surrounding the vertebral body there is a moderate increase in T2 signal intensity. Multiple other vertebral bodies are mildly misshapen and have inhomogenous T2 signal intensity and multifocal linear low signal intensities attributed to compression fractures. B — Transverse T2-weighted image at the level of L4 showing narrowing of the vertebral canal with attenuation of the subarachnoid space and loss of epidural fat. C — Transverse T2-weighted image at the level of L2 showing no attenuation of the subarachnoid space.
The diet was analyzed using computer software (Balance IT online formulation and supplements, West Sacramento, California, USA) and a homogenized sample of the diet (obtained from the owner) was sent for proximate analysis and analyses of minerals (SGS Agri-food Laboratories, Guelph, Ontario) and VitD (NF EN 12821: Determination of vitamin D by high performance liquid chromatography (HPLC) — Measurement of cholecalciferol (vitamin D3) and ergocalciferol (vitamin D2) (Table 1). Both analyses included the main recipe, the additional rice portion, and the 2 supplements. For the software analysis, since the owner was not familiar with the brands or manufacturers, commercially available supplements were added to match the recipe as closely as possible, so that it could be compared to the laboratory analysis. The computer-generated diet analysis represents an estimation of the dietary nutrient profile based on ingredient composition available in an online nutrient database [United States Department of Agriculture (USDA) National Nutrient Database for Standard Reference Release]. However, the laboratory diet analysis represents the actual nutrient composition of this puppy’s diet (all ingredients and supplements, per owner). The findings of this laboratory analysis were not available until 10 d after presentation. Therefore, an initial computer-generated diet analysis was performed and revealed deficiencies in protein, Ca, chloride, copper, iodine, iron, P, potassium, sodium, selenium and zinc, vitamin E, choline and riboflavin (the most relevant nutrients are shown in Table 2).
Table 2.
Nutrient profiles of an unbalanced homemade diet, fed to a 6-month-old intact female giant schnauzer, and of the commercial puppy dry food given to this puppy following admission. The homemade diet was assessed by both computer software and laboratory analysis. The nutrient profiles of both diets are compared to the National Research Council (2) recommended allowance for growth and American Association of Feed Control Officials (AAFCO) (3) minimum requirements for growth.
| Homemade diet | Puppy dry foodc | NRC (2) | AAFCO (3) | ||
|---|---|---|---|---|---|
|
| |||||
| Softwarea | Laboratoryb | ||||
| Moisture (%) | 58.99 | 54.07 | 8.5 | ||
| Protein (% DM) | 13.18 | 18.48 | 30.60 | 17.50 | 22.00 |
| Ca (% DM) | 0.54 | 0.09 | 1.11 | 1.20 | 1.00 |
| P (% DM) | 0.59 | 0.37 | 0.84 | 1.00 | 0.80 |
| Ca:P ratio | 1:1.09 | 1:4 | 1.32:1 | 1.2:1e | 1 to 2:1 |
| Na (% DM) | 0.15 | 0.11 | 0.56 | 0.22 | 0.30 |
| K (% DM) | 0.37 | 0.76 | 0.84 | 0.44 | 0.60 |
| Mg (% DM) | 0.08 | 0.09 | 0.09 | 0.04 | 0.04 |
| VitD (IU/100 g DM) | 58 | < LODd | 100 | 55.20 | 50.00 |
| ME (kcal/100 g DM) | 431.3 | NA | 398.5 | ||
DM — dry matter; Ca — calcium; P — phosphorus; Na — sodium; K — potassium; Mg — magnesium; VitD — vitamin D; ME — metabolizable energy; NA — not available; LOD — limit of detection.
BalanceIT, West Sacramento, California.
SGS Agri-Food Laboratories, Guelph, Ontario.
Royal Canin Maxi Puppy dry food; formulated to meet the nutritional levels established by AAFCO (3) dog food nutrient profiles for growth.
Upon laboratory analysis, the VitD content of the homemade diet was below the LOD of 100 IU/kg DM.
The ratio was calculated using the recommended allowance according to the NRC (2).
