ABSTRACT
Cytoplasmic dynein is a family of cytoskeletal motor proteins that move towards the minus-end of the microtubules to perform functions in a variety of mitotic processes such as cargo transport, organelle positioning, chromosome movement and centrosome assembly. However, its specific roles during mammalian oocyte meiosis have not been fully defined. Herein, we investigated the critical events during porcine oocyte meiotic maturation after inhibition of dynein by Ciliobrevin D treatment. We found that oocyte meiotic progression was arrested when inhibited of dynein by showing the poor expansion of cumulus cells and decreased rate of polar body extrusion. Meanwhile, the spindle assembly and chromosome alignment were disrupted, accompanied by the reduced level of acetylated α-tubulin, indicative of weakened microtubule stability. Defective actin polymerization on the plasma membrane was also observed in dynein-inhibited oocytes. In addition, inhibition of dynein caused the abnormal distribution of cortical granules and precocious exocytosis of ovastacin, a cortical granule component, which predicts that ZP2, the sperm binding site in the zona pellucida, might be prematurely cleaved in the unfertilized dynein-inhibited oocytes, potentially leading to the fertilization failure. Collectively, our findings reveal that dynein plays a part in porcine oocyte meiotic progression by regulating the cytoskeleton dynamics including microtubule stability, spindle assembly, chromosome alignment and actin polymerization. We also find that dynein mediates the normal cortical granule distribution and exocytosis timing of ovastacin in unfertilized eggs which are the essential for the successful fertilization.
KEYWORDS: Dynein, oocyte meiotic maturation, spindle assembly, actin polymerization, cortical granule
Introduction
Mammalian oocytes undergo induced arrest at the late diplotene stage of prophase I during meiosis in the ovary. Upon hormonal surge, an oocyte will exit the prophase I arrest from the germinal vesicle (GV) stage, be known as germinal vesicle breakdown (GVBD), until the next arrest, metaphase II (MII), where oocytes are fertilized.1 After GVBD, the microtubules are polymerized at microtubule organizing centers (MTOCs) to form the bipolar spindle around homologous chromosomes. Accurate control of spindle assembly and chromosome separation is obligatory for orderly meiosis during oocyte maturation, where any errors in this process can lead to the generation of aneuploid eggs.2-5 Microtubule polymerization is regulated by a number of microtubule-associated proteins including motor proteins such as cytoplasmic dynein and kinesins6,7 which can directly interact with microtubule ends to modulate the microtubule length and dynamics.7,8 Dynein, a large protein complex, drives long-range retrograde transport along microtubules and carries a wide range of cargos to play a vital role in cellular processes as diverse as spindle positioning, chromosome movement, Golgi dynamics and centrosome assembly etc.9-11 It has been shown that either inhibition or overexpression of dynein disrupts the mitotic spindle assembly.12,13 Dynein also takes part in the inactivation of mitotic spindle checkpoint after all chromosomes have bi-oriented at the equatorial plate.14,15 In addition, a previous study has reported that dynein plays an important role in actin remodeling during neurite outgrowth.16 Dynein light chain Tctex-1-mediated neurite development relies on its ability to modulate actin dynamics.16 Actin cytoskeleton mediates a broad range of essential biological functions in eukaryotic cells by providing the driving force for cells to move and to divide.17 During oocyte meiosis, actin cytoskeleton participates in spindle positioning, cortical polarization and asymmetric cell division.18,19
Cortical granules are regulatory secretory organelles found within oocytes and are most associated with the polyspermy prevention after fertilization.20 Cortical granules continuously arise from the Golgi apparatus and require actin microfilaments to migrate to the periphery during oocyte growth and their migration is considered an indication of oocyte maturity and organelle organization in mammals.20 Cortical granules become fully competent for exocytosis just before ovulation and are triggered at fertilization to migrate to the plasma membrane, where they fuse and exocytose their contents.21,22 Ovastacin, an oocyte-specific member of the astacin family of metalloendoproteases, is the first identified component of mammalian cortical granules, encoded by the single-copy gene Astl.21,22 Female mice lacking ovastacin could not cleave ZP2, the sperm recognizing site in the zona pellucida, after fertilization, leading to polyspermy and embryonic lethal.23-25 However, premature exocytosis of ovastacin in unfertilized eggs brings about failure of sperm binding and fertilization.21
In the present study, we used porcine oocytes as a research model to investigate the function of dynein during oocyte meiotic maturation because they are developmentally and physiologically more similar to the humans.26,27 Our findings reveal that dynein participates in porcine oocyte meiotic progression by regulating the cytoskeleton dynamics including microtubule stability, spindle assembly, chromosome alignment and actin polymerization. We also find that dynein mediates the normal cortical granule localization and exocytosis timing of ovastacin in unfertilized eggs to ensure the successful fertilization.
Result
Dynein is required for the porcine oocyte meiotic maturation
To investigate the functional roles of dynein during porcine oocyte maturation, a cell-permeable and specific blocker of AAA+ ATPase motor cytoplasmic dynein, Ciliobrevin D, was supplemented to the culture medium. As shown in Fig. 1, Ciliobrevin D treatment obviously caused the porcine oocyte meiotic failure by showing the poor expansion of cumulus cells surrounding COCs and lower proportion of first polar body extrusion (PBE) (Fig. 1A). The quantitative results revealed that treatment with different concentrations of Ciliobrevin D (5 μM, 50 μM and 100 μM) could lead to the reduced rates of PBE in varying degree in oocytes cultured for 44 h in vitro and supplementation with 50 μM and 100 μM inhibitor significantly decreased the PBE rate compared to controls (37.5% ± 3.3%, n = 122 VS 12.5% ± 3.6%, n = 113 VS 68.3% ± 2.5%, n = 139, p < 0.05; Fig. 1B). 50 μM Ciliobrevin D was used for subsequent studies because this concentration not only resulted in the meiotic arrest but also allowed a proportion of oocytes to develop to MII stage for investigations. Collectively, these observations suggest that dynein is required for normal porcine oocyte meiotic progression.
Figure 1.
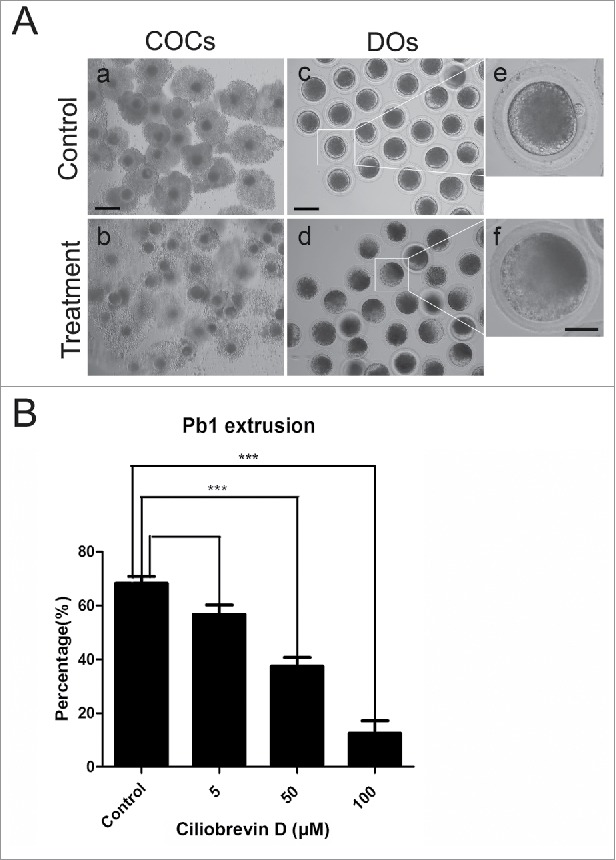
Effect of dynein inhibition on the porcine oocyte maturation. (A) Representative images of oocyte meiotic progression in control and Ciliobrevin D-treated oocytes. Cumulus cell expansion of COCs and polar body extrusion of DOs were imaged by confocal microscope. Scale bar, 150 μm (a, b); 80 μm (c, d); 20 μm (e, f). (B) The rates of polar body extrusion were recorded in control and different concentrations of Ciliobrevin D groups (5 μM, 50 μM and 100 μM) after culture for 44 h in vitro. Data were presented as mean percentage (mean ± SEM) of at least three independent experiments. Asterisk denotes statistical difference at a p < 0.05 level of significance.
Inhibition of dynein disturbs the spindle organization and chromosome alignment in porcine oocytes
To ask whether the meiotic arrest induced by inhibition of dynein is mediated by the spindle/chromosome abnormalities, oocytes were immunostained with anti-α-tubulin-FITC antibody to observe the spindle morphologies and counterstained with PI (Propidium Iodide) to visualize the chromosome alignment. The staining results showed that a great mass of control oocytes displayed a typical barrel-shape spindle apparatus with a well-aligned chromosome on the equatorial plate (14.3% ± 2.9%, n = 101, spindle; 14.0% ± 1.6%, n = 101, chromosome; Fig. 2A, B, C). In striking contrast, a majority of abnormal spindles with misaligned chromosomes were observed in Ciliobrevin D -treated oocytes (42.3% ± 0.6%, n = 117, p < 0.05, spindle; 58% ± 1.2%, n = 117, p < 0.05, chromosome; Fig. 2A, B, C).
Figure 2.