Based on the dog’s clinical presentation, initial diagnostics (abnormalities on blood analysis, radiographs and MRI), and the presence of nutrient deficiencies assessed by computer software (Table 2), the presumptive diagnosis was NSH (18,19). The prognosis for this patient was guarded to poor, and euthanasia was considered. However, the owner decided to pursue treatment.
Previous studies have demonstrated malnutrition, especially energy excess, suboptimal dietary VitD, Ca, and P content, as well as a reverse Ca:P ratio, to be a risk factor for DODs in large breed puppies (6,9). Accordingly, on the day of presentation the puppy was immediately transitioned to a balanced commercial large breed puppy dry food (Table 2), accounting for the key nutritional factors mentioned. The daily energy requirement (DER) was calculated, based on a current ideal body weight of 20.36 kg, to be 1476 kcal/day using a DER factor of 2.2 [DER = 70 × (20.36)^0.75 × 2.2 = 1476 kcal/day] (20,21). The DER factor was chosen for a growing puppy that reached more than 50% of adult weight, but accounted for the dog being cage rested for 6 wk due to vertebral compression fractures, and was therefore lower than the recommended 2.5 for this growth period (20).
Prior to discharge, 2 d following admittance, a blood sample was taken for analysis of parathyroid hormone (PTH), 25-hydroxyvitamin D [25(OH)D], and ionized Ca (iCa) (Diagnostic Centre for Population and Animal Health, Lansing, Michigan). The serum PTH concentration was within the reference range, the serum 25(OH)D concentration was low, and the serum iCa concentration was slightly elevated (Table 1). At the time the blood sample was taken, the dog had been consuming a commercial puppy food for 48 h.
The results of the laboratory diet analysis became available 10 d after presentation. This analysis focused on fewer nutrients due to costs, so comparison to the computer-generated diet analysis was restricted to the nutrients assessed by the laboratory. Differences between the 2 analyses included higher Ca, P, Ca:P, Na, VitD and lower protein with the computer software analysis compared with the laboratory analysis. These differences resulted from average nutrient profiles of the ingredients based on information from an online nutrient database (USDA — National Nutrient Database for Standard Reference Release) used within the computer software, and the addition of generic supplements to the recipe by the authors, as detailed information from the owner was lacking. Still, similar end results were obtained (Table 2), i.e., minimal dietary Ca and P concentrations, reversed Ca:P ratio, and dietary VitD content that was below the detection level of 100 IU/kg DM. These results suggested the diagnosis of NSH and also that the nutritional VitD deficiency most likely resulted in rickets. This was based on the dog’s young age and the consumption of an unbalanced diet since 2 months of age, although X-rays of the long bones were not performed to confirm this clinical diagnosis (4,18).
Two months after discharge, the puppy was re-checked at the OVC-HSC Neurology Service and the Clinical Nutrition Service. The puppy was bright, alert, and responsive, and the owner noted the positive trend for her recovery approximately 3 wk after discharge. At that time, neurological examination revealed marked improvement with only mild residual ataxia. The dog had also regained strength and had improved activity. On screening nutritional assessment (11), the patient weighed 24 kg and had a BCS of 6/9 (12) with mildly underdeveloped muscle (13). The owner confirmed feeding of the recommended commercial large breed puppy dry food (Table 2). Treats and unbalanced human foods (e.g., salmon skin, apple, carrot, and table scraps) were also given, but the exact amounts were unknown. The risks of excessive energy intake resulting in rapid growth rate and/or obesity during the growth period were explained to the owner, and the link to DODs was emphasised (22). Also, the risks of unbalanced nutrition were reiterated to the owner. The owner agreed to reduce the treats and unbalanced human foods to no more than 10% of the recommended DER (23). The dog’s DER was recalculated to be 1446 kcal/day based on the estimated ideal body weight, which was reassessed to be 22.5 kg [(24 kg × 75% lean mass)/80% lean mass] (24). The DER factor was reduced from 2.2 to 2 because of inactivity and overweightness [DER = 70 × (22.5)^0.75 × 2.0 = 1446 kcal/day] (20). The diet remained the same, and a gradual increase in activity was recommended to improve muscle mass.