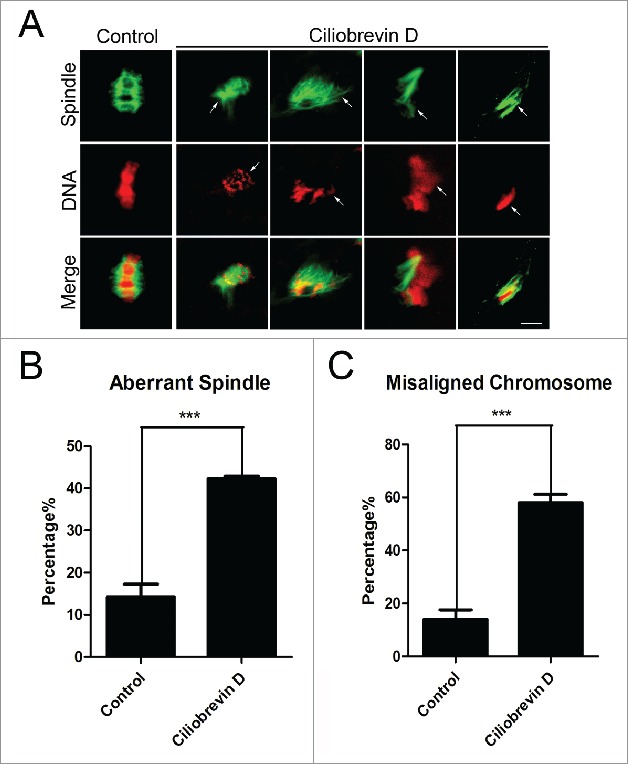
Effect of dynein inhibition on the spindle assembly and chromosome alignment in porcine oocytes. (A) Representative images of spindle morphologies and chromosome alignment in control and Ciliobrevin D-treated oocytes. Oocytes were immnunostained with anti-α-tubulin-FITC antibody to visualize the spindles and counterstained with Hoechst to visualize the chromosomes. Scale bar, 5 μm. Arrows indicate the abnormalities. (B) The rate of aberrant spindles was recorded in control and Ciliobrevin D-treated oocytes. (C) The rate of misaligned chromosomes was recorded in control and Ciliobrevin D-treated oocytes. Data in (B) and (C) were presented as mean percentage (mean ± SEM) of at least three independent experiments. Asterisk denotes statistical difference at a p < 0.05 level of significance.
Inhibition of dynein reduces the acetylation level of α-tubulin in porcine oocytes
The spindle assembly defects prompted us to examine the microtubule dynamics in oocytes treated with dynein inhibitor. Since previous studies have reported that acetylated α-tubulin is an indicator of the stabilized microtubules in both mitotic and meiotic cell,2,3,5,28 we thus assessed the stability of microtubules in Ciliobrevin D-treated oocytes. We found that Ciliobrevin D treatment remarkably lowered the signals of acetylated α-tubulin in porcine oocytes, and the fluorescent intensity had a significant reduction in the treatment group compared to the controls (18.2 ± 1.6, n = 87 VS 48.9 ± 1.5, n = 91; Fig. 3A, B). The hypoacetylation of α-tubulin suggests that microtubules are less stable in the absence of dynein, which hence impairs the microtubule dynamics and spindle organization.
Figure 3.
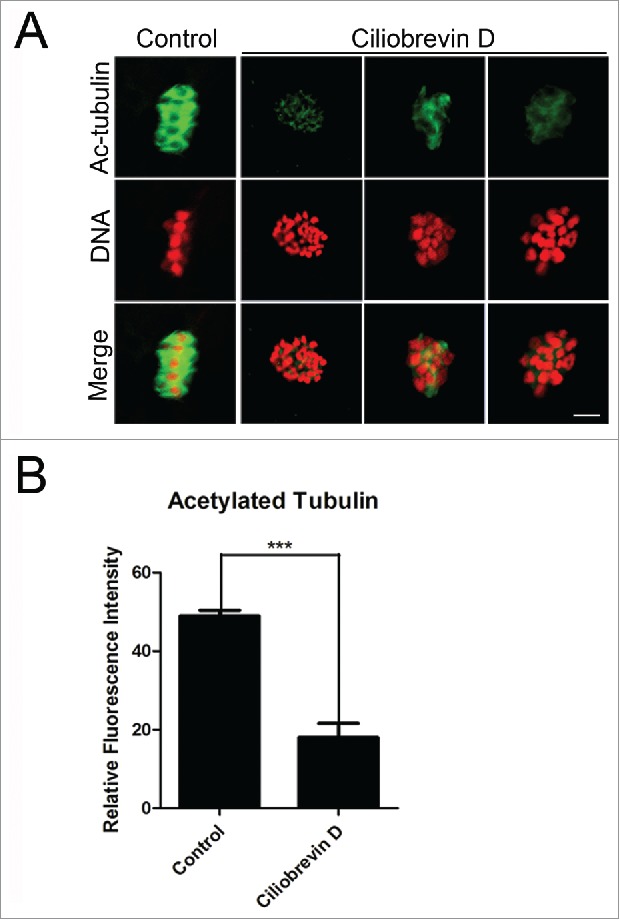
Effect of dynein inhibition on the microtubule stability in porcine oocytes. (A) Representative images of acetylated α-tubulin in control and Ciliobrevin D-treated oocytes. Oocytes were immnunostained with anti-acetyl-α-tubulin (Lys-40) antibody to assess the acetylation level of α-tubulin. Scale bar, 5 μm. (B) Quantitative analysis of the fluorescence intensity of acetylated α-tubulin in control and Ciliobrevin D-treated oocytes. Data were presented as mean percentage (mean ± SEM) of at least three independent experiments. Asterisk denotes statistical difference at a p < 0.05 level of significance.
Inhibition of dynein compromises the actin cytoskeleton in porcine oocytes
Dynamic actin polymerization drives meiotic spindle migration, spindle positioning, intracellular transportation and cytokinesis in cells.29 To further investigate whether dynein is required for the oocyte development through influencing the actin cytoskeleton, phalloidin was used as a probe to stain the F-actin. In control oocytes, actin was distributed evenly in the plasma membrane with strong signals (Fig. 4A). However, Ciliobrevin D-treated oocytes displayed a discontinuous distribution of actin filaments with weak signals (Fig. 4A). Furthermore, quantitative measurement of fluorescence intensity showed that actin signals were substantially reduced when dynein was inhibited compared to controls (7.34 ± 0.9, n = 69 VS 47.6 ± 1.3, n = 97; Fig. 4B), indicating that dynein is implicated in the actin dynamics to promote the oocyte meiotic maturation.
Figure 4.
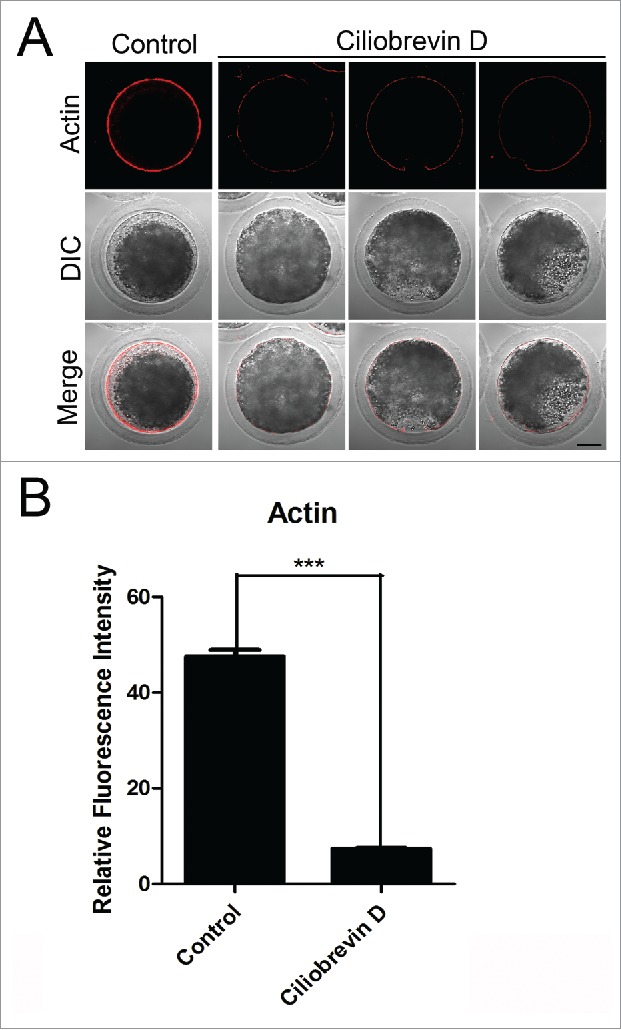
Effect of dynein inhibition on the actin dynamics in porcine oocytes. (A) Representative images of actin filaments in control and Ciliobrevin D-treated oocytes. Oocytes were immnunostained with anti-phalloidin-TRITC antibody to visualize the actin filaments. Scale bar, 20 μm. (B) The fluorescence intensity of actin was measured in control and Ciliobrevin D-treated oocytes. Data were presented as mean percentage (mean ± SEM) of at least three independent experiments. Asterisk denotes statistical difference at a p < 0.05 level of significance.
The normal localization of CGs and ovastacin requires dynein in porcine oocytes
CGs (cortical granules) are Golgi apparatus-derived and oocyte-specific vesicles which are well known for their function in the block to polyspermy. Notably, CGs are usually considered as one of the most important indicators of oocyte cytoplasmic maturation. Thus, the localization of CGs was detected with their marker LCA-FITC and imaged by confocal microscope. The staining analysis showed that Ciliobrevin D treatment considerably impaired the normal localization of CGs in the subcortical region and the fluorescent intensity of CGs had an marked decline compared to the controls (10.1 ± 1.8, n = 112 VS 34.4 ± 1.7, p < 0.05, n = 109; Fig. 5A, B). In addition, ovastacin is the first identified component of CGs in mammals and is responsible for the post-fertilization cleavage of sperm recognizing site ZP2 to block sperm binding and polyspermy. We also examined its localization and protein level after inhibition of dynein. Premature exocytosis of ovastacin was observed in Ciliobrevin D-treated oocytes by showing the loss of localization and lower signals of fluorescent intensity than those in control oocytes (6.2 ± 1.2, n = 89 VS 16.4 ± 0.6, n = 87, P < 0.05; Fig. 5C, D), implying that sperm binding site might be prematurely lost in dynein-inhibited oocytes.
Figure 5.