Follow-up with the owner 1 year after discharge revealed that the dog was doing well, although she became tired easily. Once rested, she resumed her normal activity. Her hunched posture was permanent. The owner was still feeding her the recommended commercial large breed puppy dry food (Table 2); however, he was about to transition her to an adult food, from the same manufacturer. The dog still received various treats in small amounts (e.g., cooked turkey necks, vegetables, fruits, and some of the muffin mixture that was given to her before the initial admittance to the hospital). The owner was well aware of the risks for unbalancing the nutrient profile of the diet with excessive treats and the risks associated with feeding cooked bones. A recheck appointment with both the OVC-HSC Neurology and Clinical Nutrition Services was recommended, but the owner declined.
Discussion
Nutritional recommendations for large breed puppies take into consideration key nutritional factors, such as energy, VitD, Ca, P, and Ca:P ratio (7). Energy excess results from offering food free choice or above caloric needs, especially when a diet has a high fat content, and therefore high energy density. Excess energy during growth not only promotes obesity (25) and reduced longevity (26), but also promotes rapid growth, and hence DODs (27), such as hip dysplasia and subsequent osteoarthritis (28). Therefore, reduced energy density (350 to 400 kcal/100 g) and a moderate fat level [< 12% on a dry matter (DM) basis], are recommended (6,24).
Passive Ca absorption occurs from 6 wk to 6 mo of age, and corresponds directly to dietary Ca intake (29). When the hormonal and intestinal regulation mature, active Ca absorption represents 90% of Ca uptake (30). Excessive Ca intake was shown to promote occurrence of DODs, even with normal Ca:P ratio (6). Also, 1,25-dihydroxyvitamin D [1,25(OH)2D] may play a role in passive absorption of Ca in the small intestines and modulates active intestinal Ca absorption, as well as renal Ca reabsorption (18,30–32). Other calcitropic hormones, PTH, growth hormone, and calcitonin, are associated with active Ca absorption, and bone mineralization and remodelling (18). In puppies, excessive dietary Ca intake is negatively correlated to P absorption (29). Contrarily, a diet deficient in Ca, but high in P causes a reverse Ca:P ratio, resulting in NSH (18,22). Unbalanced homemade diets that contain a significant amount of meat commonly have high P concentrations (33). For growing puppies 0.8% to 1.2% Ca on DM is recommended (2,3), and P is based upon Ca concentrations, to achieve an appropriate Ca:P ratio (22), of 1.2:1 calculated based on the National Research Council (NRC) recommended allowance for both minerals (2), or 1 to 2:1 based on the American Association of Food Control Officials (AAFCO) recommendations for growth (3). For large breed puppies the Ca:P ratio becomes more narrow at 1.1 to 1.3:1 (6). Therefore, if a homemade diet contains insufficient Ca and a reverse Ca:P ratio (above 1:2) occurs (4), this leads to NSH, demineralization of the bones, and skeletal deformity (34). Skeletal abnormalities manifest in 1 to 3 mo, affecting cancellous bone located in the epiphyseal plate of long bones or within the center of the vertebral bones. These areas become very thin, allowing the spiculae to collapse, causing compression fractures (18). Vitamin D and calcitropic hormones, such as PTH, and calcitonin, strongly influence bone and Ca metabolism in the growing dog. Vitamin D3 is formed in most mammals in the skin, under UV-B radiation. However, in dogs, it is an essential vitamin, absorbed from the diet (VitD3— cholecalciferol, animal origin; VitD2 — ergocalciferol, plant origin), bound to VitD binding protein and transported to the liver for its first hydroxylation into 25(OH)D. A second hydroxylation occurs in the kidneys into either the most biologically active metabolite, 1,25(OH)2D, or 24,25-dihydroxyvitamin D (18). Impaired metabolism or inappropriate dietary intake of VitD will lead to low concentration of serum 25(OH)D and its metabolites. Nutritional VitD deficiency combined with dietary Ca deficiency can manifest as both rickets and fibrous osteodystrophy. In fibrous osteodystrophy, fibrous connective tissue replaces mature bone due to osteoclastic resorption (35). However, in young mammals with open epiphyseal plates, these deficiencies will cause improper bone formation (defective mineralization/calcification), potentially leading to deformities and fractures, i.e., rickets (18).