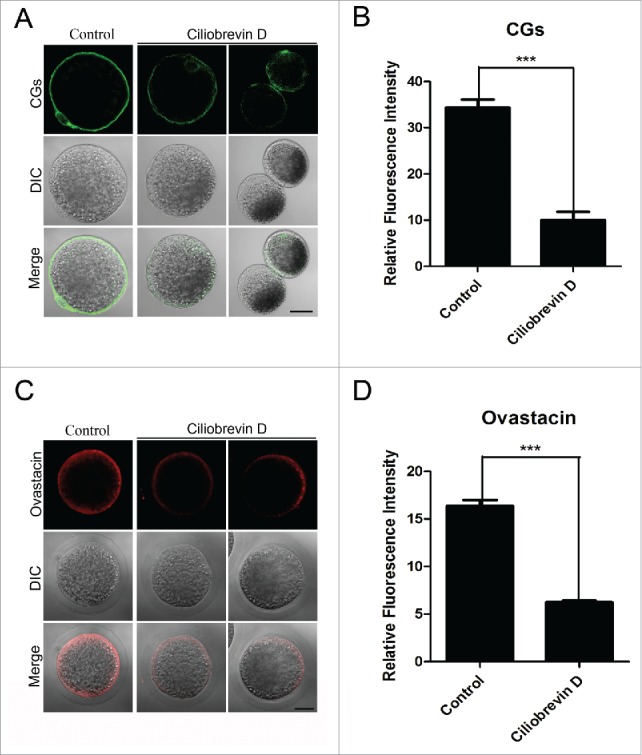
Effect of dynein inhibition on the distribution of cortical granules and ovastacin in porcine oocytes. (A) Representative images of cortical granule localization in control and Ciliobrevin D-treated oocytes. Oocytes were immnunostained with LCA-FITC to visualize the cortical granules. Scale bar, 20 μm. (B) The fluorescence intensity of cortical granules was measured in control and Ciliobrevin D-treated oocytes. (C) Representative images of ovastacin localization in control and Ciliobrevin D-treated oocytes. Ovastacin was immunostained with rabbit polyclonal anti-human ovastacin antibody and imaged by confocal microscopy. Scale bar, 20 μm. (D) The fluorescence intensity of ovastacin was measured in control and Ciliobrevin D-treated oocytes. Data in (B) and (D) were presented as mean percentage (mean ± SEM) of at least three independent experiments. Asterisk denotes statistical difference at a p < 0.05 level of significance.
Discussion
Cytoplasmic dynein is a family of cytoskeletal motor proteins that move towards the minus-end of the microtubules to perform functions in various biological processes such as cargo transport, organelle positioning, chromosome movement and centrosome assembly.30,31 To explore the exact roles of dynein during oocyte meiotic maturation, we selected pig oocytes as a model because they are physiologically more similar to human oocytes than mouse oocytes, and thus the data could provide a more solid basis for human reproductive research.
We first investigated the effects of dynein inhibitor Ciliobrevin D on the first polar body extrusion in porcine oocytes. With the use of increasing concentrations of inhibitor, the treated oocytes displayed a remarkably decreased rate of polar body extrusion with poor expansion of cumulus cells, suggesting that inhibition of dynein impairs the meiotic progression. We finally applied a mild treatment concentration of Ciliobrevin D (50 μM) for our subsequent study. Previous studies by us and others have indicated that oocyte meiotic arrest is largely caused by the activation of spindle assembly checkpoint induced by the defective spindle assembly and chromosome alignment.2,3,5,28 Meanwhile, it has been reported that dynein is required for the association of chromosomes with astral microtubules during in vitro meiosis I spindle assembly in the cytoplasmic extracts of Spisula solidissima oocytes.32 Dynein light intermediate chain 1 participates in the spindle assembly checkpoint silencing via removal of Mad1/2 and Zw10 but not BubR1 from kinetochores in human cells.33 Also, dynein/dynactin contribute to the targeting of Kif2a to spindle poles, suggesting a model in which dynein/dynactin regulate spindle length and coordinate flux by maintaining microtubule depolymerizing activities at spindle poles.34 Consistently, our findings revealed that in the dynein-inhibited oocytes spindle morphologies and chromosome alignment were severely compromised. The involvement of dynein in the spindle assembly further prompted us to test its possible roles in microtubule dynamics. Tubulin acetylation is a post-translational modification that occurs on Lys-40 of the α-tubulin subunit.35,36 Acetylated α-tubulin is abundant in stable microtubules but is absent in dynamic subcellular structures.37 Recent studies have also shown that acetylated α-tubulin is found in stabilized microtubules in mouse oocytes.2,4,5 Thus we detected acetylated α-tubulin as the indicator of microtubule stability in Ciliobrevin D-treated oocytes. Our observations revealed that the acetylation level of α-tubulin was significantly reduced when inhibited of dynein, indicating that the loss of microtubule stability might be the leading cause resulting in the defective spindle assembly we observed before.
Next we determined the distribution of actin filament caused by dynein inhibition. The previous reports have showed that dynein plays a role in actin remodeling during neurite outgrowth, and dynein light chain Tctex-1 mediated neurite development relies on its ability to modulate actin dynamics.38 Our findings further validate that dynein is required for the actin dynamics, showing that dynein inhibition significantly reduced the fluorescence intensity of actin filaments in the porcine oocytes.
Mammalian cortical granules are Golgi apparatus-derived, membrane-bound vesicles (0.2-0.6µm) that accumulate during oogenesis and form a uniform layer in the subcortical region of fully grown eggs.22 The normal distribution of cortical granule is often regarded as a symbol of oocyte cytoplasmic maturation. We therefore ask if inhibition of dynein would disrupt the cortical granule dynamics. The immunostaining and quantitative data revealed that cortical granules were lost from the subcortex of the Ciliobrevin D-treated oocytes, suggesting that cytoplasmic maturation is impaired under this circumstance. Furthermore, ovastacin, an oocyte-specific member of the astacin family of metalloendoproteases, is a pioneer cortical granule component that is responsible for post-fertilization cleavage of ZP2 at the N-terminus for definitive block to sperm binding to the zona pellucida surrounding fertilized eggs and embryos.22,39,40 In agreement with the defect of cortical granule dynamics, the localization of ovastacin was compromised and the amount was reduced in dynein-inhibited oocytes, implying that ovastacin might be prematurely exocytosed to cleave ZP2 in the zona pellucida surrounding unfertilized eggs so as to cause the fertilization failure.
Taken together, we provide several lines of evidence demonstrating that cytoplasmic dynein plays a part in the porcine oocyte meiotic maturation via maintaining the normal cytoskeleton assembly and organelle arrangement, which might be related to the involvement of dynein-mediated intracellular transport.
Materials and methods
Antibodies
Mouse monoclonal anti-α-tubulin FITC antibody, anti-phalloidin-TRITC antibody, anti-acetyl-α-tubulin (Lys-40) antibody and LCA-FITC were purchased from Sigma (St. Louis, MO, USA); Rabbit polyclonal anti-human ovastacin antibody was obtained from Dr. Jurrien Dean. FITC-conjugated goat anti-mouse IgG (H + L) and TRITC-conjugated goat anti-mouse IgG (H + L) were purchased from Zhongshan Golden Bridge Biotechnology Co., LTD (Beijing, China).
Inhibitor treatment
Ciliobrevin D (Calbiochem, San Diego, CA, USA) was dissolved in DMSO and diluted into a final concentration of 5 μM, 50 μM and 100 μM, respectively, with maturation medium, with the final concentration of the solvent not more than 0.1% of the culture medium.
Porcine oocyte collection and in vitro maturation
For IVM (in vitro maturation), porcine ovaries were collected from pubertal gilts at a local slaughterhouse and then transported to our laboratory in a physiological saline (0.9% NaCl) containing 500IU/ml of both penicillin and streptomycin, maintained at 30–35°C within 2 h in a thermos bottle after slaughter. Soon afterwards ovaries were washed twice with sterile phosphate-buffered saline (PBS), the COCs (cumulus-oocyte complexes) were subsequently aspirated from medium-sized follicles (3-6 mm in diameter) using a 20-gauge needle attached to a 20-ml disposable syringe. COCs surrounded by a compact cumulus mass with evenly granulated cytoplasm were washed three times with maturation medium, separated from the cellular debris, and then transferred to the maturation medium. The basic maturation medium was improved TCM-199 supplemented with 75 μg/ml of penicillin, 50 μg/ml of streptomycin, 0.5 μg/ml of LH, 0.5 μg/ml of FSH, 10 ng/ml of epidermal growth factor (mouse EGF; Sigma) and 0.57 mM cysteine (Sigma). To prepare mature oocyte in vitro, a group of 80 COCs was transferred to 500 ul of maturation medium and then covered with 200 ul paraffin oil to culture at 38.5°C in a humidified atmosphere of 5% CO2.
Immunofluorescent and confocal microscopy
Denuded oocytes (DOs) were washed in PBS, and then fixed in 4% paraformaldehyde in PBS for 1 h at room temperature. Oocytes were washed 3 times in PBS, and then rehydrated and transferred to the permeabilization solution (1% Triton X-100, 20 mM HEPES, PH 7.4, 3 mM MgCl2, 50 mM NaCl, 300 mM sucrose, 0.02% NaN3 in PBS) for 8–12 h. After blocking with 3% BSA for 1 h at room temperature, oocytes were incubated with anti-α-tubulin-FITC antibody (1:200), anti-acetylated tubulin antibody (1:100), anti-phalloidin-TRITC antibody (1:200) or rabbit polyclonal anti-mouse ovastacin antibody (1:100) at 4°C overnight, followed by incubation with an appropriate secondary antibody for 1 h and counterstaining of PI (Propidium Iodide) for 10 min at room temperature. Finally, oocytes were mounted on glass slides and observed under a laser-scanning confocal fluorescent microscope (Zeiss LSM 700 META confocal system).
For measurement of fluorescence intensity, the signals from both control and treatment oocytes were acquired by performing the same immunostaining procedure and setting up the same parameters of confocal microscope. The average fluorescence intensity per unit area within the region of interest (ROI) was applied to quantify the fluorescence of each oocyte images. Fluorescence intensity was randomly measured by plot profiling using ImageJ software (NIH, USA).
Statistical analysis
For each treatment, at least three biological replicates were done and the data were expressed as mean ± SEM and analyzed by one-way ANOVA, followed by LSD's post hoc test, which was provided by SPSS 16.0 statistical software. The level of significance was accepted as p < 0.05.
Disclosure of potential conflicts of interest
None declared.
Acknowledgment
We thank Dr. Jurrien Dean for providing antibodies.
Funding
This work was supported by the National Natural Science Foundation (31571545) and Natural Science Foundation of Jiangsu Province (BK20150677).
Authors' roles
Y.M. and B.X. designed the research; Y.M., C.Z., Z.C., L.T., X.SY., Y.L., M.Z. and X.D. performed the experiments; Y.M. and B.X. analyzed the data; Y.M. and B.X. wrote the manuscript.