In NSH, an increase of 1,25(OH)2D is expected to promote intestinal absorption and renal reabsorption of Ca and P (18). However, if the diet contains insufficient amounts of VitD2 or VitD3, then low concentrations of serum VitD metabolites are inevitable, worsening the concurrent NSH. Nutritional VitD deficiency can also initiate NSH on its own, due to the reduced Ca absorption caused by hypovitaminosis D (18,36).
In this report, a 6-month-old large breed puppy was presented with vertebral compression fractures, suspected rickets, and NSH due to nutritional deficiencies caused by consumption of an unbalanced homemade diet, confirmed by computer- generated and laboratory diet analyses. The computer-generated diet analysis gives an estimation of nutrient composition based on average ingredient composition available in an online nutrient database. In addition, since no information was available from the owner regarding the VitD and bone meal supplements he used, common commercial supplements were added according to the amounts the owner gave, in order to have an approximate initial assessment of the dog’s nutrient intake. This type of analysis was helpful as an initial assessment, and assisted in convincing the owner to have the laboratory analysis performed. The laboratory diet analysis assessed the actual nutrient profile of this puppy’s diet. Although the software analysis overestimated the content of Ca, P, Ca:P, Na, and VitD, and underestimated protein content (Table 2), both agreed on imbalances of the nutrients of concern. Serum total Ca measured on the day of presentation was lower than the reference range, and could be indicative of low dietary intake or reduced absorption, as a high correlation exists in puppies younger than 6 mo (Table 1) (29). Alkaline phosphatase is a membrane-associated enzyme which can originate from several tissues, including bone, and indicates damage. Creatine kinase (CK) is a cytosolic enzyme, highly active in muscular tissues, brain, and nerves. Increased serum CK concentrations are indicative of muscle damage, and if persistent, indicate on-going muscle damage (37), which in this case, could occur due to the vertebral fractures and the patient’s decreased mobility. An increase in creatinine was observed likely for the same reasons. The hypophosphatemia detected in the serum is a result of insufficient dietary calcium for intestinal passive absorption, reverse Ca:P ratio, and insufficient VitD, which resulted in deficient 1,25(OH)2D levels, and NSH; hence bone resorption (18). Unfortunately, it was difficult to convince the owner that further diagnostics should be done; therefore, serum samples for analysis of PTH, 25(OH)D, and iCa were not submitted on the day of presentation. A tentative diagnosis of nutritional VitD deficiency and NSH was made based on abnormalities on serum biochemical profile, the presence of vertebral compression fractures, muscle damage, and generalized osteopenia found on radiographs and MRI, and further supportive findings resulting from the computer-generated diet analysis (Table 2). In this case, the spinal radiographs supported the diagnosis of NSH, but the degree of spinal cord compression associated with the mild vertebral malalignment could not be determined on radiographs alone. An MRI examination was performed as a more sensitive and specific diagnostic test to determine the site and degree of spinal cord compression. The MRI was also used to assess for any lesions that could require surgical decompression. Since no clinical signs of swelling or discomfort were observed in the long bones, which are commonly affected by NSH, no further diagnostics were performed to evaluate them.
On the day of discharge (48 h after presentation), the owner agreed to perform more in-depth blood analysis (Table 1). Serum iCa concentrations at discharge were mildly higher than the reference range, while serum PTH was within the reference range. Serum 25(OH)D concentrations were low compared with the reference range. In puppies younger than 6 mo, Ca absorption is in direct proportion to the Ca in the diet (29) and passive absorption can represent up to 70% of Ca uptake (30). It is most likely that serum iCa and PTH concentrations normalized (Table 1) by the time of discharge due to passive Ca absorption, especially since the dog was fed a commercial large breed puppy diet following admission (Table 2). However, 25(OH)D might have been too depleted to respond in just 2 d (Table 1). Continuing this diet was recommended as it seemed sufficient to normalize the serum Ca concentration without additional Ca supplementation. The dog continued to improve and recover on this diet after hospitalization, which was observed at the follow-up appointment.