References
- [1].Petronczki M, Siomos MF, Nasmyth K. Un menage a quatre: The molecular biology of chromosome segregation in meiosis. Cell. 2003;112:423-40. doi: 10.1016/S0092-8674(03)00083-7. [DOI] [PubMed] [Google Scholar]
- [2].Dai X, Zhang M, Lu Y, Miao Y, Zhou C, Xiong B. Cullin9 protects mouse eggs from aneuploidy by controlling microtubule dynamics via Survivin. BBA-Mol Cell Res. 2016;1863:2934-41. [DOI] [PubMed] [Google Scholar]
- [3].Miao Y, Zhou C, Cui Z, Dai X, Zhang M, Lu Y, Xiong B. Smc1beta is required for activation of SAC during mouse oocyte meiosis. Cell Cycle. 2017;16:536-44. doi: 10.1080/15384101.2017.1282583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zhang K, Lu Y, Jiang C, Liu W, Shu J, Chen X, Shi Y, Wang E, Wang L, Hu Q, et al.. HDAC8 functions in spindle assembly during mouse oocyte meiosis. Oncotarget. 2017;8:20092-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhang M, Dai X, Sun Y, Lu Y, Zhou C, Miao Y, Wang Y, Xiong B. Stag3 regulates microtubule stability to maintain euploidy during mouse oocyte meiotic maturation. Oncotarget. 2017;8:1593-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cassimeris L. Accessory protein regulation of microtubule dynamics throughout the cell cycle. Curr Opin Cell Biol. 1999;11:134-41. doi: 10.1016/S0955-0674(99)80017-9 [DOI] [PubMed] [Google Scholar]
- [7].Gaetz J, Kapoor TM. Dynein/dynactin regulate metaphase spindle length by targeting depolymerizing activities to spindle poles. J Cell Biol. 2004;166:465-71. doi: 10.1083/jcb.200404015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wittmann T, Hyman A, Desai A. The spindle: A dynamic assembly of microtubules and motors. Nat Cell Biol. 2001;3:E28-E34. doi: 10.1038/35050669 [DOI] [PubMed] [Google Scholar]
- [9].Carter AP, Diamant AG, Urnavicius L. How dynein and dynactin transport cargos: A structural perspective. Curr Opin Struc Biol. 2016;37:62-70. doi: 10.1016/j.sbi.2015.12.003 [DOI] [PubMed] [Google Scholar]
- [10].Roberts AJ, Kon T, Knight PJ, Sutoh K, Burgess SA. Functions and mechanics of dynein motor proteins. Nat Rev Mol Cell Bio. 2013;14:713-26. doi: 10.1038/nrm3667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cianfrocco MA, DeSantis ME, Leschziner AE, Reck-Peterson SL. Mechanism and regulation of cytoplasmic dynein. Annu Rev Cell Dev Bi. 2015;31:83-108. doi: 10.1146/annurev-cellbio-100814-125438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Vaisberg EA, Koonce MP, Mcintosh JR. Cytoplasmic dynein plays a role in mammalian mitotic spindle formation. J Cell Biol. 1993;123:849-58. doi: 10.1083/jcb.123.4.849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Echeverri CJ, Paschal BM, Vaughan KT, Vallee RB. Molecular characterization of the 50-kD subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. J Cell Biol. 1996;132:617-33. doi: 10.1083/jcb.132.4.617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Howell BJ, McEwen BE, Canman JC, Hoffman DB, Farrar EM, Rieder CL, Salmon ED. Cytoplasmic dynein/dynactin drives kinetochore protein transport to the spindle poles and has a role in mitotic spindle checkpoint inactivation. J Cell Biol. 2001;155:1159-72. doi: 10.1083/jcb.200105093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhang D, Yin S, Jiang MX, Ma W, Hou Y, Liang CG, Yu LZ, Wang WH, Sun QY. Cytoplasmic dynein participates in meiotic checkpoint inactivation in mouse oocytes by transporting cytoplasmic mitotic arrest-deficient (Mad) proteins from kinetochores to spindle poles. Reproduction. 2007;133:685-95. doi: 10.1530/rep.1.01167 [DOI] [PubMed] [Google Scholar]
- [16].Chuang J-Z, Yeh T-Y, Bollati F, Conde C, Canavosio F, Caceres A, Sung C-H. The dynein light chain Tctex-1 has a dynein-independent role in actin remodeling during neurite outgrowth. Dev Cell. 2005;9:75-86. doi: 10.1016/j.devcel.2005.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509-14. doi: 10.1126/science.279.5350.509 [DOI] [PubMed] [Google Scholar]
- [18].Yi KX, Rubinstein B, Li R. Symmetry breaking and polarity establishment during mouse oocyte maturation. Philos T R Soc B. 2013;368. doi: 10.1098/rstb.2013.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zhang Y, Wang QC, Han J, Cao R, Cui XS, Kim NH, Rui R, Sun SC. Involvement of Dynamin 2 in actin-based polar-body extrusion during porcine oocyte maturation. Mol Reprod Dev. 2014;81:725-34. [DOI] [PubMed] [Google Scholar]