This patient’s debilitating diet-induced diseases and permanent hunched posture emphasize the importance of not only nutritional assessment but also of client compliance, based on good client communication. The client had fed the puppy an unbalanced homemade diet since she was 2 mo old, believing that it would improve the dog’s health and strengthen their bond, as many pet owners do. Compassionate communication is required to relay medical findings to the owner, allowing the owner a better understanding of the diet-induced diseases, without blame, anger, or guilt (38). It was also important to set expectations that the dog would be permanently crippled, but could maintain a good quality of life if her pain could be managed. Once the owner understood the severity of the diet-induced diseases, he was willing to cooperate and change the dog’s diet based on the OVC-HSC Clinical Nutrition Service’s recommendations. At the time of the recheck, and also during the follow-up telephone conversation, the owner was satisfied with the puppy’s improvement; however, it was still difficult for him to maintain a strict regimen for treats, snacks, and human foods of no more than 10% of her daily energy requirements (23).
In conclusion, this report presented a case of nutritional VitD deficiency and NSH causing vertebral compression fractures, due to a deficient homemade diet fed to a growing large breed puppy. Successful treatment was attributed to an appropriate nutritional assessment and diagnosis. However, a key to this success was the client’s compliance, which resulted from good communication and empathy, encouraging the owner not to focus on guilt and self-blame, but rather on promoting the dog’s recovery. CVJ
Footnotes
This case report won 1st place in the American Academy of Veterinary Nutrition (AAVN) Case Report Contest 2016, and will be published as an abstract in the AAVN newsletter.
Disclaimer: Adronie Verbrugghe is the Royal Canin Veterinary Diets Endowed Chair in Canine and Feline Clinical Nutrition at the Ontario Veterinary College.
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Kirk CA. New concepts in pediatric nutrition. Vet Clin North Am Small Anim Pract. 2001;31:369–392. doi: 10.1016/s0195-5616(01)50210-7. [DOI] [PubMed] [Google Scholar]
- 2.NRC. Nutrient Requirements of Dogs and Cats. Washington, DC: The National Academies Press; 2006. pp. 357–358. [Google Scholar]
- 3.AAFCO. Association of American Feed Control Officials: Association of American Feed Control Officials Incorporated. 2014. p. 591. [Google Scholar]
- 4.Kawaguchi K, Braga IS, Takahashi A, Ochiai K, Itakura C. Nutritional secondary hyperparathyroidism occurring in a strain of German shepherd puppies. Jpn J Vet Res. 1993;41:89–96. [PubMed] [Google Scholar]
- 5.Lauten SD, Cox NR, Brawner WR, et al. Influence of dietary calcium and phosphorus content in a fixed ratio on growth and development in Great Danes. Am J Vet Res. 2002;63:1036–1047. doi: 10.2460/ajvr.2002.63.1036. [DOI] [PubMed] [Google Scholar]
- 6.Lauten SD. Nutritional risks to large-breed dogs: From weaning to the geriatric years. Vet Clin North Am Small Anim Pract. 2006;36:1345–1359. viii. doi: 10.1016/j.cvsm.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Demko J, McLaughlin R. Developmental orthopedic disease. Vet Clin North Am Small Anim Pract. 2005;35:1111–1135. v. doi: 10.1016/j.cvsm.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Sanderson SL. Taurine and carnitine in canine cardiomyopathy. Vet Clin North Am Small Anim Pract. 2006;36:1325–1343. vii–viii. doi: 10.1016/j.cvsm.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 9.Richardson DC, Toll PW. Relationship of nutrition to developmental skeletal disease in young dogs. Vet Clin Nutr. 1997;4:6–13. [Google Scholar]
- 10.Weese JS, Blondeau JM, Boothe D, et al. Antimicrobial Use Guidelines for Treatment of Urinary Tract Disease in Dogs and Cats: Antimicrobial Guidelines Working Group of the International Society for Companion Animal Infectious Diseases. Vet Med Int. 2011 doi: 10.4061/2011/263768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freeman L, Becvarova I, Cave N, et al. WSAVA Nutritional Assessment Guidelines. J Small Anim Pract. 2011;52:385–396. doi: 10.1111/j.1748-5827.2011.01079.x. [DOI] [PubMed] [Google Scholar]
- 12.Laflamme D. Development and validation of a body condition score system for dogs. Canine Pract. 1997;22:10–15. [Google Scholar]
- 13.Baldwin K, Bartges J, Buffington T, et al. AAHA nutritional assessment guidelines for dogs and cats. J Am Anim Hosp Assoc. 2010;46:285–296. doi: 10.5326/0460285. [DOI] [PubMed] [Google Scholar]
- 14.German AJ. Promoting healthy growth in pets. Proceedings of the WALTHAM International Nutritional Sciences Symposium (WINNS); Chicago, Illinois. 2016. [Google Scholar]
- 15.von Dehn B. Pediatric clinical pathology. Vet Clin North Am Small Anim Pract. 2014;44:205–219. doi: 10.1016/j.cvsm.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Gear RNA, Neiger R, Skelly BJS, Herrtage ME. Primary hyperparathyroidism in 29 dogs: Diagnosis, treatment, outcome and associated renal failure. J Small Anim Pract. 2005;46:10–16. doi: 10.1111/j.1748-5827.2005.tb00268.x. [DOI] [PubMed] [Google Scholar]
- 17.Stillion JR, Ritt MG. Renal secondary hyperparathyroidism in dogs. Compendium (Yardley, PA) 2009;31:E8. [PubMed] [Google Scholar]
- 18.Rijnberk A. Clinical Endocrinology of Dogs and Cats. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1996. pp. 167–175. [Google Scholar]
- 19.Dwivedi DK, Kushwaha RB, Gupta AK. Clinical management of pathological fractures due to rickets and osteopenia in a dog. Intas Polivet. 2012;13:361–363. [Google Scholar]
- 20.Debraekeleer J, Gross KL, Zicker SC. Feeding growing puppies: Postweaning to adulthood. In: Hand MS, Thatcher CD, Remillard RL, Roudebush P, Novotny BJ, editors. Small Animal Clinical Nutrition. 5th ed. Topeka, Kansas: Mark Morris Institute; 2010. pp. 311–319. [Google Scholar]
- 21.Debraekeleer J. Appendices: Appendix F — Body Weights and Feeding Guides for Growing Dogs and Cats. In: Hand MS, Thatcher CD, Remillard RL, Roudebush P, editors. Small Animal Clinical Nutrition. 4th ed. Topeka, Kansas: Mark Morris Institute; 2000. pp. 1020–1026. [Google Scholar]
- 22.Richardson DC, Zentek J, Hazewinkel HAW, Nap RC, Toll PW, Zicker SC. Developmental orthopedic disease of dogs. In: Hand MS, Thatcher CD, Remillard RL, Roudebush P, Novotny BJ, editors. Small Animal Clinical Nutrition. 5th ed. Topeka, Kansas: Mark Morris Institute; 2010. pp. 667–713. [Google Scholar]
- 23.Yaissle JE, Holloway C, Buffington TCA. Evaluation of owner education as a component of obesity treatment programs for dogs. J Am Vet Med Assoc. 2004;224:1932–1935. doi: 10.2460/javma.2004.224.1932. [DOI] [PubMed] [Google Scholar]