- [20].Liu M. The biology and dynamics of mammalian cortical granules. Reprod Biol Endocrin. 2011;9:149. doi: 10.1186/1477-7827-9-149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Xiong B, Zhao Y, Beall S, Sadusky AB, Dean J. A unique egg cortical granule localization motif is required for ovastacin sequestration to prevent premature ZP2 cleavage and ensure female fertility in mice. Plos Genet. 2017;13:e1006580. doi: 10.1371/journal.pgen.1006580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Burkart AD, Xiong B, Baibakov B, Jimenez-Movilla M, Dean J. Ovastacin, a cortical granule protease, cleaves ZP2 in the zona pellucida to prevent polyspermy. J Cell Biol. 2012;197:37-44. doi: 10.1083/jcb.201112094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Quesada V, Sanchez LM, Alvarez J, Lopez-Otin C. Identification and characterization of human and mouse ovastacin: A novel metalloproteinase similar to hatching enzymes from arthropods, birds, amphibians, and fish. J Biol Chem. 2004;279:26627-34. doi: 10.1074/jbc.M401588200 [DOI] [PubMed] [Google Scholar]
- [24].Avella MA, Baibakov B, Dean J. A single domain of the ZP2 zona pellucida protein mediates gamete recognition in mice and humans. J Cell Biol. 2014;205:801-9. doi: 10.1083/jcb.201404025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Avella MA, Xiong B, Dean J. The molecular basis of gamete recognition in mice and humans. Mol Hum Reprod. 2013;19:279-89. doi: 10.1093/molehr/gat004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Schatten H, Sun QY. The functional significance of centrosomes in mammalian meiosis, fertilization, development, nuclear transfer, and stem cell differentiation. Environ Mol Mutagen. 2009;50:620-36. doi: 10.1002/em.20493 [DOI] [PubMed] [Google Scholar]
- [27].Sathanathan AH. Ultrastructural-changes during meiotic maturation in mammalian oocytes – Unique aspects of the human oocyte. Microsc Res Techniq. 1994;27:145-64. doi: 10.1002/jemt.1070270208 [DOI] [PubMed] [Google Scholar]
- [28].Zhang M, Dai X, Lu Y, Miao Y, Zhou C, Cui Z, Liu H, Xiong B. Melatonin protects oocyte quality from Bisphenol A-induced deterioration in the mouse. J Pineal Res. 2017;62. doi: 10.1111/jpi.12396. [DOI] [PubMed] [Google Scholar]
- [29].Field CM, Lenart P. Bulk cytoplasmic actin and its functions in meiosis and mitosis. Curr Biol. 2011;21:R825-30. doi: 10.1016/j.cub.2011.07.043 [DOI] [PubMed] [Google Scholar]
- [30].Cianfrocco MA, DeSantis ME, Leschziner AE, Reck-Peterson SL. Mechanism and regulation of cytoplasmic dynein. Annu Rev Cell Dev Bi. 2015;31:83-108. doi: 10.1146/annurev-cellbio-100814-125438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Roberts AJ, Kon T, Knight PJ, Sutoh K, Burgess SA. Functions and mechanics of dynein motor proteins. Nat Rev Mol Cell Bio. 2013;14:713-26. doi: 10.1038/nrm3667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Palazzo RE, Vaisberg EA, Weiss DG, Kuznetsov SA, Steffen W. Dynein is required for spindle assembly in cytoplasmic extracts of Spisula solidissima oocytes. J Cell Sci. 1999;112:1291-302. [DOI] [PubMed] [Google Scholar]
- [33].Sivaram MV, Wadzinski TL, Redick SD, Manna T, Doxsey SJ. Dynein light intermediate chain 1 is required for progress through the spindle assembly checkpoint. Embo J. 2009;28:902-14. doi: 10.1038/emboj.2009.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gaetz J, Kapoor TM. Dynein/dynactin regulate metaphase spindle length by targeting depolymerizing activities to spindle poles. J Cell Biol. 2004;166:465-71. doi: 10.1083/jcb.200404015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bulinski JC, Richards JE, Piperno G. Posttranslational modifications of alpha tubulin: Detyrosination and acetylation differentiate populations of interphase microtubules in cultured cells. J Cell Biol. 1988;106:1213-20. doi: 10.1083/jcb.106.4.1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Piperno G, LeDizet M, Chang XJ. Microtubules containing acetylated alpha-tubulin in mammalian cells in culture. J Cell Biol. 1987;104:289-302. doi: 10.1083/jcb.104.2.289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Robson SJ, Burgoyne RD. Differential localisation of tyrosinated, detyrosinated, and acetylated alpha-tubulins in neurites and growth cones of dorsal root ganglion neurons. Cell Motil Cytoskel. 1989;12:273-82. doi: 10.1002/cm.970120408 [DOI] [PubMed] [Google Scholar]
- [38].Chuang JZ, Yeh TY, Bollati F, Conde C, Canavosio F, Caceres A, Sung CH. The dynein light chain Tctex-1 has a dynein-independent role in actin remodeling during neurite outgrowth. Dev Cell. 2005;9:75-86. doi: 10.1016/j.devcel.2005.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Herrero MB, Mandal A, Digilio LC, Coonrod SA, Maier B, Herr JC. Mouse SLLP1, a sperm lysozyme-like protein involved in sperm-egg binding and fertilization. Dev Biol. 2005;284:126-42. doi: 10.1016/j.ydbio.2005.05.008 [DOI] [PubMed] [Google Scholar]
- [40].Mandal A, Klotz KL, Shetty J, Jayes FL, Wolkowicz MJ, Bolling LC, Coonrod SA, Black MB, Diekman AB, Haystead TA, et al.. SLLP1, a unique, intra-acrosomal, non-bacteriolytic, c lysozyme-like protein of human spermatozoa. Biol Reprod. 2003;68:1525-37. doi: 10.1095/biolreprod.102.010108 [DOI] [PubMed] [Google Scholar]