- 24.Toll PW, Yamka RM, Schoenherr WD, Hand MS. Obesity. In: Hand MS, Thatcher CD, Remillard RL, Roudebush P, Novotny BJ, editors. Small Animal Clinical Nutrition. 5th ed. Topeka, Kansas: Mark Morris Institute; 2010. pp. 501–541. [Google Scholar]
- 25.Grandjean D, Paragon B-M. Dietary induced disorders in the puppy. Rec Med Vet. 1996;172:519–530. [Google Scholar]
- 26.Kealy RD, Lawler DF, Ballam JM, et al. Effects of diet restriction on life span and age-related changes in dogs. J Am Vet Med Assoc. 2002;220:1315–1320. doi: 10.2460/javma.2002.220.1315. [DOI] [PubMed] [Google Scholar]
- 27.Zentek J, Meyer H, Dämmrich K. The effect of a different energy supply for growing Great Danes on the body mass and skeletal development. 3. Clinical picture and chemical studies of the skeleton. Zentralbl Veterinarmed. Reihe A. 1995;42:69–80. [PubMed] [Google Scholar]
- 28.Kealy RD, Olsson S-E, Monti KL, et al. Effects of limited food consumption on the incidence of hip dysplasia in growing dogs. J Am Vet Med Assoc. 1992;201:857–857. [PubMed] [Google Scholar]
- 29.Dobenecker B. Influence of calcium and phosphorus intake on the apparent digestibility of these minerals in growing dogs. J Nutr. 2002;132:1665S–1667S. doi: 10.1093/jn/132.6.1665S. [DOI] [PubMed] [Google Scholar]
- 30.Tryfonidou MA, van den Broek J, van den Brom WE, Hazewinkel HA. Intestinal calcium absorption in growing dogs is influenced by calcium intake and age but not by growth rate. J Nutr. 2002;132:3363–3368. doi: 10.1093/jn/132.11.3363. [DOI] [PubMed] [Google Scholar]
- 31.Brickman AS, Reddy CR, Coburn JW, Passaro EP, Jowsey J, Norman AW. Biologic action of 1,25-dihydroxyvitamin D3 in the rachitic dog. Endocrinology. 1973;92:728–734. doi: 10.1210/endo-92-3-728. [DOI] [PubMed] [Google Scholar]
- 32.Puschett JB, Moranz J, Kurnick WS. Evidence for a direct action of cholecalciferol and 25-hydroxycholecalciferol on the renal transport of phosphate, sodium, and calcium. J Clin Invest. 1972;51:373–385. doi: 10.1172/JCI106823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Remillard RL, Paragon BM, Crane SW, Debraekeleer J, Cowell CS. Making pet foods at home. In: Hand MS, Thatcher CD, Remillard RL, Roudebush P, editors. Small Animal Clinical Nutrition. 4th ed. Topeka, Kansas: Mark Morris Institute; 2000. pp. 163–181. [Google Scholar]
- 34.Palmer N. Metabolic diseases of bones. In: Jubb KVF, Kennedy PC, Palmer N, editors. Pathology of Domestic Animals. 4th ed. Vol. 1. San Diego, California: Academic Press; 1993. pp. 55–92. [Google Scholar]
- 35.Dittmer KE, Thompson KG. Vitamin D metabolism and rickets in domestic animals. Vet Pathol. 2011;48:389–407. doi: 10.1177/0300985810375240. [DOI] [PubMed] [Google Scholar]
- 36.Hazewinkel HAW, Tryfonidou MA. Vitamin D3 metabolism in dogs. Mol Cell Endocrinol. 2002;197:23–33. doi: 10.1016/s0303-7207(02)00275-7. [DOI] [PubMed] [Google Scholar]
- 37.Côté E. Laboratory tests. In: Roth-Johnson L, editor. Clinical Veterinary Advisor Dogs and Cats. 1st ed. St Louis, Missouri: Mosby, Elsevier; 2007. pp. 1424–1504. [Google Scholar]
- 38.Shaw JR, Adams CL, Bonnett BN, Larson S, Roter DL. Veterinarian-client-patient communication during wellness appointments versus appointments related to a health problem in companion animal practice. J Am Vet Med Assoc. 2008;233:1576–1586. doi: 10.2460/javma.233.10.1576. [DOI] [PubMed] [Google Scholar]